The light-induced transcription factor FtMYB116 promotes accumulation of rutin in Fagopyrum tataricum
Abstract
Tartary buckwheat (Fagopyrum tataricum) not only provides a supplement to primary grain crops in China but also has high medicinal value, by virtue of its rich content of flavonoids possessing antioxidant, anti-inflammatory, and anticancer properties. Light is an important environmental factor that can regulate the synthesis of plant secondary metabolites. In this study, we treated tartary buckwheat seedlings with different wavelengths of light and found that red and blue light could increase the content of flavonoids and the expression of genes involved in flavonoid synthesis pathways. Through coexpression analysis, we identified a new MYB transcription factor (FtMYB116) that can be induced by red and blue light. Yeast one-hybrid assays and an electrophoretic mobility shift assay showed that FtMYB116 binds directly to the promoter region of flavonoid-3′-hydroxylase (F3'H), and a transient luciferase activity assay indicated that FtMYB116 can induce F3'H expression. After transforming FtMYB116 into the hairy roots of tartary buckwheat, we observed significant increases in the content of rutin and quercetin. Collectively, our results indicate that red and blue light promote an increase in flavonoid content in tartary buckwheat seedlings; we also identified a new MYB transcription factor, FtMYB116, that promotes the accumulation of rutin via direct activation of F3′H expression.
1 INTRODUCTION
Tartary buckwheat (Fagopyrum tataricum), an annual herb belonging to the family Polygonaceae, grows mainly in mountainous alpine areas at altitudes above 2,500 m. Given that this plant is rich in protein, lipids, vitamin B, and dietary fibre, it is considered a potential “functional food” (Bonafaccia, Marocchini, & Kreft, 2003; Qin, Wang, Shan, Hou, & Ren, 2010). Notably, buckwheat seeds have high flavonoid content, the largest class of secondary metabolites, which are beneficial to human health (Fabjan et al., 2003; Winkel-Shirley, 2001). Previous studies have shown that rutin, quercetin, and other flavonoids have antioxidant, anti-inflammatory, and anticancer properties (Conesa et al., 2005; P. Jiang et al., 2007; Tomotake et al., 2007). Moreover, these flavonoids play important roles in the prevention of capillary fragility and weakened permeability, as well as in reducing the risk of hardening of the arteries (Yildizoğlu-Ari, Altan, Altinkurt, & Öztürk, 1991). Furthermore, it has been reported that the content of flavonoids in seedlings is also very high at 6 to 10 days after germination (Kim et al., 2008) and also that the accumulation of metabolic products can be regulated by different light qualities (Tsurunaga et al., 2013). Buckwheat seedlings represent a new type of vegetable from which rutin content can be more readily absorbed. Flavonoid content in buckwheat seedlings therefore has important nutritional implications.
Environmental factors have important effects on plant secondary metabolism. When subjected to biotic or abiotic stresses, plants produce a large number of secondary metabolites to cope with adversity (Aziz et al., 2003; Korn, Peterek, Mock, Heyer, & Hincha, 2008; Pezet et al., 2003; Tsurunaga et al., 2013). Light is an important environmental factor that regulates the entire cycle of plant growth and development (Jiao, Lau, & Deng, 2007; Lau & Deng, 2010; Wang & Deng, 2003). Light can be divided based on wavelength into far-red, red, blue, and ultraviolet (UV) light. UV light can further be divided into UV-A, UV-B, and UV-C. It has been reported that UV-B can promote the accumulation of phenolic compounds such as flavonoids, tannins, and hydroxycinnamic acid in tartary buckwheat (Landrey, Chapple, & Last, 1995). Further, the accumulation of flavonoids in mature grape berries can be induced by UV-C irradiation (Adrian, Jeandet, Douillet-Breuil, Tesson, & Bessis, 2000; Nishikawa, Tominori, Wada, & Kondo, 2011; Takayanagi, Okuda, Mine, & Yokotsuka, 2004; Versari, Parpinello, Tornielli, Ferrarini, & Giulivo, 2001). Yoneda, Nakashima, Miyasaka, Ohdoi, and Shimizu (2017) found that, compared with white light, blue light can promote increases in the stevioside and rebaudioside-A content in Stevia rebaudiana by 2.5 and 2.7 times, respectively. Similarly, D. Zhang, Sun, et al. (2018) reported that red and blue light promote the accumulation of artemisinin in Artemisia annua L.
Although analysis of new metabolic pathways and the discovery of new enzymatic reactions are routine in the field of plant secondary metabolism, fewer studies have focused on transcriptional factors, which can simultaneously regulate multiple synthase genes in a single metabolic pathway and play important roles in secondary metabolic biosynthesis (Patra, Schluttenhofer, Wu, Pattanaik, & Yuan, 2013). Transformation of transcription factors via genetic engineering is an effective approach for enhancing secondary metabolism in plants. At present, in addition to the discovery of key synthase genes in the flavonoid biosynthesis of tartary buckwheat, certain transcription factors regulating the expression of key enzyme genes have also been identified (Falcone Ferreyra, Rius, & Casati, 2012; Hichri et al., 2011). For example, the transcriptional complex MYB-bHLH-WD40 is found to regulate the synthesis of flavonoids (Feller, Machemer, Braun, & Grotewold, 2011; Stracke et al., 2007, 2010; Xue, Charest, Devantier, & Rutledge, 2003), and in Antirrhinum majus, the transcription factors AmMYB305 and AmMYB340 can both activate phenylalanine ammonia lyase (PAL), chalcone isomerase (CHI), and flavonoid-3′-hydroxylase (F3′H) expression (Moyano, Martinez-Garcia, & Martin, 1996). Furthermore, Pattanaik et al. (2010) have reported that the MYB transcription factor NtAN2 regulates anthocyanin biosynthesis in the flowers of Nicotiana tabacum.
In this study, we undertook a comprehensive analysis of the effects of light of different wavelengths on the synthesis of flavonoids in buckwheat using a combination of transcriptomic and metabolomic methods. Our metabolomic analyses indicate that light plays a prominent role in increasing flavonoid content in buckwheat seedlings, with blue light being the most effective in this regard, followed by red light and then far-red light. Further, we found that light regulates the synthesis of flavonoids, particularly rutin, via a MYB transcription factor, FtMYB116. FtMYB116 was demonstrated to promote the expression of F3′H by binding directly to its promoter and thereby subsequently promoting the synthesis of rutin. The findings of this study provide an important basis for further research on the light-induced transcriptional regulation of flavonoid synthesis in plants.
2 MATERIALS AND METHODS
2.1 Plant material and growth conditions
Tartary buckwheat seeds were soaked in water for 20 min, after which the seed coats were peeled off. Peeled seeds were initially washed with 75% ethanol for 1 min and then soaked in 0.5% mercury for 8 min, followed by six 1-min washes with sterile water. Thereafter, the seeds were blotted on filter paper to remove excess moisture and then sown onto Murashige and Skoog (MS) medium containing 1% sucrose and 0.8% agar and were incubated in darkness for 4 days at 25°C, followed by various continuous light treatments for 2 days. Plants were illuminated with light-emitting diodes (LEDs) emitting the following wavelengths: red light (670 nm), blue light (470 nm), and far-red light (735 nm). For all treatments, the light intensity was 50 ± 5 μmol m−2 s−1, and each treatment comprised three biological replicates. Following these light treatments, plant samples were ground into powder and stored in liquid nitrogen. The flavonoid content in these preparations was determined using a 0.1-g amount of the powder, whereas a further 0.1-g sample was used to extract RNA for sequencing.
2.2 RNA sequencing
Plant total RNA was isolated using an RNA Extraction Kit (Tiangen, Beijing, China) according to the manufacturer's instructions. RNA quality was assessed using a Bioanalyzer 2100 instrument (Agilent, USA), and the RNA integrity number of all RNA samples was found to be >7.0. Sequencing libraries were prepared according to the manufacturer's instructions (Illumina, USA), and RNA sequencing was performed using an Illumina X Ten sequencing system (Illumina Inc., San Diego, CA, USA).
2.3 RNA-seq data analysis
After removing Illumina adapter sequences and trimming low-quality bases, approximately 46.9 G bases of clean data were used in our analysis. These reads were subsequently mapped to the tartary buckwheat reference genome (L. Zhang, Li, et al., 2017) using STAR v2.5 software. Differentially expressed genes (DEGs) were screened based on their FPKM (reads per kilobase of exon per million mapped reads) values (Mortazavi, Williams, Mccue, Schaeffer, & Wold, 2008). FPKM values were calculated using Htseq software. For DEG analysis, we used DESeq2 software (log2FoldChange ≥ 1).
2.4 Transcription factor identification and coexpression analysis
Transcription factor families were identified using hmmscan software. To select the possible transcription factors regulating genes involved in flavonoid biosynthesis, four highly expressed genes, F3'H (FtPinG0002353900), flavone-3-hydroxylase (F3H; FtPinG0006662600), 4-coumarate:CoA ligase (4CL; FtPinG0005072700), and cinnamate-4-hydroxylase (C4H; FtPinG0001575100), were used in coexpression analysis with a default value 0.05 (Ariani & Gepts, 2015). Paired genes showing a Pearson correlation coefficient (r) greater than 0.95 were considered as significantly coexpressed and selected to build a coexpression network using the Perl script (T. Zhang, Song, et al., 2017).
2.5 Quantitative RT-PCR
Plant total RNA was isolated using an RNA Extraction Kit (Tiangen), and first-strand cDNA was synthesized using oligo d(T)18 by reverse transcriptase (Invitrogen, Carlsbad, CA, USA) and then diluted (1:100) for use in quantitative reverse transcription polymerase chain reaction (qRT-PCR) with SYBR Premix Ex Taq Mix (Takara, Dalian, Liaoning, China) in a total volume of 20 μl. Real-time PCR was performed using a LightCycler 480 System (Roche, Basel, Switzerland), following the manufacturer's instructions. Three biological replicates were used for each sample, and the expression level was normalized to that of actin, which was used as the control gene.
2.6 Yeast hybrid assays
The AD-FtMYB116 effectors were cotransformed with the F3′Hp:LacZ reporters into yeast strain EGY48, and transformants were selected and grown on SD/-Trp-Ura dropout media. The transformants were further grown on SD/-Trp-Ura dropout media containing 20-mg ml−1 X-gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) for colour development.
2.7 Electrophoretic mobility shift assay
The MBP-FtMYB116 recombinant proteins were expressed and purified from Escherichia coli. Electrophoretic mobility shift assay experiments were performed as previously described (Z. Jiang, Xu, Jing, Tang, & Lin, 2016).
2.8 Transient luciferase expression assays
The F3′Hp:LUC reporter plasmid and FtMYB116 effector construct were transformed into Agrobacterium. The Agrobacterium suspension was slowly injected into the whole leaves of N. tabacum. After injection, the recipient N. tabacum plants were initially left in the dark overnight and thereafter grown under light/dark cycle of 16/8 hr. At 48 hr after injection, LUC fluorescence in leaf blades was observed using NightSHADE LB 985 (Berthold, Germany).
2.9 Transformation of the hairy roots of tartary buckwheat
The hypocotyls and cotyledons of 7- to 10-day tartary buckwheat seedlings were selected as explants. Cotyledons were cut into small squares, and hypocotyls were cut into approximately 0.5-cm segments, and these samples were precultured on MS solid medium for 1 day. A suspension of bacteria harbouring exogenous gene plasmids was poured into the triangular bottles containing the leaf and hypocotyl explants for 10 min and then poured out. Thereafter, the explants were transferred to sterile triangular bottles and cocultured with a bacterial solution for 3 days in the dark. The explants were rinsed with sterile water 4–5 times until the water was clear and were then placed on MS medium. The appearance of hairy roots was observed 2 weeks later. Those hairy roots showing rapid growth were selected, cut into 2- to 3-cm pieces, and propagated in liquid MS medium (containing 100-mg L−1 carbenicillin) at 25°C on a shaking table rotating at 80 rpm.
2.10 Sample treatment for secondary metabolite analysis
Fresh seedlings were ground into powder, 0.1 g of which was extracted overnight at 4°C with 1.0-ml pure methanol (or 70% aqueous methanol) containing 0.1-mg L−1 lidocaine. Lipid-soluble extracts were absorbed, and 0.4 ml of each extract was mixed and filtrated (SCAA-104; 0.22-μm pore size) before liquid chromatography–mass spectrometry (LC-MS) analysis.
2.11 LC-MS analysis
The analytical conditions used for LC-MS were as follows: column, Agilent Eclipse Plus C18 column (250 mm × 4.6 mm, 5 μm; Santa Clara, CA, USA); solvent system, water (0.1% formic acid) and acetonitrile (0.1% formic acid); gradient programme, 5:95 v/v, from 0 to 12 min and 95:5 v/v from 12 to 15 min; flow rate, 0.2 ml min−1; temperature, 45°C; and injection volume, 1 μl.
3 RESULTS
3.1 Morphology and flavonoid content of tartary buckwheat during sprout development under far-red, red, and blue light
Tartary buckwheat seedlings were initially grown in the dark for 4 days and then subjected to various light treatments for a further 2 days. The hypocotyls of seedlings in dark and under LED red light were slightly longer than those under LED far-red and blue light. Further, in the dark, the cotyledons of seedlings were closed and yellow in colour, whereas the hypocotyl was pale white. Under LED red light, the cotyledons of seedlings were slightly open and deep green in colour and the hypocotyl was light red. Under LED far-red light, the cotyledons of seedlings were wide open and light green in colour and the hypocotyl was light red. Under LED blue light, the cotyledons of seedlings were closed and deep green in colour and the hypocotyl was deep red (Figure 1a). The different morphology of seedlings indicated that the various wavelengths of light have different effects on tartary buckwheat seedlings.
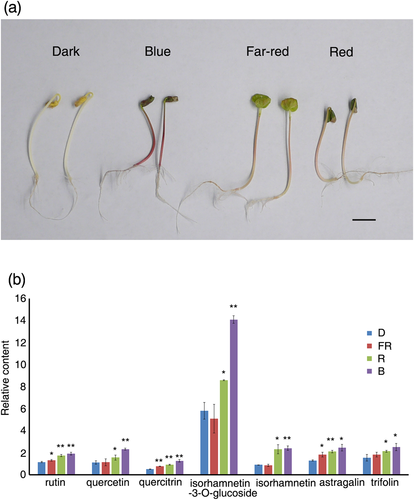
Using LC-MS analysis, we analysed the content of 28 compounds (Table S1). Compared with the dark control, the provision of far-red, red, and blue light all promoted increases in rutin, astragalin, and quercitrin content, with blue light having the most pronounced effect, followed by red and then far-red light (Figure 1b). Although we found that red and blue light promoted increases in the content of quercetin, isorhamnetin-3-O-glucoside, isorhamnetin, and trifolin, far-red light had no effect. Again, the effect of blue light was observed to be stronger than that of red light (Figure 1b). We also identified the presence of orientin, vitexin, isovitexin, and catechin-O-β-d-glucoside under various wavelengths of light, but no obvious changes were observed (Figure S1). These results showed that light can promote increases in the content of flavonoids in buckwheat seedlings and that blue light is the most effective in this regard, followed by red light and then far-red light.
3.2 Comparative analysis of transcriptional profiles
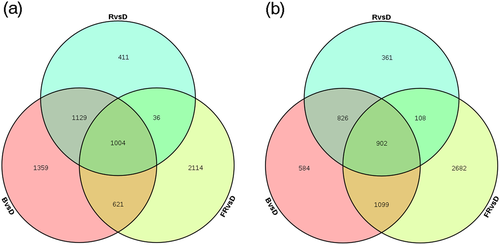
Samples of plants illuminated under different light treatments were subjected to Illumina X Ten paired-end sequencing. The sequencing data have been deposited in the National Center for Biotechnology Information database (accession number: SRP157461). To identify DEGs in the different treatment groups, fragments per kilobase of exon per million mapped reads (FPKM) values were calculated; complete data can be found in Table S2. The DEGs are listed in Table S3. We found that under red, blue, and far-red light treatments, 2,580, 4,113, and 3,775 genes, accounting for 7.7%, 12.3%, and 11.3% of the total genes (33,366), respectively, were significantly up-regulated by at least two-fold (Figure 2a). Conversely, under red, blue, and far-red light, 2,197, 3,411, and 4,791 genes, accounting for 6.6%, 10.2%, and 14.4% of the total genes, respectively, were significantly down-regulated by at least two-fold (Figure 2b). Venn diagrams constructed for the number of up-regulated and down-regulated genes among different light treatments display overlapping and uniquely expressed genes under each condition. Far-red light had the most prominent influence on gene expression, resulting in the up-regulation and down-regulation of 11.3% and 14.4% of DEGs, respectively, whereas red light had the least effect, leading to up-regulation and down-regulation of only 7.7% and 6.6% of the total DEGs, respectively. Overall, among the three treatment groups, 1,004 and 902 genes were commonly up-regulated or down-regulated between at least two treatments (Figure 2).
3.3 Genes related to flavonoid biosynthesis in tartary buckwheat
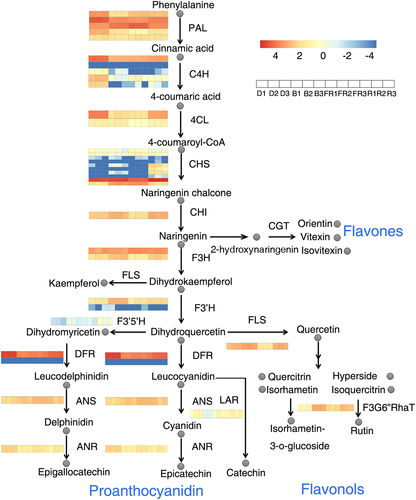
We determined the expression of genes involved in flavonoid synthesis pathways according to our annotation of the tartary buckwheat genome (Figure S2). Through the phenylpropanoid pathway, flavonoids are synthesized from several individual branches and can be classified into different groups of derivatives (Figure 3). Phenylalanine is converted to 4-coumaroyl-CoA via the catalytic action of PAL, C4H, and 4CL (Dixon & Lamb, 1979; Russell, 1971). Subsequently, chalcone synthase (CHS) catalyses the conversion of 4-coumaroyl-CoA to naringenin chalcone (Koes, Spelt, van den Elzen, & Mol, 1989). The CHS gene was found to be induced most strongly by red light. Subsequently, CHI catalyses the conversion of naringenin chalcone to naringenin (Mona & Christopher, 1987), which is then converted to flavones, such as orientin and vitexin, through CGT catalysis (Nagatomo et al., 2014). Naringenin can also be converted to dihydrokaempferol and dihydroquercetin via F3H and F3′H, respectively (Cheng, Han, Wu, & Lou, 2014; Hagmann, Heller, & Grisebach, 1983). At this point, the pathway divides into two branches. In one branch, dihydroquercetin is converted to proanthocyanidin through the catalysis of dihydroflavonol reductase (DFR), whereas in the other branch, flavonol synthase (FLS) converts dihydroquercetin to quercetin, which subsequently is converted to rutin by F3G6″RhaT (Holton, Brugliera, & Tanaka, 1993; Koja et al., 2018). The expression of CHI, F3H, and FLS was induced by red and blue light, whereas F3′H was specifically induced by blue light. In contrast, the expression of DFR was inhibited by light.
3.4 Validation of differential expression via real-time qRT-PCR
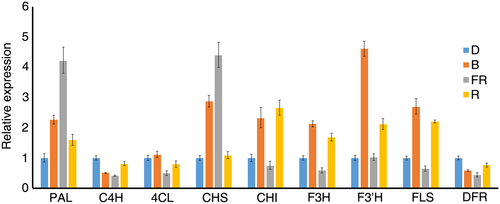
The expression levels of differentially regulated genes were validated using qRT-PCR. For the selected genes, the qRT-PCR expression profile showed positive correlations with the transcriptome data (Figure 4) and the primers used in the qRT-PCR were listed in Table S4. PAL and CHS were strongly induced, whereas C4H and DFR were inhibited by far-red and blue light, and red light had weak effects on all these genes. Furthermore, CHI, F3H, F3′H, and FLS were strongly induced by red and blue light, with the effect of blue light being more obvious than that of red light. None of these genes were induced by far-red light.
3.5 Identification of coexpressed transcription factors that directly regulate the synthesis of flavonoids
To identify the specific transcription factors that may play roles in regulating flavonoid biosynthesis gene expression, we performed coexpression analysis and results are presented in Table S5. Through this analysis, we identified 10, 33, 29, and 21 transcription factors that were coexpressed with F3′H, F3H, 4CL, and 4CH, respectively (Figure 5 and Table S6). Among these transcription factors, we found that one MYB transcription factor (FtPinG0007711300.01), which we named FtMYB116, was coexpressed with all four flavonoid biosynthesis genes (Figure 5). We accordingly focused subsequent efforts on FtMYB116 as the transcription factor most likely to directly regulate the synthesis of flavonoids.
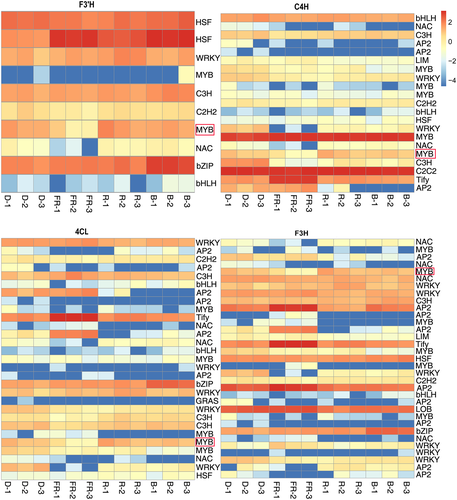
3.6 The expression pattern of FtMYB116
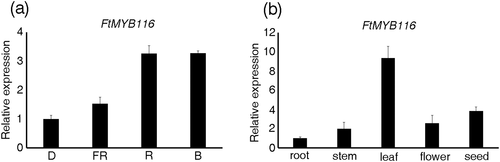
To further characterize FtMYB116, we determined the expression pattern of this transcription factor in different tissues and under different light conditions. The results showed that, compared with darkness, red and blue light significantly promoted the expression of FtMYB116, whereas far-red light was virtually ineffective in this regard. We observed that the expression of FtMYB116 was highest in leaves, followed by seeds, flowers, and stems, with the lowest expression being detected in the roots (Figure 6).
3.7 FtMYB116 directly activates the expression of F3′H
To study the direct effect of FtMYB116 on rutin synthesis, we performed yeast one-hybrid assays. Fragments (2,000 bp) of the promoters of CHS, CHI, F3H, F3′H, and FLS were cloned into pLacZ-2μ reporter vectors, and FtMYB116 was cloned into pB42AD (GAL4 activation domain [AD] fused). As shown in Figure 7b, FtMYB116 bound to the promoter of F3′H, but not to the promoters of the other four examined genes (data not shown). Subsequent bioinformatic analysis of the promoter of F3'H indicated that there are three potential motifs (MBS, MBSI, and MRE) to which FtMYB116 could bind (Figure S3). Because MRE is light induced and Hartmann has previously reported that MYB transcription factors can bind to the MRE (TACCTACC) motif (Hartmann, Sagasser, Mehrtens, Stracke, & Weisshaar, 2005), we conducted a further detailed analysis of MRE in yeast. As shown in Figure 7a,b, when TACCTACC was mutated to GCAAGCAA, FTMYB116 could not bind to the mutated F3′H promoter in yeast.
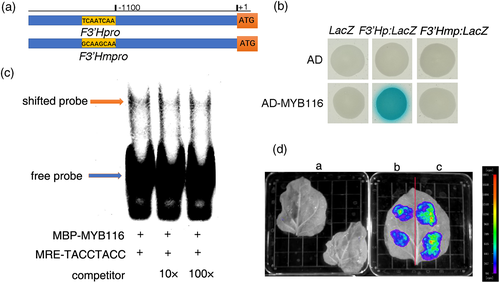
To verify the direct interaction between FtMYB116 and the F3′H promoter in vitro, we expressed MBP-FtMYB116 (fused with the maltose-binding protein) recombinant protein and performed an electrophoresis mobility shift assay. As shown in Figure 7c, the MBP-FtMYB116 fusion protein promotes a shift in the band of the biotin-labelled DNA fragments containing MRE motifs. The intensity of this shifted band was decreased with the addition of excess levels of unlabelled wild-type fragments.
A transient luciferase activity assay was performed in N. tabacum to further examine the regulation of F3′H by MYB116. As shown in Figure 7d, no luciferase fluorescence was observed in two leaves of N. tabacum injected with water (control). When leaves were injected with Agrobacterium transformed only with the F3′H promoter, there was background fluorescence. However, when leaves were injected with Agrobacterium cotransformed with the F3′H promoter and FtMYB116, there was a significant increase in the fluorescence value of leaves, indicating that FtMYB116 can activate F3′H expression.
3.8 Overexpression of FtMYB116 promotes rutin biosynthesis in tartary buckwheat hairy roots
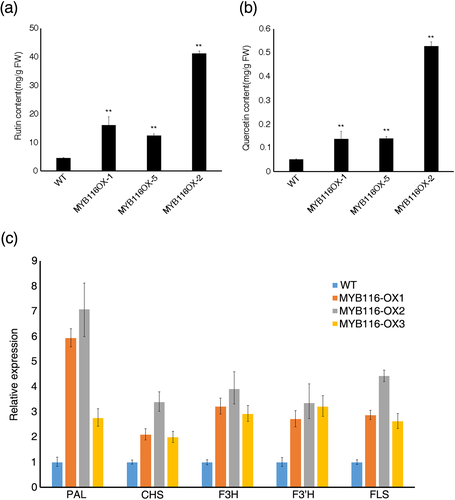
Transcriptome data revealed that FtMYB116 induces the expression of CHI, FLS, and F3H, and thus, FtMYB116 may promote the accumulation of flavonoids. To verify whether FtMYB116 affects the synthesis of flavonoids, we generated transgenic hairy roots of tartary buckwheat overexpressing FtMYB116. Empty vector was also introduced into hairy roots as a control. Three independent transgenic hairy root lines expressing FtMYB116 were used for further functional analysis, in which we investigated the expression levels of FtMYB116 (Figure S4). The expression of FtMYB116 in these lines was significantly higher than that in the control, indicating that FtMYB116 was expressed in hairy roots. Subsequently, we determined the content of flavonoids in the three transgenic lines. As shown in Figure 8, the levels of rutin and quercetin were significantly higher in these lines than in control plants containing an empty vector, indicating that FtMYB116 promotes flavonoid biosynthesis. Subsequently, qRT-PCR was performed to investigate the expression patterns of five rutin biosynthesis genes (PAL, F3H, FLS, CHS, and F3′H) in tartary buckwheat hairy root lines. The transcripts of all the genes were significantly up-regulated in the FtMYB116-overexpressing lines (Figure 8c).
4 DISCUSSION
Light not only provides energy for plant photosynthesis but also acts as an important environmental signal that contributes to the regulation of diverse aspects of plant growth and development (Jiao et al., 2007; Lau & Deng, 2010; Wang & Deng, 2003). For example, light influences the transition from heterotrophic growth to autotrophic growth, a process involving chlorophyll synthesis, chloroplast development, and photomorphogenesis. Light can also act as a stress factor to regulate plant secondary metabolism. Many studies have reported that UV-B can promote flavonoid accumulation in buckwheat (Landrey et al., 1995). However, UV light tends to be harmful to organisms, via the generation of DNA base mutations. Consequently, it is important to study the effects of other wavelengths of light on the synthesis of flavonoids. Here, we found that blue and red light (particularly the former) promote the accumulation of flavonoids in tartary buckwheat. These findings have potentially important implications for the cultivation of plants that produce elevated levels of certain desirable metabolic compounds.
The Venn diagrams depicted in Figure 2 indicate that among the light sources examined, far-red light induces the expressions of the largest number of DEGs (approx. 8,566 genes), accounting for 25.7% of the total number of genes. By comparison, the DEGs induced by red light and blue light accounted for 14.3% and 22.5% of the total number of genes, respectively. Red and blue light caused more gene up-regulation than down-regulation. Gene Ontology annotation of the up-regulated genes revealed that these genes are generally closely related to photosynthesis. This is consistent with the findings of previous studies, which have indicated that red and blue light are the main light sources for photosynthesis (Moore, Clark, Kingsley, & Vodopich, 1995).
The heat maps we generated revealed that blue, far-red, and red light promoted the up-regulation of numerous flavonoid biosynthesis genes, although some genes were specifically up-regulated by a certain type of light (Figure S2). For example, red light promotes the expression of most of the CHS genes, but some of them are induced by far-red light. PAL, 4CL, DFR, and C4H are expressed at high levels in the dark, indicating that light inhibits their expression. CHI, F3H, F3′H, FLS, and F3G6″RhaT genes are specifically promoted by blue and red light, especially blue light, but blue light represses LAR expression. Most of the GTR genes and F3′5′H are expressed at high levels upon exposure to red light. These observations, revealing that different flavonoid biosynthesis genes are induced by different wavelengths of light, indicate that the promotion of flavonoid synthesis by light is a complex process. Accordingly, maximizing the content of these compounds can only be achieved by taking these factors into consideration. In this study, we showed that buckwheat irradiated using blue and red light has a bright appearance and higher content of flavonoids, and we thus anticipate that the quality of buckwheat sprouts can be further improved by optimizing the combination of light of different wavelengths.
The transcriptional regulation of metabolic pathway genes has long been an active area of research, and numerous transcriptional factors that mediate transcriptional regulation have been identified and characterized. Members of the MYB (myeloblastosis) transcription factor family are found in almost all eukaryotes. An oncogene found in the avian myeloid tumour virus was the first MYB gene to be identified (Klempnauer, Gonda, & Bishop, 1982). The MYB transcription factor family plays an important role in plant development, secondary metabolism, hormone signalling, and stress resistance. MYB transcription factors have been reported to play an important role in flavonoid biosynthesis (Feller et al., 2011; Stracke et al., 2007, 2010; Xue et al., 2003). Here, we identified a light-induced MYB transcription factor (MYB116) that can promote the accumulation of rutin. As shown in Figure 9, red and blue light activate their respective receptors, phys and crys. Once activated, these photoreceptors can promote the expression of FtMYB116, which can bind directly to the promoter of F3'H and activate its expression. Thus, high expression of F3'H promotes the synthesis of rutin. Moreover, activated photoreceptors can activate FLS and other rutin-related genes through unknown transcription factors, which can also promote the synthesis of rutin. The existence of several MYB transcription factors has been reported in tartary buckwheat. We found that FtMYB116 is the same factor previously reported as FtMYB1. Bai et al. (2014) reported that overexpression of FtMYB1 and FtMYB2 significantly enhanced the accumulation of proanthocyanidins in N. tabacum. FtMYB116 is responsive to changes in light quality, but FtMYB9, FtMYB10, FtMYB12, FtMYB17, FtMYB21, and FtMYB22 respond to abiotic stresses such as high salinity, drought stress, and cold (Gao, Yao, et al., 2016; Gao, Zhao, et al., 2016; Gao et al., 2017; Zhou et al., 2015). Moreover, FtMYB116 acts as a positive regulator, but FtMYB11, FtMYB13, FtMYB14, FtMYB15, and FtMYB16 act as negative regulators of flavonoid biosynthesis (K. Zhang, Logacheva, et al., 2018; Zhou et al., 2017).
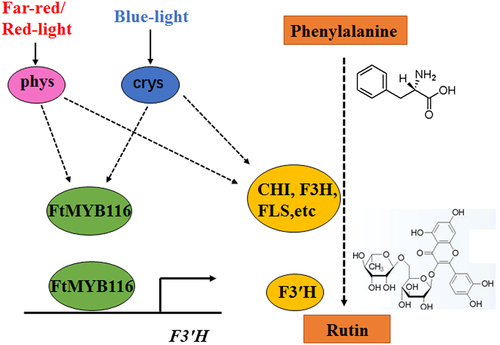
In conclusion, we found that red and blue light, which are not detrimental to the growth and development of tartary buckwheat, can promote the synthesis of rutin. Furthermore, we identified a new MYB transcription factor that can bind directly to the promoter of F3'H and activate its expression. The provision of light of certain wavelengths is an environmentally friendly and convenient treatment, and cultivating tartary buckwheat under light of appropriate wavelengths may have beneficial effects in terms of elevating the levels of desirable nutrient and/or pharmaceutical content.
ACKNOWLEDGEMENTS
The authors thank Qinggang Yin and Yujun Zhang from the Institute of Chinese Materia Medica and the China Academy of Chinese Medical Sciences for helpful discussions and assistance with language editing. This work was supported by the following grants and projects: National Natural Science Foundation of China (31501368 and 81573518) and Research and Demonstration of Imitation Ecological Cultivation Techniques of Cold Temperate Medicinal Materials (special plan for social welfare research); National Special Public Welfare Industry Special (201404718); and Major New Drug Creation “Study on the Innovative Drugs of Artemisinin and its Derivatives” (2017ZX09101002-003-001).




