ISPAD Clinical Practice Consensus Guidelines 2022: Assessment and management of hypoglycemia in children and adolescents with diabetes
1 WHAT IS NEW OR DIFFERENT?
- Updated recommendations of maximum permissible time in hypoglycemia, as defined by continuous glucose monitoring (CGM) metrics as well as details for treatment of hypoglycemia.
- Added descriptions of newer easy-to-use formulations of glucagon approved for use, which have variable availability across different regions of the world.
- Updated details from studies of newer insulin analogues and technology (CGM and advances in hybrid closed-loop therapy) on reducing the time spent in hypoglycemia.
2 EXECUTIVE SUMMARY AND RECOMMENDATIONS
- Hypoglycemia and fear of hypoglycemia (FOH) are major physiological and psychological barriers to achieving optimal glycemia and may result in significant emotional morbidity for children with type 1 diabetes (T1D) and their caregivers. B
- Monitoring for hypoglycemia is a key component of diabetes care as is education about its causes, prevention, and treatment. Hypoglycemia is detected by self-monitoring of blood glucose (SMBG) or CGM. A
-
Hypoglycemic events include all episodes of a plasma glucose concentration low enough to cause symptoms and/or signs, including impaired brain functioning and expose the individual to potential harm. While there is no single numerical definition of hypoglycemia, clinical thresholds have been defined to aid assessment. E
- Glucose value <3.9 mmol/L (70 mg/dl) is used as the clinical alert or threshold value for initiating treatment for hypoglycemia because of the potential for glucose to fall further and avoid consequences of glucose levels below 3 mmol/L. Children with T1D should spend less than 4% of their time <3.9 mmol/L (70 mg/dl). E
- Glucose value <3.0 mmol/L (54 mg/dl) is defined as clinically important or serious hypoglycemia as neurogenic symptoms and cognitive dysfunction can occur below this level. Children with T1D should spend less than 1% of their time <3.0 mmol/L (54 mg/dl). E
- Severe hypoglycemia is defined as an event with severe cognitive impairment (including coma and convulsions) requiring assistance by another person to administer carbohydrates, glucagon, or intravenous dextrose. Hypoglycemic coma is a subgroup of severe hypoglycemia defined as an event associated with a seizure or loss of consciousness. E
- The incidence of hypoglycemic coma has fallen over the last two decades with a current rate of 3 to 7 per 100 patient-years across international registries from developed countries. Although lower glycated hemoglobin (HbA1c) was considered a risk factor for severe hypoglycemia, this association is no longer observed with contemporary intensive insulin therapy. B
- Symptoms of hypoglycemia in the young result from adrenergic activation (shakiness, pounding heart, sweatiness) and neuroglycopenia (headache, drowsiness, difficulty in concentrating). In young children, behavioral changes such as irritability, agitation, quietness, and tantrums may be prominent. B
- Symptoms of hypoglycemia and physiological hormone responses may occur at a higher glucose level in children compared to adults. Thresholds for activation of hormonal responses may be altered by chronic hyperglycemia (i.e., occur at a higher glucose level) or repeated hypoglycemia (i.e., occur at a lower glucose level). B
- Common precipitants for hypoglycemia include excessive insulin dose, missed meals, exercise, sleep, and in adolescents, alcohol ingestion. Risk factors include previous severe hypoglycemic events and reduced hypoglycemia awareness. B
- Hypoglycemia with exercise may occur at the time of activity or may be delayed. B Education focused on insulin adjustment with exercise should be provided to enable people with T1D to exercise safely and avoid hypoglycemia.
- Glucose levels are recommended to be monitored overnight particularly if there is an additional risk factor that may predispose to nocturnal hypoglycemia. E
- Impaired hypoglycemia awareness can occur in children with diabetes and when present, is associated with a significantly increased risk of severe hypoglycemia. The determination of hypoglycemia awareness should be a component of routine clinical review. Impaired awareness may be corrected by avoidance of hypoglycemia. B
- Hypoglycemia can be detected using SMBG or CGM. Newer factory-calibrated CGM devices are approved to make diabetes-related decisions. However, a blood glucose test is recommended if there is a suspected mismatch between clinical expectations and the sensor glucose level. Likewise, glucose should always be measured if the child is symptomatic or shows signs of hypoglycemia. B
- Hypoglycemia should be treated with oral glucose. An immediate source of glucose must always be available to young people with diabetes. Depending on circumstances, rapid-acting glucose should be followed by additional carbohydrates to prevent recurrence of hypoglycemia. B
- Treatment of hypoglycemia should increase blood glucose level by ~3 to 4 mmol/L (54 to 72 mg/dl). This can be accomplished by administering ~0.3 g/kg glucose orally, which equates to 9 g of glucose for a 30 kg child and 15 g for children >50 kg. C
- If on automated insulin delivery systems, the current approach of standard hypoglycemia management can cause rebound hyperglycemia, and hence consideration should be given to treat hypoglycemia with less glucose (e.g., 5 to 10 grams). E
- Following initial hypoglycemia treatment, blood glucose should be retested in 15 min. If there is no response or an inadequate response, repeat hypoglycemia treatment. Retest glucose in another 15 min to confirm that target glucose has been reached. E
- If on standard pump therapy (no suspend or automated insulin delivery) and glucose level <3 mmol/L, suspend insulin delivery until glucose >4 mmol/L. E
-
Severe hypoglycemia requires urgent treatment.
- In the ambulatory setting, SC or IM glucagon should be given (1 mg for children >25 kg and 0.5 mg for children <25 kg. Other preparations, more recently introduced and are easier to administer, including a single 3 mg dose of nasal glucagon for children >4 years, dasiglucagon, a stable glucagon analog, available as 0.6 mg ready-to-use pen SC for children ≥6 years and Gvoke (stable liquid glucagon) 0.5 or 1 mg autoinjector for children >2 years of age. A
- In a hospital setting, intravenous glucose (10% dextrose, 2 ml/kg) can be administered. B
- Glucagon should be readily accessible to all parents and caregivers. Education on the technique of administration of glucagon is essential. E.
- Hypoglycemia should be prevented, as it is often associated with significant psychosocial dysfunction. It can rarely lead to long-term sequelae and may be potentially life-threatening. A
- Diabetes education is critical in the prevention of hypoglycemia. A.
- Education about the risk factors for hypoglycemia should be given to children and their families to alert them as to times and situations when increased glucose monitoring is required and when treatment regimens need to be changed. E
- Particular attention should be given to training children, parents, school teachers, and other caregivers to recognize the early warning signs of hypoglycemia and treat low blood glucose immediately and appropriately. E
- Devices for blood glucose measurement must be available to all children with diabetes for immediate confirmation and safe management of hypoglycemia. E
- Glucose monitoring should be performed prior to exercise, and extra carbohydrates may be consumed based on the glucose level and the expected intensity and duration of exercise. B
- Children and their parents should be trained to contact their diabetes care provider if hypoglycemia is documented without symptoms or if the symptoms are those of neuroglycopenia and not autonomic symptoms (i.e., impaired hypoglycemia awareness). E
- Regular screening for FOH is important to understand who will need interventions through educational and/or behavioral strategies, although evidence in children is limited. E
- Blood glucose goals may need to be adjusted upwards in children with recurrent hypoglycemia and/or impaired hypoglycemia awareness. B
- If unexplained hypoglycemia is frequent, evaluation for unrecognized celiac and Addison's disease should be considered. E
- Children and adolescents with diabetes should wear some form of identification or alert indicating that they have T1D. E
- Currently available technologies like CGM, automated insulin suspensions (Low Glucose Suspend, Predictive Low Glucose Suspend) and hybrid closed loop systems have reduced the duration of hypoglycemia. A
3 INTRODUCTION
Hypoglycemia is a common occurrence in the management of T1D. It interferes with daily activities and poses a constant perceived threat to the individual and their families. It is a recognized limiting factor in achieving optimal glycemia1 with an impact on quality of life.2 Minimizing hypoglycemia is an important objective of diabetes management that can be addressed by acknowledging the problem, evaluating the risk factors and applying the principles of intensive glycemic management.3 Therefore, it is vital to address this important clinical concern during diabetes education and institute appropriate management. The last two decades have experienced a paradigm shift in diabetes management through the availability of improved insulin analogues, insulin pump therapy and advent of CGM with algorithms incorporated in sensor-augmented pump therapy (SAP) to reduce and prevent hypoglycemia. There is increasing evidence to suggest that the time spent in hypoglycemia4-6 and the rates of severe hypoglycemia have declined in recent years in developed countries with newer intensive therapies.7-11 Unfortunately, hypoglycemia continues to be a problem in countries with limited resources, where many children are treated with insulin injections, with minimal access to technology and resources.
4 DEFINITION AND INCIDENCE
4.1 Definition
Hypoglycemic events include all episodes of a plasma glucose concentration low enough to cause symptoms and/or signs, including impaired brain functioning and expose the individual to potential harm. It is difficult to assign a numerical value to hypoglycemia. Nonetheless, it is important to identify and record a level of hypoglycemia that needs to be avoided because of its immediate and long-term impact on the individual. The definitions as below, incorporated in Table 1, are intended to guide clinical care and reporting and are based on glucose values detected by SMBG, CGM or a laboratory measurement of plasma glucose.12 These definitions have informed the standardization of CGM metrics to set clinical targets for CGM data interpretation.13
| Definition | Clinical hypoglycemia alert | Clinically important or serious hypoglycemia | Severe hypoglycemia Coma/convulsions/severe cognitive impairment |
|---|---|---|---|
| Threshold | < 3.9 mmol/L or < 70 mg/dl | <3.0 mmol/L or < 54 mg/dl | No specific glucose threshold |
| Action | Requires hypoglycemia treatment | Requires hypoglycemia treatment | Requires third-party assistance to administer carbohydrates, glucagon, or intravenous dextrose |
| Acceptable CGM targets for hypoglycemia | <4% or <1 h/day | <1% or < 15 min/day | - |
1. Clinical hypoglycemia alert
A glucose value of <3.9 mmol/L (70 mg/dl) is an alert value that requires attention to prevent more serious hypoglycemia. The alert can be used as the threshold value for identifying and treating hypoglycemia in children with diabetes because of the potential for glucose levels to drop further.
2. Clinically important or serious hypoglycemia
A glucose value of <3.0 mmol/L (54 mg/dl) indicates clinically important or serious hypoglycemia. These low levels may lead to defective hormonal counter regulation14 and impaired awareness of hypoglycemia (IAH). Neurogenic symptoms and cognitive dysfunction occur below this level15, 16 with subsequent increased risk of severe hypoglycemia. This level should be recorded in routine clinical care and reported in audits and in clinical trials of interventions directed toward reducing hypoglycemia.
3. Severe hypoglycemia
Severe hypoglycemia is defined as an event associated with severe cognitive impairment (including coma and convulsions) requiring assistance by another person to administer carbohydrates, glucagon, or administer IV dextrose. This aligns with the definition of severe hypoglycemia in adults in accordance with the American Diabetes Association (ADA) guidelines.17 This will also enable complete recording of events. Furthermore, if severe hypoglycemia is defined by coma or convulsions alone, the frequency of severe hypoglycemia in children can be underestimated. However, as young children require assistance to correct even mild hypoglycemia, the event requires an assessment by the caregiver and clinician as to the presence (or not) of hypoglycemia-induced cognitive dysfunction. A subgroup of severe hypoglycemia is hypoglycemic coma which is described as a severe hypoglycemic event resulting in coma or convulsion requiring parenteral therapy. These events should be recorded independently as these are unequivocally significant clinical outcomes.
4.2 Incidence
The exact incidence of hypoglycemia is difficult to ascertain but mild hypoglycemia is common. Asymptomatic events are more likely to be unrecognized and underreported while symptomatic hypoglycemia occurs on an average of two episodes per week with multiple such episodes in the lifetime.18 The ADA workgroup on hypoglycemia in 2005 recommended the reporting of both the proportion (percentage) of people with T1D affected and the event rates (episodes per patient-year or 100 patient-years) for each of the categories of hypoglycemic events as these provide complementary information.19
Although there was a significant improvement in glycemia and reduction of diabetes-related complications in individuals with T1D on intensive glycemic therapy compared to conventional management in the Diabetes Control and Complications Trial (DCCT), there was a threefold increased risk of severe hypoglycemia events in individuals randomized to the intensive management arm of the study.20 The incidence of severe hypoglycemia requiring treatment assistance was 61 per 100 patient-years in intensively treated versus 19 per 100 patient-years in those conventionally treated with an incidence of coma and/or seizure of 16 per 100 patient-years and 5 per 100 patient-years, respectively. Similar high rates were reported in observational cohorts in Western Australia21 and Colorado.22 Historically, these high rates of severe hypoglycemia were associated with lower HbA1c20-24 although this relationship weakened with time.25, 26 Significant reduction in the frequency of severe hypoglycemia was observed in Germany and Austria (Diabetes-Patienten-Verlaufsdokumentation—DPV), Western Australia, and Denmark with minimal/no association of severe hypoglycemia with glycemic status.26-28 The incidence of hypoglycemic coma has fallen over the last two decades with a rate of 3 to 7 per 100 patient years across international registries.29 The decreasing trends for the occurrence of severe hypoglycemia in youth have continued.7-11 Unfortunately, hypoglycemia continues to be a problem in countries with limited resources as evidenced by high rates of severe hypoglycemia in Brazil30 and India.31 The cohorts in these studies were predominantly on insulin injections, with minimal access to technology and resources.
Younger age and low HbA1c were historical risk factors for severe hypoglycemia, however low HbA1c is no longer a strong predictor of severe hypoglycemia in pediatric T1D cohorts on contemporary therapy.7, 25, 29, 32, 33 The T1D Exchange and the DPV registry did not find increased rates of hypoglycemic coma in those <6 years of age with HbA1c <7.5% (58.5 mmol/mol) compared with those with higher HbA1c levels.32 No differences in HbA1c were also reported from an Indian study assessing children with or without severe hypoglycemia.31 This change can be attributed to a number of factors including increased use of insulin analogues and insulin pump therapy,27, 34, 35 and improved hypoglycemia education.36 These studies highlight the important observation that optimal glycemia can be achieved without an increase in severe hypoglycemia.
5 MORBIDITY AND MORTALITY WITH HYPOGLYCEMIA
5.1 Mortality
In the pre- and immediate post-DCCT period up to more than a decade ago, hypoglycemia was assigned as the cause of death in 4%–10%37-39 in population-based cohorts and international registries of childhood-onset diabetes. Most deaths attributed to hypoglycemia occurred in adults. Hypoglycemia can be difficult to ascertain with certainty as the cause of death.40, 41 The global burden of hypoglycemia-related mortality was ascertained from an analysis of death certificates from 109 countries from 2000 to 2014. The study reported global differences with high rates of hypoglycemia-related deaths in South and Central America and lower rates in Europe, North America, and Australasia.42
Hypoglycemia is also proposed to play a role in the “dead-in-bed” syndrome, which is more prevalent in people with T1D than in the general population. In a coroner's case series, dead-in-bed syndrome accounted for ~15% of deaths in young adult males (≤40 years) with diabetes.43 Although the etiology is not well established, it has been postulated that it may be secondary to prolongation of QT interval caused by number of factors: acute hypoglycemia44 on a background of autonomic neuropathy45 and possible genetic influences.46 In addition to hypoglycemia-induced abnormal QTc prolongation, hypokalemia and adrenergic activation increase the risk for ventricular arrhythmias.47 Alterations in cardiac repolarization can lead to fatal ventricular arrhythmias and may contribute to the sudden nocturnal death of young individuals with T1D.48 It is likely that widespread use of CGM and the increased use of population-based databases will clarify the true incidence of deaths caused by hypoglycemia in the future. Although the role of hypoglycemia in “dead-in-bed” syndrome remains unclear, it is important to recognize that this continues to be a source of distress for parents of children with diabetes.49
5.2 Morbidity
Neurological sequelae of hypoglycemia
Previous studies have shown that early onset of diabetes predicts poorer cognitive function and hypoglycemia plays a critical role in causing brain dysfunction.50 Severe hypoglycemia, particularly in children51-53 under the age of 6 years, was associated with cognitive deficits and was believed to contribute to the neurotoxic milieu affecting brain development.54 However, the role of acute hypoglycemia in causing long-term impairment has gained less traction, whereas repetitive, chronic hyperglycemia is now viewed as more injurious to the brain.55
Transient cognitive dysfunction occurs with hypoglycemia with recovery generally complete within 1 h of correcting glucose levels, although recovery from severe events can take up to 36 h.56 The long-term implication of severe hypoglycemia on cognitive function was reported as part of the Epidemiology of Diabetes Interventions and Complications (EDIC) follow-up of the original DCCT cohort. There were no significant cognitive abnormalities observed even after 18 years in the entire cohort57 and also among adolescents who participated in the trial,58 while the 32-year follow-up demonstrated an overall decline in cognition with age.59
The association of structural brain abnormalities with severe hypoglycemia has received significant attention with neuropathological evidence suggesting that severe hypoglycemia may preferentially harm neurons in the medial temporal region, including the hippocampus.60 Mesial temporal sclerosis,61 larger hippocampal volumes,62 and reduced gray and white matter volumes have been reported in children who experienced hypoglycemic seizures.51 However, gray and white matter neurological changes are not seen only with hypoglycemia, but also in children with hyperglycemia.63, 64
Psychological impact of hypoglycemia
Severe hypoglycemic episodes may have negative psychosocial consequences and undesirable compensatory behaviors arising from hypoglycemia.65 These hypoglycemic symptoms can be distressing and embarrassing and impact academic, social, and physical activities. While this fear can induce anxiety, avoidance of these episodes may be adaptive, leading to appropriate vigilance in glucose management. Elevated levels of anxiety can lead to disruptions in daily activities impacting diabetes management.66 This FOH impacts the child and family unit. The strongest predictor of parental FOH across studies is the experience of a severe hypoglycemic event with their child.66 Given the negative consequences associated with severe hypoglycemic episodes, individuals with T1D and their parents are at risk for increased anxiety, poor sleep, and reduced quality of life.2, 67-69 FOH may lead families and/or physicians to accept higher glucose levels with behaviors directed toward avoiding hypoglycemia leading to suboptimal glycemic status.2, 70-72 A progressive and lasting increase in HbA1c occurs with episodes of severe hypoglycemia, contributing to an increase in long-term complications.73 Routine FOH screening is important to recognize those who would benefit from intervention.66 The Hypoglycemia Fear Survey (HFS) has been adapted for use for parents of young children,74, 75 as well as adolescents and children.76 The Children's Hypoglycemia Index (CHI), developed independently of the HFS, has the added benefit of including a scale assessing FOH in specific situations, such as only at night or only at school.77
Behavioral interventions (cognitive behavioral therapy) and psychoeducation have been shown to reduce FOH in adults; this intervention may be of benefit in older children but pediatric studies are not available.66 Pilot data, using a group-based behavioral intervention in caregivers of children with diabetes reduced FOH and parenting stress.78 Apart from the behavioral intervention, the availability of real-time CGM79 and algorithms with automated insulin suspension and delivery4, 80 has the potential to reduce FOH, although studies are limited in this field.
6 SIGNS AND SYMPTOMS
Hypoglycemia is often accompanied by signs and symptoms of autonomic (adrenergic) activation and/or neurological dysfunction from glucose deprivation in the brain (neuroglycopenia),81 as shown in Table 2. As the blood glucose concentration falls, initial symptoms result from activation of the autonomic nervous system and include shakiness, sweating, pallor, and palpitations. In healthy individuals without diabetes, these symptoms occur at a blood glucose level of ~3.9 mmol/L in children and 3.2 mmol/L in adults.82 However, this threshold in individuals with diabetes will depend on their glycemic levels83-86 with an adaptive shift of the glycemic threshold for symptom onset to a higher glucose level with chronic hyperglycemia and lower glucose level with chronic hypoglycemia. Neuroglycopenic symptoms result from brain glucose deprivation and include headache, difficulty concentrating, blurred vision, difficulty hearing, slurred speech, and confusion. Behavioral changes such as irritability, agitation, quietness, stubbornness, and tantrums may be the prominent symptoms particularly for the preschool child, and may result from a combination of neuroglycopenic and autonomic responses.87 In this younger age group, observed signs are more important, and at all ages there is a difference between reported and observed symptoms or signs. The dominant symptoms of hypoglycemia tend to differ depending on age, with neuroglycopenia more common than autonomic symptoms in the young.88
| Autonomic signs and symptoms |
| Shakiness |
| Sweatiness |
| Trembling |
| Palpitations |
| Pallor |
| Neuroglycopenic signs and symptoms |
| Poor concentration |
| Blurred or double vision |
| Disturbed color vision |
| Difficulty hearing |
| Slurred speech |
| Poor judgment and confusion |
| Problems with short-term memory |
| Weakness |
| Numbness |
| Dizziness |
| Lack of coordination and unsteady gait |
| Loss of consciousness |
| Seizure |
| Behavioral signs and symptoms |
| Irritability |
| Erratic behavior |
| Agitation |
| Nightmares |
| Inconsolable crying |
| Nonspecific symptoms |
| Hunger |
| Headache |
| Nausea |
| Tiredness |
Physiological responses in children and adolescents
It is well recognized that although many physiological responses are similar across the age groups, there can be significant developmental and age-related differences in children and adolescents. The DCCT reported a higher rate of severe hypoglycemia in adolescents as compared to adults; 86 versus 57 events requiring assistance per 100 patient-years20 despite higher HbA1c in adolescents. Several physiological and behavioral mechanisms may contribute to this difference. Firstly, there are behavioral factors such as variable engagement with diabetes care which are associated with sub-optimal glycemia in adolescents.89 Secondly, during puberty, adolescents with or without T1D are more insulin resistant than adults.90 Adolescents also have quantitative differences in counter regulatory hormone responses. In healthy individuals without diabetes, hypoglycemia symptoms occur at a blood glucose level of ~3.9 mmol/L in adolescents and 3.2 mmol/L in adults82 In adolescents with suboptimal glycemia, this glucose level may be higher, reported at 4.9 mmol/L in one study.82 Intensively treated young adults with T1D counter-regulate and experience hypoglycemia symptoms at a lower glucose level than those on treatment with twice daily injections.85 To date, nearly all studies have been conducted in adolescents and young adults primarily due to difficulty in studying a younger age group. As a result, little is known about responses in pre-adolescents as to whether younger children demonstrate a similar or different response to hypoglycemia although there is evidence that a developing brain is more susceptible to the influence of glycemic excursions.91
7 HYPOGLYCEMIA AWARENESS
In healthy individuals without diabetes, endogenous insulin secretion is shut down and counter regulatory hormones (glucagon, epinephrine, and norepinephrine) are released in response to hypoglycemia.92 However, in people with diabetes, there is a progressive loss of glucagon response to insulin-induced hypoglycemia. This has been demonstrated as early as 12 months after diabetes onset and is lost in most people with T1D by 5 years.93, 94 Individuals with diabetes are therefore primarily dependent on the epinephrine response to counteract the hypoglycemic effect of insulin. Recurrent episodes of hypoglycemia contribute to the development of defective counter regulatory hormone responses to subsequent reductions in glucose levels and may further exacerbate the problem, whereby “hypoglycemia begets hypoglycemia.”
IAH is a syndrome in which the ability to detect hypoglycemia is diminished or absent, reported in ~25% of adults with T1D.92 In children and adolescents, a similarly high prevalence (33%) of IAH was reported in 2002, which declined to 21% in 2015.95, 96 Although the prevalence of IAH has decreased, it remains a concern in a substantial proportion of adolescents.
IAH is associated with lowering of glycemic thresholds for the release of counter regulatory hormones and generation of symptoms. A two to threefold reduction in the epinephrine responses contributes to the impaired adrenergic warning symptoms during hypoglycemia.97 Clinically, this is manifested as loss of some of the symptoms of hypoglycemia over time. The loss of autonomic symptoms precedes the neuroglycopenic symptoms and individuals are less likely to seek treatment for low blood glucose levels. As the awareness of low blood glucose level is impaired, hypoglycemia is prolonged. These episodes, if unrecognized and prolonged, can lead to seizures.98 Individuals with IAH have a sixfold increase in severe hypoglycemic episodes.99 This highlights the need to evaluate for IAH as part of clinical management. The identification of IAH is limited by the availability of tools to measure hypoglycemia awareness. It is not practical to measure the adrenergic responses during hypoglycemia to identify IAH and questionnaires have been developed as surrogate measures that can be administered to older children who are able to self-report. The single-question Gold,99 the eight-question Clarke100 and six-question modified Clarke95, 96 have been used to screen children with IAH(Appendices A to C). The Clarke questionnaire has higher specificity than Gold in predicting clinically significant hypoglycemia.101, 102 Although a score of ≥4 implies IAH on these measures, it is important to acknowledge that IAH is not an “all or none phenomenon” but reflects a continuum in which differing degrees of impaired awareness can occur.
The blood glucose threshold for activation of autonomic signs and symptoms is related to glycemic status, antecedent hypoglycemia, antecedent exercise, and sleep. Tight glycemic management leads to adaptations that impair counter regulatory responses103 with a lower glucose level required to elicit an epinephrine response.86 An episode of antecedent hypoglycemia may reduce the symptomatic and autonomic response to subsequent hypoglycemia, which in turn further increases the risk of subsequent severe hypoglycemia.104 Likewise, moderate-intensity exercise also blunts the hormonal response to subsequent hypoglycemia.105 Most severe episodes of hypoglycemia occur at night as sleep further impairs the counter regulatory hormone responses to hypoglycemia in people with diabetes and in normal subjects.106 On the other hand, the blood glucose threshold for neuroglycopenia does not appear to vary as much with the level of glycemia or with antecedent hypoglycemia.82, 107, 108 The glycemic threshold for cognitive dysfunction may be triggered before autonomic activation and hence are the symptoms associated with IAH.
The cause of IAH is not well understood. “Hypoglycemia-associated autonomic failure” resulting from a failure of centrally-mediated counter regulation is one of the proposed mechanisms109 although the term may be misleading as the autonomic system does not fail. Rather, recurrent hypoglycemia causes a process of adaptation referred to as habituation; that is, IAH may represent a habituated response to hypoglycemia.110, 111 A habituated response can also be temporarily reversed by the introduction of a novel (heterotypic) stimulus (dishabituation). Preliminary results of a recent study demonstrated that a single burst of high-intensity exercise restored counter regulatory responses to hypoglycemia induced the following day in a rodent model112 and in people with T1D and IAH.113
IAH can be reversed by avoiding hypoglycemia for two to three weeks114 but this may be difficult to accomplish and, until recently, has not been practical in a clinical setting with current therapies. Therapeutic options are limited although some individuals benefit from structured education.115 Technological advances could potentially have a role to play with the use of CGM,116 SAPT with low glucose suspend functions4, 117 or hybrid closed loop systems.118
8 RISK FACTORS FOR HYPOGLYCEMIA
Risk factors for hypoglycemia can be classified as modifiable and non-modifiable; most are modifiable. Younger age (due to the inability to communicate symptoms) and prolonged diabetes duration (due to its association with IAH) increase the risk of hypoglycemia. A higher risk of hypoglycemia has also been reported with limited access to private insurance and lack of nationalized schemes to access technology.22 The main risk factor for hypoglycemia is a mismatch between administered insulin and consumed food. Insulin excess could result from increased doses due to poor understanding of insulin type and action, accidental delivery, reduced food intake or missed meals, and in situations where glucose utilization is increased (during exercise) or endogenous glucose production is decreased (after alcohol intake).
Recurrent hypoglycemia
Majority of children with T1D who experience severe hypoglycemia have isolated events; however, a few experience recurrent episodes. After an episode of severe hypoglycemia, the risk of recurrent severe hypoglycemia remains higher up to 4 years compared with children who have never experienced severe hypoglycemia.73 When hypoglycemia is recurrent, it is important to exclude IAH and rule out coexisting autoimmune disorders like subclinical hypothyroidism,119 celiac disease,120 and Addison's disease.121, 122 Rarely, surreptitious self-administration of insulin causes repeated and unexplained severe hypoglycemia and should be considered as a sign of psychological distress123, 124 with underlying risk factors such as eating disorders (anorexia and bulimia) and depression. The clinical factors associated with an increased risk of hypoglycemia are shown in Table 3.
| Precipitants |
| Excess insulin |
| Less food consumption |
| Exercise |
| Sleep |
| Alcohol ingestion |
| Risk factors |
| Impaired awareness of hypoglycemia |
| Previous severe hypoglycemia |
| Longer duration of diabetes |
| Co-morbidities |
| Coeliac disease |
| Addison's disease |
| Hypothyroidism |
| Psychological distress |
Exercise
The glucose response to exercise is affected by many factors including the duration, intensity and type of exercise, the time of day when exercise is performed, plasma glucose, and insulin levels, and the availability of supplemental and stored carbohydrates.125 The risk of hypoglycemia is increased during moderate-intensity exercise, immediately after as well as 7 to 11 h after exercise.126 The pathophysiology of post-exercise-induced hypoglycemia is multifactorial, and includes increased insulin absorption, increased insulin sensitivity, increased peripheral glucose utilization with depletion of glycogen stores and exercise-induced counter regulatory hormone deficits. Furthermore, children on fixed insulin doses are at “triple jeopardy” for hypoglycemia on nights following exercise related to: increased peripheral glucose utilization with exercise, impaired counter regulatory hormone responses during sleep, and unchanged insulin concentrations related to the treatment regimen.127 Treatment guidelines to help individuals exercise safely are updated in this edition of the ISPAD guidelines (See ISPAD 2022 Consensus Guidelines Chapter 14 for Exercise in Children and Adolescents with Diabetes).
Alcohol
Alcohol inhibits gluconeogenesis128 and hypoglycemia may occur if there is an inadequate intake of carbohydrates. Furthermore, the symptoms of hypoglycemia may be masked by the intoxicating effects of alcohol. Even moderate consumption of ethanol may reduce hypoglycemia awareness and impair the counter regulatory response to insulin-induced hypoglycemia.129 Apart from the acute effects, moderate consumption of alcohol in the evening is associated with reduced nocturnal growth hormone secretion and may increase the risk of hypoglycemia on the subsequent morning.130 Although an increase in insulin sensitivity with alcohol intake has been postulated, this remains inconclusive.131
Nocturnal hypoglycemia
The Juvenile Diabetes Research Foundation (JDRF) CGM study group in 2010 described frequent prolonged nocturnal hypoglycemia on 8.5% of nights in both children and adults but more prolonged episodes in children.132 In this study, the mean time spent in nocturnal hypoglycemia (<60 mg/dl) was 81 min. Almost half of these episodes were undetected by caregivers or individuals with diabetes.133, 134 The counterregulatory responses to hypoglycemia are attenuated during sleep106, 135, 136 and individuals with T1D are much less likely to be awakened by hypoglycemia than individuals without diabetes.106 This is significant as prolonged nocturnal hypoglycemia can lead to seizures.98 Nocturnal hypoglycemia should be suspected if pre-breakfast glucose is low, and/or the individual experiences confusion, nightmares or seizures during the night, or if impaired thinking, lethargy, altered mood, or headaches are reported on waking.137 It is recommended to monitor overnight glucose levels, particularly if there is an additional risk factor that may predispose to nocturnal hypoglycemia. Younger age, lower HbA1c levels, antecedent exercise, and hypoglycemia are associated with a greater frequency of nocturnal hypoglycemia.138
Studies of overnight hypoglycemia in children have been unable to identify a glucose value that reliably predicts a low risk of hypoglycemia. In a study using CGM to detect nocturnal hypoglycemia, there was a twofold increase, 45% versus 22%, in the incidence of hypoglycemia with a bedtime glucose ≤5.5 mmol/L (100 mg/dl).139 Similarly, the fasting glucose levels were significantly lower (6.6 mmol/L; 118 mg/dl) in those with than those without hypoglycemia (9.9 mmol/L; 179 mg/dl).140 In contrast, in children on twice daily soluble and isophane (NPH) insulin therapy, hypoglycemia was partially predicted by a midnight glucose of <7.2 mmol/L (130 mg/dl).141
To reduce nocturnal hypoglycemia, a carbohydrate snack before bed for children on insulin injections was recommended based on studies using intermediate-acting insulins with peak action 4–12 h and duration 16–24 h.142 However, use of long-acting insulin analogues such as glargine and detemir, due to their less pronounced peak effect, have reduced overnight hypoglycemia.143 Hence, extra snacks may be unnecessary144 and enforcing pre-bed meals may contribute to nocturnal hyperglycemia and add unnecessary calories contributing to weight gain. The recommendation for inclusion of pre-bed snacks is not mandatory and should be individually tailored.144 Newer insulin analogues, like the ultralong-acting basal insulin degludec, have the potential to provide similar glycemia while reducing nocturnal hypoglycemia risk.145
Nocturnal hypoglycemia is less frequent with pump therapy and there has been a further decline with use of pumps that incorporate control algorithms that suspend basal insulin with sensor-detected,146 sensor-predicted hypoglycemia4 and hybrid closed loop systems.
9 HYPOGLYCEMIA TREATMENT
Diabetes education should focus on recognition of precipitants and risk factors for hypoglycemia, the ability to detect subtle symptoms, the importance of confirming low glucose levels by monitoring, appropriate hypoglycemia treatment and approaches to prevent future events. Figure 1 outlines the management of hypoglycemia.
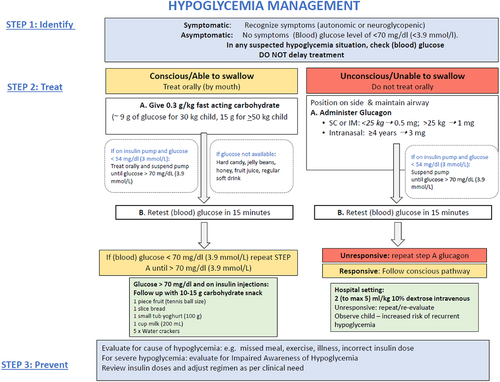
Hypoglycemia can be detected using SMBG or CGM. Newer factory calibrated CGM devices are approved to use sensor glucose data to make diabetes management decisions. However, a fingerpick blood glucose measurement is recommended if there is a suspected mismatch between clinical expectations and the sensor glucose level. Likewise, blood glucose should always be tested if the child is symptomatic or shows signs of hypoglycemia. If the glucose level is <3.9 mmol/L (70 mg/dl), remedial actions to prevent a further drop in glucose is recommended. In adults, 20 g of carbohydrate in the form of glucose tablets raised glucose levels by ~2.5 to 3.6 mmol/L (45–65 mg/dl) at 45 min.147-149 This was extrapolated to 0.3 g/kg in children, which would be approximately 9 g of glucose for a 30 kg child and 15 g for a 50 kg child. A pediatric study has confirmed that 0.3 g/kg of rapidly acting carbohydrate containing preparations (glucose tablets, orange juice), effectively resolved hypoglycemia in most children and raised median blood glucose by 1–1.3 mmol/L in 10 min and 2–2.1 mmol/L in 15 min without rebound hyperglycemia at the next meal.150 A similar weight-based approach was also found to be effective in children on insulin pumps.151 In children on insulin pump therapy, it is also recommended that basal insulin delivery is suspended if glucose level is <3 mmol/L. To date no studies have looked at the amount of carbohydrate required to treat hypoglycemia in children who use automated insulin delivery systems that suspend basal insulin delivery when hypoglycemia is predicted or use closed loop systems. The current approach of standard hypoglycemia management can cause rebound hyperglycemia152; hence consideration should be given to treat hypoglycemia with less glucose (e.g., 5–10 g)152, 153 or approximately half the amount of glucose used for standard treatment.
The choice of carbohydrate source is an important consideration for hypoglycemia treatment. In clinical practice, glucose-containing products are recommended for immediate treatment because of their rapid absorption from the intestine. It is important to review the amount of glucose in the product to ensure adequate treatment is provided. When glucose tablets are not available, dietary sugars can be used. Glucose tablets result in a higher rate of relief of symptomatic hypoglycemia 15 min after ingestion compared with dietary sugars (hard candy, table sugar, jelly beans, fruit juice). If available, glucose-based treatment should be the first choice when treating symptomatic hypoglycemia.154
Although 15 g of “carbohydrates” are recommended for first-line treatment by ADA,155 it is essential to note that the dose required for dietary sugars is not established and may be higher than glucose. For example, 40 g of carbohydrate in the form of juice results in approximately the same rise as 20 g in the form of glucose tablets.147 Likewise, sucrose requires a greater amount to provide the same increase in blood glucose concentration compared to oral glucose.148 Husband et al reported that glucose- and sucrose-based hypoglycemia treatments resulted in timely resolution of hypoglycemia, although fructose-based treatment (fruit juice) was less effective.156 Honey and fruit juice have been reported to be more effective than one sugar cube (sucrose) for treatment of hypoglycemia.157 Honey contains nearly 70% fructose and glucose as main sugars although composition differs depending on the geographical origin which may lead to variability in response. Jelly beans contain glucose syrup and although they cause glucose levels to rise, the resolution of hypoglycemia is slower.150 If a soft drink is used for treatment, ensure regular and not sugar-free/diet forms is used for treatment.
Dietary sugars are generally discouraged to avoid confusion between “treatment” and “treats” with the potential of hypoglycemia induction to receive these treats. Furthermore, complex carbohydrates, or foods containing fat (chocolate) which delay intestinal absorption and result in slow absorption of glucose should be avoided as the initial treatment of hypoglycemia. Whole milk containing 20 g of carbohydrate (435 ml) causes a minimal response with rise of ~1 mmol/L (18 mg/dl).147
After treatment, repeat a blood glucose measurement after 15 min.158 If there is no response or an inadequate response, repeat oral intake as above. It is important to be aware of the physiological time lag with rising and falling sensor glucose levels in children using CGM.159 The decision to repeat hypoglycemia treatment should not be based on a low sensor glucose level at 15 min unless a finger prick glucose measurement confirms persistent hypoglycemia. Once hypoglycemia is reversed, the child should have the usual meal or snack if due at that time; otherwise, a snack (15 g) of slower-acting carbohydrate, such as bread, milk, biscuits, or fruit should be consumed. However, this is not always required, particularly for those on insulin pump therapy.
The amount of carbohydrate required to guide treatment will also depend on the size of the child, type of insulin therapy, active insulin on board, and the timing and intensity of antecedent exercise.147, 160 It is important to educate the child/caregiver to consider the factors that led to hypoglycemia.
Severe hypoglycemia
Severe hypoglycemia requires urgent treatment. If the child is unconscious or unable to swallow, hypoglycemia can be safely reversed by administration of glucagon, a potent and effective agent that can be administered intravenously, intranasally, intramuscularly or subcutaneously.161 Table 4 shows a list of the available forms of glucagon.
| Product | Preparation | Route | Dose | Ages |
|---|---|---|---|---|
| Glucagon | 1 mg/ml lyophilized powder to be reconstituted with diluent | SC/IM | 1 mg: children >25 kg 0.5 mg: children <25 kg | All ages |
| Baqsimi | 3 mg glucagon nasal powder | Intranasal | 3 mg | >4 years |
| Dasiglucagon | 0.6 mg/0.6 ml prefilled syringe or autoinjector | SC | 0.6 mg | ≥6 years |
| Gvoke Hypopen | 0.5, 1 mg prefilled syringe or autoinjector | SC | 1 mg: children >45 kg 0.5 mg: children <45 kg | >2 years |
Recombinant crystalline glucagon is available as a lyophilized (freeze-dried) powder that is mixed with an aqueous diluent to a concentration of 1 mg/ml. Commercially available glucagon rescue kits include GlucaGen® HypoKit 1 mg (Novo Nordisk®A/S, Bagsvaerd, Denmark) and Glucagon Emergency Rescue Kit (Eli Lilly and Company, Indianapolis IN). The recommended glucagon dose is weight based: 1 mg for adults and children >25 kg and 0.5 mg for children <25 kg (according to Novo Nordisk manufacture guidelines) while Eli Lilly uses a weight cut-off of 20 kg. The evidence for these recommendations is unclear.
Glucagon often induces nausea and vomiting on regaining consciousness and hence it is important to continue close observation and glucose monitoring after treatment.162 The frequency of side effects increases with repeated doses. The efficacy of glucagon depends on having adequate hepatic glycogen. Consequently, glucagon would be predicted to be less efficacious in cases of hypoglycemia associated with prolonged fasting; in these circumstances, parenteral glucose would be the therapy of choice.162 Currently available preparations require glucagon reconstitution with sterile diluent and therefore parents and caregivers require instruction on how to prepare and administer glucagon. Because glucagon is seldom used, parents and caregivers require regular reviews about when and how to administer glucagon. The need for reinstitution of glucagon powder before use is a known challenge which can delay or prevent glucagon administration. To overcome this barrier, an intranasal single-use glucagon preparation is now available as a needle-free device that delivers glucagon for the treatment of severe hypoglycemia in children163 and adults164, 165 with T1D. A recent meta-analysis indicated that intranasal glucagon and SC/IM glucagon were equally effective in resolution of hypoglycemia in conscious individuals.166 Intranasal preparation may cause headache, upper airway discomfort, tearing, or nasal congestion in addition to the typical side effects of glucagon.163 A single dose of 3 mg is used in children (≥4 years) and adults with T1D.167 Administration of nasal glucagon is faster and has a much higher success rate for delivery of the full dose with fewer errors than injectable glucagon.168 Recently, dasiglucagon has also received regulatory approval.169 Dasiglucagon, a soluble and stable glucagon analog available as 0.6 mg ready-to-use pen, provided rapid and effective reversal of hypoglycemia in children (≥6 years)170 and adults171 with T1D; side-effects were comparable to IM glucagon. A premixed SC autoinjector Gvoke HypoPen® (liquid-stable glucagon) is also available for use in children >2 years of age.172, 173 The availability of different forms of glucagon varies across different regions of the world and access to these forms of hypoglycemia treatment may be limited in lower resource settings.
In a hospital setting, intravenous glucose or glucagon may be given. Intravenous glucose should be administered by trained personnel over several minutes to reverse hypoglycemia. The recommended dose is 0.2 g/kg of glucose which equates to 2 ml/kg of 10% dextrose with a maximum dose of 0.5 g/kg body (5 ml/kg). As high concentrations cause sclerosis of peripheral veins, the maximum concentration of dextrose that can be administered through a peripheral vein is 25% dextrose. Rapid administration or an excessive concentration (dextrose 50%) can result in a rapid osmotic change with risk of hyperosmolar cerebral injury.174 10% dextrose is effective and safe and hence recommended for management.175 In the event of recurrent hypoglycemia, the child will require additional oral carbohydrates and/or intravenous infusion of 10% dextrose to provide a glucose infusion rate of 2–5 mg/kg/min (1.2–3.0 ml/kg/h). The predisposing events that led to the severe event should be evaluated to prevent future events. Caregivers need to be aware that following a severe hypoglycemic event, the child will be at higher risk of a future event, and alterations to insulin therapy may be appropriate.
Glucagon is not readily available in countries with limited resources. Sugar or any other powdery substance or thin liquids such as a glucose solution or honey should not be given forcibly to the semi/unconscious child. The child should be put in a lateral position to prevent aspiration and a thick paste of glucose (glucose powder with a few drops of water or crushed table sugar with consistency of thick cake icing) smeared onto the dependent cheek pad; the efficacy of this practice is anecdotal. Although an earlier study in healthy adult volunteers demonstrated poor buccal absorption of glucose,176 sublingual glucose was found to be a child-friendly method of raising blood glucose in severely ill children with malaria.177 In situations where there is a danger of aspiration and intravenous access is unavailable, parenteral glucose solutions may be administered via nasogastric tubes.178
Minidose glucagon
Children with gastrointestinal illness and/or poor oral carbohydrate intake with a blood glucose ≤4.4 mmol/L can benefit from mini-dose glucagon injected by their caregivers to avoid impending hypoglycemia and hospitalizations.161, 179 The dose of reconstituted glucagon is administered subcutaneously using a 100 U insulin syringe (1 unit ~10 μg of glucagon) and is age-based: 2 units (20 μg) for children ≤2 years and 1 unit/year for children ≥3–15 years (with a maximum dose of 150 μg or 15 units). If blood glucose fails to rise over the first 30 min, a repeat injection should be given using twice the initial dose. The minidose glucagon regimen resulted in an increase of 3.3–5 mmol/L of glucose (60–90 mg/dl) within 30 min of administration with an average increase of 4.7 mmol/L.161
10 ROLE OF TECHNOLOGY IN REDUCTION OF HYPOGLYCEMIA
The rapid technological advances in diabetes management with stand-alone CGM systems or systems with integrated CGM and insulin pump use have empowered individuals with T1D to further reduce the frequency of hypoglycemia. There are many technological devices available to reduce hypoglycemia; however, the choice of device should be a decision based on a dialogue between the health care professional and the individual with diabetes. More detailed recommendations and guidelines for pump and CGM are covered in ISPAD pump and CGM chapters respectively (See ISPAD 2022 Consensus Guideline Chapter 16 on Diabetes Technologies: Glucose Monitoring and Chapters 17 on Diabetes Technologies: Insulin delivery).
Continuous subcutaneous insulin infusion
Continuous subcutaneous insulin infusion (CSII) use can reduce hypoglycemia. A meta-analysis showed that pump therapy in children may be better than injections in reducing the incidence of severe hypoglycemia.180 Compared to injection therapy, a lower risk of severe hypoglycemia was associated with CSII in the T1D exchange,181 DPV registry182 and the International Pediatric SWEET Registry.183
Continuous glucose monitoring: Stand-alone systems
Studies have shown a reduction in time spent in hypoglycemia with a concomitant decrease in HbA1c with CGM use in both children and adults.184-186 There was a trend to reduction in severe hypoglycemia in the DPV and T1D Exchange registry with CGM initiation.8, 181 Sensors with predictive alerts can further reduce hypoglycemia.187 These alerts with real-time glucose monitoring may also contribute to reduced parental FOH.79 Intermittently scanned CGM (isCGM) (without alerts) also has the potential to reduce hypoglycemia,188 however the reduction in hypoglycemia is greater with real-time CGM than with intermittently scanned isCGM189 and is potentially a better management tool for individuals at high risk of hypoglycemia.
Sensor-augmented pump therapy with low glucose and predictive low glucose suspension
The incorporation of algorithms in sensor-augmented pump therapy further reduces the time spent in hypoglycemia due to suspension of basal insulin delivery with hypoglycemia (Low Glucose Suspension)117, 190 and with prediction of hypoglycemia.4, 191 Predictive Low Glucose Management (PLGM) systems include the Medtronic PLGM system and Tandem Basal IQ. The Medtronic PLGM system suspends basal insulin when sensor glucose (SG) is at or within 3.9 mmol/L (70 mg/dl) above the patient-set low limit and is predicted to be 1.1 mmol/L (20 mg/dl) above this low limit in 30 min. Following pump suspension and in the absence of user interference, insulin infusion resumes after a maximum suspend period of 2 h or earlier if the auto-resumption parameters are met. Prospective and retrospective studies have shown that PLGM reduces hypoglycemia.4-6, 192, 193 Time spent in hypoglycemia <3.5 mmol/L was reduced from 2.8% at baseline to 1.5% during the 6-month study compared with a reduction from 3% to 2.6% with SAPT, representing close to 50% reduction in hypoglycemia.4
The Tandem Basal IQ, available with Dexcom G6® CGM and Tandem t:slim X2 pump, uses a linear regression algorithm that relies on the last four SG values to predict SG level in 30 min. It suspends basal insulin when SG is predicted to be 4.4 mmol/L (80 mg/dl) in 30 min or current SG is <3.9 mmol/L (70 mg/dl). Time spent in hypoglycemia <3.9 mmol/L decreased from 3.6% at baseline to 2.6% during the 3-week PLGM period compared with 3.2% with SAPT, representing a 31% relative reduction in hypoglycemia.191 The insulin resumption is more aggressive and hence there was no difference in the mean SG levels in the two groups or in the time spent in hyperglycemia.191
Hybrid closed-loop systems
Automated insulin delivery offers the potential to mitigate the significant glycemic excursions associated with conventional therapy. These systems utilize a control algorithm that automatically and continually increases and decreases the subcutaneous delivery of insulin based on real-time sensor glucose levels. Several systems are available but all may not have national regulatory approvals: Medtronic 670G/770G194, 195 and Medtronic 780G196 with advanced algorithm is approved for use in children 7 years and above, Control IQ (Tandem Inc., San Diego, California)197, 198 for 6 years and above and CamAPS FX interoperable app118, 199-201 (CamDiab, Cambridge, UK) for 1 year and above. These hybrid closed-loop systems require user input of insulin bolus for meals ± corrections. Both in clinical trials and in observational studies, these systems have consistently shown a reduction in time spent in hypoglycemia while improving time in target glucose range.194, 195, 197, 202, 203 Improved glycemic variability, especially overnight, with reduced hypoglycemia has the potential to improve sleep and quality of life in children and their parents.204 Individuals with IAH also have the potential to improve their hypoglycemia awareness with these systems.118 Use of closed loop systems in individuals with IAH showed reduction in hypoglycemia with higher self-reported hypoglycemia scores during controlled hypoglycemia. However, this was not associated with an improvement in counter regulatory hormonal responses which could be due to the relatively short duration (8 weeks) use of the system.118 Advancements in this field are ongoing in the pursuit of a fully automated closed loop system that can further improve glycemic outcomes and reduce the burden of disease in individuals with T1D.
11 SUMMARY
Diabetes management should optimize glycemia with minimal hypoglycemia to positively impact quality of life. Hypoglycemia education and management is fundamental in the care of children with T1D. This guideline provides an evidence-based approach to hypoglycemia management.
AUTHOR CONTRIBUTION
All authors contributed to the content of the chapter, reviewed and approved the final version of the manuscript.
ACKNOWLEDGEMENT
Open access publishing facilitated by The University of Western Australia, as part of the Wiley - The University of Western Australia agreement via the Council of Australian University Librarians.
CONFLICT OF INTEREST
Dr Dovc received speaker's honoraria from Abbott, Pfizer, Novo Nordisk, and Eli Lilly and Advisory Board honoraria from Sanofi and Pfizer. Dr Dovc is a member of the European Commission Expert panel for Medical Devices for Endocrinology and Diabetes. Dr Abraham received speaker's honoraria for educational sessions organized by Medtronic Australia and Eli Lilly.
APPENDIX A
A.1 CLARKE'S HYPOGLYCAEMIA AWARENESS QUESTIONNAIRE
(Tick only one response for each question)
1. 2. 3. 4. |
Tick the category that best describes you.
Have you lost some of the symptoms that used to occur when your blood sugar was low?
In the past 6 months how often have you had moderate hypoglycemia episodes where you may have been confused, disorientated or lethargic, and needed help to treat yourself?
In the past year, how often have you had severe hypoglycemic episodes, where you were unconscious or had a seizure and needed glucagon or intravenous glucose?
|
5. 6. 7. |
How often in the last month have you had readings < 70 mg/dl (<3.9 mmol/L) with symptoms?
How often in the last month have you had readings < 70 mg/dl (<3.9 mmol/L) without symptoms?
Scoring Q5 and Q6: R = answer to Q5 is less than answer to Q6 A = answer to Q5 is greater than or equal to answer to Q6 How low does your blood sugar go before you feel symptoms?
|
8. |
To what extent can you tell by your symptoms that your blood sugar is low?
Scoring A = aware, R = reduced awareness, U = unaware Four or more R responses = reduced awareness |
- Source: Adapted from Reference 205.
APPENDIX B
B.1 MODIFIED CLARKE'S HYPOGLYCEMIA AWARENESS QUESTIONNAIRE
(Tick only one response for each question)
|
|
A = 0 PU = 1 U = 2 |
In the last month, how many times have you tested and found a BGL less than 3.5 mmol/L (63 mg/dl) without realizing your BGL was low?
|
A = 0 PU = 1 U = 2 |
2. Tick the box that best describes you. I…
….have signs when my BGL is low. |
U = 2 PU = 1 A = 0 A = 0 |
3. How low does your BGL fall before you notice any signs?
|
U = 2 U = 2 PU = 1 PU = 1 A = 0 |
4. Can you tell your BGL is low by certain signs or behavior?
|
U = 2 A = 0 |
5. Have you lost some of the signs and symptoms that used to occur when your BGL was low?
|
- Note: Questions 2 to 6 are scored A: Aware, PU: partially unaware, U: unaware. A score of ≥4 implies impaired awareness of hypoglycemia.
- Source: Adapted from Reference 205.
APPENDIX C
C.1 GOLD SCORE
Do you know when your hypos are commencing? (circle one only).
| Always aware | Never aware | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 |
- Note: A score of ≥4 implies impaired awareness of hypoglycemia.
- Source: Adapted from Reference 99.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.



 I always have symptoms when my blood sugar is low (A)
I always have symptoms when my blood sugar is low (A) I sometimes have symptoms when my blood sugar is low (R)
I sometimes have symptoms when my blood sugar is low (R) I no longer have symptoms when my blood sugar is low (R)
I no longer have symptoms when my blood sugar is low (R) Yes (R)
Yes (R) No (A)
No (A) Never (A)
Never (A) Once or twice (R)
Once or twice (R) Every other month (R)
Every other month (R) Once a month (R)
Once a month (R) More than once a month (R)
More than once a month (R) Never (A)
Never (A) 1 to 3 times (R)
1 to 3 times (R) 4 to 7 times (R)
4 to 7 times (R) 8 to 11 times (R)
8 to 11 times (R) 12 times or more (R)
12 times or more (R) Never
Never 1 to 3 times in the month
1 to 3 times in the month Once a week
Once a week 2 to 3 times a week
2 to 3 times a week 4 to 5 times a week
4 to 5 times a week Almost every day
Almost every day Never
Never 1 to 3 times in the month
1 to 3 times in the month Once a week
Once a week 2 to 3 times a week
2 to 3 times a week 4 to 5 times a week
4 to 5 times a week Almost every day
Almost every day 60–69 mg/dl or 3.3–3.8 mmol/L (A)
60–69 mg/dl or 3.3–3.8 mmol/L (A) 50–59 mg/dl or 2.8–3.3 mmol/L (A)
50–59 mg/dl or 2.8–3.3 mmol/L (A) 40–49 mg/dl or 2.2–2.7 mmol/L (R)
40–49 mg/dl or 2.2–2.7 mmol/L (R) <40 mg/dl or <2.2 mmol/L (R)
<40 mg/dl or <2.2 mmol/L (R) Never (R)
Never (R) Rarely (R)
Rarely (R) Sometimes (R)
Sometimes (R) Often (A)
Often (A) Always (A)
Always (A) None
None One to three times in the last month
One to three times in the last month Once a week
Once a week Two to three times a week
Two to three times a week More than three times a week
More than three times a week None
None One to four times
One to four times Greater than four times
Greater than four times always
always sometimes
sometimes never
never Less than 2.5 mmol/L (45 mg/dl)
Less than 2.5 mmol/L (45 mg/dl) 2.5–3.0 mmol/L (45–54 mg/dl)
2.5–3.0 mmol/L (45–54 mg/dl) 3.0–3.5 mmol/L (54–63 mg/dl)
3.0–3.5 mmol/L (54–63 mg/dl) Greater than 3.5 mmol/L (63 mg/dl)
Greater than 3.5 mmol/L (63 mg/dl) Never
Never Rarely
Rarely Sometimes
Sometimes Usually
Usually Always
Always Yes
Yes No
No
