Cryptochromes are the dominant photoreceptors mediating heliotropic responses of Arabidopsis inflorescences
Serrano Alejandro Miguel and Vanhaelewyn Lucas contributed equally to this work.
Funding information: Consejo Nacional de Investigaciones Científicas y Técnicas, Grant/Award Number: 11220150100237CO; Fonds Wetenschappelijk Onderzoek, Grant/Award Number: G000515N; Ministerio de Ciencia, Tecnología e Innovación Productiva, Grant/Award Number: VS00715N-FW/14/02
Abstract
Inflorescence movements in response to natural gradients of sunlight are frequently observed in the plant kingdom and are suggested to contribute to reproductive success. Although the physiological and molecular bases of light-mediated tropisms in vegetative organs have been thoroughly investigated, the mechanisms that control inflorescence orientation in response to light gradients under natural conditions are not well understood. In this work, we have used a combination of laboratory and field experiments to investigate light-mediated re-orientation of Arabidopsis thaliana inflorescences. We show that inflorescence phototropism is promoted by photons in the UV and blue spectral range (≤500 nm) and depends on multiple photoreceptor families. Experiments under controlled conditions show that UVR8 is the main photoreceptor mediating the phototropic response to narrowband UV-B radiation, and phototropins and cryptochromes control the response to narrowband blue light. Interestingly, whereas phototropins mediate bending in response to low irradiances of blue, cryptochromes are the principal photoreceptors acting at high irradiances. Moreover, phototropins negatively regulate the action of cryptochromes at high irradiances of blue light. Experiments under natural field conditions demonstrate that cryptochromes are the principal photoreceptors acting in the promotion of the heliotropic response of inflorescences under full sunlight.
1 INTRODUCTION
Plants respond to external stimuli by modifying their growth and developmental patterns. For example, plants can orient their growth according to the direction of the incoming light, a process called phototropism. In green tissues, phototropic responses are important for improving photosynthetic light capture (Hohm, Preuten, & Fankhauser, 2013). Although phototropism has been described in different plant structures, most of the knowledge about phototropic mechanisms in Arabidopsis thaliana (hereafter Arabidopsis) is derived from studies focused on vegetative organs, especially hypocotyls (Liscum et al., 2014). In contrast, the mechanisms mediating phototropic responses in reproductive organs have received very little attention (Inoue et al., 2008; Kagawa, Kimura, & Wada, 2009; Kumar, Millar, & Kiss, 2011; Sato, Sasaki, Matsuzaki, & Yamamoto, 2014).
At the beginning of the past century, experiments by Johnstone (1934) demonstrated that the blue region of the light spectrum had maximum effectiveness in promoting the phototropic response of oat coleoptiles. Later, wavelengths in the UV-B region (280–315 nm) were also shown to stimulate phototropism of hypocotyls of several dicotyledonous species (Baskin & Iino, 1987; Shinkle et al., 2004). The blue-light sensing photoreceptors phototropin 1 (phot1) and phototropin 2 (phot2) are considered the main photoreceptors responsible for phototropic responses towards blue light (Briggs et al., 2001; Hohm et al., 2013; Liscum et al., 2014; Pedmale, Celaya, & Liscum, 2010), and phot1 and phot2, along with the UV-B photoreceptor UV RESISTANCE LOCUS 8 (UVR8), are the main photoreceptors involved in the response towards UV-B (Vandenbussche et al., 2014; Vanhaelewyn et al., 2016). At the molecular level, phototropins are associated to the plasma membrane, where they function as light-activated serine/threonine kinases (Huala et al., 1997; Sakamoto & Briggs, 2002). In the past decade, several endogenous substrates for phototropin kinase activity were identified (Christie et al., 2011; Demarsy et al., 2012; Takemiya et al., 2013), including the phototropins themselves (Christie et al., 1998; Sakai et al., 2001). In contrast to phototropins, UVR8 is a soluble photoreceptor that, in etiolated seedlings exposed to UV-B, monomerizes and translocates to the nucleus, where it interacts with the E3 ubiquitin ligase CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1) protein, a positive regulator of UVR8 signaling (Oravecz et al., 2006). In addition to phototropins and UVR8, studies in Arabidopsis and cucumber indicate that the red/far-red-light absorbing photoreceptor phytochrome B (phyB) plays a direct role in negative phototropic response to far-red light reflected from neighbouring vegetation (Ballaré, 1999; Ballaré, Scopel, Radosevich, & Kendrick, 1992). The presence of phytochromes and the blue light photoreceptors cryptochromes in the nucleus regulates the expression of several genes that indirectly contribute to modulate phototropic responses (reviewed by Goyal, Szarzynska, & Fankhauser, 2013; Liscum et al., 2014). In this context, several studies indicate that cryptochromes positively regulate the blue-light mediated phototropism in etiolated seedlings (Kang, Lian, Wang, Huang, & Yang, 2009; Nagashima et al., 2008; Ohgishi, Saji, Okada, & Sakai, 2004; Tsuchida-Mayama et al., 2010; Zhao et al., 2019). Moreover, the behaviour of phyA cry1 cry2 loss-of-function mutants suggests that both phyA and cryptochromes are required, to some extent, for the phototropic response (Tsuchida-Mayama et al., 2010). In contrast to the situation in etiolated seedlings, in green, photoautotrophic seedlings, phytochromes and cryptochromes negatively regulate phototropism and prevent excessive phototropic response when the plants are exposed to full sunlight (Boccaccini et al., 2020; Goyal et al., 2016).
Several plant species show persistent bending of the inflorescences towards the dominant direction of incoming solar radiation (i.e., persistent bending towards the south or the north in the Northern and Southern Hemispheres, respectively). This response is termed ‘seasonal heliotropism’ and it is proposed to contribute to the thermal regulation of flower activity (Patiño & Grace, 2002). Flower temperature can be crucial for plant reproduction because temperature influences flower growth and development, pollen and ovule viability, and emission of volatile compounds that attract pollinators (Serrano et al., 2018; van der Kooi, Kevan, & Koski, 2019). The movement of reproductive organs in seasonal heliotropism differs from diurnal heliotropism (i.e., east–west daily solar tracking). Diurnal heliotropism involves mechanisms related to the classical blue-mediated phototropic responses. For instance, flowers of Ranunculus adoneus track the sun during the day, and this response is significantly attenuated when the irradiance in the blue region of the solar spectrum is reduced. By contrast, the red portion of sunlight had just a minor effect on the heliotropic movement (Stanton & Galen, 1993). In sunflower, diurnal heliotropism results from differential elongation on opposite sides of stems, and it is regulated by blue light and the circadian clock (Atamian et al., 2016). In inflorescence stems, studies in Arabidopsis indicate that phototropins are the key photoreceptors involved in bending towards low irradiances of monochromatic blue light (Kagawa et al., 2009) and that phyE positively regulates bending (Kumar & Kiss, 2006). In addition, recent studies showed that narrowband UV-B is able to promote phototropism in inflorescences of Arabidopsis with UVR8 acting as dominant photoreceptor in this response (Vanhaelewyn et al., 2019). However, the photoreceptors that mediate seasonal heliotropism under natural conditions have not been investigated.
The aim of this work was to study the light signals and photoreceptors controlling heliotropic responses in Arabidopsis inflorescences. We show that, under natural conditions, Arabidopsis plants orient their inflorescences towards the dominant direction of incoming solar radiation, displaying seasonal heliotropism. Through a combination of laboratory and field experiments, we demonstrate that inflorescence bending is promoted by spectral regions in the range of UV and blue light (λ ≤ 500 nm). Phototropins mediate bending in response to low irradiances of blue, and cryptochromes are the principal photoreceptors acting at high irradiances. Interestingly, we found that cryptochromes are the principal photoreceptors acting in the promotion of the heliotropic response under full sunlight, linking cryptochromes with inflorescence bending in response to UV-A and blue light under natural field conditions.
2 METHODS
2.1 Plant material
Wild-type (Col-0 and Ler) seeds were from NASC, Nottingham, United Kingdom. The double mutant phot1-5 phot2-1 (Kinoshita et al., 2001), uvr8-6 (Favory et al., 2009) and cop1-4 (McNellis et al., 1994) were a gift from Professor Roman Ulm (University of Geneva). The phot1-5 phot2-1 amiRUVR8 transgenic line was described in (Vandenbussche et al., 2014). The double mutant cry1-104 cry2-1 (Mao et al., 2005) was a kind gift from Hong-Quan Yang and the quadruple mutant phot1-5 phot2(SALK_051046) cry1-104 cry2-1 (Mao et al., 2005) was a kind gift from Professor Hongtao Liu (Shangai Institutes for Biological Sciences). The cry1-104 cry2-1 uvr8-6 triple mutant with Col-0 background was obtained by crossing the cry1-104 cry2-1 mutant, with the uvr8-6 mutant. The hy4-2.23n mutant that corresponds to cry1 mutant in Landsberg erecta (Ler) background was described in Koornneef, Rolff, and Spruit (1980) and Ahmad and Cashmore (1993) and the fha-1 that corresponds to cry2 mutant in Ler background was described in Koornneef, Hanhart, and Van der Veen (1991) and Guo, Yang, Mockler, and Lin (1998). The double hy4-2.23n fha-1 that corresponds to cry1 cry2 mutant in Ler was described in Mazzella, Cerdán, Staneloni, and Casal (2001).
2.2 Growth conditions
For the experiments under controlled conditions involving Col-0, phot1-5 phot2-1, phot1-5 phot2-1 amiRUVR8, uvr8-6, cry1-104 cry2-1, cry1-104 cry2-1 phot1-5 phot2-1, cry1-104 cry2-1 uvr8-6, cop1-4 under unidirectional red, far-red, UV-B and blue 2.5, 15 and 50 μmol.m−2.s−1 and Col-0 plus phot1-5 phot2-1 amiRUVR8 under blue 25 and 40 μmol.m−2.s−1, seeds were sown on moistened Jiffy-7 peat pellets (Jiffy Stange, Norway), supplemented with nutrients N, P, K (1:500 Wuxal Super 8–8-6, OptimAgro, Diegem, Belgium). Plants were grown until flowering stage under 16 hr light/ 8 hr dark at 65 μmol m−2 s−1 PPFD (Photosynthetic Photon Flux Density) at 22°C with 70% humidity in a growth chamber (Weiss Technik). Experiments were conducted in the Laboratory of Functional Plant Biology at Ghent University (Gent, Belgium). For the experiments under controlled conditions involving the study of the response of Col-0 plus the genotypes in Col-0 background to blue 25 and 40 μmol.m−2.s−1 (in exception to phot1-5 phot2-1 amiRUVR8, which were analysed in the Laboratory of Functional Plant Biology at Gent University) and the response of Ler, cry1 (hy4-2.23n), cry2 (fha-1) and cry1 cry2 (hy4-2.23n fha-1) to unilateral blue (15, 25, 40 and 50 μmol.m−2.s−1), seeds were sown on 0.8% agar, stratified for 3–5 days at 4°C in darkness and then exposed to white light to induce germination. Seedlings were transferred to 180 cm3 pots filled with two parts of Perlite (Sustraplanta, Argentina), two parts of peat moss sphagnum and one part of sand and watered with a solution containing 0.7 g/L of Hakaphos Red (COMPO, Spain). Plants grew in growth chambers under 16 hr light/ 8 hr dark at 100 μmol m−2 s−1 PPFD (F32T8/741 fluorescent, ARK Lighting, Gardena, CA), at 22°C until they were used in the experiments. Experiments were conducted in IFAB, Bariloche, Rio Negro, Argentina.
For the experiments under sunlight, seeds were sown on 0.8% agar, stratified for 3–5 days at 4°C in darkness and then exposed to white light to induce germination. Seedlings were transferred to 180 cm3 pots filled with two parts of Perlite (Sustraplanta, Argentina), two parts of peat moss sphagnum and one part of sand and watered with a solution containing 0.7 g/L of Hakaphos Red (COMPO, Spain). Plants grew inside growth chambers under 16 hr light/ 8 hr dark at 400 μmol m−2 s−1 PPFD (Philips Twister cool white 27 W and 80 W), at 22°C until they were used in the experiments under natural conditions.
2.3 Light treatments
For indoor experiments conducted in the Laboratory of Functional Plant Biology at Ghent University, UV-B irradiance (1.3 μmol.m−2.s−1) was provided by a Philips TL01 narrow band lamp filtered through two layers of cellulose acetate film (Jürgen Rachow, Germany), as described in Vanhaelewyn, Viczián, et al. (2019). Red light (20 μmol.m−2.s−1) was provided by 660 nm Deep Red LEDs (ABI LED lighting). Blue light (2.5, 15, 25, 40 or 50 μmol.m−2.s−1) was provided by 470-nm LEDs (Osram dragontape and Philips GreenPower research module). Different fluence rates of narrowband blue light were set at 2.5, 15, 25, 40 and 50 μmol.m−2.s−1 by adjusting the distance between the plants and the light source. Far-red radiation (35 μmol.m−2.s−1) was generated by 730-nm LEDs (Philips GreenPower LED flowering lamp, the Netherlands). For indoor experiments conducted in IFAB, Bariloche, Argentina, blue light (15, 25, 40 or 50 μmol.m−2.s−1) was provided by 465 ± 5 nm LEDs (Epistar, Taiwan). Different fluence rates of narrowband blue light were set at 15, 25, 40 and 50 μmol.m−2.s−1 by adjusting the distance between the plants and the light source. Fluence rates were measured with a SKP200 light meter (Skye Instruments, Powys, UK).
For outdoor experiments, we used plastic films mounted on metallic structures (50 cm height, 65 cm width, 35 cm long) to manipulate the solar spectrum (Figures S1 and S2). All sides of the structures were covered by ultra-fine polyvinylchloride (PVC) film (Rolopac, Argentina) with >95% of solar light transmittance, leaving 5 cm at the base and 10 cm at the top of each side of the structure without film in order to allow ventilation. To evaluate the influence of the solar spectrum coming from the north side on inflorescence bending, we added a layer of specific spectral filters to each wall of the structures, with the exception of the south wall. We generated three treatments: ‘solar’ using PVC film with no additional filter; ‘–UVB,’ which carried an additional layer of polyester film (Oeste Aislante, Buenos Aires), optically equivalent to Mylar-D; 0.1 mm thick to cut off radiation below 315 nm without affecting longer wavelengths (Mazza, Izaguirre, Zavala, Scopel, & Ballaré, 2002); and ‘–UV/blue,’ which carried an additional layer of LEE767 (Oklahoma Yellow) filter in order to cut off solar radiation below 500 nm. All light treatments had highly transparent, clear food wrap (PVC film) on the south side of the plants. Light gradients, thermal profiles and experimental setup are described in Figure S1. Light spectra in Figures S1B–D and S2A were acquired near solar noon with a cosine-corrected OceanOptics USB4000 microspectroradiometer.
2.4 Experiments under natural sunlight
Outdoor experiments were conducted in the Southern Hemisphere, in the experimental field of the IADIZA institute, Technological Scientific Centre, CONICET, Mendoza (−32.892981, −68.875502, altitude: 720 m above sea level: a.s.l.) and in the experimental field of the IFAB, INTA EEA Bariloche-CONICET, Rio Negro (−41.124815, −71.250982, altitude 820 m a.s.l.), both in Argentina. Experiments were performed during autumn, winter and spring in 2014, 2016, 2017 and 2019, and validated in both locations. Temperature values during experiments were in the range between 0°C and 24°C (IADIZA, Mendoza) or 0°C and 18°C in IFAB, Bariloche. Figure S1E–G shows representative temperature profiles during the experiments. As an additional control, inflorescence bending of WT plants (Col-0) under solar light was also tested in the experimental field of Ghent University, Belgium (51.046791, 3.728605, altitude: 10 m a.s.l.).
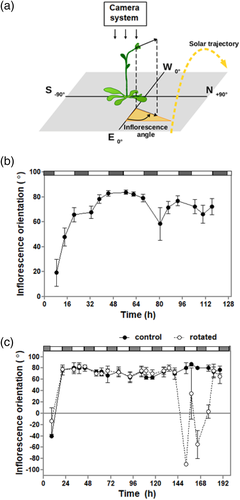
Plants bearing primary inflorescences (inflorescence length between 5 and 10 cm) were transferred from the growth chambers to the field treatments 2–3 hr after sunrise, and exposed to different treatments; solar, –UVB and –UV/blue. Col-0 plants showed a saturated response 4.5 hr after the beginning of the treatment (Figure S3). Therefore, plants were photographed at the beginning of the experiment and 5 hr later using a Canon PowerShot SX100 IS camera. Focal plane was parallel to the soil surface, with the camera located at the zenith of the inflorescence. Bending angles at the beginning and end of the experiment were quantified using the angle tool of ImageJ (National Institutes of Health). The angle of curvature was defined as the angle between the inflorescence axis and its vertical vector (Figure 1a). Inflorescence bending towards the north or south is indicated by positive or negative values, respectively.
2.5 Experiments under controlled conditions
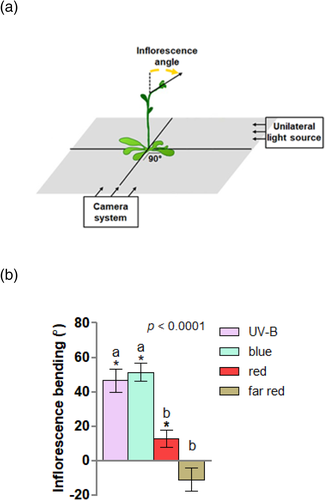
Plants bearing primary inflorescences (inflorescence length between 5 and 10 cm) were transferred from the growth chamber to a dark room at 09:00 a.m. where they were exposed to the different unilateral narrowband light treatment (UV-B, blue, red or far red light) at 22°C. Plants were photographed at the beginning of the experiment and 6 hr later using a webcam based time lapse with focal plane parallel with the incoming unilateral light as described in Vandenbussche et al. (2014) and Vanhaelewyn, Van Der Straeten, and Vandenbussche (2019). Bending angles (Figure 2a) at the beginning and end of the experiment were quantified using the angle tool of ImageJ.
2.6 Statistical analyses
We compared the initial (T0) and final (T1) angles of each treatment/genotype through unilateral Wilcoxon signed rank tests. Asterisks in figures indicate significant differences (*** = p < .001; ** = p < .01; * = p < .05) between T0 and T1. Inflorescence bending was defined as the difference between inflorescence angles (T0 – T1). This variable showed a normal distribution in KS and Shapiro–Wilk normality tests. Differences in inflorescence bending were therefore tested by one-way analysis of variance (one-way ANOVAs) followed by Tukey's post-hoc tests. Statistical analyses were carried out using INFOSTAT version 2020I (https://www.infostat.com.ar/?lang=en). Col-0 data in response to 25 and 40 μmol.m−2.s−1 of unilateral blue did not show significant differences (two-tailed t-test p > .05) between experiments performed in the Laboratory of Functional Plant Biology at Ghent University (inflorescence angle, mean ± standard error of the media [SEM]: 19.2 ± 2.15, 18.7 ± 2.1 at 25 and 40 μmol.m−2.s−1, respectively) and IFAB, Bariloche, Rio Negro, Argentina (inflorescence angle, mean ± SEM: 25.2 ± 2.2 and 24.3 ± 2.5 at 25 and 40 μmol.m−2.s−1, respectively). However, for clarity, in those cases in which experiments carried out in different laboratories are shown on the same figure [Figure 3d (blue light 25 μmol. m−2.s−1) and E (blue light 40 μmol. m−2.s−1)], the bars corresponding to the relevant wild types are noted in the graphs.
3 RESULTS
3.1 Heliotropic behaviour of Arabidopsis inflorescences in the field
When Arabidopsis plants were transferred form a growth chamber to the field, their inflorescences bent towards the north (i.e., the dominant direction from which direct solar radiation impinges on the ground in the Southern Hemisphere) (Figure 1b). The inflorescences remained oriented in this direction for the duration of the experiment, demonstrating a classic example of seasonal heliotropism (Patiño & Grace, 2002, Serrano et al., 2018). When plants having their inflorescences already pointing to the north were rotated 180°, the inflorescences returned to a north-facing position within 24 hr (Figure 1c). Qualitatively similar results were obtained in outdoor experiments carried out in Ghent, Belgium (inflorescence angle: 62.5 ± 16.2).
3.2 Inflorescence bending under controlled conditions
In order to identify the regions of the light spectrum that trigger inflorescence bending, wild-type plants were placed inside a dark room and unilaterally irradiated with narrowband UV-B (1.3 μmol.m−2.s−1), hereafter UV-B, blue (15 μmol.m−2.s−1), red light (20 μmol.m−2.s−1) and far red light (35 μmol.m−2.s−1). Inflorescences exposed to UV-B and blue light showed a conspicuous bending towards the light source; in contrast, red and far red light had little or no effect on inflorescence angles (Figure 2b). These results indicate that the UV-B and blue regions of the light spectrum are important signals promoting phototropic bending in inflorescence stems exposed to narrowband unilateral radiation.
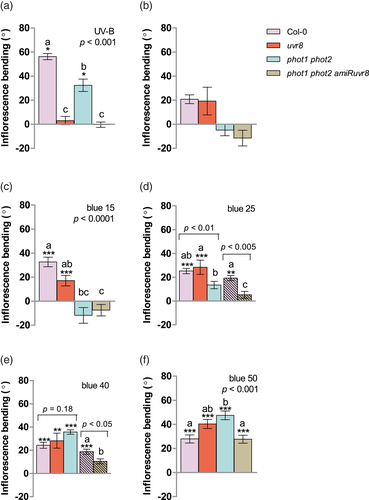
It is widely recognized that UVR8 and phototropins are the principal photoreceptors mediating phototropic responses to UV-B and blue (Briggs et al., 2001; Pedmale et al., 2010; Vandenbussche et al., 2014; Vanhaelewyn, Viczián, et al., 2019). In order to test their relevance in the bending of inflorescences in response to unilateral UV-B and blue, we exposed Col-0 plants and the loss-of-function mutants phot1 phot2, uvr8 and the line phot1 phot2 amiRUVR8, to narrowband UV-B (1.3 μmol. m−2.s−1) and blue (2.5, 15, 25, 40 or 50 μmol. m−2.s−1). The choice of different fluxes of blue light was based on the observation that phototropic responses of hypocotyls are fluence-rate dependent (Whippo & Hangarter, 2003). UVR8 was the main photoreceptor mediating bending of inflorescences under UV-B, with some minor contribution from phototropins (Figure 3a). Very low irradiances of blue light (2.5 μmol.m−2. s−1) failed to elicit a significant phototropic response (Figure 3b). At low irradiances of unilateral blue light (≤15 μmol.m−2. s−1), phototropins were the main photoreceptors involved in the promotion of inflorescence phototropism, with some minor contribution from UVR8 (Figure 3c). Interestingly, however, at intermediate irradiances of narrowband blue (25 μmol.m−2. s−1), the response of the phot1 phot2 plants to unilateral blue was nearly normal (Figure 3d) and, at relatively high irradiances (40 and 50 μmol.m−2. s−1), the phot1 phot2 mutant showed an even slightly enhanced bending response in comparison to Col-0 (Figure 3e,f). This indicates that the importance of phototropins in controlling inflorescence bending towards blue light varies with the photon flux density of the blue light stimulus. In addition, these results suggest that photoreceptors different from the phototropins and UVR8 are mediating the bending of inflorescences under high irradiances of blue light, and that phototropins may negatively regulate the action of those photoreceptors.
On the basis of these results, we tested the role of cryptochromes, by exploring the phototropic responses of inflorescences of Col-0, the double cry1 cry2, the triple cry1 cry2 uvr8, and the quadruple phot1 phot2 cry1 cry2 loss of function mutants to different irradiances of unilateral blue light. Under low to intermediate irradiance of narrowband blue, cry1 cry2 mutants showed no significant differences compared to Col-0 (Figure 4a–c). Conversely, at 50 μmol.m−2.s−1, bending of cry1 cry2 was clearly reduced (Figure 4d), indicating that CRY1, CRY2 or both are required for a full phototropic response of inflorescences to high irradiances of unilateral blue. Plants lacking both phototropins and cryptochromes showed greatly attenuated bending responses over the whole range irradiances tested, supporting the relevance of both photoreceptor families in the control of inflorescence phototropism to unilateral blue (Figure 4a–d). Moreover, the enhanced phototropic response of phot1 phot2 relative to Col-0 at 40 and 50 μmol.m−2. s−1 (Figure 3e,f) was severely reduced when cryptochromes were not functional in the phot1 phot2 cry1 cry2 mutants (Figure 4d). This result suggests that phototropins negatively regulate the action of cryptochromes at high irradiances of blue. In the cry1 cry2 mutant background, UVR8 enhanced phototropism at 15, 25 and 40 μmol.m−2. s−1 of blue (Figure 4a–c comparison between cry1 cry2 and cry1 cry2 uvr8), further suggesting a role of UVR8 in inflorescence bending at low to intermediate irradiances. The cry mutations promoted the bending response to unilateral UV-B (comparison between uvr8 and cry1 cry2 uvr8 in Figure 3a and Figure S4A, p < .05). We also examined the response of cop1 loss-of-function mutant to UV-B and different irradiances of blue light, since COP1 is an essential component of both cryptochrome- and UVR8-triggered light signalling (Favory et al., 2009; Lian et al., 2011). Compared to Col-0, cop1 inflorescences showed significantly attenuated responses to unilateral UV-B and 50 μmol.m−2. s−1 of blue (Figure S4B) and this is in agreement with the observed main role of UVR8 and cryptochromes under UV-B and high irradiances of blue light, respectively (Figures 3a and 4d).
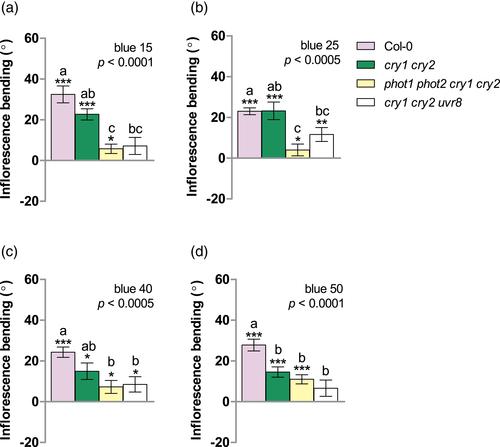
To further document the role of cryptochromes in blue light-induced inflorescence phototropism, we tested the behaviour of cry1 and cry2 mutants in the Ler background. Interestingly, ablation of CRY1 and CRY2 had an even stronger effect in Ler than in Col reducing inflorescence bending; this effect was statistically significant even at blue light irradiances as low as 25 μmol.m−2.s−1 (Figure S5B). CRY1 and CRY2 appeared to have additive effects on the bending response to high irradiances of blue light (Figure S5).
3.3 Inflorescence heliotropism under natural conditions
We conducted experiments under natural solar radiation to determine (a) the regions of the solar spectrum that are relevant for inflorescence heliotropism in the field, and (b) the roles of UV and blue light photoreceptors. For this purpose, we designed a multifactorial experiment that included plants of Col-0, phot1 phot2, uvr8, phot1 phot2 amiRUVR8, cry1 cry2, cry1 cry2 phot1 phot2 and cry1 cry2 uvr8 lines in combination with a set of three light treatments. The treatments were solar light (full solar radiation); –UVB, in which the UV-B component coming from the north was blocked using a clear polyester filter (λ > 315 nm); and –UV/blue, in which the UV and blue light photons coming from the north were blocked using a yellow filter (λ > 500 nm). In all treatments, the south wall of the structures consisted of a thin layer of clear food wrap (clear PVC film; Rolopac), which had a very high transmittance (>95%) in the UV and visible regions of the solar spectrum (see detail in Figures S1 and S2).
Inflorescences of Col-0 plants showed normal bending towards the north under full sunlight (solar light treatment), and this response was still present when the UV-B photons coming from the north were cut-off in the –UVB treatment (Figure 5a,b). Conversely, the attenuation of the UV/blue region of the solar spectrum, which inverted the direction of the UV/blue gradient (Figure S1), promoted negative bending—that is, bending towards the south in Col-0- (Figure 5c). These results indicate that the UV-A/blue region of the spectrum constitutes the main signal for the promotion of inflorescence bending under natural gradients of sunlight. The response to solar UV-A/blue was not affected in phot1 phot2, uvr8 and phot1 phot2 amiUVR8 but was severely impaired in all the genotypes lacking cryptochromes (cry1 cry2) (Figure 5a,b). Taken together, these results indicate that cryptochromes are the main photoreceptors mediating the bending of inflorescences in response to the natural gradient of sunlight under field conditions.
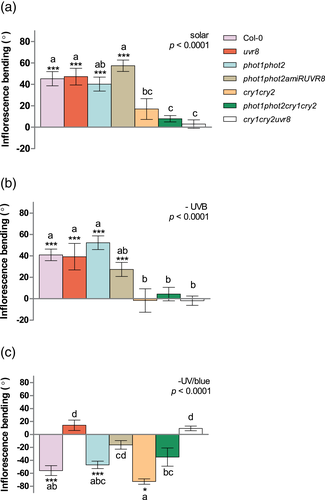
The southward bending observed in plants grown under inverted gradients of UV/blue was conserved in the cry1 cry2 mutant. In contrast, plants lacking functional UVR8, or with reduced UVR8 activity such as uvr8, phot1 phot2 amiRUVR8 and cry1 cry2 uvr8 did not show significant southward bending (Figure 5c). However, UVR8 by itself was not able to promote bending in the absence of both phototropins and cryptochromes: since phot1 phot2 cry1 cry2 showed a weak, non-significant bending response, but both phot1 phot2 and cry1 cry2 double mutants showed significant bending (Figure 5c).
4 DISCUSSION
Our results demonstrate that Arabidopsis plants display heliotropic inflorescences with spectral sensitivity in the UV and blue regions of the spectrum. Under natural conditions, heliotropism was mostly a UV-A/blue light-mediated response, principally promoted by cryptochromes. Cryptochromes were also the main photoreceptors acting in the promotion of phototropism of inflorescences in response to relatively high irradiances of narrowband blue in controlled environments.
The observed effect of narrowband UV-B and blue light in inflorescence bending under controlled conditions (Figure 2) is in agreement with Kagawa et al. (2009) and Vanhaelewyn, Viczián, et al. (2019) and the central role of UVR8 under UV-B light (Figure 3a) is in agreement with the findings of Vanhaelewyn, Viczián, et al. (2019). Previous studies indicate that inflorescences of plants lacking functional phototropins show normal phototropic response towards UV-B. However, phototropins do play a role, since bending responses to UV-B in plants lacking functional ELONGATED HYPOCOTYL 5 (HY5) and HY5 HOMOLOG (HYH) genes are dramatically reduced in the phot1 phot2 background (Vanhaelewyn, Viczián, et al., 2019). Our results show that the lack of phototropin activity reduced the phototropic response towards UV-B (Figure 3a), reinforcing the proposed roles of these photoreceptors in inflorescence stem bending (Vanhaelewyn, Viczián, et al., 2019). The phototropin-mediated effect is probably triggered by the photoexcitation of flavin mononucleotide chromophores by UV-B photons (Briggs, 2014; Kasahara et al., 2002; Knieb, Salomon, & Rüdiger, 2005). In etiolated seedlings, phototropins are the principal photoreceptors acting in the promotion of phototropism in response to low intensities of UV-B (0.002 μmol.m−2.s-1), and both UVR8 and phototropins act at higher UV-B irradiances (0.12 μmol.m−2.s-1) (Vanhaelewyn et al., 2016).
In the blue region, the irradiance range also influenced the role of photoreceptors and their hierarchical relationships in controlling inflorescence bending. Phototropins showed a prominent role in inflorescence bending in response to low irradiances of blue light, with UVR8 exerting a minor contribution (Figure 3c). In contrast, cryptochromes were the main photoreceptors promoting bending responses at high irradiances of blue light (Figure 4d and Figure S5B–D), and phototropins negatively regulated the actions of cryptochromes (Figure 3e,f). Plants lacking UVR8 also showed a slight promotion of bending (Figure 3f), and this effect could be due to a competition between the UV and blue pathways for downstream signalling components, such as COP1. The described effect of cryptochromes and phototropins in inflorescence stems contrasts with their actions in etiolated hypocotyls, where phototropins are the main promoters of bending over a wide range of intensities of blue light, including high irradiances (Sakai et al., 2001). Phototropins are also the primary photoreceptors controlling hypocotyl phototropism in green seedlings (Goyal et al., 2016), although their role at intermediate/high irradiances (above 10 μmol.m−2.s−1) has not been investigated.
In contrast to the results of laboratory experiments using narrowband illumination, our experiments in the field tested the effects of a polychromatic environment, where UV-B represents less than 0.5% of the photon irradiance received at ground level between 290 and 700 nm (Aphalo, 2018; Demkura, Abdala, Baldwin, & Ballaré, 2010; Morales et al., 2013). We show that the UV-A/blue components of the solar spectrum are the major drivers of inflorescence heliotropism under full sunlight and this response requires the presence of functional cryptochromes (Figure 5a,b). This is consistent with data of experiments under controlled conditions, which show a reduced response to high irradiances of narrowband blue in cryptochrome-deficient plants (Figure 4 and Figure S5). Recent work demonstrates that cry1 can negatively modulate phototropic responses elicited by phototropins in the hypocotyls of de-etiolated seedlings. This cry1 effect is mediated by reduction of phytochrome-interacting factor 4 (PIF4) levels (Boccaccini et al., 2020), which causes decreased auxin signalling and hence reduced phototropic response. It is tempting to speculate that, in inflorescence stems, which are thicker and optically more complex structures compared to hypocotyls, directional blue light can create a radial gradient of cryptochrome signalling across the stem, which would in turn result in higher PIF activity and auxin signalling on the shaded side, leading to inflorescence bending. When a yellow filter was placed on the north side of the plants, the total fluence rate of blue and UV light was greatly reduced, and their natural north-to-south gradient was reversed. Under these artificial conditions of low irradiance, UVR8 emerged as the principal photoreceptor driving the bending of the inflorescences towards the south (Figure 5c), This result agrees with the observed role of UVR8 in the response of inflorescences to unilateral UV-B and low to intermediate irradiances of blue under controlled conditions (Figures 3a–e and 4a–c).
Finally, it is important to note that, under full sunlight, the lack of cryptochromes had a stronger effect abolishing the bending response than under our controlled conditions, where cryptochrome mutants such as cry1 cry2 or cry1 cry2 phot1 phot2 showed reduced but significant curvature in response to gradients of narrowband blue (Figures 4 and 5). The differences between indoor and outdoor experiments suggest that novel interactions between photoreceptors could emerge under a polychromatic environment, which are not evident under controlled conditions. The recently described cross-regulation of cryptochrome and UVR8 signalling through the proteins repressor of UV-B photomorphogensis 1 and 2 (RUP1 and RUP2) and blue light inhibitors of cry1 and cry2 (BIC1 and BIC2) (Tissot & Ulm, 2020) may provide some insights into the mechanisms of light signalling crosstalk under natural environments. Additionally, BIC1/2 and RUP1/2 transcriptional activation can also be promoted by phyB (Gruber et al., 2010; Wang et al., 2017), and RUP1/2 promotes a negative feedback pathway that prevents UVR8 signalling to interfere with the aforementioned phototropin-mediated hypocotyl bending under UV-B (Vanhaelewyn et al., 2016). Inflorescence stems constitute an attractive system to study the way in which these molecular components interact under natural environments, and to explore the functional consequences of these interactions.
Heliotropism of reproductive organs is widespread in Angiosperms. In insect-pollinated species, proper floral orientation is proposed to regulate the thermal environment of flowers and favour flower visibility, enhancing attractiveness to pollinators (Sapir, Shmida, & Ne'eman, 2006; van der Kooi et al., 2019). Heliotropism, by modulating floral temperature, also has the potential to influence other traits related to sexual reproduction, such as stigmatic receptivity, pollen germination and seed production (reviewed by Serrano et al., 2018 and van der Kooi et al., 2019). In spite of its widely recognized ecological relevance, our knowledge of the physiological mechanisms underlying flower heliotropism is very limited, and based on the study of a fairly low number of species (van der Kooi et al., 2019). In this context, it is interesting to ask: are the cryptochromes the principal photoreceptors mediating heliotropism of inflorescences and flowers across plant taxa? Is there intra-specific natural variation in heliotropic responses of inflorescences? Is this variation associated with environmental variables (e.g., ambient temperature) or ecological factors (e.g., dependence on pollinators)? Is this cryptochrome response adaptive? The discovery of heliotropism in inflorescence stems of Arabidopsis and its underlying photobiological bases opens new opportunities to elucidate the molecular mechanisms controlling this physiological response, with the potential to influence components of plant fitness in natural environments.
ACKNOWLEDGMENTS
This work was supported by the research project G000515N of the Research Foundation Flanders to Filip Vandenbussche (as of October 2017 administratively transferred to Dominique Van Der Straeten), Research foundation Flanders and Ministerio de Ciencia, Tecnología e Innovación, Argentina: project MINCYT-FWO VS00715N-FW/14/02 to Carlos Luis Ballaré and Dominique Van Der Straeten, Ghent University to Dominique Van Der Straeten and the research project PIP 11220150100237CO of the Consejo Nacional de Investigaciones Científicas y Técnicas to María Verónica Arana. Alejandro Miguel Serrano was supported by a CONICET doctoral fellowship and Lucas Vanhaelewyn is a doctoral fellow of the FWO. We thank Marcos Bladauskas (Universidad Nacional del Comahue) for his technical support and Dr. Rodolfo A. Sanchez (IFEVA, Facultad de Agronomía) for his helpful comments on the manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request from the authors.




