Effect of i.v. immunoglobulin in the first 4 days of illness in Kawasaki disease
Abstract
Background
Although early treatment of Kawasaki disease (KD) with i.v. immunoglobulin (IVIG) is expected to prevent coronary artery abnormalities, the effectiveness of IVIG by day 4 of illness remains to be determined.
Methods
This was a multi-institutional, retrospective cohort study. Patients diagnosed with KD at ≤4 days of illness were divided into two groups: those who received initial IVIG before and on day 5 of illness. Baseline characteristics were adjusted using propensity scores. The primary endpoint was the need for additional treatment.
Results
Of 339 patients diagnosed with KD by day 4, 181 and 158 received IVIG before and on day 5 of illness, respectively. Patients in the early treatment group had more adverse prognostic factors: infancy, early onset of the principal symptoms, and abnormal laboratory data. We thus adjusted baseline characteristics before treatment decisions using propensity scores. Propensity score matching of the two groups yielded 100 observations. More patients required additional treatment in the matched early treatment group: 37% vs 24% (adjusted OR, 1.7; 95%CI: 1.06–2.8; P = 0.047). The difference was more pronounced for risk of relapse after initial resolution of fever: 14% vs 5.0% (adjusted OR, 3.2; 95%CI: 1.3–7.7; P = 0.02). The risk of coronary artery lesion did not differ significantly.
Conclusions
IVIG treatment by day 4 of illness is associated with the requirement for additional treatment even after adjustment of baseline characteristics. Increased resistance to IVIG when given by day 4 should be considered in order to improve the treatment regimen for early-diagnosed KD.
Kawasaki disease (KD), an acute vasculitis of childhood that preferentially affects coronary arteries,1 is still the leading cause of acquired heart disease in children in the developed world.2 Timely treatment with high-dose i.v. immunoglobulin (IVIG) is essential to reduce the incidence of coronary artery abnormalities.3, 4 The American Heart Association recommends IVIG treatment by day 7 of illness.5 Moreover, IVIG treatment on day 5 of illness or earlier is shown to reduce the risk of cardiac sequelae, suggesting the efficacy of the earlier initiation of IVIG treatment.6, 7 Several retrospective studies, however, reported that IVIG use at ≤4 days of illness had no benefit in preventing cardiac complications,8, 9 but was instead associated with increased resistance to IVIG treatment.8-12 This raises the question of when IVIG should be started in patients diagnosed with KD by day 4 of illness.
To determine the efficacy of early IVIG treatment, we need to account for the differences in patient background between those treated before and those treated on day 5 of illness. The control group should not include patients diagnosed with KD on day 5 or later, who did not have a chance to receive early IVIG. Diagnosis of KD by day 4 of illness in itself is reported to be associated with IVIG resistance;12, 13 this supports the need for limiting the subject group to early-diagnosed patients. In addition, IVIG might be more likely to be used early in severe disease, which is associated with high risk of treatment resistance. These potential confounders make it difficult to determine whether IVIG use by day 4 of illness is a cause of IVIG resistance or only reflects disease severity. A study specifically designed to estimate the effectiveness of early IVIG treatment is needed.
Here, we performed a multi-institutional, retrospective cohort study of KD. The purpose of this study was to evaluate the effectiveness of IVIG by day 4 of illness in patients with KD.
Methods
Study design, setting, and participants
This was a multi-institutional, retrospective cohort study. Detailed clinical data were obtained by retrospective chart review of KD patients consecutively admitted to the eight hospitals in Japan between August 2006 and January 2013. No other cause such as infection, allergy and collagen disease was identified in these patients. Two hospitals changed their primary treatment of severe KD from IVIG alone to IVIG plus prednisolone, following the report of the efficacy of IVIG plus prednisolone for severe KD in April 2012.14 We excluded all the KD patients admitted to those hospitals after the change in the treatment strategy. The other institutions did not change their treatment strategy.
The first day of fever was defined as day 1. Patients diagnosed with KD by day 4 of illness were included in the study (Fig. 1). Diagnosis of KD was based on the presence of at least five of the six principal findings.15 Patients with five principal features including fever for 4 days were also diagnosed with KD according to the American Heart Association guidelines.5 When a patient presented with a second or more episodes of KD diagnosed by day 4, only the first episode was included. We excluded patients who had received IVIG in the first 3 months of admission, those who had not undergone laboratory tests until day 5, those who developed coronary artery lesions by day 4 and initial IVIG use, those who did not receive IVIG, those treated with IVIG before they met the diagnostic criteria, and those who received initial treatment other than IVIG, aspirin, flurbiprofen, and dipyridamole. Patients were also excluded when the initial IVIG dose was <1.6 g/kg or was given after day 5. The remaining patients were classified into two groups: those who received the initial IVIG by day 4 of illness (the early treatment group) and those who were treated on day 5 (the conventional treatment group).
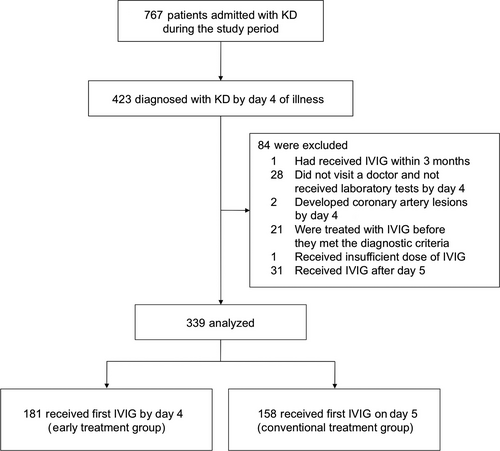
The parents of all patients gave written informed consent for the treatment. They did not receive any stipend. The institutional review board of the University of Tokyo Hospital approved this study protocol and waived the requirement for informed consent.
Adjustment for differences in baseline characteristics
Patients who received the initial IVIG by day 4 of illness (the early treatment group) were compared with those who were treated on day 5 (the conventional treatment group). We collected information on the following factors known to be associated with IVIG resistance or cardiac complications:16 infancy (<12 months of age), sex, medical history of KD, illness day at diagnosis, number of principal symptoms at diagnosis, and laboratory results by day 4 and initial IVIG use, which included white blood cell (WBC) count, neutrophil percentage, hematocrit, platelet count, and serum concentration of albumin, lactate dehydrogenase (LDH), aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, sodium, and C-reactive protein (CRP).10-13, 17-23 If laboratory tests were performed more than once, we used the highest value for WBC count, neutrophil percentage, LDH, AST, ALT, total bilirubin, and CRP and the lowest value for hematocrit, platelet count, and sodium. The missing continuous variables were imputed by multiple imputation methodology as implemented in the R package Multivariate Imputation by Chained Equations (MICE).24, 25 Propensity scores for each patient were averaged across 10 randomly imputed complete data sets.26
Propensity scores estimating the probability of receiving the initial IVIG by day 4 were calculated by logistic regression including the aforementioned variables. All the continuous variables were modeled as a linear trend. Patients in the two groups were matched 1:1 without replacement on the logit of the estimated propensity score using caliper width 0.20 of the SD of the logit of the propensity score.27 We confirmed the validity of the model by comparing the standardized differences of the covariates between the groups.
Outcomes
The primary endpoint was IVIG resistance, defined as the need for additional treatment because of persistent fever or relapsing fever associated with other KD symptoms after resolution of fever. The secondary endpoints were duration of fever, and development of coronary artery lesions during the first month of the illness, and presence of coronary artery lesions at 1 month of illness. Axillary 24 h temperature <37.5°C was defined as afebrile. Duration of fever was defined as total febrile days. Abnormal coronary artery was defined as internal luminal diameter ≥3.0 mm in children <5 years of age or ≥4.0 mm in those aged ≥5 years, when the internal diameter of any segment was ≥1.5-fold that of an adjacent segment, or when the luminal contour was clearly irregular.28
Statistical analysis
Patient characteristics and treatment frequency were compared between groups as follows. For unadjusted (whole cohort) comparison, categorical variables were compared using Fisher's exact test, and numerical values were compared using Wilcoxon rank-sum test. We compared distribution of patient characteristics and treatment frequency within the matched cohort using the standardized differences and McNemar's test, respectively.
Prevalence of IVIG resistance and coronary artery lesions were compared for the whole cohort using Fisher's exact test. Matched-pair comparisons were performed using the paired Wilcoxon signed-rank test for duration of fever and McNemar's test for prevalence of IVIG resistance and coronary artery lesions.
The following sensitivity analyses were performed. First, inverse probability weighting based on the estimated propensity score was applied to the whole cohort and to the 264 patients who did not have missing variables. Second, we compared rates of IVIG resistance between the matched and unmatched patients within each treatment group. Third, we included patients who received their initial IVIG after day 5 of illness (i.e. patients who were originally excluded). Fourth, we also included hospital identification number as a covariate in the propensity score model. Finally, to account for possible misspecification of the propensity score, forward stepwise multivariate logistic regression analysis using the Akaike information criterion was carried out with IVIG resistance as a dependent variable.
All reported P values were two-sided, and P < 0.05 was regarded as significant. Statistical analysis were performed using R version 3.0.1 (R Foundation, Vienna, Austria) and the packages Matching and MICE.24, 25, 29
Results
Subject characteristics
A total of 767 patients were diagnosed with KD during the study period (Fig. 1). Of these, 423 patients satisfied the diagnostic criteria by day 4, and had a chance to receive initial IVIG by day 4 of illness. We removed 84 patients who fulfilled the exclusion criteria from the analysis, and the remaining 339 patients were studied. Subject characteristics are listed in Table 1. Median age was 21 months, and 38% were female. In the present cohort, 181 patients (53%) received the initial IVIG by day 4 of illness (the early treatment group). The remaining 158 (47%)s were observed until day 5, followed by initial IVIG treatment on day 5 of illness (the conventional treatment group).
| Unadjusted | Propensity score matching | ||||||
|---|---|---|---|---|---|---|---|
| Missing (%) | Early treatment group %, median (IQR) or mean ± SD | Conventional treatment group %, median (IQR) or mean ± SD | P-value | Early treatment group %, median (IQR) or mean ± SD | Conventional treatment group %, median (IQR) or mean ± SD | Standardized difference (%) | |
| n | 181 | 158 | 100 | 100 | |||
| Age (months) | 0 | 19 (9–41) | 22 (13–37) | 0.11 | 19.5 (10–36.5) | 21 (11–35) | 5.5 |
| Infants | 0 | 33 | 20 | 0.010 | 29 | 29 | 0 |
| Male | 0 | 66 | 58 | 0.12 | 65 | 63 | 4.1 |
| Past medical history of KD | 0 | 4.4 | 1.9 | 0.23 | 3.0 | 2.0 | 6.4 |
| Days of illness at diagnosis | 0 | 3.6 ± 0.7 | 3.9 ± 0.3 | <0.001 | 3.9 ± 0.4 | 3.9 ± 0.4 | 2.7 |
| No. principal symptoms at diagnosis | 0 | 5.61 ± 0.49 | 5.56 ± 0.50 | 0.29 | 5.50 ± 0.50 | 5.49 ± 0.50 | 2.0 |
| Laboratory data before day 5 of illness and first IVIG | |||||||
| WBC (×109/L) | 0 | 15.3 (12.3–18.5) | 14.3 (11.8–16.4) | 0.057 | 14.7 (11.5–17.9) | 15.0 (12.6–17.0) | 5.7 |
| Neutrophil (%) | 10 | 70.4 (60.0–81.0) | 70.0 (62.0–80.5) | 0.83 | 70.0 (61.6–79.5) | 70.0 (60.0–80.9) | 1.9 |
| Hematocrit (%) | 0 | 34.0 (32.1–35.7) | 34.0 (32.2–35.5) | 0.93 | 34.0 (31.8–35.8) | 34.0 (32.4–35.5) | 2.2 |
| Platelet (×109/L) | 0 | 323 (254–375) | 307 (265–379) | 0.78 | 329 (275–374) | 312 (265–386) | 3.4 |
| Albumin (g/L) | 2.4 | 37 (35–40) | 38 (36–41) | 0.020 | 38 (36–41) | 37 (35–40) | 7.9 |
| LDH (U/L) | 0.59 | 287.5 (225–373) | 294.0 (244.0–358) | 0.71 | 290 (233–378) | 301 (253–363) | 2.1 |
| AST (U/L) | 0 | 50 (28–198) | 45 (27–104) | 0.070 | 45 (28–166) | 49 (29–131) | 3.8 |
| ALT (U/L) | 0 | 139 (24–292) | 59 (15–246) | 0.004 | 101 (22–267) | 104 (19–259) | 1.2 |
| Total bilirubin (mg/dL) | 12 | 0.65 (0.50–1.34) | 0.60 (0.50–0.90) | 0.34 | 0.60 (0.46–0.99) | 0.61 (0.50–1.1) | 0.99 |
| Sodium (mmol/L) | 0 | 133 (132–135) | 133 (132–135) | 0.61 | 133 (132–135) | 133 (131–135) | 0.33 |
| CRP (mg/L) | 0 | 71.8 (45.8–102.3) | 75.5 (48.8–109.4) | 0.68 | 75.1 (45.3–107.0) | 75.8 (48.5–109.9) | 0.83 |
- ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; IVIG, i.v. immunoglobulin; KD, Kawasaki disease; LDH, lactate dehydrogenase; WBC, white blood cell count.
Patients in the early treatment group had more adverse prognostic factors than those in the conventional treatment group: infancy, early onset of the principal symptoms, high serum ALT concentration, and low albumin concentration (Table 1). This supported the hypothesis that patients with severe disease are more likely to be treated with early IVIG. We thus adjusted differences in baseline characteristics between the treatment groups using propensity scores. Propensity score matching of the two groups yielded 100 observations. Adequate balance in baseline variables was achieved at this time, with all of the standardized differences being <10% (Table 1).
Treatment outcome
Unadjusted analysis
Treatment details are listed in Table 2. All the patients were first treated with 2 g/kg IVIG. Flurbiprofen and dipyridamole were sometimes used as an alternative to aspirin in patients with liver damage. Other therapies including prednisolone were not used in the initial treatment. No patient required four or more doses of IVIG.
| Unadjusted | Propensity score matching | |||||
|---|---|---|---|---|---|---|
| Early treatment group (n = 181) (%) | Conventional treatment group (n = 158) (%) | P-value | Early treatment group (n = 100) (%) | Conventional treatment group (n = 100) (%) | P-value | |
| IVIG | 100 | 100 | 1 | 100 | 100 | 1 |
| Aspirin | 93 | 91 | 0.55 | 93 | 89 | 0.35 |
| Flurbiprofen | 0.55 | 5.1 | 0.014 | 1.0 | 6.0 | 0.06 |
| Dipyridamole | 5.0 | 5.7 | 0.81 | 2.0 | 7.0 | 0.10 |
| Additional treatment | ||||||
| Second dose of IVIG | 33 | 23 | 0.035 | 37 | 24 | 0.047 |
| Third dose of IVIG | 4.4 | 5.1 | 0.80 | 3.0 | 6.0 | 0.26 |
| PSL or mPSL | 12 | 10 | 0.61 | 13 | 10 | 0.49 |
| Infliximab | 0.55 | 0.63 | 1 | 0 | 1.0 | 0.32 |
| Plasma exchange | 0.55 | 0 | 1 | 0 | 0 | NA |
- IVIG, i.v. immunoglobulin; KD, Kawasaki disease; mPSL, methyl-prednisolone; NA, not assessed; PSL, prednisolone.
Information on treatment outcomes was available for all the patients. On unadjusted analysis, the early treatment group had a significantly higher rate of IVIG resistance than the conventional treatment group (33% vs 23%; OR, 1.7; 95%CI: 1.04–2.7; P = 0.04; Table 3). We classified the IVIG resistance group into two classes: those with persistent fever, and those with relapsing fever after resolution of fever. While no significant difference was found in the rate of persistent fever (20% vs 18%; OR, 1.1; 95%CI: 0.67–2.0; P = 0.68; Table 3), the difference was pronounced in the risk of relapse after initial resolution of fever (13% vs 4.4%; OR, 3.1; 95%CI: 1.3–7.5; P = 0.007; Table 3). The risk of coronary artery lesions did not differ significantly (Table 3).
| Early treatment group (%) | Conventional treatment group (%) | OR | 95%CI | P-value | |
|---|---|---|---|---|---|
| Whole cohort | |||||
| IVIG resistance | 33 | 23 | 1.7 | 1.04–2.7 | 0.04 |
| Persistent fever after first dose of IVIG | 20 | 18 | 1.1 | 0.67–2.0 | 0.68 |
| Relapse of fever after first dose of IVIG | 13 | 4.4 | 3.1 | 1.3–7.5 | 0.007 |
| Coronary artery lesions during the first month | 14 | 15 | 0.94 | 0.51–1.7 | 0.88 |
| Coronary artery lesions at 1 month | 7.7 | 4.4 | 1.8 | 0.71–4.6 | 0.26 |
| Propensity score-matched cohort | |||||
| IVIG resistance | 37 | 24 | 1.7 | 1.06–2.8 | 0.047 |
| Persistent fever after first dose of IVIG | 23 | 19 | 1.2 | 0.68–2.0 | 0.48 |
| Relapse of fever after first dose of IVIG | 14 | 5.0 | 3.2 | 1.3–7.7 | 0.02 |
| Coronary artery lesions during the first month | 12 | 14 | 0.92 | 0.50–1.7 | 0.67 |
| Coronary artery lesions at 1 month | 9.0 | 5.0 | 1.86 | 0.73–4.7 | 0.29 |
- IVIG, i.v. immunoglobulin; KD, Kawasaki disease.
We next compared the duration of fever. The total duration of fever was significantly shorter in the early treatment group (mean, 6.0 vs 6.4 days; P < 0.001; Fig. 2) despite the higher rate of IVIG resistance. The total duration of fever was divided into duration of initial fever and that of relapsing fever. The duration of the initial fever was significantly shorter in the early treatment group (mean, 5.5 vs 6.3 days; P < 0.001; Fig. 2). The shorter duration of the initial fever was slightly offset by the significantly longer duration of the relapsing fever in the early treatment group (mean, 0.42 vs 0.11 days; P = 0.003; Fig. 2). Although patients in the conventional treatment group had relapsing fever associated with other KD symptoms ≤4 days after resolution of the initial fever, relapses occurred for >10 days in the early treatment group (Fig. 3). The total duration of fever, however, remained significantly shorter in the early treatment group.
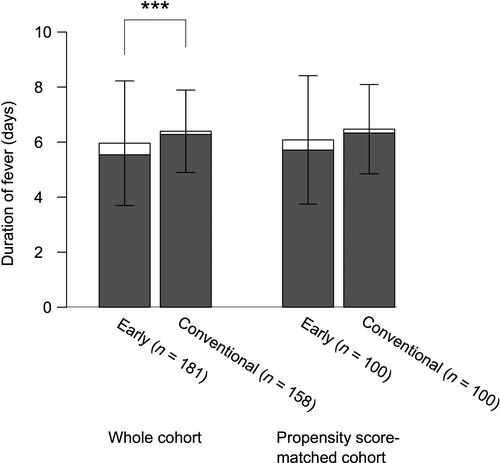
 ) Initial fever; (
) Initial fever; ( ) relapsing fever. Error bars, SD. ***P < 0.001.
) relapsing fever. Error bars, SD. ***P < 0.001.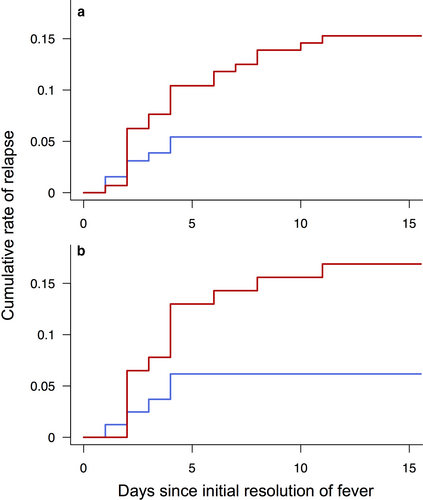
 ) early treatment group and (
) early treatment group and ( ) conventional treatment group.
) conventional treatment group.Propensity score-matching analysis
We next compared treatment outcomes in the propensity score-matched cohort. The rate of IVIG resistance was higher in the early treatment group even after propensity score matching (37% vs 24%; adjusted OR, 1.7; 95%CI: 1.06–2.8; P = 0.047; Table 3). The difference was again more prominent for the risk of relapse of fever (14% vs 5.0%; adjusted OR, 3.2; 95%CI: 1.3–7.7; P = 0.02; Table 3). No difference in the risk of coronary artery lesions was found between the two groups (Table 3).
The duration of the initial fever was again significantly shorter in the early treatment group (mean, 5.7 vs 6.3 days; P = 0.004; Fig. 2). Due, however, to the longer, but not significant, duration of relapsing fever (mean, 0.37 vs 0.14 days; P = 0.21; Fig. 2), the total duration of fever did not differ significantly between the early and conventional treatment groups (mean, 6.1 vs 6.5 days; P = 0.06; Fig. 2).
We conducted the following sensitivity analyses. First, inverse probability weighting based on the propensity score was used to adjust for differences between the two treatment groups. This analysis also confirmed a higher rate of IVIG resistance in the early treatment group than in the conventional treatment group (34% vs 22%; OR, 1.81; 95%CI: 1.12–2.92; P = 0.02). OR did not change significantly even when 264 patients who did not have missing variables were analyzed (OR, 1.7; 95%CI: 1.0–3.0; P = 0.048). This indicates that the main result using multiple imputation is robust. Second, we compared the rates of IVIG resistance between the unmatched patients and matched patients in each treatment group. Although the unmatched patients in the early treatment group (n = 81) were expected to have more severe disease, they did not have a higher risk of treatment resistance as compared with the matched patients (28% vs 37%; OR, 0.68; 95%CI: 0.36–3.69; P = 0.27). In the conventional treatment group, the prevalence of treatment resistance did not differ between the unmatched and matched patients (21% vs 24%; OR, 0.83; 95%CI: 0.38–5.00; P = 0.70). Third, we included 31 patients who received initial IVIG after day 5 of illness (i.e. patients who were originally excluded). The rate of IVIG resistance was again higher in the early treatment group even after propensity score matching (34% vs 21%; adjusted OR, 2.0; 95%CI: 1.3–3.2; P = 0.03). Fourth, we also recalculated propensity scores including hospital identification number as a covariate. A higher rate of IVIG resistance in the early treatment group was confirmed in the propensity score matched cohort: 35% vs 22% (adjusted OR, 1.7; 95%CI: 1.04–2.7; P = 0.03). IVIG by day 4 was not associated with the risk of coronary artery lesions in all of the aforementioned analyses. Finally, we performed a forward stepwise multivariate logistic regression analysis. Higher serum AST and CRP concentration, lower ALT concentration, lower platelet count, and initial IVIG by day 4 of illness (OR, 1.7; 95%CI: 1.00–2.7; P = 0.05) were selected in the regression model to predict IVIG resistance (Table 4).
| Logistic coefficient | OR | 95%CI | Wald statistic | P-value | |
|---|---|---|---|---|---|
| AST (U/L) | 0.0023 | 1.0023 | 1.0008–1.0039 | 8.84 | 0.0030 |
| CRP (mg/dL) | 0.064 | 1.066 | 1.014–1.12 | 6.22 | 0.013 |
| Initial IVIG by day 4 of illness | 0.50 | 1.66 | 1.00–2.74 | 3.84 | 0.050 |
| Platelets (×109/L) | −0.0027 | 0.997 | 0.996–0.999 | 3.93 | 0.047 |
| ALT (U/L) | −0.0016 | 0.998 | 0.997–1.00 | 3.62 | 0.057 |
- ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; IVIG, i.v. immunoglobulin; KD, Kawasaki disease.
Discussion
This study shows that initial IVIG by day 4 of illness is associated with a requirement for additional treatment, especially due to recrudescent fever, even after adjustment of baseline characteristics. This indicates that IVIG treatment by day 4 of illness is associated with a requirement for additional treatment independently of early diagnosis of KD. This suggests that early IVIG use in itself might be a cause of IVIG resistance. In assessing the risk of IVIG resistance for treatment stratification of KD, it might be better to consider increased resistance to IVIG when given by day 4.
The risk factors of IVIG resistance as well as coronary aneurysms have been extensively studied. Although IVIG treatment by day 4 of illness was reported to be associated with IVIG resistance, the differences in patient background between those treated before and those treated on day 5 of illness should be taken into account.8-12 We adjusted differences in the baseline characteristics to better infer a causal relationship between early IVIG use and treatment resistance. We investigated when principal symptoms appeared in each case to identify patients who were diagnosed early but who were not treated with IVIG until day 5. Patients who were diagnosed with KD on day 5 or later were not candidates for early IVIG use, and were excluded from the control group (Fig. 1). It should be noted that patients who did not visit a doctor or did not undergo laboratory tests until day 5 were also excluded from the analysis. Moreover, physicians are expected to start treatment early in the case of severe KD. A recent paper addressed this issue by stratifying patients according to the need for additional treatment, followed by comparison of the risk of cardiac complications between the early and conventional treatment groups.30 This approach is based on a hypothesis that treatment timing does not affect risk of treatment resistance, which should be tested separately. We thus adjusted known confounders using propensity scores. Adjustment of baseline characteristics was made based on variables before the decision to start treatment, not on those before treatment. Variables before treatment included laboratory data on day 5 or later, but they were available only in the late treatment group. We found considerable overlap in the baseline characteristics between the early and conventional treatment groups. This overlap enabled us to adequately adjust patient characteristics.
A higher rate of treatment resistance in the early treatment group suggests that response to IVIG changes during the disease course. A higher incidence of relapsing fever after transient remission raises the possibility that early IVIG is more likely to lose its effect before the inflammation subsides. Serum Ig concentration changes significantly after i.v. administration. An initial sharp rise in serum Ig concentration after i.v. administration is followed by a rapid waning for 1–4 days due to catabolism of Ig and distribution to extravascular spaces.31 Meanwhile, a concentration-dependent immunomodulatory effect of IVIG in KD has been demonstrated.3, 32-34 We speculate that early IVIG treatment might have resulted in an earlier reduction in Ig levels. In addition, the immunological profile, that is, the activation status of monocytes/macrophages, lymphocyte profile, and level of serum cytokines changes dramatically during the acute phase of KD.35 These changes could affect the immunomodulatory effect of IVIG. Future research should investigate the precise pharmacokinetics of IVIG as well as the influence of immunological changes during the acute phase of KD on treatment response.
Approximately one-third of the patients with KD rapidly develop symptoms and are diagnosed by day 4 of illness in Japan.8 They are known to be at high risk of IVIG resistance and coronary artery abnormalities.11, 13 The optimal timing of initial IVIG in these patients has long been discussed in order to improve outcome.5 In the present study, initiation of IVIG by day 4 was associated with a requirement for additional treatment. Although the present results do not provide a definitive answer to the question of when to administer the initial IVIG in early-diagnosed KD, some clinical implications can be drawn from this study. The more frequent recrudescent fever in the early treatment group suggests the need for a careful clinical approach. After early IVIG, patients need to be closely monitored for relapse. It might also be better to carefully taper aspirin or steroids, when used. Predictive factors of relapsing fever, which will refine treatment stratification, should also be investigated.
This study has several limitations. First, although adjustment of baseline characteristics achieved adequate balance between the two groups, the potential remains for unmeasured confounders to have influenced the accuracy of the comparisons. The criteria for when to provide IVIG are unclear and differ between physicians. We addressed this limitation inherent to observational studies by adjusting for as many known risk factors as possible. Considering that adjustment did not largely reduce the differences in outcome, it would be difficult to assume the major influence of unmeasured confounders. Second, the type of additional treatment after the first dose of IVIG was not standardized. This could have influenced the duration of fever and the incidence of coronary artery lesions. Nevertheless, IVIG resistance was an event before the additional treatment and was not affected by additional treatment. Third, we did not evaluate coronary dimensions based on z score, because z score for distal coronary arteries was not available. Mild dilatation of coronary arteries might have not been detected, but this is unlikely to have affected the relative incidence of coronary artery lesions between the two treatment groups. Finally, this study was based on the data before wide acceptance of the efficacy of IVIG plus prednisolone in severe KD,14 but, owing to the uniform treatment strategy, we were able to assess the effectiveness of early IVIG without being confounded by other treatments.
In conclusion, IVIG treatment by day 4 of illness is associated with a requirement for additional treatment even after adjustment of baseline characteristics. Increased resistance to IVIG when given by day 4 should be considered in order to improve the treatment regimen for early-diagnosed KD.
Acknowledgments
All phases of this study were supported by institutional and departmental sources at the Department of Pediatrics, University of Tokyo Hospital, Japan. We deeply appreciate the physicians and nurses at the participating hospitals for their detailed medical records and generous support of this study.
Disclosure
The authors declare no conflict of interest.
Author contributions
Y.S. and R.I. conceptualized and designed the study; Y.S., J.I., Y.Y., Y.N., Y.O., M.T., and A.O. coordinated and supervised data collection; Y.S. carried out all the analyses; T.K. supported for statistical analysis; Y.S. and R.I. wrote the manuscript; T.S., R.M., T.H., Yo.Hi., N.S., Yu.Ha., and T.K. gave conceptual advice and critically reviewed the manuscript; All authors read and approved the final manuscript.




