Pharmacokinetics of tacrolimus granules in pediatric de novo liver, kidney, and heart transplantation: The OPTION study
Funding information:
This study was funded by Astellas Pharma Global Development, Inc.
Abstract
Tacrolimus granules were developed for patients who are unable to swallow capsules. Therapeutic drug monitoring (TDM) is required to optimize efficacy and safety, which is based on Ctrough for tacrolimus capsules. Pharmacokinetic (PK) data for tacrolimus granules are required to establish the basis for TDM in those who are unable to swallow capsules. In this phase IV study (NCT01371331) of children undergoing liver, kidney, or heart transplantation, patients received tacrolimus granules 0.15 mg/kg twice daily; first dose was administered within 24 hours of reperfusion. PK analysis samples were collected after reperfusion, after first dose of tacrolimus (Day 1), and at steady state (Day 7; >4 days stable dose). Of the 52 transplant recipients enrolled, 38 had two evaluable PK profiles. Mean AUCtau after first dose of tacrolimus was 211, 97, and 224 hour*ng/mL in liver, kidney, and heart transplant recipients, respectively; corresponding mean AUCtau at steady state was 195, 208, and 165 hour*ng/mL. Ctrough and AUCtau were positively correlated after first dose of tacrolimus and at steady state (Pearson's coefficients: r = 0.81 and r = 0.87, respectively). This study demonstrated that Ctrough is a reliable marker for TDM in pediatric transplant recipients treated with tacrolimus granules, consistent with TDM for other tacrolimus formulations.
Abbreviations
-
- AUC
-
- area under the blood concentration–time curve
-
- AUCtau
-
- area under the blood concentration–time curve for a dosing interval
-
- Cmax
-
- maximum blood concentration
-
- C trough
-
- trough blood concentration
-
- HPLC/MS/MS
-
- high-performance liquid chromatography tandem mass spectrometry
-
- PK
-
- pharmacokinetic
-
- PKAS
-
- pharmacokinetic analysis set
-
- SAF
-
- safety analysis set
-
- SD
-
- standard deviation
-
- TDM
-
- therapeutic drug monitoring
-
- TEAE
-
- treatment-emergent adverse event
-
- t last
-
- time of last non-zero concentration
-
- t max
-
- time to maximum blood concentration
1 INTRODUCTION
Transplantation, now a routine procedure for end-stage liver, kidney, or heart disease, is associated with excellent long-term survival rates (>80%) in pediatric populations.1 Approximately 25% of patients undergoing liver or heart transplantation are aged ≤1 year, with 39% of pediatric liver and 22% of pediatric heart transplantations occurring in children aged 1-5 years.1, 2
Successful transplantation is dependent on an effective immunosuppression regimen and is often based on tacrolimus.2, 3 Although extemporaneously prepared suspensions of the tacrolimus capsule contents have been used in children, they have inherent limitations in terms of batch-to-batch variability and short stability.3-5
Tacrolimus granules (Modigraf®) were developed to address an unmet medical need, specifically for patients unable to swallow intact capsules. The mean oral bioavailability of tacrolimus granules has been shown to be 26% ± 23% (range 4%–80%).6 Tacrolimus in granule formulation was approved in Japan in 1998 and in Europe in 2009, and is available as 0.2 and 1 mg sachets that can be suspended in water and administered orally.6
Clinical studies have established that achieving a sufficient systemic exposure to tacrolimus (AUC) is an important variable associated with efficacy. However, monitoring tacrolimus AUC to optimize the dose is not practical in routine clinical care, thus Ctrough is used as a surrogate marker of AUC for TDM.7, 8 The relationship between Ctrough and AUC has been established for capsule formulations of tacrolimus in adult and pediatric populations;8-12 corresponding data for tacrolimus granules are limited. Here, we report PK data for tacrolimus granules in children undergoing de novo liver, kidney, or heart transplantation.
2 MATERIALS AND METHODS
2.1 Study design
This was a multicenter, single-arm, phase IV PK study of tacrolimus granules in children undergoing de novo liver, kidney, or heart transplantation. The study was conducted across 10 sites in six countries: United Kingdom, Spain, Germany, Belgium, Poland, and France (NCT01371331, https://clinicaltrials.gov). The Independent Ethics Committee/Institutional Review Board reviewed the ethical, scientific, and medical appropriateness of the study, and approval for the study protocol was obtained from the relevant competent authorities at each study site, prior to study initiation. For all study subjects, the patient's parent(s) or their legal representative(s) had been fully informed and had given written informed consent to participate in the study. The patient had given assent where applicable. All procedures performed during this study were in accordance with the 1975 Declaration of Helsinki and its later amendments or comparable ethical standards.
2.2 Patients
Eligible patients were ≤12 years of age and first-time recipients of a liver, kidney, or heart transplant. Patients were permitted to have received a multiorgan transplant. Exclusion criteria included: high immunologic risk (panel reactive antibody score >50% in the previous 6 months for kidney transplant recipients); significant renal impairment (serum creatinine ≥230 μmol/L [≥2.6 mg/dL] pretransplantation in liver and heart transplant recipients); or liver disease (elevated alanine aminotransferase, and/or aspartate aminotransferase, and/or total bilirubin levels three times the upper limit of normal during the 28 days prior to transplantation) in kidney and heart transplant recipients.
2.3 Intervention
Transplant recipients were treated with tacrolimus granules (Astellas Pharma, Tokyo, Japan) at an initial daily dose of 0.3 mg/kg per day, given as two doses (0.15 mg/kg twice daily) postoperatively. When the total daily dose was not divisible by 0.2 (the smallest sachet size), the dose was rounded up by 0.1 mg. In cases where it was not possible to divide the daily dose exactly, the higher dose was given in the morning. The first dose of tacrolimus 0.15 mg/kg was administered in the morning within 24 hours of reperfusion (this period could be extended up to 5 days for heart transplant recipients, if required). Subsequent oral tacrolimus doses were adjusted based on clinical evidence of efficacy and the occurrence of TEAEs, aided by TDM, to maintain Ctrough in the recommended range of 5-20 ng/mL using site-specific assays.
2.4 Preparation of tacrolimus granules suspension for dosing
Centers were advised to prepare the tacrolimus granules suspension by adding the contents of the tacrolimus granule sachets to a measured volume of water (maximum of 50 mL) in a glass or cup; materials containing polyvinyl chloride were avoided for all dosing procedures. The mixture was then stirred gently until the granules were completely suspended. The suspension was drawn up with a syringe and administered orally; alternatively, the contents of the glass or cup were swallowed directly by the transplant recipient. The glass or cup was then rinsed and filled with an equal amount of water to the suspension to be consumed by the transplant recipient. Other methods of delivery, such as through a gastrostomy tube, were not permitted.
2.5 Concomitant medications
Monoclonal antibodies, polyclonal antibodies (thymoglobulin), mycophenolate mofetil (CellCept®), and corticosteroids were permitted as concomitant immunosuppressive medications. Concomitant medications with known drug interactions with tacrolimus, which may increase or decrease tacrolimus exposure, were prohibited throughout the study and 7 days prior to the first dose of tacrolimus. The following concomitant medications were prohibited within 24 hours prior to the first administration of tacrolimus granules and throughout the study: omeprazole, esomeprazole, and lansoprazole (pantoprazole or ranitidine were permitted as alternatives). The following concomitant medications were prohibited within 7 days prior to the first administration of tacrolimus granules and throughout the study: ketoconazole, fluconazole, itraconazole, clotrimazole, voriconazole, erythromycin, clarithromycin, josamycin, telithromycin, danazol, ethinyl estradiol, calcium antagonists (except nifedipine), nefazodone, phenobarbital, phenytoin, rifampicin, rifabutin, antacids (eg, aluminium hydroxide, oral sodium bicarbonate, or sucralfate), St John's wort, rapamycin, everolimus, and mycophenolic acid.
2.6 Pharmacokinetic evaluation
Whole blood samples (0.5 mL) were collected in tubes containing ethylenediaminetetraacetic acid as an anticoagulant, to provide two blood concentration–time profiles for PK evaluations. Blood samples for PK analyses were taken after the first dose of tacrolimus (Day 1), and at steady state (Day 7; after a minimum of 4 days without a dose change), 0 hours (predose), and 0.5, 1, 2, 4, 8, and 12 hours (postdose). A 10% deviation from nominal time points was permitted for blood samples taken up to 4 hours after administration with tacrolimus granules. A 30-minute deviation was permitted for blood samples taken at the 8- and 12-hour time points. Samples were frozen and transported to a central laboratory for bioanalysis.
Blood samples were thawed, and 0.2 mL aliquots were taken for analysis. The assay was based on the method developed by Alak et al.13 Aliquots were extracted using protein precipitation and solid-phase extraction using C18 200 mg/3 mL cartridges. Elutes were evaporated to dryness under a stream of nitrogen at 40°C, and residues were re-dissolved in a 50:50 mix (vol/vol) of acetonitrile and water, mixed and centrifuged, before being submitted for HPLC/MS/MS. An analog of tacrolimus (ascomycin) was used as an internal standard. The lower limit of quantification was 0.5 ng/mL. All procedures were performed in compliance with the Principles of Good Laboratory Practice.
Tacrolimus concentrations for TDM were determined using a site-specific HPLC/MS/MS assay or immunoassay at local laboratories.
2.7 Outcomes
The primary objective of this study was to determine the PK of tacrolimus granules after the initial dose and at steady state in children undergoing de novo liver, kidney, or heart transplantation. The PK parameters evaluated were AUCtau, Cmax, tmax, and Ctrough. All PK parameters were calculated using noncompartmental methods from concentration–time profiles of tacrolimus in whole blood. Using the WinNonlin software (version 5.0 or later), AUC was estimated using the linear trapezoidal rule with linear interpolation. Cmax, tmax, and Ctrough were obtained directly from concentration–time profiles.
Secondary objective measures included rejection episodes (according to the histological grading of liver biopsies for rejection, the Banff 97 diagnostic categories for renal allograft biopsies, or the standardized nomenclature of the International Society of Heart and Lung Transplantation), transplant recipient and graft survival, and TEAEs over a 2-week time frame.
A post hoc analysis was conducted to examine the relationship between age and AUCtau after the first dose of tacrolimus 0.15 mg/kg for all transplant recipients.
2.8 Statistical analyses
No power calculations were performed; however, based on previous experience, and assuming a drop-out rate of 40%, approximately 60 transplant recipients were required to achieve 36 evaluable transplant recipients (12 [±2] from each transplant group), each with two evaluable PK profiles.
The SAF consisted of all transplant recipients who took at least one dose of study medication. The PKAS included all transplant recipients who provided two evaluable PK profiles (after the first dose of tacrolimus and at steady state).
For continuous variables, descriptive statistics included the number of transplant recipients, mean, SD, median, minimum, and maximum. In addition, for the PK parameters AUCtau and Cmax (and any other PK parameter determined, except tmax), the coefficient of variation and geometric mean were calculated. If Cmax occurred at more than one time point, the first time it occurred was considered for tmax. Frequencies and percentages were displayed for categorical data.
Data were analyzed by type of organ transplant and overall. All data processing, summarization, and analyses were performed using SAS® version 9.1.3 or higher on UNIX.
3 RESULTS
3.1 Patients
Trial enrollment commenced on June 9, 2011; 53 patients were screened, of whom 52 received transplants and treatment with tacrolimus granules (Figure 1). The study was completed by 46 transplant recipients (88.5%), and the last evaluation occurred on February 3, 2015. Six transplant recipients discontinued the study (three liver and three kidney transplant recipients). Of those undergoing liver transplantation, one discontinued owing to taking prohibited concomitant medication, one owing to intolerable TEAEs (vomiting after administration and toxicity to tacrolimus), and one owing to medication therapy. Of those undergoing kidney transplantation, two discontinued owing to withdrawal of consent, and one discontinued because they did not have an evaluable PK profile after the first dose of tacrolimus.
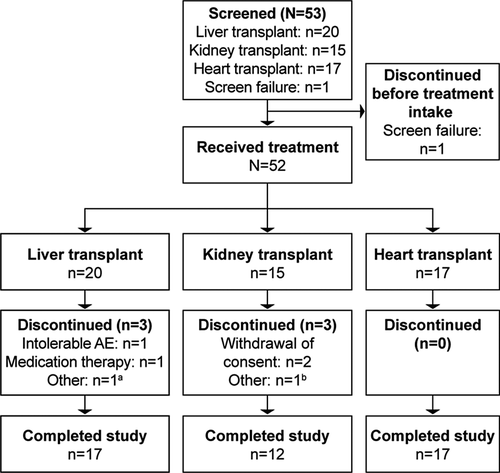
Baseline characteristics were generally consistent across each transplant group (Table 1). There were more male transplant recipients (35 [67.3%]) than female (17 [32.7%]), and 49 transplant recipients were white (94.2%; there was one Asian kidney transplant recipient and two liver transplant recipients whose ethnicities were recorded as “other”). A total of 36 transplant recipients (69.2%) were aged <5 years, and 17 transplant recipients (32.7%) were aged <2 years. The mean age distribution and weight of transplant recipients was lower in the liver transplant group than in the kidney and heart transplant groups (Table 1). All kidney transplant recipients were aged between 2 and 11 years, and no patients were multiorgan transplant recipients.
| Parameter | Transplantation group | |||
|---|---|---|---|---|
| Liver (n = 20) | Kidney (n = 15) | Heart (n = 17) | Total (N = 52) | |
| Recipient sex, n (%) | ||||
| Male | 10 (50.0) | 12 (80.0) | 13 (76.5) | 35 (67.3) |
| Female | 10 (50.0) | 3 (20.0) | 4 (23.5) | 17 (32.7) |
| Recipient race, n (%) | ||||
| White | 18 (90.0) | 14 (93.3) | 17 (100) | 49 (94.2) |
| Black or African American | 0 | 0 | 0 | 0 |
| Asian | 0 | 1 (6.7) | 0 | 1 (1.9) |
| Other | 2 (10.0) | 0 | 0 | 2 (3.8) |
| Recipient age (y), mean (SD) | 2.6 (3.2) | 5.4 (3.0) | 5.2 (4.2) | 4.3 (3.7) |
| Recipient age subgroup, n (%) | ||||
| <5 y | 17 (85.0) | 10 (66.7) | 9 (52.9) | 36 (69.2) |
| ≥5 y | 3 (15.0) | 5 (33.3) | 8 (47.1) | 16 (30.8) |
| ≥0 d – ≤27 d (newborn) | 0 | 0 | 0 | 0 |
| ≥28 d – ≤23 mo (infants and toddlers) | 12 (60.0) | 0 | 5 (29.4) | 17 (32.7) |
| ≥2 y – ≤11 y (children) | 8 (40.0) | 15 (100) | 11 (64.7) | 34 (65.4) |
| ≥12 y – ≤17 y (adolescents) | 0 | 0 | 1 (5.9) | 1 (1.9) |
| Recipient weight (kg), mean (SD) | 12.4 (8.4) | 16.7 (5.3) | 17.2 (9.0)a | 15.2 (8.0)b |
- a n = 16.
- b n = 51.
3.2 PK profile and exposure
Overall, 38 of 52 transplant recipients had two evaluable PK profiles and were included in the PKAS (14 [70.0%] liver transplant recipients, 12 [80.0%] kidney transplant recipients, and 12 [70.6%] heart transplant recipients). Within the PKAS, 66% of transplant recipients were aged <5 years.
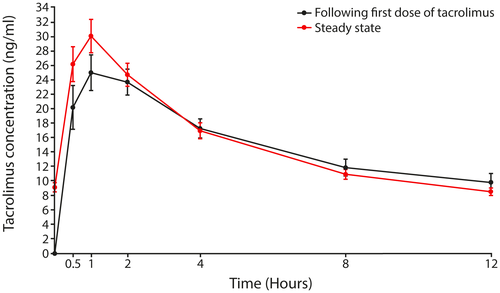
Mean (±SD) whole blood concentration–time profiles after the first dose of tacrolimus and at steady state are shown in Figure 2. Cmax was observed approximately 1 hour after drug dosing for all transplant groups after the first dose of tacrolimus and at steady state. In the PKAS, the mean initial dose of tacrolimus was 0.15 mg/kg for all transplant recipients; corresponding values at steady state were 0.18 mg/kg for liver, 0.16 mg/kg for kidney, and 0.14 mg/kg for heart transplant recipients.
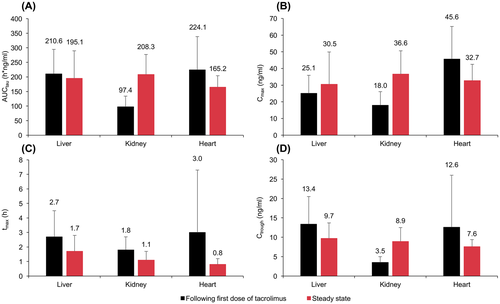
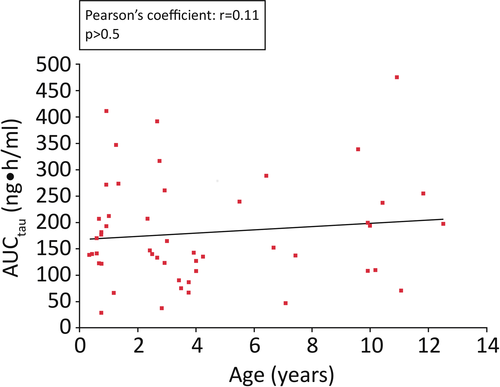
After the first dose of tacrolimus, mean AUCtau was approximately twofold higher in children who received a liver or heart transplant (211 and 224 hour*ng/mL, respectively) compared with those who received a kidney transplant (97 hour*ng/mL) (Figure 3). Overall, mean AUCtau was 163 hour*ng/mL in children aged <5 years and 209 hour*ng/mL in those aged ≥5 years (Table 2). At steady state, mean AUCtau was 195 hour*ng/mL in children who received a liver transplant, 208 hour*ng/mL in children who received a kidney transplant, and 165 hour*ng/mL in children who received a heart transplant (Figure 3). Mean AUCtau at steady state was 185 hour*ng/mL in children aged <5 years and 199 hour*ng/mL in those aged ≥5 years (Table 3). A post hoc analysis found a low correlation (Pearson's coefficient: r = 0.11; P > 0.5) between age (range: 0.3-12.5 years) and AUCtau at a fixed dose of tacrolimus 0.15 mg/kg (Figure 4).
| AUCtau (h*ng/mL) | Cmax (ng/mL) | tmax (h) | Ctrough (ng/mL) | |
|---|---|---|---|---|
| Liver transplant | ||||
| <5 y (n = 12) | 200 (86.6) | 25.0 (11.7) | 2.2 (0.9) | 11.5 (5.7) |
| ≥5 y (n = 2) | 272 (23.9) | 26.0 (2.0) | 6.1 (2.9) | 24.8 (0.4) |
| Kidney transplant | ||||
| <5 y (n = 8) | 104 (39.6) | 19.4 (7.7) | 1.5 (0.6) | 3.6 (1.6) |
| ≥5 y (n = 4) | 83.9 (30.5) | 15.2 (9.3) | 2.3 (1.3) | 3.4 (1.4) |
| Heart transplant | ||||
| <5 y (n = 5) | 170 (106.7) | 36.5 (21.5) | 1.3 (1.5) | 6.5 (4.0) |
| ≥5 y (n = 7) | 263 (110.1) | 52.1 (16.6) | 4.1 (5.4) | 17.0 (16.3) |
| Overall | ||||
| <5 y (n = 25) | 163 (87.5) | 25.5 (14.0) | 1.8 (1.0) | 8.0 (5.6) |
| ≥5 y (n = 13) | 209 (118.0) | 36.8 (21.7) | 3.9 (4.1) | 14.0 (13.9) |
- All values are mean (SD).
- For one transplant recipient, AUC was extrapolated to 12 h as tlast = 11.38 h for the Day 1 PK profile. For another transplant recipient, AUC was interpolated to 12 h as tlast = 12.5 h for the Day 1 PK profile.
| AUCtau (h*ng/L) | Cmax (ng/L) | tmax (h) | Ctrough (ng/L) | |
|---|---|---|---|---|
| Liver transplant | ||||
| <5 y (n = 12) | 197 (102.7) | 32.6 (20.2) | 1.7 (1.2) | 9.1 (4.1) |
| ≥5 y (n = 2) | 185 (9.4) | 18.1 (0.9) | 2.0 (0) | 13.3 (1.5) |
| Kidney transplant | ||||
| <5 y (n = 8) | 187 (56.9) | 34.3 (14.8) | 0.9 (0.5) | 7.7 (2.3) |
| ≥5 y (n = 4) | 251 (78.8) | 41.4 (12.7) | 1.4 (0.8) | 11.4 (4.8) |
| Heart transplant | ||||
| <5 y (n = 5) | 152 (29.5) | 33.2 (11.3) | 0.9 (0.7) | 5.9 (0.8) |
| ≥5 y (n = 7) | 175 (44.5) | 32.3 (9.5) | 0.8 (0.3) | 8.8 (1.3) |
| All ages | ||||
| <5 y (n = 25) | 185 (78.9) | 33.3 (16.5) | 1.3 (1.0) | 8.0 (3.3) |
| ≥5 y (n = 13) | 199 (61.8) | 32.9 (12.1) | 1.2 (0.6) | 10.3 (3.1) |
- All values are mean (SD).
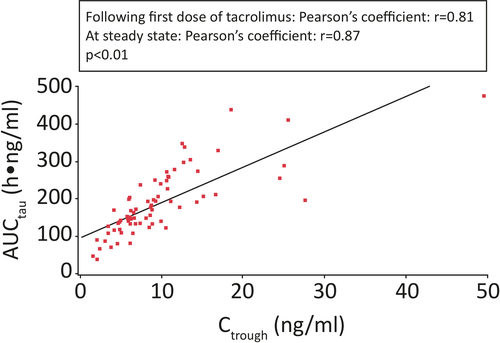
Overall, there was a strong correlation between the Ctrough of tacrolimus in whole blood and AUC after the first dose of tacrolimus and at steady state (Pearson's coefficient: r = 0.81 and r = 0.87, respectively; P < 0.01; Figure 5).
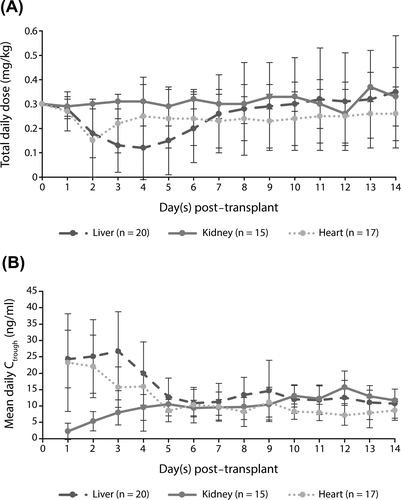
The mean (SD) Ctrough level of tacrolimus over the 2-week study period was 11.7 (4.2) ng/mL (14.9 [4.5] ng/mL in liver, 9.6 [2.1] ng/mL in kidney, and 9.8 [2.9] ng/mL in heart transplant recipients). In the SAF, the mean (SD) Ctrough level of tacrolimus was similar in both age subgroups (11.6 [3.9] ng/mL in transplant recipients aged <5 years and 11.9 [5.1] ng/mL in transplant recipients aged ≥5 years). The mean daily dose of tacrolimus was reduced from Day 2 in liver and heart transplant recipients to maintain required Ctrough levels (Figure 6).
Secondary end-points are summarized in Table 4. Overall, five transplant recipients experienced acute rejection episodes, and no graft loss or deaths occurred. No unexpected clinically relevant differences in secondary end-points between the age categories were identified. Across all transplant groups, 34 transplant recipients (65.4%) reported at least one drug-related TEAE. The most commonly reported drug-related TEAE was hypertension (10 transplant recipients [19.2%]), followed by toxicity to various agents (six transplant recipients [11.5%]). The most commonly reported serious TEAEs were increased blood creatinine (three liver transplant recipients [5.8%]), and toxicity to various agents (three kidney transplant recipients [5.8%]) due to high exposure to tacrolimus after the starting dose. One liver transplant recipient reported two TEAEs (vomiting after administration and toxicity to tacrolimus) resulting in treatment discontinuation. The incidence of TEAEs was similar in transplant recipients aged <5 years compared with those aged ≥5 years (32 [88.9%] and 14 [87.5%], respectively).
| Liver (n = 20) | Kidney (n = 15) | Heart (n = 17) | |
|---|---|---|---|
| Acute rejection | 3 (15.0) | 0 | 2 (11.8) |
| Spontaneously resolving acute rejectionsa | 1 (5.0) | 0 | 1 (5.9) |
| Corticosteroid-sensitive acute rejectionsb | 2 (10.0) | 0 | 1 (5.9) |
| Incidence of any TEAE | 20 (100) | 12 (80.0) | 14 (82.4) |
| Drug-related TEAEsc | 18 (90.0) | 6 (40.0) | 10 (58.8) |
| Drug-related TEAEs occurring in ≥2 transplant recipientsc | |||
| Hypertension | 9 (45.0) | 0 | 1 (5.9) |
| Toxicity to various agents | 5 (25.0) | 1 (6.7) | 0 |
| Metabolic acidosis | 4 (20.0) | 0 | 0 |
| Renal impairment | 2 (10.0) | 0 | 2 (11.8) |
| Blood creatinine increased | 0 | 1 (5.9) | 2 (13.3) |
| Diarrhea | 0 | 2 (13.3) | 1 (5.9) |
| Hyperglycemia | 3 (15.0) | 0 | 0 |
| Tremor | 2 (10.0) | 1 (6.7) | 0 |
| Hypomagnesemia | 1 (5.0) | 0 | 1 (5.9) |
| Renal failure | 2 (10.0) | 0 | 0 |
| Renal injury | 0 | 0 | 2 (11.8) |
| Vomiting | 1 (5.0) | 1 (6.7) | 0 |
| Serious TEAEsd | 9 (45.0) | 4 (26.7) | 4 (23.5) |
| Serious TEAEs occurring in ≥2 transplant recipientsd | |||
| Blood creatinine increased | 0 | 3 (20.0) | 0 |
| Toxicity to various agents | 3 (15.0) | 0 | 0 |
| Drug-related serious TEAEs | 5 (25.0) | 2 (13.3) | 1 (5.9) |
| Blood creatinine increased | 0 | 1 (6.7) | 0 |
| Pneumonia | 0 | 1 (6.7) | 0 |
| Renal failure | 1 (5.0) | 0 | 0 |
| Renal impairment | 0 | 0 | 1 (5.9) |
| Sepsis | 1 (5.0) | 0 | 0 |
| Toxicity to various agents | 3 (15.0) | 0 | 0 |
| Death | 0 | 0 | 0 |
- All values are n (%).
- a A spontaneously resolving rejection is defined as a rejection episode not treated with any new or increased corticosteroid medication, antibody, or any other medication, which resolved irrespective of any tacrolimus dose changes.
- b A corticosteroid-sensitive acute rejection is defined as a rejection episode treated with new or increased corticosteroid medication only, which has resolved irrespective of any tacrolimus dose changes.
- c Possible or probable, as assessed by the investigator, or records where relationship is missing.
- d Includes serious TEAEs upgraded by the sponsor based on review of the sponsor's list of always serious terms, if any upgrade was done.
4 DISCUSSION
Tacrolimus granules were developed to address an unmet clinical need for dosing tacrolimus in transplant recipients who are unable to swallow capsules. This study sought to define the PK profile of tacrolimus granules when administered as an oral suspension, after the first dose and at steady state, in pediatric liver, kidney, and heart transplant recipients. The observed PK parameters were consistent with those observed for the immediate-release oral capsule formulation.14
In adult and pediatric transplant recipients, tacrolimus Ctrough values are considered an appropriate surrogate marker of systemic exposure (AUCtau), a significant variable of acute rejection.14-16 Our study has shown a strong correlation between Ctrough and AUCtau (after the first dose of tacrolimus and at steady state; Pearson's coefficients: r = 0.81 and r = 0.87, respectively). Findings from this study suggest that Ctrough is also a reliable measure of systemic exposure to tacrolimus granules in pediatric transplant recipients. A similar relationship between tacrolimus Ctrough values and AUC has been observed with the oral capsule formulation of tacrolimus (Prograf®) in pediatric renal (r = 0.90)10 and liver (r = 0.90)9 transplant recipients. In adults, Pearson's coefficients for Ctrough–AUC correlation have been reported to be r = 0.80-0.97,11 r = 0.89-0.93,12 and r = 0.938 in liver, kidney, and heart transplant recipients, respectively. In this study of tacrolimus granules in pediatric transplant recipients, the mean AUCtau after the first dose of tacrolimus was approximately twofold higher in liver and heart transplant recipients than in kidney transplant recipients, suggesting a higher clearance or lower oral bioavailability of tacrolimus in children with kidney transplants.14 Observations from studies in adult liver, kidney, and heart transplant recipients have reported similar differences in systemic exposure among these recipients. Furthermore, studies in adult transplant recipients receiving intravenous tacrolimus showed a higher clearance in kidney transplant recipients compared with liver transplant recipients.17, 18
The Cmax of tacrolimus was observed approximately 1 hour after dosing across all transplant types, indicating that tacrolimus granules were rapidly absorbed. This is similar to the tmax of oral tacrolimus (~2 hours) observed in other studies in pediatric liver and kidney transplant recipients.9, 10
The safety and efficacy of tacrolimus granules was established in a 12-month, open-label, parallel-group, randomized phase III study of children who had undergone liver transplantation.19 Treatment with tacrolimus granules, combined with corticosteroids, showed superiority over treatment with a ciclosporin A microemulsion combined with corticosteroids and azathioprine (acute rejection-free rate at Month 12 in the tacrolimus granules vs ciclosporin A groups was 55.5% vs 40.2%, respectively; P = 0.03) with a similar incidence of TEAEs.19 In our study, the efficacy and TEAE profile of tacrolimus granules were similar to other tacrolimus studies over the first 2 weeks of exposure. However, owing to the short duration of observation, it is difficult to interpret the safety of tacrolimus granules in this study. A long-term study is underway to evaluate the safety and efficacy of tacrolimus granules in pediatric solid allograft recipients.
5 CONCLUSIONS
In this study, the PK profile of tacrolimus granules in de novo pediatric transplant recipients has demonstrated a strong correlation between Ctrough and AUCtau. In pediatric transplantation, Ctrough may be used as a reliable marker for monitoring tacrolimus exposure within the first 2 weeks post-transplantation.
ACKNOWLEDGMENTS
Medical writing support in the development of this manuscript was provided by Helen Farrington and Matthew Reynolds from SuccinctChoice Medical Communications, London, UK. This study was funded by Astellas Pharma Global Development, Inc., and was supported by the NIHR Manchester Clinical Research Facility. The authors would like to acknowledge the contributions to the conception of the study design, acquisition, analyses and evaluation of data, and clinical conduct of this study from other investigators of the OPTION study group; Dr. Luis Garcia-Guereta1, Prof. Burkhard Tönshoff2, Dr. Raymond Reding3, Dr. Jacek Rubik4, Prof. Sylvie Di-Filippo5, and Prof. Georges Deschenes6.
1Hospital Universitario La Paz, Madrid, Spain; 2Universitätsklinikum Heidelberg, Heidelberg, Germany; 3Cliniques Universitaires Saint-Luc UCL, Brussels, Belgium; 4Children's Memorial Health Institute, Warsaw, Poland; 5Groupement Hospitalier EST, Bron, France; 6Hopital Robert Debré, Paris, France.
CONFLICT OF INTEREST
NW reports receiving personal fees from AbbVie, Alexion, Astellas Pharma Global Development, Inc., Quintiles, and Raptor. UB reports receiving grants and personal fees from Astellas Pharma Global Development, Inc., and Alexion Pharmaceuticals, Inc., and reports receiving personal fees from Novartis. MC and EF declare no conflict of interest. NU reports employment with Astellas Pharma EMEA.
AUTHORS’ CONTRIBUTIONS
Nicholas J. A. Webb, Ulrich Baumann, Manuela Camino, Esteban Frauca, and Nasrullah Undre conceived or designed the study, acquired the data, drafted the manuscript, critically reviewed the manuscript for intellectual content, provided final approval of the manuscript, and agreed to be accountable for all aspects of the work.




