Melanomas of unknown primary have a mutation profile consistent with cutaneous sun-exposed melanoma
Summary
Melanoma of unknown primary (MUP) is an uncommon phenomenon whereby patients present with metastatic disease without an evident primary site. To determine their likely site of origin, we combined exome sequencing from 33 MUPs to assess the total rate of somatic mutations and degree of UV mutagenesis. An independent cohort of 91 archival MUPs was also screened for 46 hot spot mutations highly prevalent in melanoma including BRAF, NRAS, KIT, GNAQ, and GNA11. Results showed that the majority of MUPs exhibited high somatic mutation rates, high ratios of C>T/G>A transitions, and a high rate of BRAF (45 of 101, 45%) and NRAS (32 of 101, 32%) mutations, collectively indicating a mutation profile consistent with cutaneous sun-exposed melanomas. These data suggest that a significant proportion of MUPs arise from regressed or unrecognized primary cutaneous melanomas or arise de novo in lymph nodes from nevus cells that have migrated from the skin.
Significance
Little is known about the mechanism of development of melanomas of unknown primary (MUPs). To address this issue, we performed a large comprehensive genetic analysis of MUP and show that the majority has a mutation profile consistent with cutaneous sun-exposed melanomas. As our data suggest that MUPs arise via regressed lesions or develop de novo in lymph nodes, the common practice to perform exhaustive searches to identify the primary site may be costly, time-consuming, and could be considered redundant. Furthermore, the majority of these patients should be responsive to current and emerging therapeutic modalities (including BRAF inhibitor strategies).
Introduction
Melanoma of unknown primary (MUP) is an uncommon phenomenon whereby a patient presents with metastatic melanoma without a clinical history of previous melanoma and no evidence of a concurrent or regressed primary tumor (Dasgupta et al., 1963). Existing literature regarding MUP is sparse and often contradictory, in part due to its unusual presentation, its infrequency of occurrence, and variability in its clinical definition (Kamposioras et al., 2011).
Theories explaining the occurrence of MUP include spontaneous regression of the primary melanoma after it has metastasized (Smith and Stehlin, 1965), de novo development of melanoma in lymph nodes from nodal nevus cells (Ridolfi et al., 1977; Shenoy et al., 1987), metastasis from an unrecognized primary tumor, metastasis from a previously excised but misdiagnosed lesion, or metastasis from an unusual or concealed primary site. A better understanding of the mechanism of MUP development would help guide optimal therapeutic approaches and appropriate clinical investigative practices.
Recent advances in sequencing technologies have led to significant progress in understanding the genetic diversity of melanoma (Berger et al., 2012; Hodis et al., 2012; Krauthammer et al., 2012; Nikolaev et al., 2012; Pleasance et al., 2010; Stark et al., 2012; Wei et al., 2011). In particular, exome sequencing studies have shown that melanomas arising in sun-exposed sites display high rates of somatic mutations with characteristic signatures of ultraviolet radiation (UVR) mutagenesis, which results in preferential C>T/G>A DNA alterations (Hodis et al., 2012; Krauthammer et al., 2012; Pleasance et al., 2010). In contrast, sun-shielded acral, mucosal, and uveal melanomas harbor a lower mutation rate and tend to lack the UVR damage signature (Furney et al., 2012; Harbour et al., 2010; Hodis et al., 2012; Krauthammer et al., 2012; Turajlic et al., 2012; Van Raamsdonk et al., 2009). These genetic differences suggest distinct key drivers and regulators of sun-exposed and sun-shielded melanomas.
Melanoma subtypes differ in their profile of somatic mutations, with different genes tending to drive melanomagenesis (Curtin et al., 2005; Dutton-Regester and Hayward, 2012). Cutaneous melanomas, representing ~90% of all diagnosed melanomas, frequently exhibit mutually exclusive recurrent mutations in BRAF and NRAS resulting in the constitutive activation of the mitogen-activated protein kinase (MAPK) pathway. In contrast, MAPK pathway activation in acral and mucosal melanomas is more frequently driven by mutation or amplification of KIT (Curtin et al., 2006), while mutually exclusive mutations in GNAQ and GNA11 are responsible for MAPK activation in uveal melanoma (Van Raamsdonk et al., 2009, 2010).
These relatively distinctive somatic mutation profiles can provide information on the origin of a melanoma metastasis. As such, we performed an extensive genetic characterization of a large, carefully defined patient cohort of MUP to identify their somatic mutation burden and therefore their association with sun exposure and likely primary site of origin of these tumors.
Results
Exome sequencing performed on 10 early passage MUP cell lines and their matched normal counterparts revealed a total somatic mutation rate between 18 and 14 559 mutations, while the total number of non-synonymous mutations varied between 13 and 9001 (Table S1). Nine of the ten MUP cell lines had >100 somatic non-synonymous mutations (Figure 1A). Furthermore, those MUP cell lines with a higher rate of mutation typically exhibited a higher proportion of C>T/G>A transitions, indicative of a UVR mutagenesis signature (Figure 1B). Exome sequencing data from an additional 22 publicly available MUP tumors revealed a total somatic non-synonymous mutation rate ranging from 0 to 680, with only two of 22 having fewer than 50 mutations (Table 1).
| Cell line | Age at onset | Sex | Data set | No. somatic non-synonymous mutation | Cutaneous | Acral and non-cutaneous | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| BRAF | NRAS | KIT | GNAQ | GNA11 | BAP1 | |||||
| YUFUZZ | 85 | F | 3 | 0 | ||||||
| C092 | 51 | M | 1 | 13 | ||||||
| YUPORCH | 75 | F | 3 | 31 | ||||||
| Ma-mel-15 | 39 | F | 2 | 63 | ||||||
| Ma-mel-28 | 65 | F | 2 | 88 | Q61R | |||||
| Ma-mel-108 | 35 | F | 2 | 94 | V600E | |||||
| YUWHIM | 65 | M | 3 | 94 | V600E | |||||
| YUSEL | 53 | M | 3 | 111 | V600K | |||||
| YUWALI | 41 | M | 3 | 114 | V600E | |||||
| C037 | 27 | F | 1 | 127 | ||||||
| YUZEST | 55 | M | 3 | 129 | V600E | |||||
| YUFARCI | 54 | M | 3 | 152 | Q61K | |||||
| YULLON | 56 | F | 3 | 178 | Q61H | |||||
| Ma-mel-122 | 47 | M | 2 | 209 | V600E | |||||
| YUDIALE | 53 | M | 3 | 211 | V600E | |||||
| D38-T | 48 | M | 1 | 214 | E85K | |||||
| Me037 | 45 | M | 2 | 248 | V600K | |||||
| C004 | 42 | F | 1 | 283 | V600E | |||||
| Me020 | 28 | M | 2 | 321 | V600E | |||||
| YULAPE | 75 | M | 3 | 386 | Q61H | |||||
| Ma-mel-63 | 44 | F | 2 | 399 | V600E | |||||
| Me024 | 48 | M | 2 | 402 | V600E | A895T | ||||
| D01 | 54 | F | 1 | 414 | Q61K | |||||
| Me049 | 47 | M | 2 | 445 | Q61R | |||||
| YUDUTY | 80 | F | 3 | 640 | Q61H | R183C | ||||
| YURTHE | 81 | F | 3 | 642 | ||||||
| C089 | 50 | F | 1 | 658 | V600E | |||||
| Ma-mel-85 | 38 | M | 2 | 680 | V600E | |||||
| C058 | 39 | M | 1 | 762 | L597S | |||||
| D28 | 66 | M | 1 | 1030 | V600K | |||||
| C008 | 65 | F | 1 | 1243 | L349F | |||||
| C086 | 27 | F | 1 | 9001 | R827K | A343T | L216F | |||
- Meta-analysis of MUPs that have been exome sequenced both in this report and from publicly available data sets. The majority of MUPs have >50 non-synonymous mutations and a high prevalence of cutaneous melanoma-associated mutations in BRAF and NRAS mutations. There is a distinct lack of driver mutations in genes associated with acral, mucosal, and uveal melanomas (KIT, GNAQ, GNA11, and BAP1), suggesting that most MUPs develop from cutaneous origins. Data set references: 1. data generated in this project; 2. Hodis et al., 2012;. Cell 150(20):251-263; 3. Krauthammer et al., 2012. Nature Genetics 44(9):1006-1014.
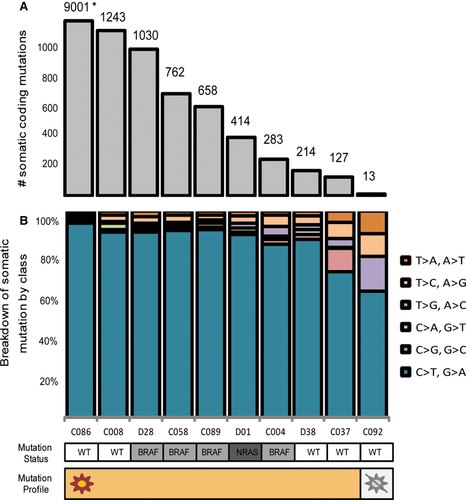
As different genetic mutational processes have been identified as being implicated in the pathogenesis of cutaneous and non-cutaneous subtypes of melanoma (Dutton-Regester and Hayward, 2012), we screened the MUP exome data to determine whether there was a preferential selection of mutations for specific melanoma subtypes (Table 1 and Table S2). Exome sequencing of MUP cell lines revealed profiles typical of cutaneous melanoma origin including mutually exclusive mutations in BRAF (16 of 32, 50%) and NRAS (7 of 32, 22%) and few mutations associated with mucosal, acral, and uveal origin (KIT, GNAQ, GNA11, and BAP1). Although a small number of GNAQ and KIT mutations were identified, these mutations lie outside of the Ras-like domains (Curtin et al., 2006; Van Raamsdonk et al., 2009). The functional significance of these mutations remains unclear, and they may represent passenger mutations rather than bona fide driving events. In addition, the KIT A895T and GNA11 R183C mutations occurred simultaneously with known driver mutations: BRAF V600E and NRAS Q61H, respectively.
Mutation frequencies of key driver genes involved in melanomagenesis were compared between MUP and publicly available exome sequencing data from 151 cutaneous melanomas of known primary (MKPs) (Table S3). Similar rates of non-synonymous point mutations were observed between most genes (BRAF, NRAS, TP53, and ERBB4); however, NF1, CDKN2A, and PTEN were mutated less frequently in MUP compared with cutaneous MKP.
To confirm the high prevalence of cutaneous melanoma-associated driver mutations in MUP observed in our exome sequencing results, we analyzed an independent cohort of FFPE MUP tumors with a targeted melanoma-specific mutation panel (Dutton-Regester et al., 2012b). This independent cohort of samples consisted of 91 MUPs from patients who underwent therapeutic lymph node dissections (TLNDs) at Melanoma Institute Australia between 1993 and 2010 (A. Van der Ploeg, L.E. Haydu, R.A. Scolyer, M.J. Quinn, R.P.M. Saw, K.F. Shannon, A.J. Spillane, J. R. Stretch, J.F. Thompson, submitted), in addition to the original 10 MUP cell lines that were exome sequenced. Results from the melanoma-specific mutation panel were compared with a panel of 247 melanomas with a known primary (MKPs) to identify any significant trends or differences between subgroups (Dutton-Regester et al., 2012b).
As in our exome sequencing results, MUP exhibited a high frequency of BRAF (45 of 101, 44%) and NRAS (32 of 101, 32%) mutations (Table 2 and Tables S4 and S5). A distinct lack of non-cutaneous melanoma mutations was observed in MUP, with only three mutations occurring in GNA11. All other genes screened had comparable rates of mutation between MUP and MKP, except those occurring in PPP6C and IDH1, which were significantly higher in MUP (P < 0.01).
| Gene | MUP (n = 101) | Cutaneous (n = 232) | Uveal (n = 9) | Acral/mucosal (n = 6) | ||||
|---|---|---|---|---|---|---|---|---|
| No. mutated | % | No. mutated | % | No. mutated | % | No. mutated | % | |
| BRAF | 45 | 44.5 | 140 | 60.3 | 2 | 22.2 | 1 | 16.6 |
| NRAS | 33 | 32.6 | 48 | 20.7 | 0 | 0.0 | 1 | 16.6 |
| RAC1 | 6 | 5.9 | 8 | 3.4 | 0 | 0.0 | 0 | 0.0 |
| CDK4 | 6 | 5.9 | 6 | 2.6 | 1 | 11.1 | 0 | 0.0 |
| PTK2B | 8 | 7.8 | 6 | 2.6 | 0 | 0.0 | 0 | 0.0 |
| PPP6C* | 13 | 12.8 | 5 | 2.1 | 0 | 0.0 | 0 | 0.0 |
| ERBB4 | 6 | 5.9 | 5 | 2.1 | 0 | 0.0 | 0 | 0.0 |
| TRRAP | 0 | 0.0 | 5 | 2.1 | 0 | 0.0 | 0 | 0.0 |
| IDH1** | 8 | 7.9 | 4 | 1.7 | 0 | 0.0 | 0 | 0.0 |
| MET | 6 | 5.9 | 3 | 1.3 | 0 | 0.0 | 0 | 0.0 |
| AKT3 | 2 | 1.9 | 2 | 0.9 | 0 | 0.0 | 0 | 0.0 |
| MEK | 3 | 2.9 | 2 | 0.9 | 0 | 0.0 | 0 | 0.0 |
| EPHA10 | 2 | 1.9 | 1 | 0.4 | 0 | 0.0 | 0 | 0.0 |
| GNA11 | 3 | 2.9 | 1 | 0.4 | 1 | 11.1 | 0 | 0.0 |
| KRAS | 4 | 3.9 | 1 | 0.4 | 0 | 0.0 | 0 | 0.0 |
| NEK10 | 0 | 0.0 | 1 | 0.4 | 0 | 0.0 | 0 | 0.0 |
| PDGFRA | 1 | 1.0 | 1 | 0.4 | 0 | 0.0 | 0 | 0.0 |
| PIK3CA | 0 | 0.0 | 1 | 0.4 | 0 | 0.0 | 0 | 0.0 |
| CTNNB1 | 2 | 1.9 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| CXCR4 | 1 | 1.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| EPHB6 | 2 | 1.9 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| IDH2 | 2 | 1.9 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| GNAQ | 0 | 0.0 | 0 | 0.0 | 3 | 33.3 | 0 | 0.0 |
| KIT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Wild type | 12 | 11.9 | 32 | 13.8 | 3 | 33.3 | 4 | 66.8 |
- Melanoma-specific mutation panel results from an independent cohort of MUP compared with melanomas of known primary (MKPs). MUP had a high rate of BRAF and NRAS. Similar to the exome sequencing results, there was a distinct lack of mutations associated with uveal, acral, and mucosal melanomas in MUP. Interestingly, mutation of PPP6C and IDH1 occurred more frequently in MUP compared with cutaneous MKP, suggesting a putative role of these genes in the development of MUP. *P < 0.001, **P < 0.01.
As the majority of MUPs in this independent cohort originated from lymph nodes, we decided to group these tumors into three subgroups based on anatomical location (axilla, groin, and head and neck) to determine whether any significant trends were present. BRAF mutations were most prevalent in MUP of the head and neck (12 of 19 or 63%), while NRAS mutations occurred more frequently in MUP of axilla or groin (Table 3). BRAF V600K mutations were over-represented within tumors arising on the head and neck, consistent with their known association with severe sun damage (Menzies et al., 2012). When total numbers of each respective group of origin were compared with numbers expected by chance calculated on the percentage of skin surface area, MUPs were significantly over-represented in the head and neck region (P < 0.001).
| Characteristic | Male (n = 66) | Female (n = 25) | Total (n = 91) | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Age (years) average | 54.5 | 59.1 | 55.8 | |||
| Age (years) median | 56.5 | 60 | 57 | |||
| Range | 18–87 | 38–79 | 18–87 | |||
| BRAF mutation | 28 | 42 | 12 | 48 | 40 | 44 |
| NRAS mutation | 23 | 35 | 10 | 40 | 33 | 36 |
| BRAF/NRAS wild type | 15 | 23 | 3 | 12 | 18 | 20 |
| Lymph origin | 59 | 90 | 22 | 88 | 81 | 89 |
| Visceral origin | 2 | 3 | 1 | 4 | 3 | 3 |
| Subcutaneous origin | 5 | 7 | 2 | 8 | 7 | 8 |
| MUP of lymph node origin | Axilla (n = 43) | Groin/inguinal/pelvic (n = 19) | Neck/parotid (n = 19) | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| BRAF | 19 | 44 | 6 | 32 | 12 | 63 |
| V600E | 16 | 84 | 5 | 94 | 6 | 50 |
| V600K | 0 | 0 | 1 | 6 | 5 | 41 |
| Other (L597, G466, K601) | 3 | 16 | 0 | 0 | 1 | 8 |
| NRAS | 17 | 40 | 10 | 53 | 4 | 21 |
| BRAF/NRAS wild type | 8 | 19 | 3 | 16 | 3 | 16 |
| Number expected by chance (based on% skin surface area) | 44 | 54 | 44 | 37 | 7 | 9 |
| Number observed* | 43 | 53 | 19 | 24 | 19 | 24 |
- Clinical characteristics and mutation data associated with the independent retrospective cohort of FFPE MUP. When location of presentation was separated into three broad categories (axilla, groin/inguinal/pelvic and neck/parotid), MUP occurred more frequently within the head and neck region than expected by chance (*P < 0.001). Tumors from this region also had a higher proportion of BRAF V600K mutations suggesting chronic sun damage.
Discussion
We describe the most comprehensive genetic analysis of MUP performed to date, combining the use of exome sequencing and oncogenic mutation profiling in order to shed new light on their mechanism of development. Our results indicate that the majority of MUPs have a mutation profile consistent with sun-exposed cutaneous melanomas exhibiting high somatic mutation rates, increased C>T/G>A alterations, frequent oncogenic mutations in BRAF and NRAS, and a propensity for tumors to arise in lymph nodes draining sun-exposed sites.
Although existing literature regarding the genetics of MUP is relatively sparse, studies reporting BRAF and/or NRAS mutation status in large institutional cohorts confirm our findings of comparable rates of mutation between MUP and cutaneous melanomas. A prospective study from an Australian cohort correlating clinicopathologic and prognostic associations of BRAF status in metastatic melanoma found a BRAF mutation frequency of 48% (14 of 29) to 55% (23 of 42) in occult melanomas (Long et al., 2011; Menzies et al., 2012). These findings are similar to a large retrospective analysis of BRAF and NRAS mutation status in 677 patients with melanoma, which revealed NRAS mutation as an independent prognostic factor of shorter survival at stage IV diagnosis (Jakob et al., 2012). Within this cohort, 110 MUPs had an analogous mutation frequency of BRAF (n = 58 or 53%) and NRAS (n = 21 or 19%) status to that of 516 melanomas of cutaneous origin (253 or 49% and 109 or 21.1%, respectively).
Using the results from the genetic screening of MUP, insights into the mechanism of development of this disease can be addressed. Existing theories explaining MUP include spontaneous regression of a primary melanoma, de novo development within lymph nodes from ectopically located nevus cells, origin from an unrecognized or previously excised melanoma misdiagnosed as a nevus, or origin from unusual concealed sites within the body. Careful examination of patient history, general clinical examination, and review of the pathology of any previously excised lesions can exclude most unrecognized primary melanomas. Additional investigations can be used to exclude concealed primary melanomas, but in our experience, these are rarely fruitful; furthermore, the current results demonstrate that the majority of MUPs appear to have a mutation profile consistent with sun-exposed cutaneous melanomas rather than from melanomas of acral, mucosal, or uveal origins.
Our results indicate that the most likely mechanism for the development of MUP is through a model of spontaneous regression of an unrecognized cutaneous primary or the de novo transformation of ectopic nodal nevus cells. The two most commonly proposed mechanisms by which nevus cells may come to be present in lymph nodes are mechanical transportation from cutaneous nevi and aberrant migration from neural crest tissues during embryogenesis. In our view, the former theory is more plausible based on the occasional observation of nevus cells within cutaneous lymphatics and the usual location of nodal nevus cells in the subcapsular space (near the site of entry of afferent lymphatics). Our data would support this theory from the observation of a frequent UVR mutagenesis signature and the high frequency of MUP involving skin-draining lymph nodes. Further evidence comes from a significant increase in the median number of positive involved lymph nodes observed in melanoma patients presenting with stage III disease with a known primary compared with stage III presenting with MUP (A. Van der Ploeg, L.E. Haydu, R.A. Scolyer, M.J. Quinn, R.P.M. Saw, K.F. Shannon, A.J. Spillane, J. R. Stretch, J.F. Thompson, submitted); in this sense, transformation occurring within the lymph node means that the MUP may have a reduced ability to spread to neighboring lymph nodes as it is in an earlier phase of acquiring metastatic capabilities as compared to a nodal metastatic tumor from a primary melanoma.
Interestingly, mutations in PPP6C were significantly enriched in MUP compared with cutaneous MKP, possibly suggesting a novel role in the pathology of MUP. PPP6C or ‘protein phosphatase 6, catalytic subunit’ functions in a dominant negative fashion, reducing levels of CCND1 to preclude cells transitioning from G1 to S phase during cell division (Stefansson and Brautigan, 2007). The mutations of PPP6C analyzed in this study occurred within the catalytic domain, and although not yet functionally characterized, have been suggested to be inactivating mutations (Krauthammer et al., 2012); in this sense, the mutation may result in subsequent activation of the RB pathway leading to cellular proliferation. Krauthammer et al. alluded to the possibility of PPP6C mutation acting in a cooperative pathway for overcoming oncogene-induced senescence in BRAF/NRAS mutant melanocytes (Krauthammer et al., 2012). If true, it is interesting to speculate that the increased frequency of PPP6C mutations may be a result of transformed nevi, providing further support to the concept of MUP arising from cutaneous nevus cells migrating to lymph nodes.
Mutations in IDH1 were also observed more frequently in MUP compared with cutaneous MKP. Isocitrate dehydrogenases (IDH1/2) are involved in the metabolic conversion of isocitrate to α-ketoglutarate with concurrent reduction of nicotinamide dinucleotide phosphate (NADP+ to NADPH) and are most frequently mutated in progressive glioma and acute myeloid leukemia (Mardis et al., 2009; Yan et al., 2009). In contrast, IDH1/2 mutations occur infrequently in melanoma, and to date, including data generated in this report, all samples with a mutation have been identified in metastatic lesions and frequently co-occur with either BRAF, NRAS, or KIT mutations (Hodis et al., 2012; Lopez et al., 2010; Shibata et al., 2011). It has been postulated that IDH1/2 mutation confers a cell growth advantage after the acquisition of early oncogenic drivers (BRAF, NRAS, or KIT) because IDH1 R132H was shown to enhance signaling of the MAPK pathway in a BRAF mutant melanoma in vivo tumorigenicity assay (Shibata et al., 2011). Similarly to PPP6C, it is interesting to speculate whether the over-representation of IDH1 mutations in MUP compared with cutaneous MKP may be explained by the nevus cells migrating to lymph node hypothesis of MUP pathogenesis.
Notably, we observed a lower frequency of non-synonymous point mutations occurring in NF1, PTEN, and CDKN2A in MUP compared with cutaneous MKP. Although it is possible that the decreased rate of mutation may describe an inherent difference in the etiology of these two melanoma subtypes, it is difficult to eliminate that the discrepancy may be explained by alternative mechanisms of genomic aberrations. Besides single nucleotide mutation events, both CDK2NA and PTEN frequently undergo homozygous deletion and loss of heterozygosity (LOH) in melanoma (Stark and Hayward, 2007). Further genomic analysis to assess chromosomal copy number through use of SNP arrays would be useful in addressing either possibility.
The alternate MUP pathogenesis theory of spontaneous regression stems from the well-described observations of its occurrence in melanoma (Everson, 1964; Smith and Stehlin, 1965). Although reports have varied, recent large-scale analyses of patients with MUP have shown an increased survival advantage to patients with similarly staged MKP (Cormier et al., 2006; Lee et al., 2008; Prens et al., 2011; A. Van der Ploeg, L.E. Haydu, R.A. Scolyer, M.J. Quinn, R.P.M. Saw, K.F. Shannon, A.J. Spillane, J. R. Stretch, J.F. Thompson, submitted). It has been proposed that this effect may be explained by spontaneous regression of the primary melanoma because the immune system would also be directed against any metastatic deposits that existed (Azimi et al., 2012).
This evidence that suggests MUP frequently arises from sun-exposed cutaneous sites may provide supporting evidence for the management of these patients in the clinic. Upon presentation of a patient with MUP, in some institutions, it is common practice to perform an exhaustive search for a primary site including extensive radiological imaging and internal endoscopies. In our experience, this is rarely fruitful, and at MIA, it is not routinely performed. Furthermore, a recent retrospective study showed that of 103 patients with MUP presenting between 1986 and 2006 who had undergone thorough examinations including sigmoidoscopy, rectoscopy, and gynecological and oto-rhino-laryngology screens, only one primary tumor was identified (Tos et al., 2011). The authors concluded that such investigations are costly, time-consuming, and intrusive and may be considered unfeasible and/or redundant. They recommended obtaining a detailed history, performing a standard physical examination, and histopathological review with PET/CT scanning for staging as appropriate for the initial work-up of patients presenting with MUP.
It should be noted that because MUPs have a mutation profile consistent with tumors arising from the skin, the majority of these patients should be responsive to current and emerging therapeutic modalities. In particular, the high proportion of BRAF mutations in MUP indicates that BRAF inhibitor strategies will be effective in a similarly high proportion of patients with MUP (Falchook et al., 2012; Flaherty et al., 2012; Hauschild et al., 2012; Long et al., 2012). It is also interesting to speculate that if spontaneous regression accounts for a proportion of MUP, this subset of tumors will be more responsive to immune-based interventions. Retrospective survival analysis of patients with MUP enrolled in clinical trials with CTLA-4, PD-1, and PD-L1 antibodies is warranted (Brahmer et al., 2012; Camacho et al., 2009; Hodi et al., 2010; Topalian et al., 2012).
In conclusion, we have performed the largest genetic analysis of MUP to date and shown that the majority of MUP have a mutation profile consistent with sun-exposed cutaneous melanomas. Furthermore, these results shed light on their mechanism of development, supporting the theories that MUPs arise via spontaneously regressed lesions or through de novo development in lymph nodes from nevus cells that have migrated from the skin and not frequently from unusual or concealed sites of the body. These findings provide supporting evidence against extensive specialized clinical and endoscopic examinations upon patient presentation with MUP and may explain the survival advantage observed in MUP compared to MKP patients.
Methods
Patient selection
Melanoma of unknown primary was defined as a patient diagnosed with a metastatic melanoma deposit at either a lymph node, visceral, or subcutaneous site for which there was no confirmed prior diagnosis of melanoma.
Melanoma of unknown primary cell lines used for exome sequencing were established at the Queensland Institute of Medical Research (QIMR) as previously described (Dutton-Regester et al., 2012a). These cell lines were profiled to confirm authenticity and matching status using an AmpFISTR Profiler Plus PCR amplification kit (Applied Biosystems, Foster City, CA, USA) and analyzed on a 3100 Genetic Analyzer (Applied Biosystems). Public data available from previous melanoma exome sequencing reports (Hodis et al., 2012; Krauthammer et al., 2012) were interrogated to identify further samples that met the same MUP definition; those samples listed with an ‘unknown’ status were not considered for analysis. A further 10 and 12 MUP samples were ascertained from the Hodis et al., 2012 and Krauthammer et al., 2012 reports, respectively (Hodis et al., 2012; Krauthammer et al., 2012). The comparison cohort of cutaneous MKP used to analyze differences in mutation rates of key genes of melanomagenesis consisted of 95 and 56 exomes from Hodis et al., 2012 and Krauthammer et al., 2012, respectively.
A cohort of 248 MKPs site had been screened with a melanoma-specific mutation panel, previously described (Castellano et al., 1997; Dutton-Regester et al., 2012b; Mann et al., 2013; Pavey et al., 2004; Rizos et al., 1999). It comprised of 232 stage III and IV melanomas of cutaneous origin and 15 of acral, mucosal, or uveal origin, ascertained from a variety of sources. The independent cohort of 91 MUPs screened with the melanoma-specific mutation panel was selected from a prior retrospective study investigating survival outcome in patients with MUP presenting with macroscopic lymph node disease compared with similarly staged patients with a known primary melanoma (manuscript submitted) (A. Van der Ploeg, L.E. Haydu, R.A. Scolyer, M.J. Quinn, R.P.M. Saw, K.F. Shannon, A.J. Spillane, J. R. Stretch, J.F. Thompson). Patients were selected based on availability of formalin-fixed, paraffin-embedded (FFPE) blocks with sufficient tumor content as described in the DNA extraction methods below.
DNA extraction
Cell line DNA was extracted using QIAamp Blood Maxi Kits (QIAGEN, Valencia, CA, USA) according to the manufacturer's instructions. MUP tumors from FFPE blocks required a minimum of 80% tumor content with minimal tumor-infiltrating lymphocytes (TILs) for selection within this study. Blocks were core punched and pellet extracted using a NucleoSpin® FFPE DNA kit according to the manufacturer's instructions (MACHERY-NAGEL, Duren, Germany).
Exome sequencing
DNA from MUP cell lines and matching normal lymphoblastoid cell lines (LCLs) were sent to Macrogen Inc. (Axeq Technologies-South Korea) for exome sequencing, with the exception of C037 and C037-LCL which had their complete genome sequenced. The TruSeq EZ oligo exome capture (Illumina, Inc, San Diego, CA, USA) was used for library preparation, following sequencing on the Illumina Hiseq platform following standard procedures. A minimum of 100X coverage was achieved for each sample (refer to Table S6 for coverage statistics). Reads were mapped to UCSC hg19 genome using BWA (Li and Durbin, 2009) and variants called using SAMTOOLS (Li et al., 2009). All variants were screened with dbSNP v131 and the 1000 genomes database.
Somatic variants were identified with SAMTOOLS (Li et al., 2009) in paired mode requiring a CLR>60 and subsequently annotated using ANNOVAR (Wang et al., 2010). To reduce false positives, we applied stringent filtering criteria similar to criteria described by Kobalt et al. (Koboldt et al., 2009). Variants with significant strand bias (P < 10−4), tail distance bias (P < 10−4), great difference in average mapping quality between reference and variant reads (>30), significant variant allele frequency in normal sample (VAF > 5%), variants with average read position close the read start/end (ARP > 10), and variants falling into segmentally duplicated regions (>90%) were excluded (Bailey et al., 2001).
Melanoma-specific oncogenic mutation analysis
Samples were genotyped for a set of 39 oncogenic mutations covering 20 genes using a melanoma-specific mutation panel (Dutton-Regester et al., 2012b). This panel, MelaCarta v1.0 (Sequenom, Inc, San Diego, CA, USA), screens for frequently occurring mutations in cutaneous and non-cutaneous melanoma using the Sequenom MassARRAY system (Sequenom, Inc). A threshold of 10% was used to call the presence of a mutant allele, and all such calls were manually reviewed. Additional assays covering recently published melanoma-specific mutations (RAC1, PPP6C, TRRAP, IDH1, and IDH2) were designed using the somatic mutation assay design suite through MySequenom (Sequenom, Inc) (Hodis et al., 2012; Krauthammer et al., 2012; Wei et al., 2011).
Statistics
Comparisons between the gene mutation frequency of MUP and cutaneous MKP from mutation hot spot analysis were performed using a two-tailed Fisher's exact test. A chi-square test was used to calculate the probability of distributions of the appearance of MUP in lymph nodes compared with the expected numbers based on total skin surface area. P-values < 0.05 were considered statistically significant.
Acknowledgements
This project was funded by the Rio Tinto Ride to Conquer Cancer fund benefitting the Queensland Institute of Medical Research (NH and KDR). RAS is supported by the Cancer Institute New South Wales and National Health and Medical Research Council Fellowship programs. We thank the Nichols family for their support during this project.