Pepper RING-Type E3 Ligase CaFIRF1 Negatively Regulates the Protein Stability of Pepper Stress-Associated Protein, CaSAP14, in the Dehydration Stress Response
Yeongil Bae and Chae Woo Lim contributed equally to the study.
ABSTRACT
As part of the cellular stress response in plants, the ubiquitin–proteasome system (UPS) plays a crucial role in regulating the protein stability of stress-related transcription factors. Previous study has indicated that CaSAP14 is functionally involved in enhancing pepper plant tolerance to dehydration stress by modulating the expression of downstream genes. However, the comprehensive regulatory mechanism underlying CaSAP14 remains incompletely understood. Here, we identified a RING-type E3 ligase, CaFIRF1, which interacts with and ubiquitinates CaSAP14. Pepper plants with silenced CaFIRF1 exhibited a dehydration-tolerant phenotype when subjected to dehydration stress, while overexpression of CaFIRF1 in pepper and Arabidopsis resulted in reduced dehydration tolerance. Co-silencing of CaFIRF1 and CaSAP14 in pepper increased sensitivity to dehydration, suggesting that CaFIRF1 acts upstream of CaSAP14. A cell-free degradation analysis demonstrated that silencing of CaFIRF1 led to decreased CaSAP14 protein degradation, implicating CaFIRF1 in the regulation of CaSAP14 protein via the 26S proteasomal degradation pathway. Our findings suggest a mechanism by which CaFIRF1 mediates the ubiquitin-dependent proteasomal degradation of CaSAP14, thereby influencing the response of pepper plants to dehydration stress.
1 Introduction
Modern challenges, such as erratic rainfall and soil desertification, pose threats to plant survival, leading to dehydration stress and consequent adverse impacts on plant growth and productivity (Muhammad Aslam et al. 2022; Zhang et al. 2022; Zaib et al. 2023). Dehydration stress elevates cellular levels of reactive oxygen species, causing oxidative damage that impairs various elements of plant cells, including DNA, proteins and lipids. This ultimately results in DNA and protein deterioration, lipid peroxidation, and cell death (Apel and Hirt 2004). To cope with this severe threat, plants have evolved a diverse range of morphological, physiological and molecular mechanisms. Abscisic acid (ABA) serves as a major phytohormone governing various plant stress responses, particularly dehydration (Muhammad ASLAM et al. 2022). Within the array of ABA signalling pathways, the ‘PYR/PYL/RCAR-PP2Cs-subclass lll SnRK2s’ pathway stands out as central, regulating numerous factors, including channels, transcription factors, or kinases, from the transcriptional to post-translational levels (Fujita et al. 2011; Nakashima and Yamaguchi-Shinozaki 2013; Chen et al. 2020). ABA signalling triggers stomatal closure and activates stress-responsive gene expression, inducing morphological and physiological changes aimed at minimizing water loss (Kamiyama, Katagiri, and Umezawa 2021; Soma et al. 2021). Recent research has extensively focused on the post-translational modifications (PTMs) of these factors to enhance the ability of plants to respond more effectively to dehydration stress (Joo, 2021; Lim, Lim, and Lee 2022; Shao et al. 2023; Zhou et al. 2021). However, despite these efforts, significant knowledge gaps persist, underscoring the need for further research.
Among the numerous PTMs, the protein ubiquitination and ubiquitin-mediated proteasomal degradation systems have been of interest in plant stress response for decades (Bachmair et al. 2001; Lyzenga and Stone 2012; Stone 2014; Sharma et al. 2016). Three main enzymes are responsible for protein ubiquitination: ubiquitin-activating enzymes (E1s), ubiquitin-conjugating enzymes (E2s) and ubiquitin-protein ligases (E3s) (Bachmair et al. 2001). E3 ligases are particularly notable due to their ability to selectively recognize and bind substrates, making them a research focus. E3 ligases are broadly classified into two types: monomeric and multimeric. Monomeric E3 ligases comprise a Really Interesting New Gene (RING), U-box, homologous to E6-AP carboxyl terminus (HECT) and RING-between-RING (RBR) domains. Four different cullin-based E3s, including Skip1-Cullin1-F-box (SCF), Cullin3-RING (CRL3), Cullin4-RING (CRL4) and anaphase-promoting complex/cyclosome (APC/C), are multimeric E3 ligases (Morreale and Walden 2016; Al-Saharin, Hellmann, and Mooney 2022). Among them, RING-type E3 ligases comprise the largest proportion of plant E3 ligases—approximately 300–500 different RING members—thus, RING E3 ligases are expected to be responsible for diverse abiotic stress responses in plants (Jiménez-López et al. 2018; Metzger et al. 2014). In the dehydration stress response, RGLG1 and RGLG2 negatively regulate MAP3K18, and RGLG2 also negatively modulates AtERF53 in Arabidopsis (Cheng et al. 2012; Yu et al. 2021). Moreover, OsDIRP1 downregulates the drought stress response in rice (Cui et al. 2018), and CaAIR1 and CaDIR1 act as negative regulators in the drought stress response in hot pepper (Park et al. 2015; Joo et al. 2017). Although RING E3 ligases have been functionally characterized in several species, their substrates in the context of the dehydration stress response remain to be fully elucidated.
For the past two decades, stress-associated proteins (SAPs) have been known as novel regulators involved in hormone synthesis and various biotic and abiotic stresses in plants (Giri et al. 2013; Kothari et al. 2016; Zhao et al. 2020; Li et al. 2022). SAPs, a member of the zinc finger proteins, are classified into two types: type 1, which has an N-terminal A20 and/or C-terminal AN1 domain; type 2, which has an N-terminal AN1 and/or C-terminal C2H2 domain (Vij and Tyagi 2006; Solanke et al. 2009; Bae, Lim, and Lee 2021). Since the structural features of SAPs are highly conserved among plant species, functional studies in different plant species are well advanced. Most SAPs function as transcription factors, while others act as E3 ubiquitin ligases, redox sensors or undetermined regulators (Ströher et al. 2009; Kang et al. 2011; Giri et al. 2013; Zhang et al. 2017; Baidyussen et al. 2021; Bae, Lim, and Lee 2023). As transcription factors, several studies have shown that SAPs play important roles in the dehydration stress response in various plant species. For example, OsiSAP1 and OsiSAP8 confer tolerance to dehydration stress, and OsSAP11 enhances water deficit tolerance by regulating the expression of endogenous stress-responsive genes in rice (Mukhopadhyay, Vij, and Tyagi 2004; Kanneganti and Gupta 2008; Giri et al. 2011). In addition, PpSAP1 improves water retention under drought stress by regulating downstream genes in Prunus persica (Lloret et al. 2017). In soybean (Glycine max), GmSAP16 positively modulates the dehydration stress response by altering stress-related genes, including GmNCED3, GmRD22 and GmDREB2 (Zhang et al. 2019). Although SAPs play important roles in the dehydration stress response, studies on their PTMs that could act as switches for SAPs are poorly understood.
Previously, we reported that type 2 stress-associated protein CaSAP14 positively modulates the dehydration stress response via the transcriptional regulation of CaRAB18 and CaOSR1 in hot pepper (Bae, Lim, and Lee 2023). In this study, we observed a significant inhibition of CaSAP14 protein degradation in response to dehydration stress, with mediation by the 26S proteasomal degradation system. Notably, we found that the RING E3 ligase CaFIRF1 interacts with CaSAP14, leading to its ubiquitination, thereby indicating the role of CaFIRF1 in modulating CaSAP14 protein stability. Additionally, our results demonstrate that CaFIRF1 negatively regulates the dehydration stress response and acts upstream of CaSAP14. These findings suggest that CaFIRF1 serves as a negative regulator of the dehydration stress response by controlling CaSAP14 protein stability.
2 Materials and Methods
2.1 Plant Materials
For the experiments, Arabidopsis thaliana (ecotype Col-0), Capsicum annuum (hot pepper; cv. Nock-wang) and Nicotiana benthamiana (tobacco) were used. Arabidopsis seeds were seeded on 0.5× Murashige and Skoog (MS) salt medium with 0.8% agar and 1% sucrose (pH 5.7) and vernalized at 4°C for 2 days. For hot peppers, seeds were soaked and incubated under dark conditions at 28°C for 4 days. Arabidopsis seedlings and pepper and tobacco seeds were planted and grown in soil at 24 ± 1°C under a 16 h/8 h light/dark cycle, as previously described (Bae, Lim, and Lee 2023; Bae et al. 2024).
2.2 Generation of CaFIRF1-Overexpressing Arabidopsis Transgenic Plants
A full-length coding sequence of the CaFIRF1 gene (CA02g30250) was cloned into a pCR8/GW/TOPO vector (Invitrogen, Waltham, MA, USA) and integrated into a pro35S-GFP vector using an LR reaction (Nakagawa et al. 2007). Using the floral dip method, Arabidopsis plants were transformed using Agrobacterium tumefaciens (strain GV3101) harbouring a pro35S-CaFIRF1-GFP construct (Clough and Bent 1998), and harvested seeds were seeded and selected on 0.5× MS salt medium containing 50 μg mL⁻1 phosphinothricin.
2.3 Reverse Transcription-Quantitative Polymerase Chain Reaction Analyses (RT-qPCR)
For analyses, total RNA was isolated from the first and second leaves of pepper or Arabidopsis plant rosette leaves, after which 1 μg of quantified total RNA was used to synthesize cDNA using an iScript™ cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA, USA), as previously described (Lim, Bae, and Lee 2022; Bae, Lim, and Lee 2023, Bae et al. 2024). The synthesized cDNA was used as a template for RT-qPCR analyses, with CaPP2A (CA11g01360) and Actin8 (AT1g49240) as internal control genes (Wang et al. 2014; Petriccione et al. 2015; Fernández-Calvo et al. 2011).
2.4 Virus-Induced Gene Silencing and Gene Overexpression in Pepper
The CaFIRF1- and CaSAP14-silenced pepper plants were used according to previous studies (Bae, Lim, and Lee 2023, Bae et al. 2024). To generate virus-induced gene overexpressing (VOX) pepper plants, a full-length CaFIRF1 coding sequence (CDS) was cloned into the SPDK3876 vector (pTRV2-pPEBV-MCS) (Ellison et al. 2020). A. tumefaciens (strain GV3101) harbouring pTRV1, pTRV2-empty (pTRV2:00), pTRV2:CaFIRF1, pTRV2:CaSAP14, or SPDK3876:CaFIRF1 were co-infiltrated into the cotyledons of pepper plants (2-week-old) in five combinations (pTRV2:00, negative control; pTRV2:CaFIRF1 or CaSAP14, single gene silencing model; pTRV2:CaFIRF1/CaSAP14, double gene silencing model; SPDK3876:CaFIRF1, single gene overexpressing model). Infiltrated pepper plants were then incubated under dark conditions for 2 days. Two weeks after infiltration, the expression levels of CaFIRF1 and CaSAP14 were measured by RT-qPCR, and phenotypic analyses were also performed.
2.5 Phenotypic Analyses
The methods for the phenotypic analyses of dehydration stress have been described previously (Bae, Lim, and Lee 2023) and were used in the present study with some modifications. To test dehydration tolerance, pepper and Arabidopsis plants were exposed to drought stress by withholding water for 14 days, followed by rewatering for 3 and 2 days, respectively. To evaluate leaf water retention capacity, the fresh weights of the first and second leaves of pepper and Arabidopsis plant shoots were measured at designated time points. For leaf thermal imaging analysis, pepper leaves or Arabidopsis shoots were treated with 100 µM ABA and incubated for 4 h. In addition, Arabidopsis shoots were detached and subjected to dehydration stress for 15 min. Thermal images were obtained before and after treatment using a T420 infrared camera (Teledyne FLIR LLC, Wilsonville, OR, USA). Average temperatures for each plant were calculated using FLIR software (Teledyne FLIR LLC). To assess ABA-induced stomatal closure, pepper and Arabidopsis leaf peels were floated in stomatal opening solution (SOS) buffer (10 mM MES-KOH, 50 mM KCl and 10 μM CaCl2; pH 6.15) and incubated for 2.5 h. Thereafter, the peels were incubated with SOS buffer containing different ABA concentrations (0, 10 or 20 μM). Images of stomata (> 150) were obtained randomly using a microscope (Nikon, Tokyo, Japan), and stomatal apertures (width/length) were determined using ImageJ software (National Institutes of Health, Bethesda, MD, USA). To analyse the expression levels of stress-responsive genes, pepper and Arabidopsis shoots were detached, and the first and second leaves of pepper and Arabidopsis rosette leaves were collected at designated time points. Subsequently, total RNA was isolated from the samples, followed by cDNA synthesis and RT-qPCR.
ABA sensitivity was evaluated using previously described methods (Bae, Lim, and Lee 2023; Koh et al. 2023) with slight modifications. To determine germination percentage, 36 wild type and CaFIRF1-OE transgenic line seeds were seeded on 0.5× MS salt agar medium containing different ABA concentrations (0, 0.1, 0.2 or 0.3 µM) and vernalized at 4°C for 2 days. Thereafter, the number of seeds with radicle emergence was measured daily for 7 days, after which the percentage of cotyledon greening and primary root length were measured. To determine post-germinative root elongation, 36 germinated seeds of each plant line were seeded on 0.5× MS salt agar medium containing 0, 5 or 10 μM ABA. Next, the germinated seeds were incubated at 24°C under a 16 h/8 h light/dark cycle for 7 days.
2.6 Protein–Protein Interaction
For the one-by-one yeast two-hybrid (Y2H) assay, the full-length CDSs of CaSAP14 and CaFIRF1 were cloned into pGBKT7 and pGADT7 vectors, respectively. Yeast cells (Saccharomyces cerevisiae, strain AH109) were co-transformed using the lithium acetate yeast transformation method.
In the luciferase complementation imaging (LCI) assay, CaSAP14 and CaFIRF1 were fused to the N- and C-terminal halves of firefly luciferase, respectively (Gehl et al. 2011). A. tumefaciens (strain GV3101) harbouring the aforementioned constructs in different combinations were infiltrated into tobacco leaf epidermal cells using p19 strain cells and incubated for 72 h. After 72 h, 1 mM luciferin was infiltrated into tobacco leaves, which were subsequently incubated under dark conditions for 30 min. Signals were observed using a NightShade LB 985 In Vivo Plant Imaging System (Berthold Technologies, Bad Wildbad, Germany).
For the co-immunoprecipitation (Co-IP) assay, A. tumefaciens (strain GV3101) harbouring the pro35S:3xFLAG-CaFIRF1 construct was co-infiltrated into tobacco leaves with pro35S:CaSAP14-GFP or pro35S:empty-GFP constructs using p19 strain cells. After 72 h of co-infiltration, the leaves were collected, and total protein extraction and IP assays were conducted as previously described (Bae et al. 2024; Baek, Lim, and Lee 2021). Immunoblotting analyses were performed with GFP and FLAG antibodies.
In the bimolecular fluorescence complementation (BiFC) assay, A. tumefaciens (strain GV3101) harbouring pro35S:CaFIRF1-CYCE or pro35S:CaSAP14-VYNE were infiltrated into tobacco leaves in different combinations using p19 strain cells, and the leaf samples were incubated for 72 h. Two hours before observation, the leaves were treated with 4′,6-diamidino-2-phenylindole (DAPI; 1 µg mL⁻1) and FM4-64 (50 µM), and the signals were observed using an LSM700 confocal microscope (Carl Zeiss, Oberkochen, Germany).
2.7 In Vitro Ubiquitination Assay
For the in vitro ubiquitination assay, glutathione S-transferase (GST)-CaSAP14 and maltose-binding protein (MBP)-CaFIRF1 or -CaFIRF1CC (C441S/C444S) recombinant proteins were used as previously described (Bae, Lim, and Lee 2023; Bae et al. 2024). The assay procedure was as previously described with some modifications (Bae et al. 2024). Briefly, recombinant proteins (50 ng) were mixed in various combinations with reaction buffer (50 mM Tris-HCl [pH 7.5], 5 mM MgCl2, 2 mM ATP and 2 mM dithiothreitol [DTT]), recombinant human E1 (UBE1; Boston Biochem, Cambridge, MA, USA), human E2 (UbcH5b; Enzo Life Sciences, Farmingdale, NY, USA) and bovine ubiquitin (Sigma-Aldrich, St. Louis, MO, USA). Thereafter, the mixtures were incubated at 30°C for 6 h, boiled at 65°C for 10 min, and immunoblotted using GST, MBP and ubiquitin antibodies.
2.8 Cell-Free Degradation Assay
The methods for producing crude extracts and the cell-free degradation assay were according to previous studies with some modifications (Koh et al. 2023; Bae et al. 2024). The first and second leaves of non-transgenic and CaFIRF1-silenced and VOX pepper plants were used to produce crude extracts. In the case of non-transgenic peppers, plants were subjected to dehydration stress by detaching the shoot or spraying the leaves with 100 µM ABA for 6 h before harvesting the leaves. Next, the harvested leaves were used to produce crude extracts by mixing with extraction buffer (25 mM Tris-HCl [pH 7.5], 10 mM ATP, 10 mM MgCl2, 10 mM NaCl, 5 mM DTT and 0.1% Triton X-100). Subsequently, GST-CaSAP14 recombinant proteins (500 ng) were mixed with crude extracts (50 µg of total proteins) and incubated for 90 and 180 min with or without MG132 (50 µM) treatment. Immunoblotting analyses were conducted using GST antibodies.
3 Results
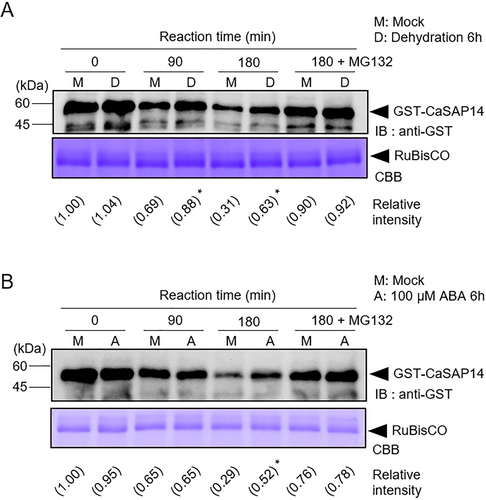
3.1 CaSAP14 Protein Is Less Degraded under ABA-Dependent Dehydration Stress Conditions
Previously, CaSAP14 was reported to positively regulate ABA-dependent dehydration resistance by controlling transpirational water loss via the regulation of stomatal closure and stress-responsive gene expression (Bae, Lim, and Lee 2023). We speculated how CaSAP14 protein stability is regulated during this stress response and what factors may be involved. To test our hypothesis, we initially conducted a cell-free degradation assay to monitor changes in the protein stability of CaSAP14 with/without dehydration and ABA treatments. GST-tagged CaSAP14 recombinant proteins were incubated with pepper leaf crude extracts from mock, dehydration and ABA treatments (Figures 1 and S1). CaSAP14 protein degradation was significantly attenuated in response to both dehydration and ABA treatments compared with that in the mock treatment. In addition, CaSAP14 degradation was inhibited by MG132, a known 26S proteasome inhibitor (Dou et al. 2021), indicating that CaSAP14 protein is degraded via the 26S proteasome pathway. Based on these findings, we suggested that reduced CaSAP14 degradation under dehydration stress could potentially enhance plant resistance to dehydration stress through an ABA-dependent pathway.
3.2 CaSAP14 Interacts With CaFIRF1
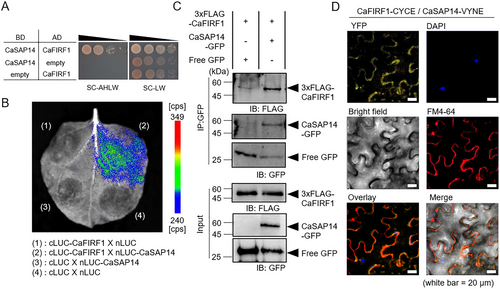
To identify candidate proteins responsible for modulating CaSAP14 protein stability in response to dehydration stress, we conducted a Y2H screening assay using CaSAP14 as bait and the pepper RING E3 ligase cDNA library as prey. Among the candidate CaSAP14-interacting RING E3 ligases, we selected the CA02g30250 gene encoding CaFIRF1, which negatively influences high-salt stress responses (Bae et al. 2024). The interaction between CaSAP14 and CaFIRF1 was re-confirmed by a one-by-one Y2H (Figure 2A). Furthermore, the interaction was confirmed by several in vivo assays, including LCI, Co-IP and BiFC assays (Figure 2B–D). Particularly, the BiFC assay revealed that signals for the interaction between CaSAP14 and CaFIRF1 were predominantly detected in the cytoplasm (Figures 2D and S2). Collectively, these results indicate that CaSAP14 physically interacts with CaFIRF1.
3.3 CaFIRF1 Ubiquitinates CaSAP14
Our previous study showed that CaFIRF1 exhibits E3 ligase activity in vitro and in vivo (Bae et al. 2024). Based on the interaction between CaSAP14 and CaFIRF1 (Figure 2), we questioned whether CaSAP14 could be a substrate for CaFIRF1. To test this supposition, we first performed an in vitro ubiquitination assay using GST-CaSAP14 recombinant protein as a substrate. As shown in Figure 3A, weak CaSAP14 smear bands were detected when GST-CaSAP14 was incubated with all components, including ubiquitin, UBE1, UbcH5b and MBP-tagged CaFIRF1 (lane 4). This CaSAP14 ubiquitination was mediated by the E3 ligase activity of CaFIRF1. However, the GST-CaSAP14 ubiquitination signal could not be detected in samples incubated without E3 ligase (Figure 3B, lane 1) or with the inactive form of MBP-CaFIRF1 (C441S/C444S, CaFIRF1CC; Figure 3B, lane 3). In contrast, incubation with the intact form of CaFIRF1 produced a GST-CaSAP14 ubiquitination signal (Figure 3B, lane 2). These findings indicate that CaFIRF1 can recognize and ubiquitinate CaSAP14 as a substrate.
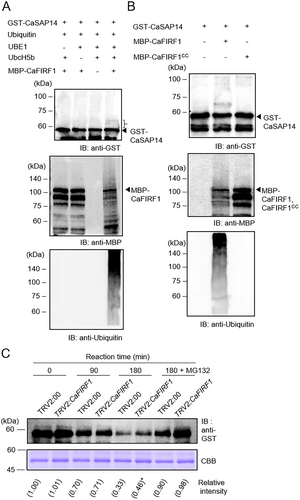
Given the CaFIRF1-mediated ubiquitination of CaSAP14, we speculated whether CaFIRF1 could modulate CaSAP14 stability. To test this hypothesis, we performed a cell-free degradation assay with CaFIRF1-silenced pepper plants (TRV:CaFIRF1), as described previously (Bae et al. 2024), and CaFIRF1-overexpressing pepper plants using the VOX system, which has been tested in several plant species (Jeong, Lim, and Lee 2023; Cheuk and Houde 2017; Rössner, Lotz, and Becker 2022; Yang et al. 2022; Yue et al. 2022) (Figure S3A). The GST-CaSAP14 proteins were gradually degraded during incubation with crude extracts from control TRV2:00 and TRV:CaFIRF1 (or CaFIRF1-VOX) plants. However, GST-CaSAP14 protein degradation was influenced by the expression levels of CaFIRF1. Specifically, higher residual GST-CaSAP14 protein levels were evident in TRV:CaFIRF1 plants (Figure 3C), whereas slightly lower levels were observed in CaFIRF1-VOX plants (Figure S3B) relative to those in control plants. This effect was abolished upon treatment with MG132, indicating the involvement of the 26S proteasomal pathway. Taken together, these results suggest a regulatory role for CaFIRF1 in facilitating CaSAP14 protein degradation via the 26S proteasomal pathway.
3.4 CaFIRF1 Silencing Enhances Dehydration Stress Tolerance in Pepper Plants
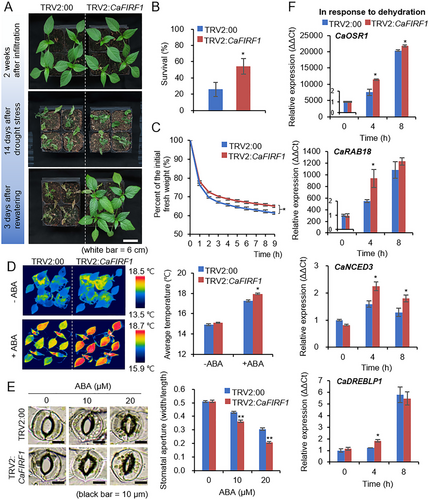
To examine the biological role of CaFIRF1 in response to dehydration stress, we performed phenotypic analyses using TRV2:CaFIRF1 and TRV2:00 plants (Figure 4). First, we examined the responses of the two plant lines to dehydration stress (Figure 4A). Under well-watered conditions, the two plant lines displayed no significant phenotypic differences (Figure 4A, top image). However, when plants were subjected to drought and subsequent rewatering, TRV2:CaFIRF1 pepper plants exhibited a less wilted phenotype than that of TRV2:00 plants (Figure 4A, bottom image). In addition, 3 days after rewatering, 54.29% of the TRV2:CaFIRF1 pepper plants survived, compared with the 25.92% survival rate of TRV2:00 plants (Figure 4B). To analyse whether this phenomenon is related to differences in water retention capacity, we examined the transpirational water loss by measuring the fresh weight of detached first and second leaves (Figure 4C). As expected, at respective time points, we observed less reduction in the fresh weight of TRV2:CaFIRF1 pepper leaves than that of TRV2:00 leaves. Given that ABA modulates stomatal opening/closing and that transpiration is modulated by stomatal states, we investigated ABA sensitivity by measuring leaf surface temperatures and stomatal apertures in the presence or absence of ABA (Figure 4D,E). In the absence of ABA, we observed no significant differences between TRV2:CaFIRF1 and TRV2:00 pepper plants in leaf surface temperatures or stomatal apertures. However, under ABA treatment, we detected higher leaf surface temperatures and a larger proportion of stomatal closing in TRV2:CaFIRF1 plants than those in TRV2:00 plants. To confirm whether these phenomena are related to the differential expression of drought-responsive genes, including CaOSR1, CaRAB18, CaNCED3 and CaDREBLP1, we conducted an RT-qPCR analysis as described previously (Baek et al. 2023, 2024; Lim, Bae, and Lee 2022). At the initial time point, the control and CaFIRF1-silenced pepper plants did not significantly differ, but over time, CaOSR1, CaRAB18, CaNCED3 and CaDREBLP1 levels in TRV2:CaFIRF1 pepper plants were significantly higher compared with those in TRV2:00 plants (Figure 4F). These results indicate that the enhanced drought tolerance of TRV2:CaFIRF1 pepper plants is associated with enhanced ABA sensitivity.
To further validate the biological role of CaFIRF1 in response to dehydration stress, we also examined drought stress responses using CaFIRF1-VOX pepper plants (Figure S4). Under well-watered conditions, no significant phenotypic differences were observed between control and CaFIRF1-VOX pepper plants (Figure S4A, top image). However, when plants were subjected to drought and subsequent rewatering, CaFIRF1-VOX pepper plants exhibited a more wilted phenotype and a lower survival percentage than those of control plants (Figure S4A, bottom image, B). Also, in response to dehydration stress, we identified the significantly lower expressions of stress-related genes, including CaOSR1, CaRAB18 and CaSAP14 in the CaFIRF1-VOX pepper plants (Figure S4C). Collectively, these findings suggest that CaFIRF1 may negatively affect plant resistance to dehydration stress.
3.5 CaFIRF1 Overexpression Reduces Dehydration Stress Tolerance in Arabidopsis Plants
A further phenotypic evaluation was conducted using CaFIRF1-overexpressing (OE) transgenic Arabidopsis plants (Figures 5 and S5). First, these plants were used to evaluate dehydration stress resistance (Figure 5A). Wild type and transgenic lines did not significantly differ under well-watered conditions (Figure 5A, top image). When plants were exposed to drought stress for 14 days, both wild type and transgenic lines showed wilted phenotypes (Figure 5A, middle image). However, 2 days after rewatering, transgenic lines exhibited more wilted phenotypes and lower survival rates than those of wild-type plants (Figure 5A, bottom image and percentage of survival). To determine whether this drought-sensitive CaFIRF1-OE phenotype was associated with alterations in the transpirational water loss of leaves, we measured the fresh weight of detached shoots from wild type and transgenic lines (Figure 5B). As expected, at indicated time points, we observed a more rapid reduction in the fresh weight of the transgenic lines than that of the wild-type plants. In addition, when shoots from the two plant lines were detached, we observed significantly lower surface temperatures in the transgenic lines than those in the wild-type plants (Figure 5C). Since transpirational water loss is linked to ABA-mediated stomatal closure, we evaluated the leaf surface temperatures and stomatal apertures with or without ABA treatment (Figure 5D,E). Without ABA treatment, wild type and transgenic lines did not significantly differ. However, under ABA treatment, we observed lower leaf surface temperatures (Figure 5D) and less stomatal closing (Figure 5E) in transgenic lines compared with wild-type plants. Furthermore, we investigated the expression of ABA-dependent/independent stress-responsive genes, including RD29B, RAB18, NCED3, KIN1, RD29A, DREB2A, RD20 and COR15A through RT-qPCR analysis as described previously (Figures 5F and S5B) (Bae, Lim, and Lee 2023, Yoshida, Mogami, and Yamaguchi-Shinozaki 2014, Liu et al. 2018). At the initial time point, wild type and transgenic lines did not differ. However, when subjected to dehydration stress for 4 h, CaFIRF1-OE plants exhibited lower expression levels of both ABA-dependent (Figure 5F) and -independent (Figure S5B) stress-responsive genes compared with those of the wild-type plants. These findings suggest a potential role for CaFIRF1 in modulating plant responses to drought stress, accompanied by the alteration of ABA sensitivity and stress-responsive gene expression.
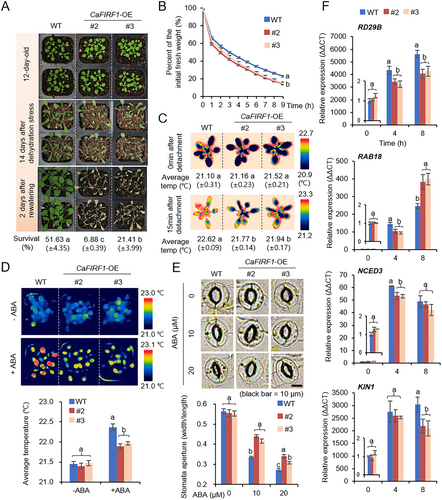
3.6 CaFIRF1 Overexpression Decreases ABA Sensitivity in Arabidopsis Plants
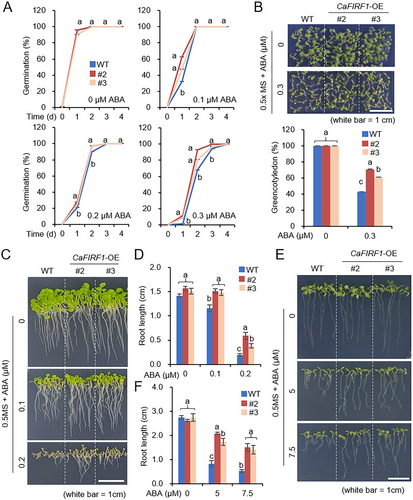
In light of our previous study wherein ectopic CaSAP14 expression enhanced ABA sensitivity at the germinative stage (Bae, Lim, and Lee 2023), we sought to explore the potential regulatory involvement of its interacting counterpart, CaFIRF1, in ABA signalling during both seed germination and seedling growth stages (Figure 6). We first evaluated the germination percentages of CaFIRF1-OE and wild-type plants in response to exogenous ABA (Figure 6A). No significant differences were observed between the two plant lines without ABA treatment. However, upon treatment with various concentrations of exogenous ABA (0.1, 0.2 and 0.3 µM) in 1/2 MS media, CaFIRF1-OE transgenic lines showed notably higher germination percentages than those of wild-type plants. Consistently, CaFIRF1-OE plants also exhibited a higher cotyledon greening percentage than wild-type plants (Figure 6B). Furthermore, we assessed the primary root elongation of the wild type and transgenic lines at the germinative and post-germinative stages (Figure 6C–F). At the germinative stage, the primary root lengths of CaFIRF1-OE transgenic lines were longer (Figure 6C,D), and this pattern was similarly observed at the post-germinative stage (Figure 6E,F). These findings suggest that CaFIRF1 may negatively regulate ABA signalling at the germinative and post-germinative stages.
3.7 CaSAP14 Acts Downstream of CaFIRF1
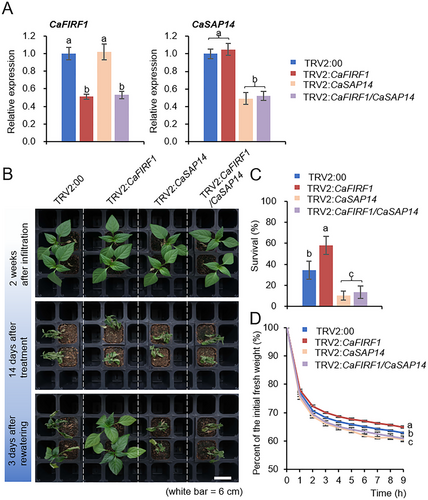
To determine the functional relationship between CaFIRF1 and CaSAP14 in response to dehydration stress, we attempted to generate double gene knockdown pepper plants using the double VIGS system, as previously described (Joo, Lim, and Lee 2019b; Jeong, Lim, and Lee 2023; Bae et al. 2024). As shown in Figure 7A, RT-qPCR analysis revealed that the transcript levels of both CaFIRF1 and CaSAP14 genes were significantly lower in CaFIRF1/CaSAP14 co-silenced pepper (TRV2:CaFIRF1/CaSAP14) than those in the control plants (TRV2:00). We then examined the responses of TRV2:00, TRV2:CaFIRF1, TRV2:CaSAP14 and TRV2:CaFIRF1/CaSAP14 plants to drought stress (Figure 7B,C). Under well-watered conditions, we could not detect any significant differences among them (Figure 7B, top image). However, when they were exposed to drought stress for 14 days and re-watered for 3 days, TRV2:CaFIRF1 plants exhibited less wilted phenotypes (Figure 7B, bottom image) and a significantly higher survival percentage of (58.13%) compared with TRV2:00 plants (34.58%). In contrast, 10.42% of TRV2:CaSAP14 plants and 13.54% of TRV2:CaFIRF1/CaSAP14 plants survived (Figure 7C). This similar pattern was also observed in the alteration of transpirational water loss by measuring the fresh weights of the first and second leaves from the four pepper lines (Figure 7D). These results suggest that CaSAP14 functions downstream of CaFIRF1 in response to dehydration stress.
4 Discussion
Protein ubiquitination and the subsequent ubiquitin-mediated proteasomal degradation system are widely accepted as integral to plant stress responses, and particularly, RING-type E3 ligases have been the subject of intensive investigation (Belknap and Garbarino 1996; Bachmair et al. 2001; Stone 2014; Stone 2019). Numerous studies have reported that various RING E3 ligases play a role in regulating the dehydration stress response across diverse plant species (Cheng et al. 2012; Li et al. 2020; Yu et al. 2021; Park et al. 2015; Joo et al. 2017; Cui et al. 2018). Despite their prevalence compared with other types of E3 ligases, RING-type E3 ligases remain poorly understood. In the present study, we determined the inhibitory role of the RING-type E3 ligase CaFIRF1 and its impact on CaSAP14 protein stability during dehydration stress.
SAP protein family members are known to be associated with plant response to various abiotic stresses, including drought and high-salt stress (Bae, Lim, and Lee 2021; Dixit et al. 2018; Giri et al. 2013; Kang et al. 2011; Li et al. 2019). Consistent with this observation, our previous studies demonstrated that CaSAP14 positively modulates the ABA-dependent dehydration stress responses in pepper plants by regulating transpirational water loss and the transcription of stress-responsive genes such as CaOSR1 and CaRAB18, with CaSAP14, not an E3 ligase, possibly functioning as a transcription factor (Bae, Lim, and Lee 2023). However, the mechanism by which these SAP protein members contribute to stress tolerance is not fully understood, and in particular, few studies have been conducted on the regulation of SAPs through the ubiquitin–proteasome pathway. Among SAPs, type I SAP proteins, such as AtSAP5, AtSAP9, OsiSAP7 and TASAP5, exhibit E3 ligase activity in vitro (Choi et al. 2012; Sharma, Giri, and Tyagi 2015; Kang et al. 2017; Zhang et al. 2017). The E3 ligase activity of most proteins is derived from the A20-type zinc finger domain, but CaSAP14 lacks this domain. Alternatively, we showed that CaSAP14 proteins were degraded via the 26S proteasomal degradation system; this process was attenuated in response to ABA and dehydration stress (Figure 1). Since E3 ligases play a critical role in the 26S proteasomal degradation system as a factor that recognizes and ubiquitinates the substrate, our present study focused on identifying the candidate E3 ligase. As a novel interacting partner of CaSAP14, we identified the RING-type E3 ligase CaFIRF1 and demonstrated that it physically interacts with and ubiquitinates CaSAP14 (Figures 2 and 3). Furthermore, the manipulation of CaFIRF1 expression levels in pepper plants revealed its regulatory role in modulating CaSAP14 degradation (Figures 3C and S3B). These findings highlight the importance of CaFIRF1-mediated ubiquitination in modulating CaSAP14 stability.
Previously, we elucidated the involvement of CaFIRF1 in the ubiquitination-mediated degradation of CaFAF1, a positive modulator essential for plant resilience against high-salt stress, and consequently demonstrated that this regulatory cascade negatively modulates the high salinity stress response (Lim, Bae, and Lee 2022, Bae et al. 2024). Consistent with reports of numerous RING E3 ligases exhibiting multifaceted functions across diverse abiotic stresses (Cui et al. 2018; Pei et al. 2019; Fang et al. 2015), the current study unveils the additional participation of CaFIRF1 in dehydration responses. CaFIRF1 was induced by several environmental stimuli, including dehydration, high salt and mannitol and ABA (Bae et al. 2024). Using CaFIRF1-silenced and -VOX pepper as well as CaFIRF1-OE Arabidopsis plants, we revealed that CaFIRF1 plays a crucial role in modulating plant responses to dehydration stress and ABA signalling (Figures 4-6 and S4). Despite the changes in CaFIRF1 gene expression, there were no differences in phenotypes between control and CaFIRF1-manipulated plants under normal conditions. This suggests that, while CaFIRF1 may regulate CaSAP14 levels under these conditions, such regulation does not significantly alter the basal phenotype of plants. However, under dehydration stress conditions, CaSAP14 degradation is attenuated, leading to its accumulation and triggering stress responses. It seems that the regulation of CaSAP14 at the translational level may play a more critical role than transcriptional regulation in the response of plants to stress. Notably, CaFIRF1-silenced pepper plants exhibited a dehydration-tolerant phenotype, whereas CaFIRF1-VOX and -OE pepper and Arabidopsis plants were highly sensitive to dehydration stress (Figures 4, 5, and S4). The following two main factors appear to contribute to these phenotypes. First, CaFIRF1 negatively regulates ABA-induced stomatal closure, leading to increased leaf transpirational water loss (Figures 4 and 5). This reduced ABA sensitivity was also observed in the early growth stages of CaFIRF1-OE Arabidopsis plants (Figure 6), indicating that CaFIRF1 negatively modulates ABA signalling. Second, CaFIRF1 negatively modulated the expression levels of several stress-responsive genes in both an ABA-dependent and -independent manner (Figures 4, 5, S4C, and S5B) (Yoshida, Mogami, and Yamaguchi-Shinozaki 2014). These findings indicate that CaFIRF1 may exert its regulatory influence on the plant response to dehydration stress through multiple pathways. Furthermore, we demonstrated that CaSAP14 acts downstream of CaFIRF1 in the dehydration stress response by co-silencing these two genes in pepper plants (Figure 7). In light of these findings, CaFIRF1 appears to be involved in the ubiquitination-mediated degradation of at least two substrates in response to different stresses. Consistently, previous studies have reported numerous RING E3 ligases targeting multiple substrates. For example, the RING E3 ligase CaASRF1 has been shown to mediate the degradation of CaAIBZ1, CaATBZ1 and CaJAZ1-03 in drought stress response and ABA signalling (Joo, Lim, and Lee 2019a, 2019b; Koh et al. 2023). Similarly, RGLG1, homologous to CaFIRF1, regulates PP2CA and MAPKKK18 degradation in response to ABA and drought signals, respectively (Belda-Palazon et al. 2019; Yu et al. 2021). However, the intricate mechanisms underlying these regulatory processes remain to be fully elucidated.
In conclusion, we found that CaFIRF1 interacts with and ubiquitinates CaSAP14, exerting a negative regulatory impact on the dehydration stress response, probably by modulating CaSAP14 proteolysis via the 26S proteasomal protein degradation pathway. It is conceivable that under dehydration stress, intricate regulatory processes may hinder CaFIRF1-mediated CaSAP14 degradation, resulting in the accumulation of CaSAP14 and the subsequent enhancement of dehydration tolerance in pepper plants. Due to the complexity of these processes, the functional relationship between CaFIRF1 and CaSAP14 could not be fully elucidated in the present study. Therefore, our future research will focus on unravelling the complexities and relationships underlying stress responses. Our findings broaden knowledge of the regulatory mechanisms governing SAPs, which play important roles in the plant stress response.
Acknowledgements
This work was supported by a grant from the Agriculture & Technology Development (Project No. RS-2024-00322140) and a National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIT) (No. RS-2024-00343006), Republic of Korea.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All data are available in the manuscript or Supplementary files. The raw data that support the findings of this study are available from the corresponding author upon reasonable request.
Data sharing is not applicable to this article as no new data were created or analysed in this study.




