Dynamic thermal imaging confirms local but not fast systemic ABA responses
Funding information: Leverhulme Trust, Grant/Award Number: RPG-2016-274
Abstract
Abscisic acid (ABA) signals regulating stomatal aperture and water loss are usually studied in detached leaves or isolated epidermal peels and at infrequent timepoints. Measuring stomatal ABA responses in attached leaves across a time course enables the study of stomatal behaviour in the physiological context of the plant. Infrared thermal imaging is often used to characterize steady-state stomatal conductance via comparisons of leaf surface temperature but is rarely used to capture stomatal responses over time or across different leaf surfaces. We used dynamic thermal imaging as a robust, but sensitive, tool to observe stomatal ABA responses in a whole plant context. We detected stomatal responses to low levels of ABA in both monocots and dicots and identified differences between the responses of different leaves. Using whole plant thermal imaging, stomata did not always behave as described previously for detached samples: in Arabidopsis, we found no evidence for fast systemic ABA-induced stomatal closure, and in barley, we observed no requirement for exogenous nitrate during ABA-induced stomatal closure. Thus, we recommend dynamic thermal imaging as a useful approach to complement detached sample assays for the study of local and systemic stomatal responses and molecular mechanisms underlying stomatal responses to ABA in the whole plant context.
1 INTRODUCTION
Stomata, small pores in leaf epidermis, control the balance between CO2 uptake for photosynthesis and water loss via transpiration. Stomatal apertures are adjusted through changes in guard cell turgor in response to changes in various environmental and endogenous factors. Abscisic acid (ABA) is the key hormone that triggers stomatal closure and regulates drought responses in plants (Cutler, Rodriguez, Finkelstein, & Abrams, 2010). Stomatal responses to ABA are most commonly studied in isolated epidermal peels or leaf fragments floated in ABA solution (Kuhn, Boisson-Dernier, Dizon, Maktabi, & Schroeder, 2006; McAinsh, Brownlee, Hetherington, & Mansfield, 1991; Medeiros et al., 2018; Uraji et al., 2012; Yamamoto et al., 2016). Alternatively, stomatal responses have been studied in detached leaves with petiole-fed ABA followed by gas exchange analysis (Brodribb & McAdam, 2011; Ceciliato et al., 2019; Müller et al., 2017; Pantin et al., 2013; Raschke, 1975; Schäfer et al., 2018; Tõldsepp et al., 2018), gravimetry (Shatil-Cohen, Attia, & Moshelion, 2011), chlorophyll fluorescence (Meyer & Genty, 1998) or thermal imaging (Viger, Rodriguez-Acosta, Rae, Morison, & Taylor, 2013). ABA has also been supplied via nutrient solution to roots while monitoring gas exchange in leaves with a custom-built device (Leymarie, Lascève, & Vavasseur, 1998; Leymarie, Vavasseur, & Lascève, 1998). Foliar ABA spray or leaf smearing with ABA solution followed by analysis of detached leaf water loss by gravimetry has also been used (Pantin et al., 2013; Pantin, Monnet, et al., 2013; Shatil-Cohen et al., 2011). These methods have the disadvantages of low throughput and/or the inability to study the stomatal ABA response in a whole plant context. Stomatal responses to ABA applied by spraying have been studied in whole plants via gas exchange analysis (for example, Merilo, Jalakas, Kollist, & Brosché, 2015), but this often requires specific custom-built equipment. Applying a readily available, fast and high-throughput method that captures stomatal ABA responses in intact plants would be useful to fully understand the molecular mechanisms of ABA-induced stomatal regulation.
As leaf temperature depends on transpiration and thus on stomatal aperture, thermal imaging of leaves gives insight into stomatal conductance and water use efficiency, as well as heterogeneity of these parameters within plants (Jones, 1999; McAusland, Davey, Kanwal, Baker, & Lawson, 2013; Meyer & Genty, 1998; Page, Liénard, Pruett, & Moffett, 2018; Sweet, Peak, & Mott, 2017; Vollsnes et al., 2009; West, Peak, Peterson, & Mott, 2005). Thermal imaging has enabled isolation of various mutants deficient in stomatal regulation (Costa et al., 2015; Hashimoto et al., 2006; Merlot et al., 2002; Raskin & Ladyman, 1988; Takemiya, Yamauchi, Yano, Ariyoshi, & Shimazaki, 2013; Xie et al., 2006). In a small number of experiments, thermal imaging has been used dynamically to study stomatal responses to dehydration following leaf excision (Jones, 1999; Page et al., 2018), changes in light levels (Devireddy, Zandalinas, Gómez-Cadenas, Blumwald, & Mittler, 2018; McAusland et al., 2013; Vialet-Chabrand & Lawson, 2019) and drought (Martynenko et al., 2016). Stomatal response to ABA application has been detected in few selected time points by thermal imaging in barley (Hordeum vulgare, Raskin & Ladyman, 1988), common bean (Phaseolus vulgaris, Omasa & Takayama, 2003) and Arabidopsis (Arabidopsis thaliana) leaves (Kang et al., 2010). However, dynamic leaf temperature response to petiole fed ABA over 2 hr in detached poplar leaves (Populus deltoides and P. trichocarpa; Viger et al., 2013) remains one of the very few examples, where this technique has been applied to study stomatal responses to ABA across a time course.
Here, we use dynamic thermal imaging as a robust tool for characterizing local and systemic stomatal responses to ABA in intact plants. This approach enabled us to achieve higher throughput than most other methods used for studying stomatal ABA responses and identify within-plant heterogeneity in ABA responses. We attempted to study the systemic transmission of ABA responses between leaves as previously reported in Arabidopsis (Devireddy et al., 2018). However, we could find no evidence for fast systemic ABA-induced stomatal closure. Instead, we detected a slow and weak systemic response to ABA, which may be due to transport of the applied ABA to systemic leaves. We also found no evidence for the previously proposed exogenous nitrate requirement (Schäfer et al., 2018) for ABA-induced stomatal closure in barley.
2 MATERIALS AND METHODS
2.1 Plant material and growth conditions
Arabidopsis thaliana (Arabidopsis) Col-0 accession and the following mutants in the same genetic background were used in experiments: ost1-3 (SALK_008068, Yoshida et al., 2002), nced3nced5 (Frey et al., 2012), sid2-1 (Nawrath & Métraux, 1999), npr1-1 (Cao, Bowling, Gordon, & Dong, 1994), glr3.3aglr3.6a (Mousavi, Chauvin, Pascaud, Kellenberger, & Farmer, 2013), ait1-1 (SALK_146143, Kanno et al., 2012), abcg22-2 (SALK_113844, Kuromori, Sugimoto, & Shinozaki, 2011) and abcg22-2abcg25abcg40 (Merilo et al., 2015). Arabidopsis and barley (Hordeum vulgare, cultivars Golden Promise and Barke) plants were grown in 3:1 v/v mixture of compost (Levington M3) and perlite. Arabidopsis plants were grown in a controlled environment chamber (Conviron, http://www.conviron.com/) with 9/15 hr day/night regime (22°C day, 18°C night), 200 μmol m−2 s−1 of light and 60% relative air humidity (RH), 5- to 6-week-old plants were used for experiments. For low light experiments, Arabidopsis plants were grown at 40 μmol m−2 s−1 of light. Barley was grown in a growth room at 16/8 hr day/night regime (22–26°C day, 18–22°C night), ~400 μmol m−2 s−1 of light (Valoya R Series R150 AP67 LED Grow Light, http://www.valoya.com) and ~40% RH, 11–17 days old plants were used for experiments.
2.2 Thermal imaging experiments
Thermal imaging experiments were carried out in an air-conditioned room with temperature set at 23°C, RH ~40–50% and 160–180 μmol m−2 s−1 of light (Valoya R Series R150 AP673L LED Grow Light, http://www.valoya.com). Plants were allowed to acclimate in these conditions for 1.5–2 hr before treatment, whereas barley plants or leaves were kept horizontally throughout the experiments. After acclimation period, ABA was applied. For whole-plant treatment in Arabidopsis, plants were sprayed with 0.42 ± 0.04 μl cm−2 ABA [Sigma, https://www.sigmaaldrich.com; 1, 5, or 10 μM ABA, 0.012% Silwet L-77 (De Sangosse, http://www.desangosse.com), 0.01–0.05% ethanol] or control solution (0.012% Silwet L-77, 0.05% ethanol). To contain treatment and avoid spraying adjacent plants, plants were separated into trays by treatment group and moved to the other end of experiment table for treatment before placing in the field of view of the thermal imaging camera. For systemic ABA response experiments, 40.3 ± 1.8 μl cm−2 ABA (50, 100 or 200 μM ABA, 0.012% Silwet L-77, 0.05–0.2% ethanol) or control solution (0.012% Silwet L-77, 0.1–0.2% ethanol) was applied by paintbrush to either leaf 8 or to young leaves in the centre of Arabidopsis rosette. In barley, 18.9 ± 1.1 μl cm−2 5 μM ABA, 5 mM KNO3, 5 μM ABA in 5 mM KNO3 or control solution all containing 0.012% Silwet L-77 and 0.05% ethanol were applied to plants by paintbrush. Mean applied ABA amounts for the three experiment types were determined by weighing plant/leaf models before and directly after ABA application. In detached leaf assays, barley leaves were cut and placed with sheath in water or nitrate solution in 1.5 ml experiment tubes and leaf blades lightly attached to a plastic plate above the imaging table by transparent tape to ensure horizontal stable leaf position; 25 μM ABA and 5 mM KNO3 were applied via transpiration stream. Thermal images of intact plants or detached leaves were captured with 1-min intervals with a thermal imaging camera (FLIR SC660 or FLIR T650sc [resolution, 640 × 480 pixels; spectral range, 7.5–13 μm for both cameras], FLIR Systems, https://www.flir.com/) throughout the experiments.
2.3 Leaf gas exchange analysis
For analysing systemic stomatal response to ABA, stomatal conductance was measured at the widest part of a mature Arabidopsis leaf with LI-COR LI-6400XT portable photosynthesis system (Lincoln, NE, https://www.licor.com/) attached to a leaf chamber fluorometer with a 2 cm2 leaf area (LI-COR 6400-40). When conductance had stabilized, another mature leaf of the same rosette was treated with 100 μM ABA or control solution while continuing the measurement of stomatal conductance in the systemic leaf. Conditions for measuring stomatal conductance corresponded to plant growth conditions with ~60% RH, 200 μmol m−2 s−1 of light, 400 ppm CO2 and 22°C block temperature. Leaf area was measured from images with ImageJ (Schneider, Rasband, & Eliceiri, 2012) for leaves that did not fully cover the 2 cm2 area.
2.4 Data analysis
Leaf temperature analysis was carried out with ResearchIR software (FLIR Systems, https://www.flir.com/). Regions of interest (ROIs) were defined on the adaxial side of leaves and temperature data extracted. For whole-plant treatment in Arabidopsis, three ROIs per plant were defined on mature leaves, and their temperature averaged to obtain a representative leaf temperature number per plant. For measuring the temperature of young leaves, a single circular ROI was defined in the centre of the Arabidopsis rosette. For short-term systemic signalling experiments, leaf temperature was extracted for one ROI in the treated leaf and three ROIs on mature leaves to assess temperature in the systemic leaves. For long-term systemic signalling experiments, leaf temperature was extracted for one ROI in the treated leaf 8 or the centre of the rosette for local temperature and, for the centre of the rosette or five mature leaves, respectively, to assess temperature in the systemic leaves. In experiments with barley, three ROIs were defined on leaf 2 and their temperature averaged to obtain a representative leaf temperature number per plant. For calculation of differences in leaf temperature before and after treatment, the pre- and posttreatment leaf temperatures were defined as an average of leaf temperature across the 5 min before ABA application and the 5 min at the designated time point, respectively. Difference between these temperatures was calculated and defined as change in leaf temperature.
For calculating ABA-specific change in leaf temperature, subtracted time courses, where pre-treatment image was subtracted from each time point image, were generated, and thereafter, each mock-treated curve was subtracted from the paired ABA-treated curve to gain time courses of ABA specific leaf temperature responses, where response starts from x = 0, y = 0. Exponential (y = Plateau ∙ (1 − e−kx), where Plateau characterizes the magnitude and k, the rate of response with the unit min−1 and larger k values corresponding to faster response), sigmoidal (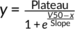 , where Plateau characterizes the magnitude of the response, V50, the time when half of the response is achieved, and Slope describes the steepness of the response with larger Slope values corresponding to slower response) or linear models (y = slope ∙ x, where higher slope corresponds to a faster response) were fitted, depending on experiment type with GraphPad Prism 8, to analyse the kinetics of ABA responses.
, where Plateau characterizes the magnitude of the response, V50, the time when half of the response is achieved, and Slope describes the steepness of the response with larger Slope values corresponding to slower response) or linear models (y = slope ∙ x, where higher slope corresponds to a faster response) were fitted, depending on experiment type with GraphPad Prism 8, to analyse the kinetics of ABA responses.
Statistical analyses were carried out with Statistica, version 7.1 (StatSoft, http://www.statsoft.com/). One or two-way ANOVA with Tukey or Tukey unequal N HSD post hoc test was used as indicated in the figure legends. ANOVA tables are presented in the Supporting Information. Slopes of linear models were compared with GraphPad Prism 8. All effects were considered significant at p < .05.
3 RESULTS
3.1 ABA induces fast concentration-dependent increase in leaf temperature in Arabidopsis
As leaf temperature is a good proxy for stomatal conductance, we were able to investigate the temporal dynamics of ABA-induced stomatal closure by detecting increases in leaf temperature using an infrared thermal imaging camera. We recorded infrared images of mature Arabidopsis plants at one-minute intervals before and after spray application of ABA or a control solution. Infrared images taken at the timepoint 1 hr after ABA application revealed a clear concentration-dependent response of leaf temperature to ABA (Figure 1a, see also time-lapse in Video S1). Next, we quantified leaf temperature every minute for 30 min before and 60 min after ABA application. The temperatures of three equal-sized areas on three mature leaves of each plant were used to calculate mean individual plant temperatures. We then calculated means for each treatment group at each time point (Figure S1). This revealed that after a transient drop in leaf temperature due to evaporation of the applied liquid, spray application of ABA resulted in a fast increase in leaf temperature of Col-0 wild-type Arabidopsis plants, which was clearly evident at 25 min after 1 μM ABA application, and larger and faster at higher concentrations of the hormone. In the experiment shown in Figure 1b,c, 1 μM ABA caused a rise in temperature of 0.7°C and 5 μM ABA caused a rise in temperature of 1.1°C in comparison to mock-treated plants 1 hr after application. Calculation of ABA-specific change in leaf temperature throughout the experiment and fitting of sigmoidal models to these data further supported a concentration-dependent increase in the magnitude of leaf temperature ABA response (Figure S2a). As might be expected, application of 5 μM ABA to the ABA-insensitive ost1-3 mutant (Mustilli, Merlot, Vavasseur, Fenzi, & Giraudat, 2002; Yoshida et al., 2002) did not cause a significant increase in leaf temperature in comparison to the control treatment (Figure 1d; Figure S2b,c).
We next asked whether leaf temperature analysis is sufficiently sensitive to detect within-plant differences in the stomatal ABA response, that is, to investigate whether the stomata of different leaves of an intact plant respond to the same extent. Recent experiments suggest that most of the water loss in the expanding leaves occurs through the cuticle (Kane, Jordan, Jansen, & McAdam, 2020) in Quercus rubra and Arabidopsis. However, experiments with detached leaves and epidermal peels in Arabidopsis have shown that the stomata of young leaves from the centre of the leaf rosette account for larger water loss of expanding leaves, as they are less sensitive to ABA than stomata of mature leaves from the rosette periphery (Pantin, Renaud, et al., 2013). We quantified leaf temperature in an area in the centre of Arabidopsis rosette (Figure S3a) to analyse the ABA response in young leaves and compared this to the mature leaf temperature response in the same plants. We found that leaf temperature of young leaves in the centre of wild-type Arabidopsis rosettes increased less than mature leaves in response to 5 μM ABA. In the experiment shown in Figure 1e, the mature leaves of intact plants increased in temperature by 1.3°C following ABA treatment, whereas young leaves increased by only 0.67°C, possibly due to larger contribution of cuticular transpiration that is not responsive to ABA in developing leaves (Kane et al., 2020) and/or due to reduced ABA-responsiveness of stomata in young leaves (Pantin, Renaud, et al., 2013). Although the magnitude of the ABA response was smaller in young leaves than in mature leaves, a significant response to 1 μM ABA concentration could still be measured through an increase in leaf temperature in the young leaves (Figure 1f; Fgure S3b,c), indicating ABA-induced stomatal closure. Thus, we could use thermal imaging to characterize ABA-induced leaf temperature increases due to stomatal closure in response to different levels of the hormone in different leaves.
3.2 ABA does not trigger fast systemic stomatal closure in Arabidopsis
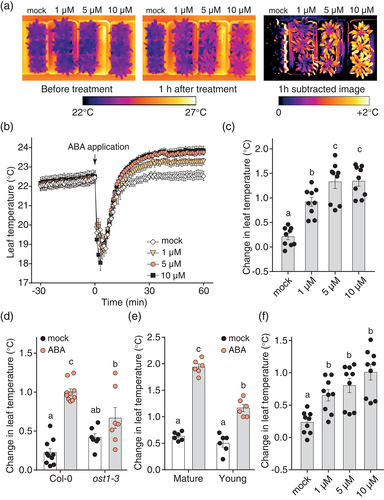
As we were able to detect within-plant differences in ABA response by thermal imaging, we used this approach to study systemic stomatal responses to ABA in intact plants. It was recently reported that ABA, when applied locally at 50 μM concentration, induces rapid stomatal closure within a few minutes in a systemic leaf (Devireddy et al., 2018). In our experiments, 50 μM ABA caused a clear and fast increase in leaf temperature in the treated leaf, but there was no rapid response in systemic mature leaves over 2 hr (Figure 2a; Figure S4a). To validate this finding with an established experimental technique, we used leaf gas exchange analysis to carry out a replicate experiment. We recorded stomatal conductance in a mature systemic leaf and applied 100 μM ABA to a local leaf, when stomatal conductance in the systemic leaf had stabilized. We found no change in stomatal conductance in the systemic leaf in response to locally applied ABA (Figure 2b), in line with our thermal imaging experiments. These data show that in systemic signalling experiments, dynamic thermal imaging can be used comparably to leaf gas exchange analysis but with higher throughput.
We next asked, whether differences in growth conditions could account for the differences between our results and previously published results (Devireddy et al., 2018). Arabidopsis was grown at a low light level (50 μmol m−2 s−1) in the study by Devireddy et al., which could have resulted in increased levels of light harvesting complex proteins (Bailey, Walters, Jansson, & Horton, 2001), and might account for the previously observed stomatal ABA responsiveness (Xu et al., 2012). To test whether low light levels are needed for the fast systemic ABA-induced stomatal closure, we grew Arabidopsis at low (40 μmol m−2 s−1) or normal (200 μmol m−2 s−1) light and studied systemic stomatal response to high levels of locally applied ABA. In our conditions, plants grown at low light levels were stunted in growth and development (Figure 2c) and showed no rapid increase in systemic leaf temperature in response to locally applied 100 μM ABA (Figure 2d; Figure S4b). The low light grown plants had consistently higher leaf temperature compared to normal light grown plants (Figure 2d), in line with their reduced stomatal density (Figure S4c). Longer monitoring of leaf temperature of the systemic leaves revealed that 3 hr after treatment, a small, but significant ABA-induced increase in leaf temperature appeared in plants grown at low but not normal light (Figure 2e). These data suggest that locally applied ABA does not induce fast systemic stomatal closure in Arabidopsis but may lead to slow systemic stomatal closure of small magnitude.
3.3 ABA induces slow systemic stomatal closure in Arabidopsis
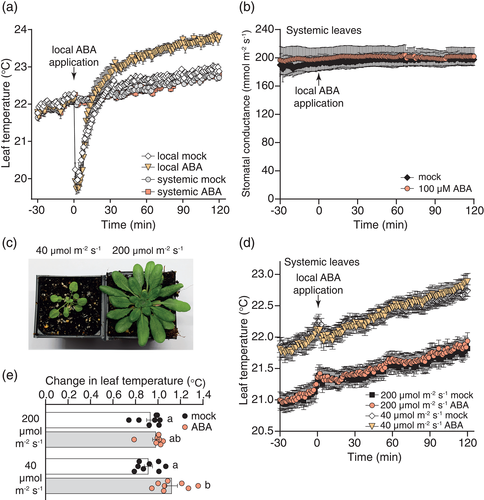
To further explore the effects of ABA on leaf temperature in systemic leaves, we carried out additional experiments with high concentrations of ABA and monitored leaf temperature for longer. Approximately, 4 hr after application of 50, 100 or 200 μM ABA to leaf 8, we noticed a large increase in temperature in the treated leaf and a much smaller increase in leaf temperature in young leaves in the centre of the rosette and closely connected leaves. The pattern of leaf temperature increase was consistent with the vascular connections of leaves (Dengler, 2006; Farmer, Mousavi, & Lenglet, 2013; Figure 3a). Thus, we quantified leaf temperature in the treated leaf (leaf 8) and in the centre of the rosette for 4 hr after treatment with different ABA concentrations. Again, we found that ABA induced a strong and fast increase in leaf temperature in treated leaves at all tested concentrations (Figure S5a), and we did not detect a fast systemic ABA-triggered increase in leaf temperature (Figure 3b). Further analysis to take into account the rise in temperature in the mock treatments (due to gradually rising room temperature across the 4 hr) revealed a small and slow systemic ABA-induced increase in leaf temperature (˂0.4°C) that was evident in young leaves at ~4 hr after treatment (Figure 3b,c). Calculation of ABA-specific change in leaf temperature of systemic young leaves throughout the experiment and fitting of linear models to these data suggested a small, slow and steady concentration-dependent increase in systemic leaf temperature in response to ABA (Figure S5b). This small systemic response was consistently present following application of 100 μM ABA to leaf 8 in three independently grown and analysed batches of plants. Representative results from one of these experiments are shown in Figures 3 and Figure S5. To test whether this systemic response was specific to signalling from mature to young leaves, we conducted a reverse experiment by treating young leaves in the centre of the rosette with ABA and monitoring the temperature of mature leaves. This again resulted in a fast leaf temperature increase in treated leaves (Figure S5c) and a slow increase of lower magnitude in systemic mature leaves (Figure 3d,e; Figure S5d). These results suggested that the long-term systemic ABA response is unspecific and might be due to movement of ABA from treated to systemic leaves.
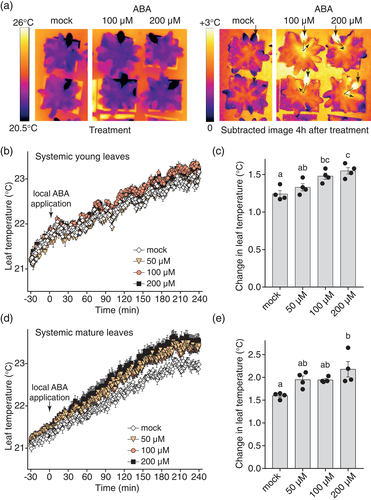
To explore any molecular mechanism(s) underpinning the slow systemic ABA response, we tested whether the response depended on de novo biosynthesis of ABA by comparing wild-type Arabidopsis with the ABA biosynthesis–deficient nced3nced5 mutant (Frey et al., 2012). A fast increase in leaf temperature in response to 100 μM ABA was detected in ABA-treated leaf 8 of both plant lines (Figure S6b,d), and the systemic ABA response appeared to be faster and stronger in young leaves of the nced3nced5 plants (Figure 4a,b; Figure S6a,c), suggesting that de novo biosynthesis of the hormone is not required for this response. Analysis of ABA-specific leaf temperature response suggested that the local ABA response in leaf 8 was slower and lower in magnitude in nced3nced5 plants (Figure S6d), whereas the systemic ABA response was stronger in the double mutant (Figure S6c).
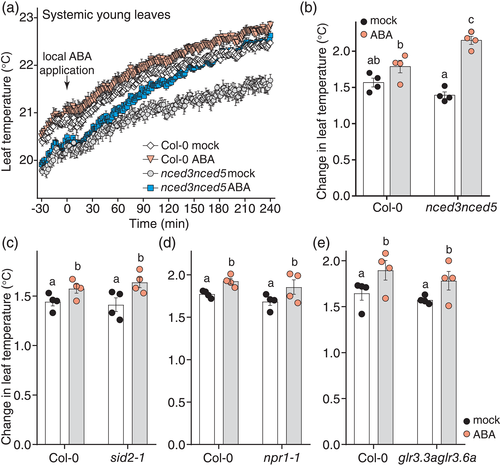
Systemic defence responses require salicylic acid (SA) signalling (Fu & Dong, 2013), thus we tested whether the long-term stomatal ABA response could employ a similar mechanism. The slow ABA-induced systemic increase in leaf temperature was also present in the SA biosynthesis–deficient sid2-1 mutant (Wildermuth, Dewdney, Wu, & Ausubel, 2001; Figure 4c; Figure S7) and plants deficient in NONEXPRESSER OF PR GENES 1 (NPR1), a key regulator of SA-responses (Cao, Glazebrook, Clarke, Volko, & Dong, 1997; Figure 4d; Figure S8), suggesting that this response is distinct from systemic defence responses. Systemic ABA response was faster, and local response appeared stronger in npr1-1 compared to wild type (Figure S8a–d), suggesting that npr1-1 may be hypersensitive to ABA.
Systemic wound signalling is mediated by GLUTAMATE RECEPTOR-LIKE (GLR) genes (Mousavi et al., 2013), thus we tested, whether slow systemic ABA-induced stomatal closure is functional in the glr3.3aglr3.6a double mutant. These plants also showed a systemic increase in leaf temperature similar to wild type (Figure 4e; Figure S9), suggesting that the GLR proteins are not required for the slow systemic stomatal ABA response.
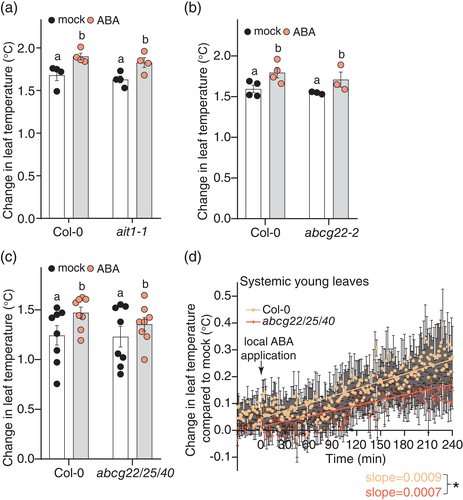
We hypothesized that the slow ABA response may be due to transport of ABA from treated to systemic leaves. In line with this, we found that the leaf temperature increase was more pronounced in smaller plants where ABA transport should be faster, such as the low light grown wild-type plants (Figure 2e) and nced3nced5 (Figure 4a,b; Figure S6a,c). In nced3nced5, ABA also seemed to reach leaves more distal to the treated leaf 8, in line with faster ABA movement either due to small plant size or higher mass flow of ABA in the vasculature, possibly resulting from the higher transpiration in the nced3nced5 mutants (Figure S6a). To assess the role of ABA transport for systemic stomatal closure, we measured systemic response to 100 μM ABA in the ait1-1 mutants that lack the vascular ABA importer ABA-IMPORTING TRANSPORTER 1 (AIT1, also known as NRT1.2; Kanno et al., 2012). The systemic response of ait1-1 was similar to wild type (Figure 5a; Figure S10), suggesting that AIT1 is not strictly required for the systemic stomatal closure response. As the ABC transporter ARABIDOPSIS THALIANA ATP-BINDING CASSETTE G22 (ABCG22) has been suggested as a candidate that may enhance ABA influx to guard cells (Kuromori et al., 2011), we analysed the slow systemic ABA response in abcg22-2 (Kuromori et al., 2011). The systemic ABA response of abcg22-2 was similar to wild type (Figure 5b; Figure S11), whereas the local ABA response was even stronger in abcg22-2 (Figure S11d), suggesting that ABCG22 is not strictly required for the systemic stomatal closure response. As ABA transporters may function redundantly, we also assessed ABA-induced systemic stomatal closure in the higher order abcg mutant abcg22-2abcg25abcg40 (Merilo et al., 2015, referred to as abcg22/25/40) that in addition to ABCG22 is also deficient in the ABA transport proteins ABCG25 (suggested to export ABA from vasculature, Kuromori et al., 2010) and ABCG40 (suggested to import ABA into guard cells, Kang et al., 2010). Like in wild-type, locally applied ABA caused a slow increase in temperature in systemic young leaves (Figure 5c,d; Figure S12), but extraction of ABA-specific temperature response curves showed that both local and systemic ABA responses were slower in abcg22/25/40 (Figure 5d; Figure S12c), in line with the expected reduced ABA uptake and transport in these mutants. Together, these data suggest that the slow systemic stomatal closure induced by locally applied high levels of ABA is, at least in part, caused by transport of the applied ABA to systemic tissues.
3.4 ABA induces fast exogenous nitrate-independent stomatal closure in barley
To test whether dynamic thermal imaging is applicable to analyse stomatal ABA responses in other species than Arabidopsis, we next studied the major cereal crop barley (Hordeum vulgare). As nitrate was recently shown to be required for cereal SLOW ANION CHANNEL-ASSOCIATED 1 (SLAC1) anion channel activation and fast and efficient ABA response in barley (Schäfer et al., 2018) and another monocot date palm (Phoenix dactylifera, Müller et al., 2017), we assessed its effect on barley leaf temperature alone and in combination with ABA. We carried out thermal imaging on intact plants placed horizontally under the light source. After placing the plants on their sides, we allowed an acclimation period of 1.5–2 hr, before applying treatment solutions by paintbrush to leaf 2. Again, we quantified leaf temperature for three areas on the treated leaf and used a mean of these measurements as representative plant temperature to calculate means for each treatment group for every time point (Figure 6a).
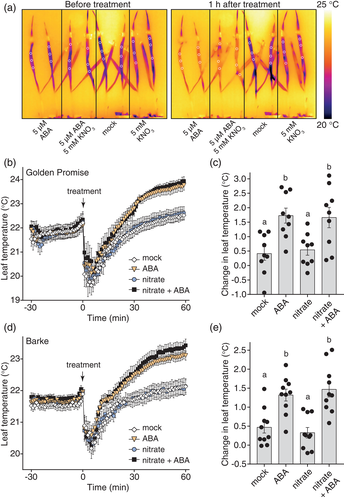
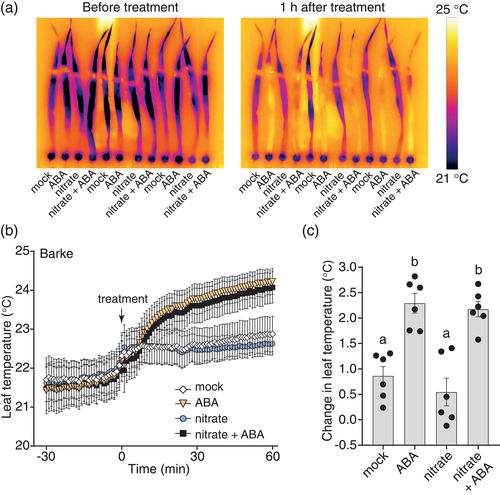
In the Golden Promise barley cultivar, application of 5 μM ABA resulted in strong and rapid increase in leaf temperature which could be discerned within 20–30 min of application. Imaging of the whole plant indicated that the leaf blades sustained much higher levels of stomatal conductance than the leaf sheath, even after ABA treatment. After 1 hr, the ABA-treated leaf blades were approximately 1°C warmer, either in the absence or presence of 5 mM potassium nitrate solution (Figure 6b,c; Figure S13a). Fitting of sigmoidal models to the ABA-specific leaf temperature response data for this experiment implied similar kinetics of ABA response both in the absence and presence of exogenous nitrate (Figure S13b), suggesting that ABA-induced stomatal closure did not require exogenous nitrate under these conditions. To test whether this divergence from the previously published requirement for nitrate in barley ABA-induced stomatal closure (Schäfer et al., 2018) could be due to our use of a different barley cultivar, we analysed stomatal response to ABA in the Barke cultivar. The application of 5 μM ABA to cv. Barke also resulted in a strong and rapid increase in leaf temperature both in the absence and presence of nitrate (Figure 6d,e, see also time-lapse in Video S2). One hour after treatment with ABA and nitrate, cv. Barke leaves had reached a slightly higher temperature than after ABA treatment alone, and fitting of sigmoidal models to the ABA-specific leaf temperature response data for this experiment also implied a slightly higher magnitude of the ABA response in the presence of nitrate (Figure S13c). However, leaf temperature response to ABA without nitrate was still fast and strong (Figure 6d; Figure S13c), suggesting that endogenous nitrate levels are sufficient to enable fast stomatal response to ABA in barley under our experimental conditions.
In our experiments, we applied both ABA and nitrate onto the leaf surface, whereas Schäfer et al. (2018) applied ABA through the transpiration stream of detached leaves. To test whether nitrate is required for fast stomatal response to ABA in detached leaves that receive ABA and nitrate through transpiration stream, we replicated the experiment as reported in Schäfer et al. (2018), in the Barke cultivar, but detected leaf temperature instead of transpiration. Detached leaves were preincubated with sheath in either water or nitrate and 25 μM ABA was supplied in solution after a stabilization period. A strong increase in leaf temperature was evident during the hour after application of 25 μM ABA to the transpiration stream of detached barley leaves both in the presence and absence of nitrate (Figure 7; Figure S14a), suggesting that independent of the application method of nitrate and ABA, in our conditions, exogenous nitrate was not required for fast ABA-induced stomatal closure in barley. Fitting of sigmoidal models to the ABA-specific leaf temperature response data for this experiment implied even a very slightly lower magnitude of the ABA response in the presence of nitrate (Figure S14d). To test whether simultaneous application of ABA and nitrate to leaves preincubated in water would enhance ABA-induced stomatal closure, we incubated the sheaths of detached barley leaves in water and applied either nitrate, ABA or nitrate and ABA after the stabilization period. As in the experiments described above, stomatal closure induced by 25 μM ABA was equally fast and strong both in the presence and absence of nitrate (Figure S14b,c,e), suggesting that under our conditions, exogenous nitrate was not required for fast ABA-induced stomatal closure neither in detached nor attached barley leaves and irrespective of the ABA and nitrate application method.
4 DISCUSSION
Thermal imaging has been successfully applied in screening for mutants with altered stomatal regulation, as well as in assessing plant responses to various abiotic and biotic stressors in both laboratory and field conditions. However, this approach has rarely been applied to study ABA responses in intact plants. Here, we successfully used dynamic thermal imaging to study systemic and local stomatal responses to ABA in Arabidopsis and barley. However, there are some limitations in measuring leaf temperature by dynamic thermal imaging as a proxy for stomatal ABA responses. For reliable results, comparisons should be made within an experiment (and repeated with independently grown batches of plants) with regular rotating of plant positions, to avoid biases resulting from fluctuations in air humidity and temperature in temperature-controlled cabinets. In experiments, where ABA is applied to the plant or leaf surface, the fast kinetics of stomatal response that occurs within the first 20–30 min cannot be reliably measured immediately after application. Nevertheless, even with this caveat, the method enabled us to get a better picture of ABA responses than measuring only the final temperature differences would give. The differences between stomatal ABA response of wild-type and the strongly ABA-insensitive ost1-3 mutant were easily detected (Figure 1d; Figure S2b), and the more subtle phenotypes of attenuated ABA response in the local ABA-treated leaves of nced3nced5 (Figure S6d) and abcg22/25/40 (Figure S12c) were discernible. Thus, dynamic thermal imaging could be used for the study of stomatal responses to ABA in parallel to detached sample methods to dissect the mechanisms of ABA-induced stomatal closure in the whole plant context. The recently described model to derive stomatal conductance from thermal imaging in a dynamic environment (Vialet-Chabrand & Lawson, 2019) will help to convert changes in leaf temperature to those in stomatal conductance and therefore allow temporal and spatial characterization of the stomatal response more directly.
The ability to detect within-plant differences in leaf temperature (Figures 1e and 2a; Figures S3–S12) makes thermal imaging a potent tool for the study of systemic stomatal responses in intact plants. However, we could not detect rapid ABA-induced systemic stomatal closure in intact Arabidopsis plants (Figure 2a,b,d; Figure S4a,b) as described previously by analysis of stomata in epidermal strips (Devireddy et al., 2018). As ABA is usually a sign of stress that accumulates over time, the adaptive value of systemic ABA-induced stomatal closure occurring in minutes is difficult to understand, especially in small plants like Arabidopsis. Currently, the evidence for fast systemic stomatal closure is mixed. Experiments using stomatal aperture measurements from isolated epidermal peels in plants grown at low light levels suggest that fast stomatal closure occurs in Arabidopsis in response to various stimuli, such as ABA, high light, wounding and heat stress (Devireddy et al., 2018; Devireddy, Arbogast, & Mittler, 2020). On the other hand, evidence from gas exchange experiments in intact plants suggests that while fast systemic stomatal closure in response to darkness and elevated [CO2] is present in trees, these systemic responses do not occur in Arabidopsis (Ehonen, Holtta, & Kangasjärvi, 2020). It is possible that fast systemic stomatal closure happens only in response to select stimuli or under certain conditions. Our results suggest that low light alone is not sufficient to enable fast ABA-induced systemic stomatal closure in Arabidopsis (Figure 2d; Figure S4b).
Although we could not detect rapid systemic stomatal closure following local ABA application, because we were able to observe leaf temperature across an extended time course, we were able to detect a relatively small systemic increase in leaf temperature over a period of hours, irrespective of whether the ABA was applied to mature or young leaves (Figure 3; Figure S5). This response did not require de novo ABA nor SA biosynthesis (Figure 4a–c; Figures S6 and S7), NPR1-dependent signalling (Figure 4d; Figure S8), GLR receptor kinases (Figure 4e; Figure S9) or the ABA transporters AIT1 (Figure 5a; Figure S10) and ABCG22 (Figure 5b; Figure S11). However, leaf temperature increase in both local and systemic leaves in response to ABA was slowed in the abcg22/25/40 triple mutant (Figure 5c,d; Figure S12), indicating that ABA transport is required for at least part of the slow systemic ABA response. We propose that it is the slow movement of some of the locally applied ABA towards systemic tissues that elicits a low level of stomatal closure manifested in the slow systemic increase in leaf temperature. In accordance with this, the systemic ABA response was faster and stronger in smaller plants (the low light grown plants, Figure 2e, and the nced3nced5 mutant, Figure 4a,b; Figure S6), where ABA movement from treated to systemic leaves in the centre of the rosette should be faster, likely leading to higher ABA levels in the systemic leaves. In the nced3nced5 mutants, the response may be further enhanced by increased mass flow of ABA in the vasculature due to high levels of transpiration in this mutant. That we were able to detect the small systemic changes in leaf temperature by thermal imaging above any background changes in temperature (Figures 2-5; Figures S5–S12) further highlights the robustness and sensitivity of the method for studying stomatal responses to ABA.
Our experiments using dynamic thermal imaging to characterize several plant genotypes (Arabidopsis wild-type and various mutants and two cultivars of barley) revealed that, despite their large differences in stomatal size, density and morphology (Bertolino, Caine, & Gray, 2019), application of 5 μM ABA elicited a remarkably similar temperature change, perhaps suggesting an approximately equivalent control of water loss. We detected ABA-induced stomatal closure in intact plants of two barley cultivars that was strong and fast irrespective of exogenous nitrate application (Figure 6; Figure S13). This contrasts with previous experiments, where five times higher ABA concentration was insufficient to trigger strong stomatal closure in the Barke barley cultivar without exogenous nitrate (Schäfer et al., 2018). The difference could not be attributed to alternative ABA application methods (transpiration stream in Schäfer et al. (2018) and leaf surface here), as ABA response assays with detached leaves under our conditions also showed fast and strong stomatal closure in response to ABA applied to transpiration stream without the need for additional nitrate (Figure 7; Figure S14). Further experiments with nitrate-deficient plants, accompanied by measurement of leaf nitrate concentrations, are required to clarify the role of nitrate in stomatal ABA response in cereals. In the context of current knowledge, the results presented here (Figures 6 and 7; Figures S13 and S14) suggest that endogenous nitrate levels are sufficient to ensure efficient ABA-induced stomatal closure in barley guard cells under our growth conditions.
Here, we use dynamic thermal imaging of leaf temperature to study stomatal responses to ABA over time in intact plants. We find no evidence for fast systemic ABA-induced stomatal closure in Arabidopsis but detect a slow and small systemic stomatal response to ABA. We also find that additional nitrate is not required for closure of grass stomata in whole plants. We suggest that dynamic thermal imaging could be used more often to study stomatal responses in intact plants in response to various triggers, such as ABA agonists and antagonists, other plant hormones, ion channel inhibitors, pathogens and pathogen-associated elicitors. So far, these signalling responses have mostly been studied in isolated epidermal peels and characterization of their role in a whole plant context would significantly contribute to a broader understanding of stomatal physiology.
ACKNOWLEDGEMENTS
We thank Marion Tout and Sarah Sommer for help with plant growth. We thank Edward Farmer for the glr3.3aglr3.6a seeds and Luke Ramsay for Barke seeds. H.H and J.E.G thank Leverhulme Trust (RPG-2016-274) for funding, and J.D. and J.L. acknowledge the BBSRC and the White Rose Universities for scholarships.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Hanna Hõrak designed experiments with Jessica A. Dunn, Joanna Landymore, Luke Fountain and Julie E. Gray; Hanna Hõrak, Luke Fountain, Jessica A. Dunn and Joanna Landymore performed experiments; Hanna Hõrak and Luke Fountain analysed data; Hanna Hõrak and Julie E. Gray wrote the manuscript, all authors commented, edited and approved the final manuscript.




