CO2-responsiveness of leaf isoprene emission: Why do species differ?
Funding information: Eesti Teadusagentuur, Grant/Award Number: PRG537; European Regional Development Fund, Grant/Award Number: Center of Excellence Ecolchange; H2020 European Research Council, Grant/Award Number: 322603
Abstract
Leaf isoprene emission rate, I, decreases with increasing atmospheric CO2 concentration with major implications for global change. There is a significant interspecific variability in [CO2]-responsiveness of I, but the extent of this variation is unknown and its reasons are not understood. We hypothesized that the magnitude of emission reduction reflects the size and changeability of precursor pools responsible for isoprene emission (dimethylallyl diphosphate, DMADP and 2-methyl-erythritol 2,4-cyclodiphosphate, MEcDP). Changes in I and intermediate pool sizes upon increase of [CO2] from 400 to 1500 μmol/mol were studied in nine woody species spanning boreal to tropical ecosystems. I varied 10-fold, total substrate pool size 37-fold and the ratio of DMADP/MEcDP pool sizes 57-fold. At higher [CO2], I was reduced on average by 65%, but [CO2]-responsiveness varied an order of magnitude across species. The increase in [CO2] resulted in concomitant reductions in both substrate pools. The variation in [CO2]-responsiveness across species scaled with the reduction in pool sizes, the substrate pool size supported and the share of DMADP in total substrate pool. This study highlights a major interspecific variation in [CO2]-responsiveness of isoprene emission and conclusively links this variation to interspecific variability in [CO2] effects on substrate availability and intermediate pool size.
1 INTRODUCTION
Isoprene is worldwide the most important reactive hydrocarbon emitted by plants (Feng et al., 2019; Fini et al., 2017; Guenther, 2013; Guenther et al., 2012). There is a large interspecific variability in the capacity for isoprene emission with some species having extremely high isoprene emission rates, followed by intermediate emitters and species emitting isoprene close to the baseline level or not at all (Fineschi, Loreto, Staudt, & Peñuelas, 2013; Fini et al., 2017; Guenther et al., 2012; Kesselmeier & Staudt, 1999; Okereke, Liu, Kaurilind, & Niinemets, 2021). The differences in isoprene emission capacity have been typically associated with interspecific variation in the activity of isoprene synthase, the terminal enzyme responsible for isoprene synthesis (Fall & Wildermuth, 1998; Lantz, Allman, Weraduwage, & Sharkey, 2019; Lehning, Zimmer, Steinbrecher, Brüggemann, & Schnitzler, 1999; Loreto et al., 2014). While the strong variation in isoprene emission capacity is widely recognized, there are also major interspecific variations in environmental responses of isoprene emission (Emmerson, Possell, Aspinwall, Pfautsch, & Tjoelker, 2020; Fini et al., 2017; Lantz et al., 2019; Niinemets et al., 2010; Niinemets, Sun, & Talts, 2015; Niinemets, Tenhunen, Harley, & Steinbrecher, 1999; Sun, Shen, & Niinemets, 2020; Wilkinson et al., 2009), but the presence of such variations is much less recognized, and the mechanistic reasons for these variations are poorly understood.
Isoprene is synthesized in chloroplasts via the 1-deoxy-d-xylulose 5-phosphate/methyl-d-erythritol phosphate (DOXP/MEP) pathway. The pathway is strongly related to photosynthesis as it directly consumes the photosynthetic product glyceraldehyde 3-phosphate (GAP) and relies on photosynthetic ATP and reductant supply (Figure 1; Lantz, Allman, et al., 2019; Monson, Grote, Niinemets, & Schnitzler, 2012; Sharkey & Monson, 2014). As the result, isoprene emission rate depends on incident light intensity according to a saturating curve similarly to photosynthesis, and on temperature according to an Arrhenius type response with an optimum (Grote, Monson, & Niinemets, 2013; Monson et al., 2012). Isoprene emission also depends on CO2 concentration ([CO2]), but differently from photosynthesis, isoprene emission rate decreases at ambient [CO2] exceeding 300–400 μmol/mol, while photosynthesis rate increases asymptotically with increasing [CO2] (Calfapietra, Scarascia Mugnozza, Karnosky, Loreto, & Sharkey, 2008; Lantz, Solomon, et al., 2019; Loreto & Sharkey, 1990; Monson, Hills, Zimmerman, & Fall, 1991; Possell & Hewitt, 2011; Rasulov, Hüve, et al., 2009; Wilkinson et al., 2009). The mechanism of the reduction of isoprene emission rate at higher [CO2] is not yet entirely elucidated. As the DOXP/MEP pathway starts with the condensation of GAP and pyruvate, one mechanism postulated to explain the reduction in isoprene emission is that higher [CO2] reduces chloroplastic pyruvate concentration (de Souza et al., 2018; Loreto et al., 2007; Monson et al., 2009; Potosnak, 2014; Wilkinson et al., 2009). Alternatively, it has been suggested that isoprene emission is reduced at higher [CO2] due to reduced ATP and NADPH availability in ribulose-1,5-bisphosphate regeneration-limited conditions when photosynthesis consumes a higher proportion of ATP and reductant or when photosynthesis is feedback-inhibited due to limited triose phosphate utilization that concomitantly suppresses ATP production (Lantz, Solomon, et al., 2019; Monson et al., 2016; Morfopoulos et al., 2014; Niinemets et al., 1999; Rasulov et al., 2016; Rasulov, Hüve, et al., 2009; Rasulov, Talts, Bichele, & Niinemets, 2018). Both mechanisms predict that higher [CO2] leads to a lower chloroplastic pool size of the immediate isoprene precursor dimethylallyl diphosphate (DMADP), and this is consistent with experimental observations (Ghirardo et al., 2014; Possell & Hewitt, 2011; Rasulov et al., 2016; Rasulov, Hüve, et al., 2009; Rosenstiel, Potosnak, Griffin, Fall, & Monson, 2003; Sun, Niinemets, Hüve, Rasulov, & Noe, 2013).
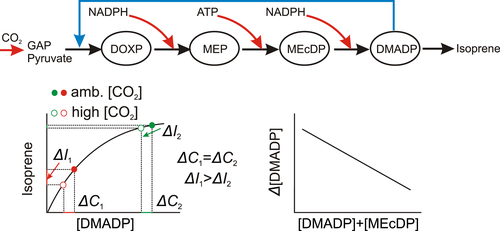
The [CO2] response of isoprene emission has been included in leaf to large scale models using empirical functions linking isoprene emission to ambient [CO2] (Arneth et al., 2007; Arneth et al., 2011; Guenther et al., 2012; Hantson, Knorr, Schurgers, Pugh, & Arneth, 2017). Incorporation of the [CO2] response is critical as without it, the models would predict a major enhancement of global isoprene emissions in the future due to raising temperature, while with the [CO2]-induced inhibition, the models predict either a constant isoprene emission or a reduced isoprene emission in future climates (Arneth et al., 2007, 2011; Bauwens et al., 2018; Emmerson et al., 2020; Hantson et al., 2017; Heald et al., 2009). So far, all the models in use incorporate a constant [CO2]-responsiveness of isoprene emission for all species, and isoprene emission is considered to be very sensitive to [CO2], much more sensitive than, for example, monoterpene emission to [CO2] (Feng et al., 2019). However, there is evidence that species can significantly differ in the responsiveness of isoprene emission to [CO2] with some species exhibiting a major reduction in isoprene emission at higher [CO2], for example, poplars, and some species only exhibiting a minor change, for example, oaks (Affek & Yakir, 2002; Lantz, Solomon, et al., 2019; Li et al., 2011; Monson et al., 2016; Possell & Hewitt, 2011; Rasulov, Hüve, et al., 2009; Sharkey, Loreto, & Delwiche, 1991; Sun et al., 2012; Wilkinson et al., 2009). So far, the variation in the sensitivity of isoprene emission to [CO2] has not been studied systematically and species differences in [CO2]-responsiveness are not understood. This is a significant limitation for accurate simulation of isoprene emissions from vegetation under global change.
Due to the low affinity of isoprene synthase with respect to its substrate DMADP (Lehning et al., 1999; Rasulov, Bichele, Hüve, Vislap, & Niinemets, 2015; Rasulov, Bichele, Laisk, & Niinemets, 2014; Schnitzler, Arenz, Steinbrecher, & Lehning, 1996), isoprene-emitting plants support a high pool size of DMADP (Li et al., 2011; Weise et al., 2013). In addition, isoprene-emitting plants also support a large pool of another DOXP/MEP pathway intermediate 2-C-methyl-d-erythritol 2,4-cyclodiphosphate (MEcDP) (Ghirardo et al., 2014; Li et al., 2011; Li & Sharkey, 2013; Rasulov et al., 2011; Rasulov, Talts, et al., 2015). Given that the increase in [CO2] ultimately results in the reduction of DMADP pool size, the question is what is the effect of a certain reduction in the pool size on isoprene emission? Isoprene emission depends on DMADP concentration according to a hyperbolic response (Figure 1). Accordingly, the effect of a given change in DMADP pool size on isoprene emission depends on the degree to which isoprene emission approaches saturation, that is, how large is the DMADP pool size relative to isoprene emission (Figure 1). Thus, species that can support a relatively larger pool size of DMADP are also expected to be less sensitive to [CO2] (Figure 1). In addition, at a given total DOXP/MEP intermediate pool size (MEcDP+DMADP), species that have a greater share of total intermediate pool in DMADP could also be less sensitive to [CO2].
We studied the variation in DOXP/MEP intermediate pool sizes, and the variation in [CO2]-responsiveness of isoprene emission in relation to the intermediate pool sizes in nine isoprene-emitting woody species and hypothesized that (a) there is a significant variation in the intermediate pool sizes and the sensitivity of isoprene emission to [CO2] and that (b) the magnitude of the [CO2]-dependent reduction in isoprene emission is quantitatively related to the reduction in intermediate pool size and (c) the variation in the sensitivity of isoprene emission to [CO2] depends on the intermediate pool size, and the share of intermediate pool size between DMADP and MEcDP pools.
The study was conducted with nine tree species from boreal to tropical ecosystems, including boreal to temperate species Populus tremula L., Populus tremula L. x P. tremuloides Michx., temperate species Eucalyptus globulus Labill., Platanus x acerifolia (Aiton) Willd., Quercus robur L., Q. rubra L., temperate to subtropical species Liquidambar formosana Hance, and subtropical to tropical species Mangifera indica L. and Phoenix dactylifera L. Populus spp., Quercus spp., P. x acerifolia and L. formosana are broad-leaved deciduous species, E. globulus and M. indica are broad-leaved evergreen simple-leaved species and the palm P. dactylifera is an evergreen compound-leaved species.
2 MATERIAL AND METHODS
2.1 Plant material
Most plants used in the experiments were grown from seed. Seeds of Eucalyptus globulus were obtained from OMC seeds Ltd. (Lithuania), and seeds of Mangifera indica cv. Tommy Atkins, and Phoenix dactylifera cv. Kenta were obtained from a local fruit shop. Seeds of Platanus x acerifolia were collected from Parc de Bruxelles, Belgium (50.84° N, 3.36° E), seeds of Liquidambar formosana from the campus of National Pingtung University of Science and Technology (22.64° N, 120.60° E) and the seeds of Q. rubra from the campus of the Estonian University of Life Sciences (58.39 °N, 26.69 °E). One-year-old plants of hybrid aspen (Populus tremula x P. tremuloides clone H200) were obtained from Juhani Puukool (Räpina, Estonia). The seeds were planted in 5 L pots, and hybrid aspen plants to 10 L pots filled with an 1:1 mixture of commercial potting soil (Biolan Oy, Kekkilä group, Finland) and quartz sand (pH of the soil water of 6.2). The plants were fertilized with a slow-release fertilizer that included essential macronutrients N (100 mg/L), P (30 mg/L) and K (200 mg/L) and micronutrients. The plants were grown in an environment-controlled plant growth room under the following conditions: light intensity at leaf surface of 500 μmol m−2 s−1 provided for 12 hr photoperiod (HPI-T Plus 400 W metal halide lamps, Philips), air temperature (day/night) of 26/24 °C, ambient CO2 concentration of 380–400 μmol/mol and relative air humidity of 60–70%. The plants were watered to soil field capacity every second day. One-year-old plants were replanted to 10 L pots. At the time of the measurements, the plants were 3-4-year-old, except for the plants of L. formosana that were 1-year-old and plants of hybrid aspen that were 1.5-year-old.
In addition to potted plants, Populus tremula and Quercus robur were sampled at the campus of the Estonian University of Life Sciences. The sampled plants of P. tremula were ca. 20-year-old and 15–20 m tall. For Q. robur, both young 4-6-year-old and 1 m tall, and mature ca. 40-year-old 15–20 m tall plants were sampled. Twigs with 2–3 mature leaves were cut under water, immediately transported to the lab and recut under water.
2.2 Experimental setup and standard protocol
All measurements were replicated with three independent plants for each species, except for Q. robur (n = 3 for both young and mature plants). In all cases, fully mature, non-senescent leaves were used in the measurements. We used an ultra-fast custom-made gas-exchange system as described in detail in Rasulov, Talts, et al. (2015). The system has a circular clip-on type leaf gas exchange chamber with 8 cm2 window area. The chamber temperature is controlled by a circulating water bath and the leaf upper side is glued to the chamber window with a starch paste for precise temperature regulation (±0.1 °C of the circulating water even at light intensities as high as 2000 μmol m−2 s−1 and during rapid temperature transients) (Laisk et al., 2002; Rasulov, Hüve, Bichele, Laisk, & Niinemets, 2010 for details). Starch paste itself did not emit any volatiles and as the stomata in all species studied were on the lower leaf surface, there was no impact of the starch paste on foliar photosynthetic characteristics and isoprene emission. Light was provided by a Schott KL 1500 light source equipped with a heat-reflecting filter (Optical Coating Laboratory, Santa Rosa, CA, USA). Measurement gas was mixed from pure N2, O2 and CO2 with custom-made mixers and the gas flow rate was maintained at 0.5 mmol/s (system response time < 0.1 s, Niinemets, 2012). The measurement system had two identical gas lines, reference and measurement line that could be rapidly swapped by electronic valves. Gas composition of the lines could be independently controlled, allowing very rapid change in chamber gas composition by switching between the lines with different gas concentration.
Chamber CO2 concentration was measured with a Li-6251 infra-red CO2 analyser (LiCor, Lincoln, NE, USA), water vapour concentration by a custom-made micropsychrometer (Rasulov et al., 2011) and chlorophyll fluorescence characteristics by a PAM 101 fluorimeter (H. Walz GmbH, Effeltrich, Germany). Isoprene concentration was measured as the parent ion with m/z 69+ by a Proton-Transfer-Reaction Time-of-Flight Mass-Spectrometer (PTR-TOF-MS 8000, Ionicon Analytik GmbH, Innsbruck, Austria) using the configuration of PTR-TOF-MS as in Rasulov, Talts, and Niinemets (2019). The PTR-TOF-MS was frequently calibrated with a custom-made isoprene calibration mixture (3.43 ppm isoprene in N2).
After leaf enclosure in the chamber, the following standard environmental conditions were established: chamber CO2 concentration of 400 μmol/mol, light intensity of 1000 μmol m−2 s−1, leaf temperature of 30 °C and water vapour pressure deficit between the leaf and the atmosphere of 1.7 kPa. The leaf was maintained under the standard conditions until stomata opened and steady-state net assimilation rate (A), transpiration rate and isoprene emission rate (I) were reached, typically 15–20 min. after leaf enclosure in the chamber. The values of A, I and transpiration rate were recorded and these measurements were followed by leaf darkening to record the dark decay kinetics of isoprene emission for estimation of the in vivo pool sizes of DOXP/MEP pathway intermediates (see the next section).
After measurements of the dark decay kinetics, light was switched on, the leaf was stabilized again until the rates of net assimilation and isoprene emission reached the pre-darkened values. The chamber CO2 concentration was further increased to 1500 μmol/mol and light remained on until a new steady-state was achieved, followed by the measurements of the dark decay kinetics. Measurements at two CO2 concentrations, 400 and 1500 μmol/mol, were conducted in all cases. In addition, in five species (L. formosana, M. indica, P. tremula, P. tremula x P. tremuloides, Q. rubra), these measurements were conducted at six different CO2 concentrations to characterize interspecific variability in the shape of the CO2 versus isoprene emission responses. Foliage gas-exchange characteristics were calculated according to von Caemmerer and Farquhar (1981) and isoprene emission rate according to Niinemets et al. (2011).
2.3 In vivo estimation of substrate pool sizes from dark decay kinetics
After the steady-state values of A and I were recorded, light was switched off and isoprene emission rate was monitored until it reached the background level. The dark release of isoprene emission is typically biphasic (Li et al., 2011; Rasulov et al., 2011). The initial isoprene release between 0 and 300 s after darkening relies on the existing pool of DMADP synthesized prior to darkening (Figure S1 for sample dark decay kinetics; Rasulov, Copolovici, Laisk, & Niinemets, 2009; Weise et al., 2013). A second burst of isoprene emission occurs between 300 and 2000 s after darkening (Figure S1). This raise of isoprene emission reflects the conversion of an upstream metabolite pool, mainly consisting of 2-C-methyl-d-erythritol 2,4-cyclodiphosphate (MEcDP) to DMADP as NADPH becomes available in the dark (Li & Sharkey, 2013; Rasulov et al., 2011). The in vivo DMADP pool size was estimated as the integral of the initial release of isoprene emission, and the in vivo MEcDP pool size as the integral of the second rise of isoprene emission. A variety of dark decay kinetics were observed across species, but deconvolution of DMADP and MEcDP pools was clearcut in all cases, except for P. dactylifera where no clear hiatus between leaf darkening and start of MEcDP conversion to DMADP was evident, resulting in less reliable separation of pools (Figure S1). The isoprene synthase rate constant, k (s−1) was estimated as the initial slope of the DMADP pool size versus I relationship. Paired values of I at time t and DMADP pool size remaining at that time were derived from the initial dark decay kinetics (Rasulov et al., 2010; Rasulov, Talts, et al., 2015).
2.4 Data analyses
To characterize the changes in isoprene emission and associated changes in substrate pool sizes in response to the increase of the ambient CO2 concentration, both absolute and relative changes were calculated. For isoprene, the absolute change was calculated as I400−I1500 and the relative change as (I400−I1500)/I400, where I400 is the isoprene emission rate at CO2 concentration of 400 μmol/mol and I1500 the emission rate at 1500 μmol/mol. Analogously, the absolute changes in pool sizes, CDMADP,400−CDMADP,1500, CMEcDP,400−CMEcDP,1500 and CTotal,400−CTotal,1500, and the relative changes, (CDMADP,400−CDMADP,1500)/CDMADP,400, (CMEcDP,400−CMEcDP,1500)/CMEcDP,400 and (CTotal,400−CTotal,1500)/CTotal,400, were calculated (CDMADP is the DMADP pool size, CMEcDP is the MEcDP pool size and CTotal is the sum of CDMADP and CMEcDP). In addition, the ratio of CDMADP/CMEcDP, and the shares of DMADP and MEcDP in total pool, CDMADP/CTotal and CMEcDP/CTotal, were calculated for different CO2 concentrations.
Within-species average values obtained at CO2 concentrations of 400 and 1500 μmol/mol were compared by paired samples t tests and the relationships between isoprene emission and substrate pool sizes and between absolute and relative changes in isoprene emission and substrate pool sizes were analysed by linear and non-linear regressions. n = 3 for all species, except for Q. robur (n = 6). In Q. robur, the measurements were conducted in mature and young plants (n = 3 for both) and the data were ultimately pooled as no significant differences among mature and young plants were observed for any of the characteristics. In the figures, we demonstrate data for both individual leaves and species averages to allow assessment of the overall variability and visual comparison of within-species and across-species responses.
3 RESULTS
3.1 Interspecific variations in net assimilation rate and isoprene emission characteristics
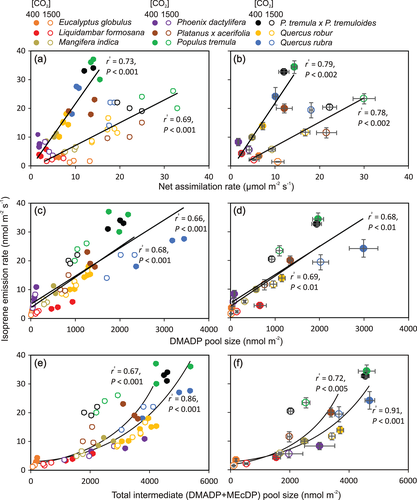
Across species, a wide range of photosynthesis and isoprene emission characteristics was uncovered. At the ambient CO2 concentration of 400 μmol/mol, species average net assimilation rate (A) varied more than eightfold, isoprene emission rate (I) more than 10-fold, isoprene synthase rate constant (k) more than eightfold, DMADP pool size (CDMADP) more than 90-fold, 2-C-methyl-d-erythritol 2,4-cyclodiphosphate (MEcDP, CMEcDP) pool size 19-fold and total substrate pool size (DMADP+MEcDP, CTotal) 25-fold (Table 1). Across leaves and species, I was positively correlated with A (Figure 2a,b), CDMADP (Figure 2c,d) and CTotal (Figure 2e,f), and the within-species responses resembled the across-species responses (cf. Figure 2a,c,e and Figure 2b,d,f). The correlations of I with CMEcDP were weaker (for all leaves pooled, r2 = .28, p < .005 for CO2 concentration of 400 μmol/mol and r2 = .16, p < .05 for 1500 μmol/mol). Isoprene emission rate was negatively correlated with k for CO2 concentration of 400 μmol/mol, but the correlation was weak (for all leaves pooled, r2 = .14, p < .05) and driven by E. globulus and P. dactylifera that had large values of k, but low isoprene emission rates (Table 1).
| Species | [CO2] (μmol mol−1) | A (μmol m−2 s−1) | I (nmol m−2 s−1) | k (s−1) | CDMADP (nmol m−2) | CMEcDP (nmol m−2) | CTotal (nmol m−2) | CDMADP/CMEcDP | CDMADP/CTotal |
|---|---|---|---|---|---|---|---|---|---|
| Eucalyptus globulus | 400 | 6.3 ± 0.5a | 3.37 ± 0.42a | 0.082 ± 0.007a | 31.7 ± 4.1a | 154 ± 12a | 186 ± 16a | 0.204 ± 0.011a | 0.169 ± 0.007a |
| 1500 | 10.6 ± 1.4b | 1.43 ± 0.18b | 0.069 ± 0.006a | 15.3 ± 1.5b | 93 ± 21a | 109 ± 22a | 0.181 ± 0.037a | 0.152 ± 0.026a | |
| Liquidambar formosana | 400 | 2.47 ± 0.48a | 4.3 ± 0.7a | 0.0107 ± 0.0005a | 670 ± 140a | 880 ± 130a | 1550 ± 270a | 0.740 ± 0.048a | 0.425 ± 0.016a |
| 1500 | 5.3 ± 1.1b | 2.1 ± 0.29a | 0.0106 ± 0.0007a | 140 ± 20b | 390 ± 70b | 530 ± 80b | 0.379 ± 0.047a | 0.273 ± 0.024a | |
| Mangifera indica | 400 | 4.8 ± 0.7a | 9.9 ± 0.8a | 0.0253 ± 0.0020a | 573 ± 45a | 1920 ± 120a | 2500 ± 150a | 0.299 ± 0.018a | 0.230 ± 0.011a |
| 1500 | 9.3 ± 0.8b | 5.67 ± 0.50b | 0.0247 ± 0.0012a | 320 ± 25b | 1360 ± 50b | 1680 ± 60b | 0.236 ± 0.018b | 0.191 ± 0.012b | |
| Phoenix dactylifera | 400 | 1.73 ± 0.12a | 8.3 ± 1.3a | 0.094 ± 0.008a | 90 ± 17a | 2920 ± 510a | 3010 ± 520a | 0.0312 ± 0.0031a | 0.0302 ± 0.0029a |
| 1500 | 4.1 ± 0.5b | 5.6 ± 1.4b | 0.082 ± 0.005a | 69 ± 18b | 1900 ± 470b | 1970 ± 480b | 0.0365 ± 0.0037b | 0.0351 ± 0.0035b | |
| Platanus x acerifolia | 400 | 12.1 ± 1.8a | 19.9 ± 1.6a | 0.0203 ± 0.0026a | 1342 ± 48a | 2050 ± 110a | 3400 ± 150a | 0.655 ± 0.023a | 0.396 ± 0.008a |
| 1500 | 21.5 ± 2.3b | 11.5 ± 1.8b | 0.0201 ± 0.0031a | 760 ± 60b | 1220 ± 70b | 1980 ± 110b | 0.627 ± 0.034a | 0.385 ± 0.013a | |
| Populus tremula | 400 | 14.3 ± 0.6a | 34.3 ± 2.2a | 0.0310 ± 0.0021a | 1970 ± 120a | 2630 ± 280a | 4600 ± 380a | 0.76 ± 0.06a | 0.430 ± 0.019a |
| 1500 | 30.0 ± 2.5b | 23.3 ± 1.8b | 0.0290 ± 0.0015a | 1100 ± 80b | 1450 ± 130b | 2550 ± 210b | 0.764 ± 0.028a | 0.433 ± 0.009a | |
| P. tremula x P. tremuloides | 400 | 12.0 ± 1.2a | 32.7 ± 0.9a | 0.0217 ± 0.0003a | 1940 ± 110a | 2620 ± 130a | 4553 ± 37a | 0.75 ± 0.08a | 0.426 ± 0.025a |
| 1500 | 22.3 ± 1.6b | 20.3 ± 0.9b | 0.0227 ± 0.0007a | 930 ± 60b | 1100 ± 80b | 2030 ± 130b | 0.848 ± 0.037a | 0.458 ± 0.011a | |
| Quercus robur | 400 | 7.20 ± 0.30a | 13.8 ± 1.3a | 0.0191 ± 0.0004a | 1160 ± 80a | 2550 ± 110a | 3710 ± 190a | 0.452 ± 0.014a | 0.311 ± 0.006a |
| 1500 | 16.8 ± 1.0b | 11.6 ± 1.3b | 0.0189 ± 0.0003a | 960 ± 80b | 2470 ± 100a | 3430 ± 170b | 0.389 ± 0.025b | 0.279 ± 0.013b | |
| Q. rubra | 400 | 10.07 ± 0.47a | 24.2 ± 3.1a | 0.0211 ± 0.0016a | 2990 ± 320a | 1710 ± 160a | 4700 ± 490a | 1.744 ± 0.025a | 0.6355 ± 0.0033a |
| 1500 | 18.10 ± 0.36b | 19.3 ± 2.7b | 0.0217 ± 0.0009a | 2030 ± 180b | 1640 ± 140a | 3670 ± 310b | 1.238 ± 0.048b | 0.553 ± 0.010b |
- Note: The measurements were conducted at an incident light intensity of 1000 μmol m−2 s−1 and leaf temperature of 30 °C. n = 3 for all, except for Q. robur (n = 6). In Q. robur, the measurements were conducted in mature and young plants (n = 3 for both) and the data were ultimately pooled as no significant differences in any of the traits were observed among trees of different age (see Material and methods [Section 2]). Within-species average trait values measured at normal (400 μmol/mol) and high (1500 μmol/mol) [CO2] were compared by paired sample t tests. Average trait values with the same lowercase letter are not significantly different between different measurement CO2 concentration.
- Abbreviations: A, net assimilation rate; CDMADP, pool size of the immediate isoprene precursor dimethylallyl diphosphate (DMADP); CMEcDP, pool size of the DOXP/MEP pathway intermediate 2-C-methyl-d-erythritol 2,4-cyclodiphosphate (MEcDP); CTotal, sum of the pools of DMADP and MEcDP; I, isoprene emission rate; k, isoprene synthase rate constant.
3.2 Responses of net assimilation rate and isoprene emission characteristics to increased [CO2]
The increase of ambient CO2 concentration from 400 to 1500 μmol/mol resulted in enhanced A in all species (Table 1), and in a reduction in I in all species except L. formosana (Table 1). Isoprene synthase rate constant (k) was not affected by ambient CO2 concentration in any of the species, but CDMADP was reduced under greater CO2 concentration in all cases (Table 1), indicating that the reduction in I was due to reduced substrate availability (I = kCDMADP). This was further supported by uniform positive correlations between I and CDMADP for both CO2 concentrations of 400 and 1500 μmol/mol (Figure 2c,d).
CMEcDP was reduced in six species and was not affected by CO2 concentration in E. globulus, Q. robur and Q. rubra (Table 1). CTotal was decreased at higher CO2 concentration in all species except E. globulus. The ratios of CDMADP to CMEcDP and CDMADP to CTotal were weakly affected by CO2 concentration, with an increase observed in P. dactylifera and a decrease in M. indica, Q. robur and Q. rubra (Table 1).
3.3 Differences in the sensitivity of isoprene emission characteristics to increased CO2 concentration
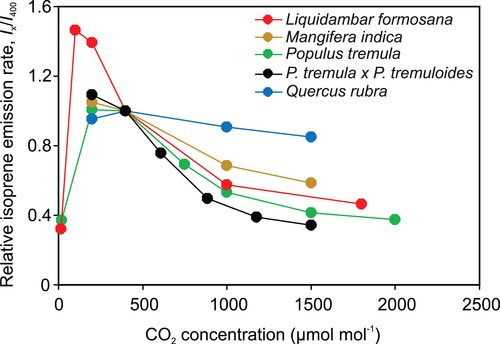
There was a large variation in the sensitivity of isoprene emission to changes in ambient CO2 concentration with some species such as Q. robur and Q. rubra having only a minor decrease in I upon increases in [CO2], and other species such as E. globulus and Populus spp. having a major reduction in I (Figure 3 and Table 1). The relative [CO2]-dependent change in I, ([I400−I1500]/I400, where I400 is the value of I measured at the CO2 concentration of 400 μmol/mol and I1500 that at 1500 μmol/mol) varied 4.5-fold across species, and almost 11-fold across individual leaves (Figure 4a,b). The absolute [CO2]-dependent change in I (I400−I1500) varied more than sixfold across species and more than 11-fold across different leaves (Figure S2a,b).
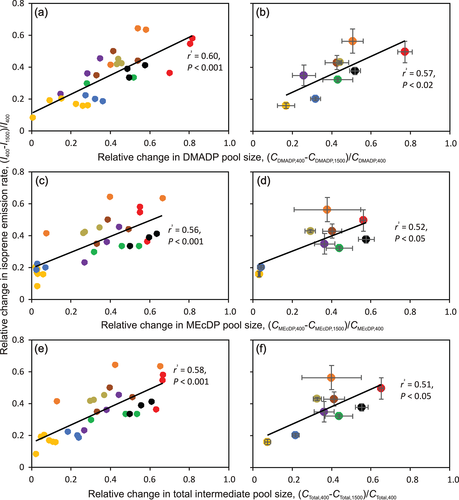
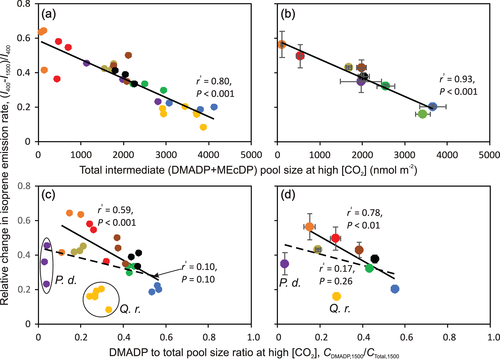
The relative [CO2]-dependent change in I was positively correlated with the relative changes in DMADP pool size ([CDMADP,400−CDMADP,1500]/CDMADP,400; Figure 4a,b), MEcDP pool size ([CMEcDP,400−CMEcDP,1500]/CMecDP,400; Figure 4c,d) and total pool size ([CTotal,400−CTotal,1500)/CTotal,400; Figure 4e,f), and the within-species responses were in most cases analogous to across species patterns (cf. Figure 4a,c,e vs. Figure 4b,d,f). The correlations of absolute [CO2]-dependent changes in I with relative pool changes were analogous to relative changes (Figure S2). The relative change in I was negatively correlated with the intermediate pool sizes with the strongest correlation observed for CTotal at high CO2 concentration (Figure 5a,b; for CDMADP, r2 = .43, p < .001 for all leaves and r2 = .56, p < .05 for species averages; for CMEcDP, r2 = .72, p < .001 for all leaves and r2 = .80, p < .002 for species averages; the patterns within species resembled across-species patterns in most cases). The relative change in I was also negatively correlated with the share of DMADP in the total substrate pool (Figure 5c,d), although P. dactylifera with a small DMADP pool and Q. robur with a large total pool (Table 1) were outliers in this relationship.
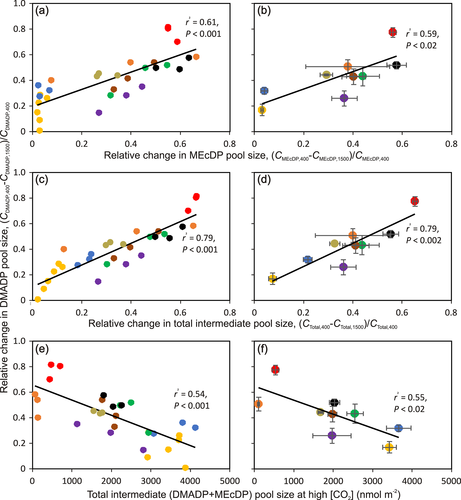
Analysis of the factors explaining the [CO2]-dependent variation in CDMADP indicated that the relative change in CDMADP correlated positively with relative changes in CMEcDP (Figure 6a,b) and total intermediate pool size (Figure 6c,d). The relative change in CDMADP also correlated negatively with the total intermediate pool size at high [CO2] (Figure 6e,f).
4 DISCUSSION
4.1 Variation in isoprene emission rates and intermediate pool sizes across species
We observed a major variation in isoprene emission rates and associated substrate pool sizes in nine studied species (Table 1). The presence of a wide variation in plant capacities for isoprene emission has been demonstrated in multiple studies (Guenther et al., 2012; Harrison et al., 2013; Keenan, Niinemets, Sabate, Gracia, & Peñuelas, 2009; Kesselmeier & Staudt, 1999), however, to our knowledge, the extensive variation in the corresponding substrate pool sizes supported by different species has not been shown before. As the end-products of the 1-deoxy-d-xylulose 5-phosphate/methyl-d-erythritol phosphate (DOXP/MEP) pathway, DMADP and isopentenyl diphosphate (IDP), can competitively inhibit DOXP synthase, the pathway intermediate pools are considered to be maintained in narrow limits (Banerjee et al., 2013; Volke, Rohwer, Fischer, & Jennewein, 2019). Nevertheless, isoprene-emitting plants are known to support a higher DMADP pool size than non-emitting species (Nogués, Brilli, & Loreto, 2006). This is associated with low affinity of isoprene synthase with respect to its substrate DMADP (a large value of the Michaelis–Menten constant) such that high substrate pool sizes are needed to support high isoprene emission rates (Köksal, Zimmer, Schnitzler, & Christianson, 2010; Rasulov et al., 2014). In particular, two key DOXP/MEP pathway intermediates can accumulate, 2-C-methyl-d-erythritol 2,4-cyclodiphosphate (MEcDP) and DMADP (Figure 1) (Ghirardo et al., 2014; Li et al., 2011; Li & Sharkey, 2013; Rasulov et al., 2011; Volke et al., 2019), and the pool sizes of these key intermediates can strongly increase in isoprene-emitting species without apparent feedback inhibition of DOXP/MEP pathway activity (Rasulov, Talts, et al., 2015).
In our study, the variation in I among leaves and species was directly associated with DMADP pool size (Figure 2c,d). A range of different dark decay kinetics was observed with some species such as P. dactylifera and E. globulus operating at very small DMADP pool sizes (Figure S1 and Table 1). However, in these species, there was apparently no clear time-dependent separation between DMADP and MEcDP pools, suggesting a rapid conversion of MEcDP to DMADP (Figure S1). In most species, the MEcDP pool was greater than DMADP pool, except for Q. rubra (Table 1), implying that the MEcDP pool can significantly buffer for changes in DMADP pool size and isoprene emission, for example, due to highly fluctuating light or temperature conditions that can occur in the field.
4.2 Reduction of isoprene emission rate at high CO2 concentration
In our study, isoprene emission rate decreased with increasing CO2 concentration in all species (Table 1 and Figure 2) and this decrease was associated with reductions in DMADP pool size in all species and in reduction in MEcDP pool size in all, except E. globulus and both Quercus species (Table 1 and Figure 2c,d). The isoprene emission versus DMADP pool size relationship was similar for both 400 and 1500 μmol/mol, only that both I and CDMADP were reduced at higher CO2 concentration (Figure 2c,d). This suggests that the decrease in I resulted from reduced isoprene substrate DMADP availability at high [CO2].
As described in the Introduction, the [CO2]-dependent reduction in CDMADP can occur due to limited availability of the DOXP/MEP pathway substrates GAP and pyruvate or limited ATP and reductant availability (Figure 1). Limited pyruvate availability has been postulated to result from enhanced phosphoenol pyruvate (PEP) carboxylase activity at higher [CO2], and concomitant drawdown of the cytosolic PEP concentration; low cytosolic PEP in turn has been predicted to limit PEP entry into chloroplasts and conversion of PEP to pyruvate, thereby ultimately curbing the whole DOXP/MEP pathway activity (Monson, 2013; Monson et al., 2009; Wilkinson et al., 2009). However, experiments with added malate suggested that cytosolic metabolites do not necessarily limit isoprene emission under different CO2 concentrations (Rasulov et al., 2016, 2018). Alternatively, photosynthetic carbon fixation becomes increasingly strongly limited by photosynthetic electron transport with increasing CO2 concentration (ribulose-1,5-bisphosphate regeneration limited photosynthesis) (Farquhar, von Caemmerer, & Berry, 1980; Sharkey, 1985). Thus, higher [CO2] has been associated with feedback-inhibited photosynthesis and reduced ATP and reductant availability, and reduced conversion of DOXP to MEcDP and MEcDP to DMADP (Figure 1; Monson et al., 2016; Morfopoulos et al., 2014; Niinemets et al., 1999; Rasulov et al., 2016, 2018). However, controversial evidence of factors controlling [CO2]-induced reduction in isoprene emission was obtained in the study of Lantz, Solomon, et al. (2019). Although the feedback inhibition of photosynthesis coincided in some cases with the reduction in isoprene emission at high [CO2], this was not always the case (Lantz, Solomon, et al., 2019). On the other hand, there was a certain reduction in PSII electron transport activity and this was associated with reductions in both MEcDP and DMADP pool sizes (Lantz, Solomon, et al., 2019) as in the study of Rasulov, Hüve, et al. (2009). We note that a reduction of the PSII or PSI electron transport rates is not the necessary condition for isoprene emission at high [CO2] to be limited by the availability of ATP and/or NADPH. Even if photosynthetic electron rate remains constant, photosynthesis consumes a greater share of photosynthetic energy and reductant at greater [CO2], drawing down the pool sizes of ATP and NADPH, and this can ultimately result in reduction in DMADP and MEcDP pool sizes (Figure 1). In the absence of viable alternative hypotheses, we suggest that the energy limitation is still the most plausible explanation for the reduction of isoprene emissions at high [CO2].
The simultaneous reduction in DMADP and MEcDP pool sizes (Table 1, Figure 6a,b) suggests that both ATP and reductant supply (reduced ferredoxin, Fd) decreased at greater [CO2]. However, in M. indica, Q. robur and Q. rubra, CMEcDP was reduced less at higher [CO2] than CDMADP (Table 1). Maintenance of a relatively greater MEcDP pool size suggests that the reductant availability limited the pathway flux more strongly at higher [CO2] in these species. As the increase of CO2 concentration suppresses photorespiration, it increases the demand of photosynthetic carbon metabolism for NADPH relative to ATP (Walker, Kramer, Fisher, & Fu, 2020; Walker, Strand, Kramer, & Cousins, 2014), and this might explain the stronger reduction of CDMADP relative to CMEcDP. Plants can achieve a balance between ATP and NADPH requirements via varying the rate of cyclic electron flow, but there are also multiple other electron and ATP consuming sinks such as nitrate reduction and fatty acid synthesis (Rasulov et al., 2018; Walker et al., 2020). Clearly, the species differences in relative changes in CDMADP and CMEcDP suggest that the balance between ATP and NADPH synthesis and consumption varied in different species.
4.3 Differences in species [CO2]-sensitivity of isoprene emission
Although the isoprene emission rate decreased with increasing [CO2] in all species, the degree of reduction strongly varied among species (Figure 3, 4, S2, and Table 1). In particular, the decrease was small, around 20% in both oak species, Q. robur and Q. rubra, and moderate, ca. 40% in P. dactylifera and M. indica and more than 40% in all other studied species (Figure 3, 4, S2, and Table 1). Such an interspecific variation in [CO2] responses of isoprene emission has been observed in several previous studies (Lantz, Solomon, et al., 2019; Sharkey et al., 1991; Wilkinson et al., 2009). In particular, isoprene emission in oaks has been known to be insensitive to changes in ambient [CO2] (Li et al., 2011; Sharkey et al., 1991), but such variations in [CO2]-sensitivity were not understood. We hypothesized that the [CO2]-sensitivity of isoprene emission is primarily driven by the sensitivity of the change of the substrate pool size to increased [CO2] and overall substrate pool size the given species can support. In particular, greater substrate pool changes were expected to result in greater changes in isoprene emission, whereas the relative change in substrate pool size was expected to be smaller in species supporting a larger substrate pool size (Figure 1). The results of the current study broadly supported these hypotheses. The [CO2]-dependent reductions in isoprene emission, both absolute and relative, were greater in species with greater changes in substrate pool size (Figure 4 and S2). A greater substrate pool size was associated with a smaller change in isoprene emission rate (Figure 5a,b), and this was because the species with greater pool size had a relatively smaller change in substrate pool size (Figure 6e,f).
Although DMADP is the substrate for isoprene synthesis, the best relationships of relative [CO2]-dependent changes in isoprene emission (Figure 5a,b) and DMADP pool size (Figure 6e,f) were observed with total, DMADP+MEcDP, intermediate pool size. This reflects the circumstance that the capacity to maintain a high MEcDP pool size is also associated with the capacity to maintain a relatively higher DMADP pool size (Figure 6a,b). However, at a given total pool size, the inability to convert MEcDP to DMADP can curb isoprene emission as the DMADP pool size decreased often relatively more than total pool size under higher [CO2] (Figure 5c,d).
As discussed above, the limited conversion of MEcDP to DMADP might reflect imbalanced ATP and reductant supply. Such imbalances can be especially relevant under varying environmental conditions as the key environmental drivers, temperature, light and [CO2], each individually and in combination, can differently alter ATP and NADPH availability. It is unclear why plants accumulate a large MEcDP pool size, but it is possible that having two large pools, which are differently controlled, one by ATP and the other by reductant availability, makes the whole pathway flux more stable under fluctuating environmental conditions. Furthermore, given that DMADP accumulation can lead to a feedback inhibition of DOXP synthesis, and thereby inhibition of the whole pathway flux (Figure 1), accumulation of MEcDP allows to support a greater total intermediate pool size compared with a situation when only DMADP accumulated. This stabilizes isoprene emission and makes it possible to support a high isoprene emission flux during stress periods such as during light- and heatflecks when the protection from isoprene could be the largest (Behnke et al., 2013; Way, Schnitzler, Monson, & Jackson, 2011). In addition, a higher total pool size allows a faster synthesis of essential isoprenoids when needed, for example, under stress conditions.
4.4 Implications for modelling isoprene emissions
The results of our study demonstrate that there is a major interspecific variation in both isoprene emission rates and substrate pool sizes. The variation in substrate pool size explains the interspecific variability in [CO2]-responsiveness of isoprene emission. The current models of isoprene emission do include the [CO2] response (Bauwens et al., 2018; Grote et al., 2013, 2014; Hantson et al., 2017; Monson et al., 2012; Morfopoulos et al., 2014), but they do not consider interspecific variability in [CO2]-sensitivity of isoprene emission. We argue that this variation has to be incorporated in leaf to global models of isoprene emission.
The question is, however, how can the differences in [CO2]-sensitivity of isoprene emission be included in practice? The species capacity for isoprene emission is a trait that is very difficult to predict, and even taxonomically closely related species might have vastly different isoprene emission potentials (Kesselmeier & Staudt, 1999; Niinemets et al., 2010). Therefore, species-level inventories of isoprene emission potentials have been used to simulate emissions at different spatial scales from ecosystem to landscape and region (Keenan, Niinemets, Sabate, Gracia, & Peñuelas, 2009; Lamb, Gay, Westberg, & Pierce, 1993; Simpson et al., 1999). To include the [CO2]-sensitivity of emissions, the substrate pool size itself could be a trait included in future species-level isoprene emission inventories.
Furthermore, a promising way of assessing the activity of isoprene emissions is the use of remote sensing products. In particular, remotely-sensed photochemical reflectance index (PRI) that characterizes the activity of xanthophyll cycle correlates with changes in isoprene emission in isoprenoid-emitting species (Balzarolo, Peñuelas, Filella, Portillo-Estrada, & Ceulemans, 2018; Harris, Owen, Sleep, & Pereira, 2016; Peñuelas et al., 2013). In addition, negative correlations between leaf carotenoid synthesis and DMADP pool size have been observed through leaf development (Owen & Peñuelas, 2013; Rasulov et al., 2014). These correlations reflect a higher rate of carotenoid synthesis at lower substrate pool size than isoprene synthesis due to high DMADP-affinity of geranyl diphosphate synthase, the first step in larger chloroplast-synthesized isoprenoids (Rasulov et al., 2014; Tholl, Croteau, & Gershenzon, 2001). In particular, younger leaves with high carotenoid synthesis rate have lower DMADP pool sizes than older leaves with lower carotenoid synthesis rate (Niinemets et al., 2015; Rasulov et al., 2014). On the other hand, overall increase in MEP/DOXP activity can be associated with both greater carotenoid contents and isoprene emission rates (Harris et al., 2016; Owen & Peñuelas, 2013). Thus, remote sensing of carotenoid pool size (Hallik et al., 2017) together with PRI could provide an insight into both isoprene emission potential and DMADP pool size. However, the remote sensing studies of isoprene emission have focused on intraspecific variability, and it is unclear how useful is the remote sensing-derived information to predict variation in isoprene emission characteristics across species. Nevertheless, similarity of [CO2]-dependent changes in DMADP pool size and isoprene emission (Figures 2, 4-6) provides encouraging evidence that the species-specific patterns observed in remote sensing studies might be applicable across species. Future studies are needed to characterize how intraspecific variation in carotenoid pool size correlates with carotenoid turnover, DMADP pool size and isoprene emission.
5 CONCLUSIONS
The wide interspecific variation in isoprene emission capacity as driven by differences in isoprene synthase activity is well-known, but why environmental responses of isoprene emission vary among species is much less understood. Our study highlights an extensive interspecific variation in substrate pool sizes supported by different species and associated variation in the sensitivity of isoprene emission to increased [CO2]. Such variation might indicate differences in overall activity of DOXP/MEP pathway in different species. It might also be indicative of differences in the sensitivity of the first step of the pathway, DOXP synthesis, to the feedback inhibition by DMADP and IDP. Further studies need to examine the species differences in the DMADP and IDP concentration dependence of feedback inhibition of DOXP/MEP pathway, and analyse how the DMADP pool size is associated with overall DOXP/MEP pathway activity and whether the pool size can be linked to remotely-sensed leaf traits for modelling [CO2]-sensitivity of isoprene emission.
ACKNOWLEDGMENTS
We acknowledge funding from the European Commission through the European Regional Development Fund (Centre of Excellence EcolChange), European Research Council (grant 322603, SIP-VOL+) and Eesti Teadusagentuur (PRG537).
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data underlying this study are provided in the supporting information (Table S1).




