Bortezomib use in a pediatric cardiac transplant center
Corresponding Author
Matthew D. Zinn
Children's Hospital of Michigan, Detroit, MI, USA
Matthew D. Zinn, Division of Pediatric Cardiology, Children's Hospital of Michigan, 3901 Beaubien Blvd, Detroit, MI 48201, USA
Tel.: 570 574 9923
Fax: 313 993 0894
E-mail: [email protected]
Search for more papers by this authorThomas J. L'Ecuyer
University of Virginia Children's Hospital, Charlottesville, VA, USA
Search for more papers by this authorOmar R. Fagoaga
Children's Hospital of Michigan, Detroit, MI, USA
Search for more papers by this authorSanjeev Aggarwal
Children's Hospital of Michigan, Detroit, MI, USA
Search for more papers by this authorCorresponding Author
Matthew D. Zinn
Children's Hospital of Michigan, Detroit, MI, USA
Matthew D. Zinn, Division of Pediatric Cardiology, Children's Hospital of Michigan, 3901 Beaubien Blvd, Detroit, MI 48201, USA
Tel.: 570 574 9923
Fax: 313 993 0894
E-mail: [email protected]
Search for more papers by this authorThomas J. L'Ecuyer
University of Virginia Children's Hospital, Charlottesville, VA, USA
Search for more papers by this authorOmar R. Fagoaga
Children's Hospital of Michigan, Detroit, MI, USA
Search for more papers by this authorSanjeev Aggarwal
Children's Hospital of Michigan, Detroit, MI, USA
Search for more papers by this authorAbstract
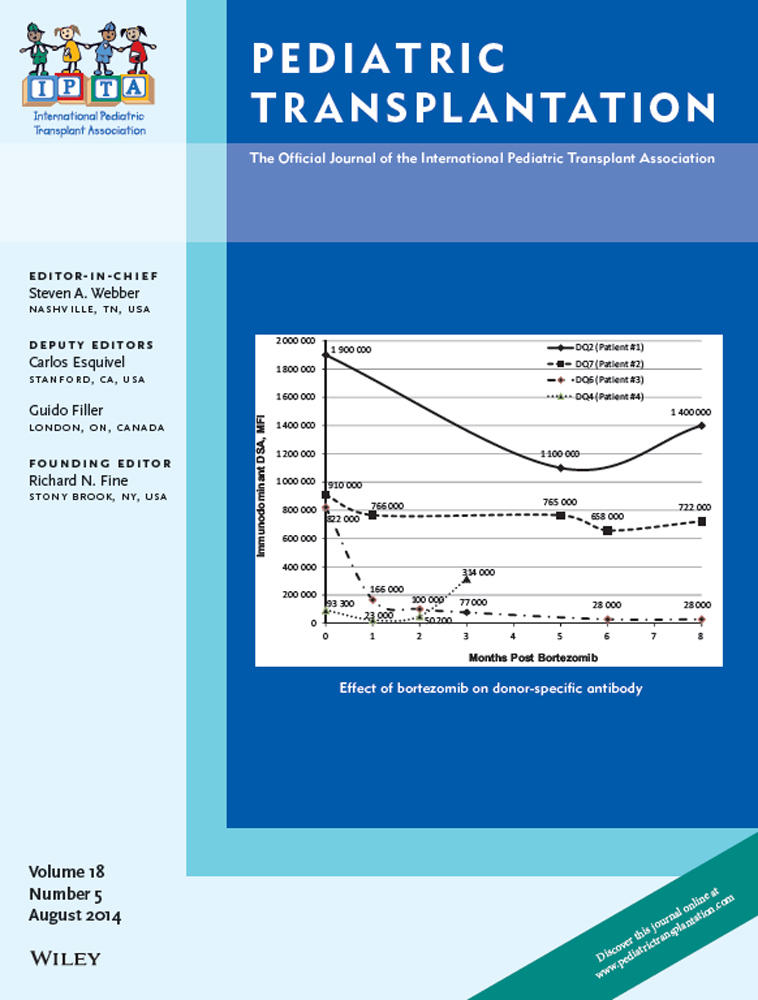
Data are limited on the efficacy and safety of bortezomib for the treatment of AMR following OHT for pediatric acquired or CHD. Retrospective chart review identified patients who received bortezomib for acute (n = 3, within two wk of diagnosis) and chronic (n = 1, three months after diagnosis) AMR or as part of a desensitization regimen (n = 1). Bortezomib was associated with a 3–66% reduction in class I DSA and a 7–82% reduction in class II DSA. Two of the three acute AMR cases resolved by the first follow-up biopsy. Two patients with AMR resolution are currently well. One patient developed a second episode of AMR, which was unresponsive to bortezomib therapy and required retransplantation for progressive coronary allograft vasculopathy. One patient died shortly after the third cycle from multi-organ failure. The desensitization patient showed transient HLA reduction with two cycles, but died five months after transplant from sepsis. Complications included infection (3/5), peripheral neuropathy (2/5), AKI (2/5), and thrombocytopenia (3/5). Adverse events appear more common in critically ill patients. Bortezomib therapy resulted in variable DSA reduction and AMR resolution in AMR in OHT secondary to pediatric acquired or CHD.
References
- 1Dipchand AI, Kirk R, Edwards LB, et al.; International Society for Heart and Lung Transplantation. The Registry of the International Society for Heart and Lung Transplantation: Sixteenth official pediatric heart transplantation report–2013; focus theme: Age. J Heart Lung Transplant 2013: 32: 979–988.
- 2Gossett JG, Canter CE, Zheng J, et al.; Pediatric Heart Transplant Study Investigators. Decline in rejection in the first year after pediatric cardiac transplantation: A multi-institutional study. J Heart Lung Transplant 2010: 29: 625–632.
- 3Feingold B, Bowman P, Zeevi A, et al. Survival in allosensitized children after listing for cardiac transplantation. J Heart Lung Transplant 2007: 26: 565–571.
- 4Holt DB, Lublin DM, Phelan DL, et al. Mortality and morbidity in pre-sensitized pediatric heart transplant recipients with a positive donor crossmatch utilizing peri-operative plasmapheresis and cytolytic therapy. J Heart Lung Transplant 2007: 26: 876–882.
- 5Michaels PJ, Espejo ML, Kobashigawa J, et al. Humoral rejection in cardiac transplantation: Risk factors, hemodynamic consequences and relationship to transplant coronary artery disease. J Heart Lung Transplant 2003: 22: 58–69.
- 6Velez M, Johnson MR. Management of allosensitized cardiac transplant candidates. Transplant Rev (Orlando) 2009: 23: 235–247.
- 7Wright EJ, Fiser WP, Edens RE, et al. Cardiac transplant outcomes in pediatric patients with pre-formed anti-human leukocyte antigen antibodies and/or positive retrospective crossmatch. J Heart Lung Transplant 2007: 26: 1163–1169.
- 8Xydas S, Yang JK, Burke EM, et al. Utility of post-transplant anti-HLA antibody measurements in pediatric cardiac transplant recipients. J Heart Lung Transplant 2005: 24: 1289–1296.
- 9Jacobs JP, Quintessenza JA, Boucek RJ, et al. Pediatric cardiac transplantation in children with high panel reactive antibody. Ann Thorac Surg 2004: 78: 1703–1709.
- 10Nwakanma LU, Williams JA, Weiss ES, Russell SD, Baumgartner WA, Conte JV. Influence of pretransplant panel-reactive antibody on outcomes in 8,160 heart transplant recipients in recent era. Ann Thorac Surg 2007: 84: 1556–1562; discussion 1562–3.
- 11Casarez TW, Perens G, Williams RJ, et al. Humoral rejection in pediatric orthotopic heart transplantation. J Heart Lung Transplant 2007: 26: 114–119.
- 12Scott V, Williams RJ, Levi DS. Outcomes of cardiac transplantation in highly sensitized pediatric patients. Pediatr Cardiol 2011: 32: 615–620.
- 13Hooper DK, Hawkins JA, Fuller TC, Profaizer T, Shaddy RE. Panel-reactive antibodies late after allograft implantation in children. Ann Thorac Surg 2005: 79: 641–644.
- 14Reed EF, Demetris AJ, Hammond E, et al.; International Society for Heart and Lung Transplantation. Acute antibody-mediated rejection of cardiac transplants. J Heart Lung Transplant 2006: 25: 153–159.
- 15Chin C. Cardiac antibody-mediated rejection. Pediatr Transplant 2012: 16: 404–412.
- 16Lemy A, Toungouz M, Abramowicz D. Bortezomib: A new player in pre- and post-transplant desensitization? Nephrol Dial Transplant 2010: 25: 3480–3489.
- 17Perry DK, Burns JM, Pollinger HS, et al. Proteasome inhibition causes apoptosis of normal human plasma cells preventing alloantibody production. Am J Transplant 2009: 9: 201–209.
- 18Everly MJ, Everly JJ, Susskind B, et al. Bortezomib provides effective therapy for antibody- and cell-mediated acute rejection. Transplantation 2008: 86: 1754–1761.
- 19Walsh RC, Everly JJ, Brailey P, et al. Proteasome inhibitor-based primary therapy for antibody-mediated renal allograft rejection. Transplantation 2010: 89: 277–284.
- 20Everly MJ. A summary of bortezomib use in transplantation across 29 centers. Clin Transpl 2009: 323–337.
- 21Woodle ES, Alloway RR, Girnita A. Proteasome inhibitor treatment of antibody-mediated allograft rejection. Curr Opin Organ Transplant 2011: 16: 434–438.
- 22Sureshkumar KK, Hussain SM, Marcus RJ, et al. Proteasome inhibition with bortezomib: An effective therapy for severe antibody mediated rejection after renal transplantation. Clin Nephrol 2012: 77: 246–253.
- 23Flechner SM, Fatica R, Askar M, et al. The role of proteasome inhibition with bortezomib in the treatment of antibody-mediated rejection after kidney-only or kidney-combined organ transplantation. Transplantation 2010: 90: 1486–1492.
- 24Trivedi HL, Terasaki PI, Feroz A, et al. Abrogation of anti-HLA antibodies via proteasome inhibition. Transplantation 2009: 87: 1555–1561.
- 25Pavlakis M. Case presentations on two patients who received bortezomib for antibody mediated rejection at BIDMC. Clin Transpl 2009: 343–345.
- 26Sberro-Soussan R, Zuber J, Suberbielle-Boissel C, et al. Bortezomib as the sole post-renal transplantation desensitization agent does not decrease donor-specific anti-HLA antibodies. Am J Transplant 2010: 10: 681–686.
- 27Argyriou AA, Iconomou G, Kalofonos HP. Bortezomib-induced peripheral neuropathy in multiple myeloma: A comprehensive review of the literature. Blood 2008: 112: 1593–1599.
- 28Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant 2005: 24: 1710–1720.
- 29Woodle ES, Walsh RC, Alloway RR, Girnita A, Brailey P. Proteasome inhibitor therapy for antibody-mediated rejection. Pediatr Transplant 2011: 15: 548–556.
- 30Richardson PG, Briemberg H, Jagannath S, et al. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol 2006: 24: 3113–3120.
- 31Batal I, Zeevi A, Lunz JG IIIrd, et al. Antihuman leukocyte antigen–specific antibody strength determined by complement-dependent or solid-phase assays can predict positive donor-specific crossmatches. Arch Pathol Lab Med 2010: 134: 1534–1540.
- 32Chung BH, Choi BS, Oh EJ, et al. Clinical impact of the baseline donor-specific anti-human leukocyte antigen antibody measured by Luminex single antigen assay in living donor kidney transplant recipients after desensitization therapy. Transpl Int 2014: 27: 49–59.
- 33Reinsmoen NL, Lai CH, Vo A, et al. Acceptable donor-specific antibody levels allowing for successful deceased and living donor kidney transplantation after desensitization therapy. Transplantation 2008: 86: 820–825.
- 34Berry GJ, Burke MM, Andersen C, et al. The 2013 International Society for Heart and Lung Transplantation Working Formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. J Heart Lung Transplant 2013: 32: 1147–1162.
- 35Eckman PM, Thorsgard M, Maurer D, Kim Y, Alloway RR, Woodle ES. Bortezomib for refractory antibody-mediated cardiac allograft rejection. Clin Transpl 2009: 475–478.
- 36Morrow WR, Frazier EA, Mahle WT, et al. Rapid reduction in donor-specific anti-human leukocyte antigen antibodies and reversal of antibody-mediated rejection with bortezomib in pediatric heart transplant patients. Transplantation 2012: 93: 319–324.
- 37Patel J, Everly M, Chang D, Kittleson M, Reed E, Kobashigawa J. Reduction of alloantibodies via proteasome inhibition in cardiac transplantation. J Heart Lung Transplant 2011: 30: 1320–1326.
- 38Patel JK, Everly MJ, Kittleson M, Kobashigawa JA. Use of bortezomib as adjunctive therapy for densensitization in combined heart and kidney transplantation–a case report. Clin Transpl 2009: 347–349.
- 39Weston M, Rolfe M, Haddad T, Lopez-Cepero M. Desensitization protocol using bortezomib for highly sensitized patients awaiting heart or lung transplants. Clin Transpl 2009: 393–399.
- 40Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: A randomised, phase 3, non-inferiority study. Lancet Oncol 2011: 12: 431–440.