The small ubiquitin-like modifier E3 ligase MdSIZ1 promotes anthocyanin accumulation by sumoylating MdMYB1 under low-temperature conditions in apple
Abstract
MdMYB1 acts as a crucial component of the MYB-bHLH-WD40 complex to regulate anthocyanin biosynthesis in red-skinned apples (Malus domestica), but little is known about its post-translational regulation. Here, a small ubiquitin-like modifier E3 ligase MdSIZ1 was screened out as an MdMYB1-interacting protein with a yeast two-hybridization approach. The interaction between MdSIZ1 and MdMYB1 was further verified with pull-down and CoIP assays. Furthermore, it was found that MdSIZ1 directly sumoylated MdMYB1 proteins in vivo and in vitro, especially under moderately low temperature (17 °C) conditions, and that this sumoylation was required for MdMYB1 protein stability. Moreover, the transcription level of MdSIZ1 gene was remarkably induced by low temperature and phosphorus deficiency, and MdSIZ1 overexpression exerted a large positive influence on anthocyanin accumulation and red fruit coloration, suggesting its important role in the regulation of anthocyanin biosynthesis under stress conditions. Our findings reveal an important role for a small ubiquitin-like modifier modification of MYB transcription factors in regulation of anthocyanin biosynthesis in plants.
Introduction
Anthocyanins greatly influence fruit quality and thus directly affect the commercial values of various fruits. Indeed, anthocyanins are present and play an important role in many other plants in addition to fruit crops: they confer colors on plant organs such as fruits, flowers, foliage and seeds (Tsao et al. 2003). For most gamogenetic plants, the bright colors endowed by anthocyanins attract pollinators to promote reproduction and distribution (Andersen & Jordheim 2006). In addition, at the cellular level, anthocyanins act as reactive oxygen species (ROS) scavengers to maintain normal metabolic processes in response to various abiotic stresses in plants (Wang et al. 1997; Gould et al. 2002).
Anthocyanin biosynthesis has been extensively studied in plants (Jaakola 2013). Many structural genes have been identified and found to play a role at different stages of the anthocyanin biosynthesis pathway, including both early biosynthetic genes CHI (chalcone isomerase), F3H (flavanone3-hydroxylase) and F3´H (flavonoid 3′-hydroxylase) and late genes UF3GT (flavonoid 3-o-glycosyl-transferase), DFR (dihydroflavonol 4-reductase) and ANS (anthocyanidin synthase) (Koes et al. 2005; Hichri et al. 2011). Furthermore, transcription factors (TFs) have been identified that bind to the promoters of these anthocyanin biosynthesis genes. For example, the R2R3 MYB TF MdMYB1 directly binds to the promoter of the MdANS gene to promote anthocyanin biosynthesis in apple fruit skin (Ban et al. 2007). Similarly, a basic helix–loop–helix (bHLH) TF, MdbHLH3, directly binds to the MdDFR and MdUFGT promoters in apple (Xie et al. 2012). The MYB and bHLH TFs coordinate with WD40 repeat proteins, usually by interacting with each other to form a large MYB-bHLH-WD40 (MBW) complex (Ramsay & Glover 2005). In addition, MdbHLH3 regulates MdMYB1 gene transcription by directly binding to its promoter, suggesting that MBW complex components modulate each other's functions (Xie et al. 2012).
Anthocyanin biosynthesis is induced by various environmental stimuli. Environmental factors influence anthocyanin accumulation by modulating the expression levels of structural or regulatory genes. For example, nitrogen and phosphorus (Pi) deficiencies remarkably induce the expression of both early biosynthetic genes (CHS, F3H and F3´H) and late genes (DFR and ANS) in Arabidopsis (Scheible et al. 2004; Misson et al. 2005; Morcuende et al. 2007; Lillo et al. 2008). Similarly, UV-B light and low temperature (LT) act together to promote anthocyanin accumulation in apple fruit skin by inducing the expression of the CHS, ANS and UFGT genes (Ubi et al. 2006). Light significantly induces the expression of the MdMYB1 gene, thereby enhancing anthocyanin content in apple skin (Takos et al. 2006). In addition, plant hormones, known as gibberellins (GAs), inhibit sucrose-induced anthocyanin accumulation in Arabidopsis seedlings by inhibiting the expression of the DFR gene, while jasmonate and abscisic acid in combination with sucrose exert a synergic effect on anthocyanin accumulation by promoting the expression of the DFR gene (Loreti et al. 2008).
In addition to transcriptional regulation, post-translational modification is one of the most common ways to regulate gene expression in organisms in response to environmental stimuli. For example, phosphorylation plays a central role in the regulation of various secondary metabolic pathways through signal transduction (Pawson & Scott 2005). In apple, 17 °C moderately LT promotes anthocyanin accumulation, partially via a putative LT-induced phosphorylation modification to the MdbHLH3 TF (Xie et al. 2012). Moreover, ubiquitination is a broad post-translational modification conferred on substrate proteins. It is involved in many biological processes via the ubiquitin-mediated degradation of target proteins (Kerscher et al. 2006). Both in apple and Arabidopsis, the ubiquitin E3 ligase COP1 negatively regulates anthocyanin accumulation in plant organs by modulating the degradation of the anthocyanin-associated MYB TFs (Li et al. 2012; Maier et al. 2013).
Small ubiquitin-like modifier (SUMO) conjugated to target proteins is also an important post-translational modification. It ubiquitously regulates various biological processes such as DNA repair, chromatin remodelling, transcriptional activity and the cell cycle in eukaryotic cells (Hay 2005). Interestingly, SUMO modification is involved in regulating anthocyanin accumulation in Arabidopsis. SIZ1 is a SUMO E3 ligase. A mutant siz1 generated lower CHS1 transcripts and anthocyanins than the wild type (WT) control in Arabidopsis (Catala et al. 2007). However, the molecular mechanism by which sumoylation modulates the biosynthesis of anthocyanins in plants is not clear.
In this study, MdMYB1 was used a bait to screen through an apple cDNA library with a yeast two-hybridization strategy. The potential interacting protein MdSIZ1, which is an SUMO E3 ligase, was verified and then characterized with functions in sumoylation and stability of MdMYB1 proteins. Finally, the molecular mechanism by which the MdSIZ1-mediated post-translational modification of MYB TF regulates anthocyanin biosynthesis was summarized and discussed.
Materials and Methods
Plant materials and growth conditions
The calli of the apple ‘Orin’ were subcultured as described by Xie et al. (2012). Transgenic calli were selected with 1.5 mg L−1 hygromycin or kanamycin in medium unless stated otherwise.
‘Gala’ apple tissue cultures were subcultured as described by Xie et al. (2012) unless stated otherwise.
Bagged apple fruits were harvested 140 d after full bloom and collected from 6-year-old ‘Red Delicious’ (Malus domestica Borkh) trees for viral vector-based transformations and other analyses.
Yeast two-hybrid assay
Yeast (Saccharomyces cerevisiae) two-hybridassays (Y2H) were performed as described by Xie et al. (2012). The MdSIZ1 coding sequence was excised by SmaI single digestion and cloned into pGAD424 to generate an in-frame fusion with the GAL4 activation domain. The domain-deleted form (1–201 aa) of MdMYB1 was excised by BamHI and SalI double digestion and cloned into pGBT9 to generate an in-frame fusion with the GAL4 DNA-binding domain. Full-length MdMYB1 was self-activated in β-galactosidase assays in yeast. The two plasmids were co-transformed into the Y2H strain using the lithium acetate method and cultured at 30 °C. Cells were plated onto selective medium lacking Trp and Leu (−Trp/−Leu), and putative transformants were subsequently transferred to medium lacking Trp, Leu, His and adenine (−Leu/−Trp/−His/−Ade).
Pull-down assay
The experiment was performed as described by Zhao et al. (2016). The MdMYB1 coding sequence was excised by BamHI and SalI double digestion and cloned into the PGEX-4T-1 vector for Glutathione S-transferase (GST) tag fusion, and the full-length cDNA of MdSIZ1 was cloned into the pEASY-Blunt E1 expression vector (Transgene, Beijing, China) for His6 tag fusion. Then, both proteins were individually expressed in and purified from Escherichia coli BL21 (DE3). Subsequently, His-MdSIZ1 was incubated with GST-MdMYB1 or GST. After immunoprecipitation (IPed) with an anti-His column, the pellet fraction was detected via immunoblotting using an anti-GST antibody.
Co-immunoprecipitation assay
A co-immunoprecipitation (CoIP) assay was performed as described by Li et al. (2012). Briefly, total protein extracts from MdMYB1-green fluorescent protein (GFP) and GFP transgenic calli were IPed using an anti-GFP antibody following the instructions of the Pierce classic IP kit (Thermo, Shanghai, China), and the eluted proteins were analysed by immunoblotting with the appropriate antibodies.
Vector construction and genetic transformation
The MdMYB1-GFP was constructed as described by Li et al. (2012). For antiMdSIZ1 and antiMdMYB1, a 350-bp fragment of MdSIZ1 (1197–1546 bp) and a 300-bp fragment of MdMYB1 (100–399 bp) were amplified using the primer pairs AsMdSIZ1-F/AsMdSIZ1-R and AsMdMYB1-F/AsMdMYB1-R, respectively (Table S1). Both fragments were cloned into the pCXSN-Myc vector under the control of the 35S promoter, which was described in detail by Chen et al. (2009), to construct antisense vectors. For MdSIZ1-MYC, the full-length cDNA of MdSIZ1 was cloned into the pCXSN-Myc vector. Subsequently, the resultant vectors were introduced into Agrobacterium tumefaciens strain LBA4404 and genetically transformed into wild or transgenic apple calli using the Agrobacterium-mediated method as described by Horsch et al. (1985).
Analysis of gene transcript levels
To carry out gene expression analysis, total RNA was isolated from plant materials using OmniPlant RNA Kit (DNase I) (Cwbiotech, Beijing, China). The RNA was then reverse-transcribed using a PrimeScript first-strand cDNA synthesis kit (Takara, Dalian, China), following the manufacturer's instructions.
For quantitative reverse transcription PCR (qRT-PCR), cDNA was diluted to 4 ng μL−1 with water, and reactions were performed in 96-well blocks with an Applied Biosystems StepOne Plus RT PCR Instrument using UltraSYBR Mixture (with ROX I) (Cwbiotech, Beijing, China) in a reaction volume of 20 μL. The primer pairs used were as follows: QM-F/QM-R for MdMYB1, QS-F/QS-R for MdSIZ1, QD-F/QD-R for MdDFR and QA-F/QA-R for MdANS (Table S1). Md18S was used as the internal control.
Sumoylation assay
For in vivo sumoylation assay, 3-week-old transgenic calli overexpressing the MdMYB1-GFP, MdMYB1-GFP/MdSIZ1-MYC and MdMYB1-GFP/antiMdSIZ1 fusions were exposed to temperatures of 17 °C for 1 h before pretreatment with light at 25 °C for 24 h. Then, the proteins of three transgenic calli were extracted with IP lysis/wash buffer (0.025 M Tris, 0.15 M NaCl, 0.001 M EDTA, 1%NP-40, 5% glycerol, pH 7.4), and soluble extracts were IPed with anti-GFP antibodies using a Pierce classic IP kit (Thermo, Shanghai, China) following the manufacturer's instructions. Immunoblot analyses were subsequently performed with anti-GFP (Abmart, Shanghai, China) or anti-SUMO1 (Abmart, Shanghai, China) antibodies.
In vitro detection of sumoylated proteins was performed in 50 μL of reaction buffer (50 mM Tris–HCl, 100 mM NaCl, 15% glycerol, 5 mM ATP, 10 mM MgCl2, pH 7.8) with or without 0.5 μg of human SAE1 full, 0.5 μg of SAE2/UBA2 peptide, 2 μg of human UBE2/UBC9 peptide, 5 μg of SUMO1 protein (Abcam, Cambridge, UK), 6 μg of GST-MdMYB1 or 8 μg of His-MdSIZ1. After incubation for 6 h at 37 °C, the reaction mixtures were separated on 10% SDS-polyacrylamide gels. Sumoylated GST-MdMYB1 was double detected with anti-SUMO1 and anti-MdMYB1 antibodies.
In vivo ubiquitination assay
For the in vivo detection of ubiquitinated MdMYB1-GFP, 3-week-old transgenic calli overexpressing MdMYB1-GFP, MdMYB1-GFP/MdSIZ1-MYC and MdMYB1-GFP/antiMdSIZ1 were pretreated with the 26S proteasome inhibitor MG132 at a concentration of 50 μM for 16 h and then underwent dark treatment. A Pierce classic IP kit (Thermo, Shanghai, China) was employed to immunoprecipitate MdMYB1-GFP with an anti-GFP antibody, and the precipitate was subsequently analysed by immunoblotting with anti-GFP or anti-ubiquitin (Sigma-Aldrich, Germany) antibodies.
Protein degradation assays
For cell-free degradation assay, proteins from MdSIZ1-MYC and antiMdSIZ1 transgenic calli, as well as WT calli, were extracted in degradation extraction buffer (25 mM Tris, 10 mM NaCl, 10 mM MgCl2, 5 mM DTT, 4 mM PMSF, 10 mM ATP, pH 7.5). After homogenization, the mixture was clarified by centrifugation at 12 000 g for 15 min. Then, the supernatant was co-incubated with GST-MdMYB1 proteins, which were expressed in and purified from E. coli, for different durations of time and sampled simultaneously to detect GST-MdMYB1 protein abundance with an anti-GST antibody (Abcam, Cambridge, UK).
Following treatment with light irradiation for 24 h, 3-week-old transgenic MdMYB1-GFP, MdMYB1-GFP/MdSIZ1-MYC and MdMYB1-GFP/antiMdSIZ1 calli were treated with 250 μM translational inhibitor cycloheximide, placed in the dark for different durations of time and sampled simultaneously for in vivo degradation assay. Then, proteins were extracted from samples at different time points and separated in 10% SDS-polyacrylamide gels to detect MdMYB1-GFP protein abundance using an anti-GFP antibody.
Determination of total anthocyanin content
Anthocyanin content in apple calli and fruit skin was extracted as described by Xie et al. (2012). The extracted anthocyanins were subjected to spectrophotometric quantification at 530, 620 and 650 nm. The relative anthocyanin content was determined with the following formula: OD = (A530 − A620) − 0.1(A650 − A620). One unit of anthocyanin content was expressed as a change of 0.1 OD (unit × 103 g−1 FW).
Viral vector-based transformation analysis in apple fruit
To construct overexpression viral vectors, the coding sequences of MdMYB1 and MdSIZ1 were inserted into the IL-60 (also designated pIR) vector under the control of the 35S promoter. Single or recombinant plasmids were subsequently used for apple fruit infiltrations.
To generate antisense viral expression vectors, MdSIZ1 and MdMYB1 fragments were amplified using the primer pairs asMdSIZ1-F/asMdSIZ1-R and asMdMYB1-F/asMdMYB1-R, respectively, and cloned into the tobacco rattle virus (TRV) vector in the antisense orientation under the control of the dual 35S promoter. Subsequently, the resultant vectors were introduced into A. tumefaciens strain LBA4404 and then used for apple fruit infiltrations.
Fruit infiltrations were performed as described by Li et al. (2012) and Xie et al. (2012). For sense co-injection, the pIR-MdSIZ1 or pIR-MdMYB1 plasmid was diluted to 10 μg mL−1 with 10 mM MgCl2 and mixed in an equal volume with the auxiliary vector IL-60-1 for injection. For sense and antisense co-injection, TRV-antiMdSIZ1 and the TRV1 Agrobacterium bacteria were suspended and diluted with the pIR-MdMYB1 plasmid at 10 μg mL−1 until the OD600 reached 0.6, then injected with the auxiliary vector IL-60-1. For antisense co-injection, TRV-antiMdSIZ1 and TRV-antiMdMYB1 were individually suspended and diluted with 10 mM MgCl2 until the OD600 reached 0.6, then mixed in equal volume to inject with the auxiliary vector TRV1.
Results
Identification of MdSIZ1 as a direct protein–protein interaction partner of MdMYB1
To identify interacting regulators of the MdMYB1 TF, Y2H screening was performed. As a result, several positive colonies were obtained. One of them contained a part of cDNA fragment of the MdSIZ1 gene, which is a homologue of AtSIZ1 and encodes a SUMO E3 ligase to facilitate the conjugation of SUMO to substrate proteins (Zhang et al. 2016). As previously reported, SIZ1 participates in regulation of anthocyanin accumulation in Arabidopsis (Catala et al. 2007); therefore, MdSIZ1 was chosen for further investigation. qRT-PCR assays demonstrated that MdSIZ1 transcription was noticeably induced by abiotic stresses such as LT (17 °C) (Fig. 1a) and Pi deficiency (Fig. S1).
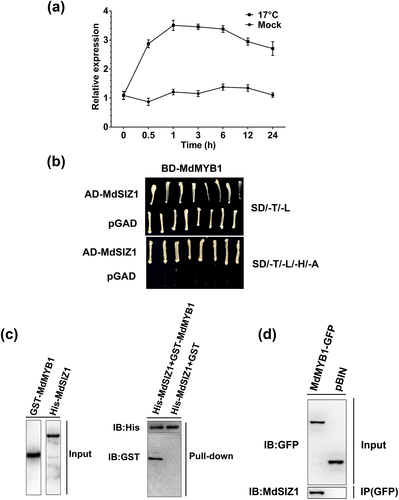
To verify the interaction between the MdSIZ1 and MdMYB1 proteins, an additional Y2H assay was conducted. Figure 1b showed that the yeast cells containing BD-MdMYB1 and AD-MdSIZ1 grew on selective medium, indicating that MdMYB1 interacted with the full length MdSIZ1 protein in yeast cells.
To verify this interaction in vitro, pull-down assays were carried out. As demonstrated in Fig. 1c, His-MdSIZ1 and GST-MdMYB1 were both detected in whole-cell lysates (Input), and an interaction between His-MdSIZ1 and GST-MdMYB1, but not GST alone, was observed in the pellet fraction, which was IPed from an anti-His column by immunoblotting with an anti-GST antibody. This result confirmed the interaction observed between the MdSIZ1 and MdMYB1 proteins.
To test whether MdSIZ1 interacts with MdMYB1 in vivo, a CoIP assay was performed. Firstly, the expression vectors MdMYB1-GFP and pBIN (35S:GFP) were constructed and genetically transformed into apple calli. qRT-PCR indicated that the expression of MdMYB1 was dramatically increased in the MdMYB1-GFP transgenic calli but not in the pBIN control (Fig. S2). Total proteins extracted from the MdMYB1-GFP and pBIN transgenic calli were used to carry out IPed with an anti-GPF antibody. As a result, the MdSIZ1 protein was detected in the pellet fraction of MdMYB1-GFP transgenic calli but not in pBIN transgenic calli (Fig. 1d), indicating that MdMYB1 interacted with MdSIZ1 in plant cells in vivo.
MdSIZ1 sumoylates MdMYB1 proteins under low temperature conditions
In Arabidopsis, SIZ1 functions as an SUMO E3 ligase to mediate the conjunction of SUMO to target proteins (Colby et al. 2006; Wang et al. 2010; Catala et al. 2007). Here, an in vitro sumoylation assay was carried out to examine if MdSIZ1 sumoylates the MdMYB1 protein. The recombinant GST-MdMYB1 proteins were incubated with the SUMO-activating enzyme E1, the SUMO-conjugating enzyme E2, SUMO1 and the His-MdSIZ1 protein, followed by immunoblotting analysis. SUMO1-MdMYB1 conjugates were not detectable with either the anti-MdMYB1 or anti-SUMO1 antibody in the absence of E1, E2 or MdSIZ1, but were detectable when all components were present (Fig. 2a), indicating that MdSIZ1 directly sumoylated the MdMYB1 protein in vitro.
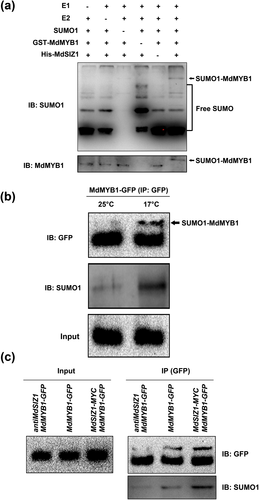
To determine whether MdSIZ1 sumoylates MdMYB1 in vivo, MdMYB1-GFP transgenic calli were employed. Under 25 °C normal temperature conditions, no significant conjugations of SUMO1 to MdMYB1-GFP were detected in the MdMYB1-GFP transgenic calli. On the contrary, the sumoylated MdMYB1 proteins were observed clearly when the calli were treated under 17 °C LT for 1 h (Fig. 2b). The result indicates that LT induces the SUMO modification of MdMYB1 proteins. To further investigate the role of MdSIZ1 in this process, the overexpression vector MdSIZ1-MYC and the suppression vector antiMdSIZ1 were genetically transformed into MdMYB1-GFP transgenic calli. Three types of transgenic calli, specifically MdMYB1-GFP, MdMYB1-GFP/MdSIZ1-MYC and MdMYB1-GFP/antiMdSIZ1, were obtained (Fig. S3). After pretreatment with 17 °C LT for 1 h, total protein extracts from MdMYB1-GFP, MdMYB1-GFP/MdSIZ1-MYC and MdMYB1-GFP/antiMdSIZ1 transgenic calli were IPed with an anti-GFP antibody, followed by a western blotting assay. Sumoylated MdMYB1 proteins were detected in the MdMYB1-GFP calli, and the conjugation of SUMO1 to MdMYB1-GFP occurred at higher levels in the MdMYB1-GFP/MdSIZ1-MYC transgenic calli than conjugation to MdMYB1 alone (Fig. 2c). Therefore, MdSIZ1 overexpression promoted the sumoylation of the MdMYB1 protein. In contrast, when MdSIZ1 was suppressed, conjugation of SUMO1 to the MdMYB1-GFP protein was notably reduced in MdMYB1-GFP/antiMdSIZ1 transgenic calli compared with the MdMYB1-GFP control (Fig. 2c). Thus, MdSIZ1 sumoylates MdMYB1 proteins under LT conditions.
MdSIZ1 stabilizes MdMYB1 proteins by inhibiting 26S proteasome-mediated degradation
To further investigate how MdSIZ1 influences MdMYB1 in apple cells, the overexpression and suppression vectors of the MdSIZ1 gene were genetically transformed into apple calli, respectively. qRT-PCR assays and western blotting analysis demonstrated that both the transcript levels and protein abundance of MdSIZ1 increased in MdSIZ1-MYC transgenic calli but decreased in antiMdSIZ1 calli compared with the WT control (Fig. 3a & Fig. S5).
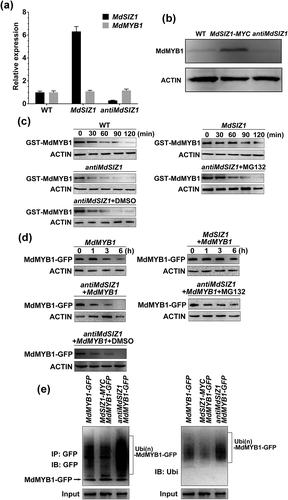
Subsequently, the MdSIZ1-MYC and antiMdSIZ1 transgenic calli were used to examine how MdSIZ1 influences MdMYB1 protein abundance in an immunoblotting assay. The protein abundance of MdMYB1 was remarkably up-regulated in MdSIZ1-MYC transgenic calli but down-regulated in antiMdSIZ1 calli compared with the WT control (Fig. 3b). However, overexpression and suppression of the MdSIZ1 gene exerted little influence on MdMYB1 gene transcript levels (Fig. 3). These results indicated that MdSIZ1 regulated MdMYB1 at the post-translational but not the transcriptional level.
Cell-free assays were carried out to examine if and how MdSIZ1 influences MdMYB1 protein degradation. The recombinant GST-MdMYB1 proteins were co-incubated for different durations of time with total proteins extracted from the MdSIZ1 and antiMdSIZ1 transgenic apple calli as well as the WT control. GST-MdMYB1 protein abundance was assayed by western blotting. The recombinant GST-MdMYB1 proteins degraded much more slowly in the total proteins from the MdSIZ1 transgenic calli but more rapidly in those from the antiMdSIZ1 transgenic calli than those from the WT control (Fig. 3c). Thus, MdSIZ1 inhibited MdMYB1 protein degradation in vitro.
To investigate the influence of MdSIZ1 on MdMYB1 proteins in vivo, three types of transgenic calli, MdMYB1-GFP, MdMYB1-GFP/MdSIZ1-MYC and MdMYB1-GFP/antiMdSIZ1, were treated at 25 °C in the dark for different durations of time after pretreatment with 250 μM translational inhibitor cycloheximide. Total proteins were then extracted and used for western blotting assays with an anti-GFP antibody. MdSIZ1 overexpression inhibited but MdSIZ1 suppression promoted MdMYB1-GFP protein degradation compared with the control (Fig. 3d).
In addition, 26S proteasome inhibitor MG132 treatment notably inhibited the MdSIZ1 suppression-induced degradation of MdMYB1 proteins, both under cell-free degradation conditions and in vivo (Fig. 3c,d), indicating that MdSIZ1 may stabilize the MdMYB1 protein by inhibiting 26S proteasome proteolysis, which is generally associated with ubiquitination modifications. To determine if MdSIZ1 influences MdMYB1 protein ubiquitination, three types of transgenic calli, MdMYB1-GFP, MdMYB1-GFP/MdSIZ1-MYC and MdMYB1-GFP/antiMdSIZ1, were used for protein extraction. MdMYB1-GFP proteins were IPed with an anti-GFP antibody, followed by a western blotting assay. The MdMYB1-GFP/antiMdSIZ1 transgenic calli generated more numerous high-molecular-mass forms of MdMYB1-GFP, while MdMYB1-GFP/MdSIZ1-MYC generated fewer than the control (Fig. 3e). Thus, MdSIZ1 stabilized the MdMYB1 protein by inhibiting its ubiquitination in apple calli.
MdSIZ1 promotes anthocyanin biosynthesis in an MdMYB1-dependent manner in the apple callus and fruit
As a component of the anthocyanin-associated MBW regulatory complex, MdMYB1 plays a central role in the regulation of anthocyanin biosynthesis in apple (Ban et al. 2007). To determine whether MdSIZ1 modulates anthocyanin biosynthesis by regulating MdMYB1 in apple, seven types of single or double transgenic calli, specifically MdSIZ1, antiMdSIZ1, MdMYB1-GFP, MdSIZ1/MdMYB1-GFP, MdSIZ1/antiMdMYB1, antiMdSIZ1/MdMYB1-GFP and an empty vector control, were obtained and employed to examine the anthocyanin contents in comparison with the WT. The results indicated that the empty vector control accumulated 12.10 nmol g−1 FW anthocyanins, similar to the WT (13.71 nmol g−1 FW), indicating that genetic transformation with the empty vector did not influence anthocyanin biosynthesis. In contrast, MdMYB1 overexpression notably promoted anthocyanin biosynthesis in MdMYB1-GFP transgenic calli (30.75 nmol g−1 FW), indicating that the apple calli represents a good material for anthocyanin analysis (Fig. 4a,b).
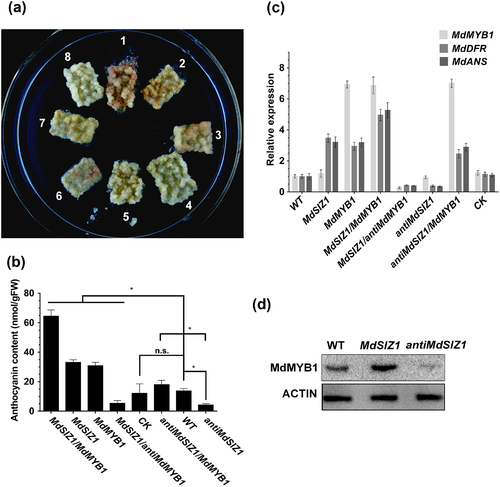
The MdSIZ1 transgenic calli produced 33.10 nmol g−1 FW anthocyanins, while the antiMdSIZ1 calli accumulated 4.18 nmol g−1 FW anthocyanins, which is significantly lower than the empty vector control (12.10 nmol g−1 FW). Furthermore, when MdMYB1 was overexpressed in the background of MdSIZ1 transgenic calli, the double transgenic MdSIZ1/MdMYB1 calli produced much greater levels of anthocyanins (64.50 nmol g−1 FW) than the MdSIZ1 and MdMYB1 calli alone. In contrast, when MdMYB1 was suppressed, MdSIZ1-mediated anthocyanin accumulation was almost completely repressed in the double transgenic MdSIZ1/antiMdMYB1 calli (5.31 nmol g−1 FW). Furthermore, antiMdSIZ1/MdMYB1-GFP calli produced 17.98 nmol g−1 FW anthocyanins, which is significantly greater than antiMdSIZ1 (4.18 nmol g−1 FW). Thus, MdSIZ1 positively regulated anthocyanin accumulation in an MdMYB1-dependent manner in apple calli (Fig. 4a,b).
MdMYB1 transcripts and proteins were detected by qRT-PCR and in immunoblotting assays, respectively. The results showed that the overexpression or suppression of MdSIZ1 exerted little influence on MdMYB1 transcript levels (Fig. 4c) but greatly influenced MdMYB1 protein abundance (Fig. 4d). In addition, the expression of anthocyanin-associated structural genes such as MdDFR and MdANS was examined by qRT-PCR. The expression of these genes was up-regulated by the overexpression of MdSIZ1 or MdMYB1 (Fig. 4c). Based on these data, MdSIZ1 promoted the expression of anthocyanin structural genes and the accumulation of anthocyanins by positively regulating MdMYB1 protein abundance in apple calli.
Because anthocyanins accumulate primarily in apple fruit peel, a viral vector-based transformation method was applied to increase or inhibit the expression of MdSIZ1 and MdMYB1 using the pIR vector for overexpression and the TRV vector for suppression. Four viral constructs, specifically pIR-MdSIZ1, TRV-antiMdSIZ1, pIR-MdMYB1 and TRV-antiMdMYB1, were obtained. Each construct, as well as three combinations (pIR-MdSIZ1 + pIR-MdMYB1, TRV-antiMdSIZ1 + pIR-MdMYB1 and TRV-antiMdSIZ1 + TRV-antiMdMYB1), was employed for fruit infiltration; the empty vectors pIR and TRV were used as controls. The results showed that the overexpression of MdSIZ1 promoted, while MdSIZ1 suppression inhibited, anthocyanin accumulation around the injection sites compared with the empty vector controls (Fig. 5a,b). As with calli, co-injection of pIR-MdSIZ1 + pIR-MdMYB1 resulted in the reddest coloration and the greatest anthocyanin accumulation in peels around the injection sites in fruit. In the pIR-MdSIZ1 + TRV-antiMdMYB1 co-injected peels, MdMYB1 gene suppression partially repressed MdSIZ1-promoted peel coloration and anthocyanin accumulation. Co-injection of TRV-antiMdSIZ1 + TRV-antiMdMYB1 suppressed the expression of both the MdSIZ1 and MdMYB1 genes, leading to the poorest peel coloration and the lowest anthocyanin accumulation (Fig. 5a,b).
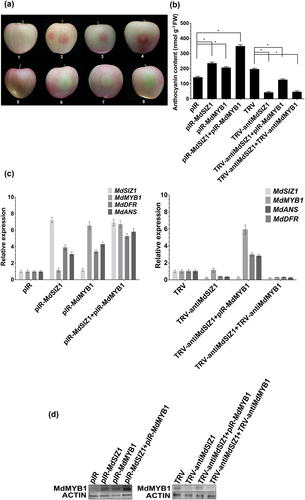
Moreover, immunoblotting and qRT-PCR assays were carried out to examine MdMYB1 protein abundance and transcript levels. MdSIZ1 gene overexpression remarkably enhanced the protein abundance of MdMYB1 but not its transcript levels (Fig. 5c,d). Subsequently, the transcript levels of MdDFR and MdANS were analysed by qRT-PCR. The overexpression or suppression of MdSIZ1 notably influenced the expression of these genes (Fig. 5c). Taken together, MdSIZ1 positively regulates the expression of anthocyanin-associated structural genes and promotes anthocyanin accumulation by modulating MdMYB1 at the post-translational level in apple fruits.
Discussion
MdSIZ1 sumoylates MdMYB1 in an E3-dependent manner to promote anthocyanin biosynthesis
We identified SUMO E3 ligase MdSIZ1 as a positive regulator of MdMYB1 TF at the post-translational level to promote anthocyanin biosynthesis. In plants, sumoylation is an important post-translational modification of substrate proteins that involves three enzymatic steps catalysed by a single E1 SUMO-activating enzyme (SAE1 and SAE2 as its subunits), a single E2-conjugating enzyme, SCE1 and two classes of E3-ligase enzymes, including MMS21 and SIZ1 (Miura et al. 2005; Huang et al. 2009; Ishida et al. 2012; Novatchkova et al. 2012). SUMO modification of substrate proteins mediated by SIZ1 participates in various biological processes related to abiotic stresses, such as the phosphate starvation response and cold acclimation processes (Miura et al. 2005, 2007). Moreover, SIZ1 mediates the abscisic acid response by sumoylating MYB30 TF in Arabidopsis (Zheng et al. 2012).
In this study, the R2R3 MYB TF MdMYB1 was sumoylated by MdSIZ1 under 17 °C LT in apple. Based on our data, MdMYB1 protein abundance, although not transcript abundance, was notably regulated by MdSIZ1. Both stable and transient transformation experiments confirmed that MdSIZ1 promotes anthocyanin accumulation in apple calli and fruits by stabilizing MdMYB1 proteins (Figs 4a & 5a); thus, SUMO modification of MdMYB1 exerts a positive effect on anthocyanin biosynthesis and accumulation.
According to previous studies, in contrast to ubiquitination, a single E2 contributes at least a certain degree of specificity by interacting directly with substrates that contain the sumoylation site consensus sequence φ-K-X-E/D (φ represents a hydrophobic amino acid, K represents the lysine that is conjugated to SUMO, X represents any amino acid and D/E represents an acidic residue) (Sampson et al. 2001). Interestingly, we found that MdMYB1 interacted with and sumoylated by MdSIZ1 in vitro and in vivo; however, no interaction between MdMYB1 and MdSCE1 (SUMO E2 conjugating enzyme1), which is a homolog of AtSCE1, was observed in either a Y2H assay or a CoIP assay (Fig. S4a,b). In certain cases, especially under in vitro conditions, the sumoylated forms of substrate proteins can be still detected even in the absence of E3 owing to the direct interaction between E2 and substrate proteins (Erica et al., 2001; Son et al. 2014). In our results, the absence of an interaction between MdSIZ1 and sumoylated MdMYB1 casts doubt on the involvement of E1 and E2, suggesting that E3, MdSIZ1, plays a decisive role in this process (Fig. 2a). Several studies have shown that SIZ1 plays an integral role in the SUMO modification of certain proteins through direct interactions with substrates. For example, the nitrate reductases NIA1 and NIA2 interact with and are sumoylated by SIZ1 in Arabidopsis (Park et al. 2011). Additionally, the ubiquitin E3 ligase COP1 interacts with and is sumoylated by SIZ1 under dark conditions (Lin et al. 2016). Therefore, MdSIZ1, as an E3 ligase, also directly interacts with target proteins and plays an important role in substrate electivity during SUMO modification, at least in certain cases.
Sumoylation participates in the environmental factors regulated anthocyanin biosynthesis
In plants, environmental factors such as drought, sufficient light and adequate LT significantly induce anthocyanin accumulation (Catala et al. 2007; Li et al. 2012, Xie et al. 2012). We found that MdSIZ1 sumoylates MdMYB1 under LT conditions, indicating adverse environmental factors induced anthocyanin biosynthesis partially through the SUMO modification of specific target proteins. Under dark conditions, the MdMYB1 protein is degraded by the ubiquitin E3 ligase MdCOP1 via the ubiquitin pathway, leading to a non-coloured apple fruit phenotype (Li et al. 2012). Our results demonstrated that MdSIZ1-mediated SUMO modification inhibited the conjugation of ubiquitin molecules to the MdMYB1 protein, and the enhanced stability of MdMYB1 further promoted the accumulation of anthocyanins. However, under dark conditions, MdSIZ1 or MdMYB1 overexpression did not effectively promote anthocyanin accumulation (data not shown), indicating that the MdSIZ1-mediated accumulation of anthocyanins is light-dependent. Thus, light plays a decisive role in the regulation of anthocyanin accumulation in apple. Recent studies found that COP1 is sumoylated by SIZ1 under dark conditions, and SIZ1-mediated SUMO modification enhances COP1 ubiquitin E3 ligase activity in Arabidopsis (Lin et al. 2016). We propose that SIZ1 plays different roles under light and dark conditions, mediating the sumoylation of different substrates. MdSIZ1 overexpression under darkness may inhibit fruit coloration to enhance MdCOP1 ubiquitin E3 ligase activity.
Ambient temperature is an important environmental factor regulating anthocyanin biosynthesis. Apple fruits accumulate more anthocyanins under 17 °C LT conditions. According to our previous study, 17 °C LT promotes anthocyanin accumulation partially via a putative LT-induced phosphorylation modification to the MdbHLH3 TF (Xie et al. 2012). In this study, we found LT responsiveness cis-elements (CCGAAA motif) in the promoter of MdSIZ1 (data not shown). We found that 17 °C LT induced elevated MdSIZ1 transcription levels (Fig. 1a). Under LT conditions, the sumoylation of MdMYB1 was induced, and the suppression of MdSIZ1 weakened the sumoylation of MdMYB1 (Fig. 2b). We proposed that, to a certain extent, this SUMO response under LTs partially enhanced anthocyanin accumulation in apple through the direct modification of MdMYB1 proteins.
In addition to LT, MdSIZ1 transcript levels notably increased under Pi deficiency stress, which also can result in anthocyanin accumulation (Henry et al. 2012; Fig. S1). It appears possible that sumoylation may also contribute to the regulation of anthocyanin accumulation under low-Pi treatment. Hence, our finding reveals new mechanism of sumoylation in regulating anthocyanin biosynthesis.
Plant undergoes various adverse environmental conditions during growth and development. For the mechanism of SIZ1-mediated sumoylation in regulating plants tolerance to abiotic stresses, most studies focused on its direct regulation to target proteins related to stresses. For example, in Arabidopsis, SIZ1-dependent sumoylation of ICE1 promotes the expression of its down-regulated genes CBF3/DREB1A, but suppress the MYB15 expression level, leading to LT tolerance (Miura et al. 2007). On the other hand, under abiotic stress conditions, plants accumulate lots of ROS, which cause significant damage to cell structures (Gill & Tuteja 2010). The biosynthesis of anthocyanin acts as an important antioxidative defence mechanism to remove excess ROS (Wang et al. 1997; Gould et al. 2002). Thus, anthocyanin accumulation is critical for plants in response to abiotic stresses, to some extent. In this study, we found sumoylation promotes anthocyanin biosynthesis via MdMYB1 under LT and Pi deficiency conditions, suggesting its important role for plants in response to stresses. Therefore, we proposed that sumoylation in plants may also participate in responses to abiotic stress via the anthocyanin biosynthesis pathway.
Sumoylation regulates apple fruit internal quality formation through MdMYB1
MdMYB1 also regulates anthocyanin and malate accumulation by directly facilitating their transport into the vacuole in apple (Hu et al. 2016). MdMYB1 binds to the promoters of MdtDT and MdALMT9 to elevate their transcription levels and thus promote the transport of malate to the vacuole, which directly affects the acidity and the quality of the apple fruit. Thus, MdMYB1-mediated transportation through the tonoplast is essential for quality characteristics such as sweetness and fleshy fruit acidity (Shiratake & Martinoia 2007; Etienne et al. 2013). In this study, MdMYB1 was sumoylated and stabilized by MdSIZ1, indicating that MdSIZ1-mediated sumoylation may also be involved in the regulation of apple fruit internal quality formation through MdMYB1.
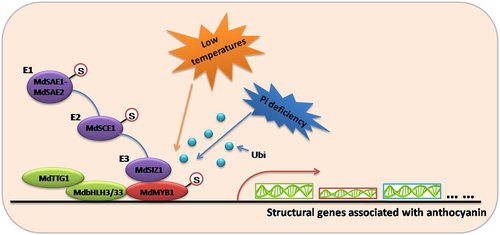
In summary, our study provides new insight into the mechanisms underlying MdSIZ1-mediated SUMO modification and abiotic stress-induced anthocyanin accumulation (Fig. 6). Several abiotic stresses, such as LTs and Pi deficiency, induce the sumoylation of anthocyanin-associated R2R3 MYB TF MdMYB1 by enhancing the transcript levels of the SUMO E3 ligase MdSIZ1. The SUMO modification of MdMYB1 inhibits the conjugation of ubiquitin molecules to the MdMYB1 protein, thus promoting MdMYB1 protein stability. Subsequently, stabilized MdMYB1 binds directly or promotes the binding of other TFs such as MdbHLH3 to the promoters of anthocyanin-specific structural genes, including MdANS (Ban et al. 2007) and MdDFR (Xie et al. 2012), to promote anthocyanin biosynthesis in apple.
Acknowledgment
This work was financially supported by the National Natural Science Foundation of China (31325024, 31301763 and 31272142).
Author Contribution
Y.J.H. and Y.Y.L. planned and designed the research. L.J.Z., Y.Y.L. and R.F.Z. performed experiments and analysed data. X.B.X., C.Z. and C.L.Z. coordinated sequencing efforts. L.J.Z., Y.Y.L. and Y.J.H wrote the manuscript.




