Root Traits: A Key for Breeding Climate-Smart Wheat (Triticum aestivum)
Funding: The authors received no specific funding for this work.
ABSTRACT
Climate change poses a serious threat to global food security by introducing uncertainty in production condition including water availability to growing crops. Technological intervention like improved crop adaptation and higher yield potential through breeding are immediately needed to ensure better availability of food to still growing low- and middle-income societies like South Asia. Root traits, such as root system architecture, root biomass, root angle, xylem diameter, root hairs, root length and root hydraulics, are crucial for plant adaptation to variable environments, but they are often overlooked in the most of crop improvement programme because of difficulty in scoring these traits. Water banking by optimization hydraulic efficiency of vascular system through reduced root density and reduced xylem diameter can play important role for adaptation for reduced water availability. The challenges of nondestructive screening in the segregating generation hampers the genetic progress Recent advances in high-throughput phenotyping facilities and identification of molecular markers has made the selection in breeding population feasible. This review explores how root morphology and anatomy influence water and nutrient uptake and how high-throughput phenotyping and genotyping can facilitate the identification of root traits associated with climate resilience. As outcome of the study, we propose an ideal wheat ideotype with deep roots, narrow root angles and low axial hydraulic conductance combined with high xylem hydraulic safety in pursuit of climate-smart wheat crops thriving under decreasing water availability throughout the growing season. In this review, we have also discussed the root-related quantitative trait loci/genes in wheat and its related species to facilitate comparative genomic analyses and their subsequent integration in the breeding programme. The review thus highlights the potential importance of optimization of metaxylem vessel size, root biomass, root length, roots hairs and understanding soil microbiota and its interaction with different root phenes in designing the better wheat ideotypes, which can offer the potential solution to climate change in the future.
1 Introduction
Ever-growing human population coupled with highly unpredictable environment conditions had raised serious concern for global food security (Gleick 2023; Yadav, Gaikwad, and Bhattacharyya 2017). With almost negligible scope for area expansion, additional demand of food is largely to be met by increasing per unit productivity (Rosegrant and Agcaoili 2010). Wheat is the most widely adapted staple crop and is grown on an area of more than 220 million hectares with production of ~770 million tonnes per year (FAO 2023). It meets 20% of daily protein and calorie requirement worldwide. Yield gains in wheat are currently estimated at about 0.5%–1% per year (Yadav, Gupta, et al. 2021) which is below the 2.4%, required to satisfy global demand (Crespo-Herrera et al. 2018).
Wheat production is greatly challenged by number of factors such as increasing competition for land resource for other purposes, rising temperature, depleting underground water, deteriorating soil health, climate change and drought (Langridge et al. 2022; Yadav et al. 2018). Short-term high-temperature events (heatwaves) during grain fillings are increasingly becoming common and more intense throughout the world leading to losses in agricultural productivity. Reports are available that for every 1°C increase in mean temperature above normal reduces the grain yield by 4.1%–6.4% at global level (Asseng et al. 2015; C. Zhao et al. 2017) and simulations shows 7%–23% of crop loss under severe climate change scenarios (Rezaei et al. 2023). To ensure food supply to every section of society under the constrains of uncertain climatic condition, resilience/stability across a range of environmental conditions beside higher yield potential is equally important.
Cultivar improvement for better adaptation to unfavourable climatic conditions by designing appropriate ideotype encompassing resilient crop growth traits have been recognized as most effective climate change adaptation strategy (Challinor et al. 2014). While developing breeding pipelines and trait panels, breeders usually ignore root traits though root play a most important role in plant plasticity to environment condition. Donald (1968) proposed a wheat ideotype focusing on above-ground traits such as a short stem, few small but erect leaves, and a large erect ear. Lynch (2013) proposed an ideotype called ‘steep, cheap and deep’ for maize root system, which can be explored in wheat as well (Nakhforoosh et al. 2021). Steep and deep root traits help in exploring the deeper layers of soil for efficient absorption of nutrients and water from the soil. Cheap ideotype helps in reducing the metabolic cost of the root and those energy can be utilized for other essential activities of the plants. However, projection of such an ideotype is environment and objective specific, for example, better root proliferation in top layer of soil for adaptation to stress caused by P deficiency (Henry et al. 2010; Lynch 2019) whereas deeper penetration to explore deeper soil layers for moisture stress (Lopes and Reynolds 2010; Rich et al. 2016). However, under no constrains for water and nutrient, lodging becomes a major limiting factor, and thereby, root plate becomes more important. It is thus clear that for yield gain under optimum and stress condition, different RSA ideotype needs to be explored.
Water and nutrient, which are fundamentally important for growth of above-ground parts of any growing plants, are mainly mediated by plant root system. Roots are also a source of chemical and hydraulic signalling that modulate shoot growth and physiology. Wheat possesses two kinds of roots: embryonically developed seminal roots and the crown roots, which develops from the crown of plants few days after germination. Majority of published studies on crop root systems usually zero down on certain functional root trait for its importance in either stabilizing or consolidating the growth of above-ground parts but without bothering, whether these traits can be integrated in breeding programme simply because of difficulties in scoring (Bishopp and Lynch 2015), more particularly in the segregating generation. Bottlenecks in high-throughput root phenotyping and limitation of resource/investment in public sector breeding programme, resulted in notions that selection on root trait is too much difficult and therefore were ignored by most of the breeding programme throughout the world. Root system architecture (RSA) (spatial organization of root structure) is the key for unlocking sustainable productivity under variable and unpredictable environments with more emphasis on specific root traits to address particular kind of environmental condition and agronomy, for example, deeper root with higher root surface area at higher depth modulated by introgression of a quantitative trait loci for DEEPER ROOTING 1 (DRO1) in IR64 resulted in more water and nutrient acquisition under their restricted supply. Surprisingly, introgression of DRO1 showed no genetic load under nondrought conditions as introgression only alters the root growth angle not the shoot or root biomass in rice (Uga et al. 2013). This suggests that improvement in root traits offers great promise for enhancing crop resilience and sustainability.
In addition to RSA, root anatomy is another major important root trait that has not really been explored by many researchers. Root anatomy can reveal the functions and adaptations of the root system, such as water and nutrient uptake, anchorage, storage and growth. Root anatomy serves as foundation for the entire physiology of the plant and its better performance. Root anatomy influences the metabolic cost of root growth, the penetration of hard soil domains, the radial and axial transport of water and solutes and the interactions with soil biota. Root morphology and/or anatomical traits help plants maintain higher grain yields under low resource availability, for example, relatively deeper distribution of roots increasing water uptake under drought conditions. Root anatomy in crop improvement is a transdisciplinary opportunity to address global challenges such as food security, climate change and environmental sustainability (Lynch et al. 2022). Root anatomical phenotypes though underutilized are attractive breeding targets for the development of efficient and resilient crops (Galindo-Castañeda et al. 2022). Lack of high-throughput phenotyping techniques restrict the use of root anatomical traits in breeding programmes; however, the development of recent advances in tangible tools and techniques facilitating high-throughput phenotyping increased the probability of integration and exploration of root anatomical traits in the breeding programme. Aim of our review is to uncover the plethora of root traits (morphological and anatomical traits) that could bestow crop performance under varying climatic conditions and how it can help us create wheat cultivars that can thrive in a changing climate.
2 Key Targets for Climate-Smart Wheat
Climate-smart crop is an extension of imagination facilitated by climate-smart practices and is a part of ‘climate-smart agriculture’—a conceptual framework developed to improve food security and rural livelihoods under changing climatic condition.
3 Role of Root Traits
3.1 RSA
Studies on RSA helps in understanding the complex process through which plant counter the stress and role of roots in these processes. It is of common knowledge that expression of all above-ground parts and physiological processes cannot be independent of their interaction with roots. Root traits have been modified, unknowingly through manipulation of above-ground traits for decades. Our understanding on plant responses to abiotic stress largely relies on above-ground responses, whereas it is largely dependent on root response to stress. RSA thus can be effectively targeted for making either quantum gain or for economizing the production and saving the natural resources. Several studies emphasized the importance of root trait plasticity and root trait–based selection along with above-ground parameters in developing the cultivars adaptable to changing climatic condition (Bainsla et al. 2020; Chandregowda et al. 2023). Historical trend analysis on corn yield in the United States evidenced the significant role of RSA in yield improvement (Hammer et al. 2009) and the role of RSA on better N, and water uptake is well established in rice (San-oh et al. 2004, 2006). Voss-Fels, Snowdon, and Hickey (2018) explained that in the context of flowering time and declining water availability throughout the growing season, deeper and narrow root angle rewards the crop the higher yield and resilience.
3.1.1 Narrow Root Angle
Water and nutrients are the major crop growth-limiting factors and are generally distributed unevenly in different profiles of the soil, and therefore, designing RSA is not a simple task. To improve the capture of limited water and nutrient resources, an ideotype featuring a steeper root growth angle (Manschadi et al. 2008), reduced production of crown or lateral roots (Y. Gao and Lynch 2016), and reduced xylem vessel diameter (Richards and Passioura 1989) has been proposed. As there is no additional metabolic cost associated with changing the root angle and it is one of the traits that do not have any trade-offs, it can be effectively integrated into selection scheme. The ease with which, root angle can be measured on few days old seedling along with its high heritability, which makes it as a character of choice for developing climate resilient varieties. Root growth angle is mainly influenced by gravitropism, hydrotropism and phototropism.
Studies of Voss-Fels et al. (2018) evidenced that VERNALIZATION1 (VRN1) gene influences the wheat root angle, length and density in addition to its key role in flowering regulation, which provides new information for wheat breeding. Deeper rooting can be achieved by moderating the root angle and therefore can lead to better capturing of N leached in deeper layer of soil. Under no-till conditions with high residue retained on the surface, implementing smart agricultural practices can support higher yield realization under no stress and contribute to resilience during periods of strong stress. Deeper rooting can enhance both soil structure and nutrient retention (Kell 2011; Yadav, Gaikwad, and Bhattacharyya 2017). Lower use of P by crop plants and its mobile nature leads to higher accumulation of P in the top layer through crop residue mineralization under conservation agriculture condition under tropical climatic condition. Moreover, Kharif maize in India and Pakistan is grown during rainy season, and therefore, growing shallow rooting maize with wider angle can help in better foraging the top P rich soil (Bonser, Lynch, and Snapp 1996; Liao et al. 2001; J. Zhao et al. 2004; J. Zhu, Kaeppler, and Lynch 2005). Sequencing the cultivation of maize with deep-rooted wheat facilitated with steep root phenotype will be highly rewarding in capturing highly mobile nutrient and water from deeper layer of the soil, more particularly during grain filling stage with declining water regime due to increased temperature. Deeper and narrow roots evidenced yield advantages in bread wheat (X. Li, Ingvordsen, et al. 2019; Lopes and Reynolds 2010), durum wheat (El Hassouni et al. 2018; Maccaferri et al. 2016), barley (Robinson et al. 2018), triticale (Severini et al. 2020), rice (Uga et al. 2013), sorghum (Mace et al. 2012) and maize (Y. Gao and Lynch 2016).
3.1.2 Root Length
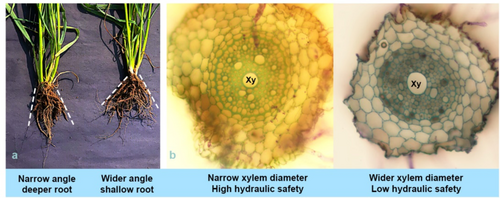
Longer root length showed significant contribution towards stable yield under water-limited conditions. Deep roots aids in exploring the deeper layers of soil and acquires stored water and nutrients from the bottom layers. Large tracts of wheat-growing areas in central India, all of Rajasthan, southern Haryana, southwestern Punjab, much of Australia, the Huang-Huai-Hai and northwestern regions of China, as well as parts of Iran, Iraq, and Pakistan, have low water productivity and declining water tables, with restricted irrigation facilities. Wheat crops grown in these areas usually face a problem in water availability at the time of grain filling, resulting in reduced crop production. Limited availability of water during grain development stage affects accumulation of starch and protein, more particularly amylopectin and B type of amyloplast (Ge et al. 2012; Lu et al. 2014). Genotype with higher root depth was able to modulate the canopy temperature favourably towards terminal stage of crop growth and higher canopy cooling results in better yield realization (X. Li, Ingvordsen, et al. 2019). Similarly, root length was found to have positive impact on area of amyloplast and protein. Therefore, root length impacts wheat grain quality positively (Y. Chen et al. 2020). Longer root length is associated with narrow root angle (Figure 1a). Deeper roots with narrow root angle will be a fascinating trait for breeding climate resilient wheat crop.
3.1.3 Root Hairs
Root hairs, single-celled tubular projections emerging out of root epidermal cells and working as first line of contact with soil, plays an important role in nutrient and water uptake from the soil. Root hairs increase the surface area of the roots, which further enhances the efficiency of roots in exploring and utilizing the available resources. Root hairs also play an important role in modulating soil health favourably by reshaping soil structure and enhancing microbial flora in root health. Agronomic relevance of root hairs has been judged through systematic study on phytohormone signalling shared by root hairs with other physiological processes in plants (Marin et al. 2021; Salazar-Henao, Vélez-Bermúdez, and Schmidt 2016).
They increase the rhizosheath of the plants which provides protection from abiotic and biotic stresses. Exudation of mucilaginous substance by plants helps in increasing the rhizosheath (Basirat et al. 2019). It also helps in efficient absorption of nutrients under low fertile conditions and as site of interaction for soil microbes. Maqbool et al. (2022) found that root hair length and root hair density had a positive correlation with yield contributing traits such as relative water content, number of seeds per spike and biomass of the wheat. Characterization of adaptive plasticity of root hairs (Schneider and Lynch 2020), any trade-off with modern agriculture practices, existence of exploitable genetic variation with in elite germplasm, role of environment and their interaction with microbes in modulating root hair phene are some of the key areas to be explored in the future for working out an ideotype for uncertain climate condition. Question such as how the root hairs perceive abiotic stress and difficulties in phenotyping for root hairs more particularly in segregating generation are some of issues which needs to be addressed before their integration in the breeding programme. The challenges in their measurement, more particularly under field condition, make them underexploited target in the current breeding programmes globally (Tsang et al. 2024); however, their role in yield enhancement and adaptation is undisputed.
3.1.4 Root Biomass
Root biomass is one of the easily decipherable root traits, which integrate number of other RSA traits, and therefore, it will always be a part of all ideotype whether designed for robustness or responsiveness (Pritchard and Rogers 2000). Root biomass contributes positively towards yield under both well-watered and drought conditions. The presence of trade-off between biomass allocation between above-ground and below-ground plant parts, which needs to be deciphered to have a better understanding. Roots with higher biomass aids in better absorption of water and nutrients and in sequestration of more carbon into the soil. Generally, breeders are continuously under confusion whether a higher biomass producing genotype with a bigger root system should be favoured or poor biomass entry with shallow root system will be more rewarding under the condition of terminal heat and water stress Breeding for conservation agriculture practices and selection of genotypes with high root biomass can help in higher yield realization (Ranjan et al. 2021) even under uncertain climatic condition.
3.2 Root Anatomical Traits
Anatomical trait is an important component of plants ability to forage particular soil domains to capture both mobile and immobile resources at reduced metabolic cost for realization of higher yield (Lynch 2019; Lynch and Wojciechowski 2015).
3.2.1 Aerenchyma
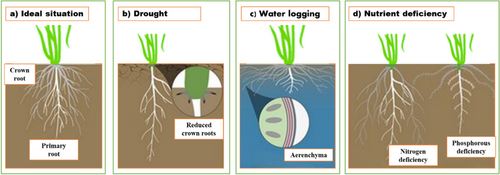
Aerenchyma is a type of tissue that contains air-filled spaces or lacunae. It is formed by the programmed cell death of parenchyma cells in the root cortex. Aerenchyma formation is induced by hypoxic or waterlogged conditions. Presence of aerenchyma helps to deliver oxygen to the roots and reduces the overall metabolic cost of the root tissue. In wheat, aerenchyma is formed at 10 mm from the root tip and increases towards the centre of the roots. Aerenchyma development in wheat roots requires nitric oxide (NO) and ethylene signalling. Aerenchyma may enhance the tolerance of wheat to flooding or waterlogging stress (Q. Xu et al. 2013).
3.2.2 Xylem Diameter—Hydraulic Conductance and Safety
Root xylem is the only motorway water and nutrients supply to the above-ground parts and thus directly decides the carbon fixing capacity of the plant. The rate of water flow (Q, m3 s−1) in relation to the biophysical force driving the flow is hydraulic conductance. The ability of root to conduct water from root surface to the xylem vessels of root and shoot tissue across a differential water potential gradient is defined as root hydraulic conductivity. Water along with nutrient dissolved in it moves first radially from soil to xylem vessels and then is transported axially towards leaves for carbon assimilation. Radial movement of water through cell wall of cortical cells and subsequently through plasma-membrane of endodermis cells is many times highly limiting the water and nutrient uptake. Movement of water in the xylem tissue may be of particular significance, particularly in cereals where xylem vessels are of small diameter, Xylem vessels support mobilization of water and dissolved minerals from the roots to the rest of the plant and provides physical support.
Axial hydraulic conductance is the ability of water to move through the xylem tissue in the axial direction. Hydraulic conductance (ks) is one of the essential vascular traits towards sustainable growth and ecological performance of crops under varying climatic conditions (McDowell, Brodribb, and Nardini 2019; Nardini and Luglio 2014; Scoffoni et al. 2016). ks is positively correlated, and the scale of water transport is found to be increasing at the magnitude of fourth power of the xylem vessel diameter (Vdia) (Ooeda, Terashima, and Taneda 2018). It is obvious that the xylem vessel diameter serves as the base for the water transport system in crop plants. Vulnerability of hydraulic failure in plants is measured by an index called hydraulic safety and is quantified by P50 (the water potential at half of the maximum hydraulic conductivity is lost). Xylem vessels with wider diameter have greater capacity to transport more water, but it also has lower hydraulic safety as there exists the greater chance of xylem embolism, vice versa (P. He et al. 2020; Santiago et al. 2018). Whether high or low root hydraulic conductivity is desirable, largely dependent upon crop production environment. Genotype with smaller xylem diameter were found highly rewarding for wheat crop under Australian dry land condition as water conserved in soil after a rain event is more slowly exploited due to poor axial conductivity during early crop growth stage and thus ensuring availability of water towards the reproductive and grain filling stage, which is more sensitive to water stress (Richards and Passioura 1989). In addition to this, Hendel et al. (2021) presented evidence that lower ks supports sustainable grain yield under terminal drought conditions. Restricting root hydraulic conductance will minimize water loss by inducing closure of stomatal pores (Vadez et al. 2014). Canopy temperature has significantly played a key role in improving yield potential in wheat (F. Gao et al. 2017; Lopes et al. 2012). Lower canopy temperature in wheat during mid-grain filling stage exhibited positive correlation with higher yield and drought tolerance (Lopes et al. 2012; Thapa et al. 2018). Higher root hydraulic conductance will reduce the canopy temperature by transpiration under moist environments. On the other side, crops with higher root hydraulic conductance are prone to be vulnerable for embolism under water-limited and adverse environmental conditions.
3.2.3 Xylem Embolism
Xylem is the transit for the water from soil to leaves, where it is required for reduction of carbon dioxide into carbohydrates. During this process, water forms a continuous chain along the vascular system. However, under conditions of drought and freezing temperatures, air can be pulled into the xylem vessels, blocking the pathway and preventing further water movement (Zimmermann 1983). Plants naturally have mechanisms to repair these blockages occurred due to embolism. During night-time, root pressure helps in repairing the embolized xylem vessels and restores the hydraulic conductance. Woody plants possess vascular cambium, which helps in replacing the old vessels and keeps the hydraulic conductance in track. Because vascular cambium is absent in cereal crops, their xylems vessels need to be functional throughout the life span of the crop. This can be achieved by vascular segmentation in root–shoot junctions. Xylem vessels in corn grass continues through the root–shoot junction. Reduction of hydraulic conductance by half as a result of embolism under arid conditions will reduce the performance of the crop (Tyree and Ewers 1991). When the crop is exposed to stress for a short period, these embolized shoot vessels can be easily removed, and hydraulic conductance will be restored by positive root pressure. In situations of long-time exposure to water or ice stress, hydraulic conductance is difficult to be restored. It is then a requirement of the cereal crop subjected to frequent stresses to develop a safety zone between root–shoot junction, which will prevent the root xylem from embolism.
Safety zone refers to the structure of xylem through which fungal spores and gaseous emboli cannot pass through. Crops adapted to mesic zones does not possess safety zones whereas crops adapted to arid regions possesses safety zones in their root–shoot junction for better survival. Crops like winter rye possesses safety zone between root–shoot junction which aids in the better performance of winter rye under adverse environmental conditions. On the other hand, crops like corn grass and sorghum lack safety zones in their root–shoot junctions which makes them more vulnerable for blockage of hydraulic conductance as their xylem vessels are continuous from root towards the shoot. From the studies of Aloni and Griffith (1991), it is obvious that vascular segmentation is not only significantly different among species but also among the cultivars of the same species. Winter wheat cultivars have more safer seminal roots than spring wheat cultivars.
4 Response of Root Traits Under Different Environmental Conditions
From breeding perspective, it is conceptualized that the combination of traits (morphological and anatomical) and physiological processes based on various biochemical reaction and compounds, which minimize the losses due to extremity of weather events and does not restrict yield maximization process to a larger extent, can be incorporated in the breeding programmes for the development of resilient crops (Yadav, Kumar, et al. 2021). Root plasticity is an important trait for optimized use and capture of edaphic resource. Plasticity is largely a reflection of phenotypic response to environmental cues and in fact creates a noise and a mean for yield consolidation under variable conditions (N. Siddiqui, Gabi, et al. 2023).
4.1 Drought and High Temperature
Moisture stress and high temperature vary strongly over timescale in majority of south Asian countries for most of the crops including cereals, pulses and oilseeds. Designing a breeding pipeline that ensures the characters on which farmers and or market do not compromise requires a robust system architecture that will be able to buffer against short-lived stress and would not incur any strong penalty on any of productive traits. Under the condition of combined short- and long-term stress, deeper growing root system (M. N. Siddiqui et al. 2021) with restricted use of water during vegetative stage ensures water supply from deeper soil layers to growing caryopsis (Figure 2) (Araus et al. 2002; Palta and Turner 2019). Winter wheat with a very long vegetative phase generally have higher root biomass and more opportunity for root growth to explore deeper profile in contrast with spring wheat with shallow root system and therefore generally ensure more better yield under dwindling water condition. Longer roots may be penalty of carbon allocation in environments with sufficient water availability, but it rewards yield stability under declining water availability along the growing season (Voss-Fels, Snowdon, and Hickey 2018). Rooting structures including rhizoids in early plants and root hairs in vascular plants have played a very important role in crop diversification and adaptation, more particularly under uncertain production condition (Kohli et al. 2022). Crops habituated in arid environments with narrow xylem diameter and high hydraulic safety under limitation of water supply ensure stable yield (Choat et al. 2018; Grossiord, Ulrich, and Vilagrosa 2020; Lens et al. 2011).
4.2 Lodging
Lodging, a consequence of permanent displacement of stem from upright position either because of failure of the anchorage system (root lodging) or bending or breakage at the basal stem internodes, causes significant losses in grain yield and quality recurringly in one or other part of the world. Failure of anchorage as a cause of lodging is more common globally (Berry et al. 2003). The anchorage strength provided by a cone of rigid crown roots emerging from the stem base is one of the important factors countering lodging under favourable conditions induced by lodging favouring agronomy (flood irrigation, high seed rate and high nitrogen application), climatic condition (sudden downpour coupled with high wind speed) and individual plant anatomy (stem strength, long internode length and heavy head). Flood irrigation loosens the contact between roots and soil. Combined with wind force rotating the soil cone and the increased weight of the moistened head causes the stem to bend downward, resulting in partial or complete lodging. Theoretically, wider root plate or root soil cone, a consequence of wider angle, provides more lodging tolerance. The genes or QTLs associated with anchorage strength are being reported on 2D, 5A, 5D and 7D for root traits (Hamada et al. 2012; Verma et al. 2005), however, with no reports of their integration in breeding programme. However, it will be very challenging to meet the requirement of high yield and resilience against lodging.
4.3 Water Logging
Aerenchyma plays a crucial role in helping wheat plants survive under flooding conditions. Aerenchyma are specialized tissues that form air channels within the roots. These channels allow the transport of oxygen from the aerial parts of the plant to the submerged roots, which is essential for maintaining aerobic respiration in waterlogged conditions (Q. Xu et al. 2013). By forming aerenchyma, wheat plants can better cope with the lack of oxygen in flooded soils, ensuring that the roots receive enough oxygen to sustain growth and nutrient uptake (Yamauchi and Nakazono 2022).
4.4 Nutrient Deficiencies
Under nutrient deficiency, wheat plants often adapt by increasing the length and density of their roots to explore a larger soil volume for nutrients. This is particularly evident under nitrogen (N) and phosphorus (P) deficiencies (Lopez et al. 2023). Nitrogen (N) deficiency leads to an increase in total seminal root and lateral root length, as well as the root/shoot ratio. In contrast, phosphorus (P) deficiency results in a greater mean root diameter and total root area compared to the control. Additionally, N deficiency reduces root and shoot dry weight and total leaf area. The root-to-shoot ratio tends to increase, meaning that a greater proportion of the plant's biomass is allocated to the roots. This helps the plant to maximize nutrient uptake from the soil (Wutthida and Karel 2015). Wheat roots develop more lateral roots and finer root hairs to increase the surface area for nutrient absorption (Rossi et al. 2024). Corroborative evidences indicate that transpiration, the loss of water from leaves through evaporation, is one of the major factors responsible for transport of inorganic nutrients through xylem in the crop plants. The continuous movement of water from roots to shoots ensures adequate supply of nutrients to the plant growth and survival. These adaptations help wheat plants to optimize nutrient uptake and maintain growth and yield even under suboptimal nutrient conditions.
5 Genetic Factors Associated With Root Traits
Standardizing the root phenotypes for different production condition and cropping system, matching with available natural resources requires exploitable genetic variation for root angle. Sufficient genetic variation has been reported to exist in maize (Bayuelo-Jiménez et al. 2011; Peñagaricano et al. 2013), sorghum (Tsuji et al. 2005), rice (Kato et al. 2006), barley (Hargreaves, Gregory, and Bengough 2009) and wheat (Manschadi et al. 2008; Oyanagi 1994). Besides, genetic variation and identification of genomic region associated with various root phenotypes including root angles can help in identification and selection of desirable root phenotypes. Genes like DRO1, a deep-rooting gene for greater water uptake in rice (Uga et al. 2013), and its orthologues (TraesCS5A02G213300, TraesCS5B02G210500 and TraesCS5D02G218700), which share 76% similarity,) have been reported in wheat (Kulkarni et al. 2017).
Root grows deep in search of water under the influence of gravity sensed by statocytes in the root columella cells through sedimentation of starch-filled grains (statoliths) to its lower side. Activation of mechanosensitive ion channels, change of pH, uneven auxin distribution in the root tip and other signals result in asymmetrical root growth (Muthert et al. 2020). Evolutionarily conserved EGT2 (ENHANCED GRAVITROPISM 2) controls root angle by encoding sterile alpha motif in barley and wheat (Kirschner et al. 2021). Several single-nucleotide polymorphisms (SNPs) including one CBL-interacting serine/threonine-protein kinase 15 (ZmCIPK15) gene (LOC100285495) associated with root angles and the plastic response of nodal root angle in maize (Schneider et al. 2020) and nodal root angle in sorghum (Mace et al. 2012) were identified. Genes associated with root traits identified were enlisted in Table 1.
| Gene | Function | Reference |
|---|---|---|
| ARF4 | ARF4 is part of the auxin signalling pathway, which is essential for enhancing root length, water and nutrient uptake | J. Wang et al. (2019) |
| BRi1 | Enhances root growth by interacting with brassinosteroids to promote cell elongation and division, which are vital for root development | Singh et al. (2016) |
| EGT2 | Encoding a STERILE ALPHA MOTIF domain-containing protein, which results in narrower and lateral root growth angle | Kirschner et al. (2021) |
| EXPA2 | Confer tolerance to drought with enhanced lateral root growth | J. Yang et al. (2020) |
| LBD16 | Responsible for variation in lateral root number | H. Wang et al. (2018) |
| LRD | Repressor of root growth under drought conditions and was proposed to control the deeper rooting phenotype under drought conditions | Placido et al. (2020) |
| MOR | Gene is part of the auxin signalling pathway, which regulates various aspects of root growth and development | B. Li et al. (2016) |
| RSL4 | Modifies root hair length by altered expression of RSL4 | Y. Han et al. (2016) |
| SERK1 | Modifies root development through auxin concentration | Singh and Khurana (2017) |
| TRIP1 | Inhibitory role of transforming growth factor-beta receptor-interacting protein-1 on root meristem size influences primary root length | X. He et al. (2014) |
| VRN1 | Spring allele of VRN1 tend to produce deeper roots and have a lower root-to-shoot ratio and influences root angle | Voss-Fels et al. (2018) |
Deeper roots efficiently uptake nitrogen from deeper layers and leads to the leachates with less nitrogen. The introgression of 1RS.1BL from rye (Secale cereale) affects RSA traits by maintaining root apical meristem activity over longer time periods. This results with deeper roots and better utilization of resources available in lower layers of soil (Ehdaie et al. 2010; Howell et al. 2018). Conversely, the wild-type bread wheat allele with reduced meristematic activity results in reduced seminal root length and root proliferation.
Introgression of chromosome segment 7DL from the wild wheat relative Agropyron elongatum, shows increased root biomass under drought stress and aids in better performance (Placido et al. 2013). Lines introgressed with fragment of the 5D chromosome connected with the molecular marker Xgwm292 and the Vrn-D1 gene had significantly longer root length and weight under all conditions of irrigation (Pshenichnikova et al. 2020). Rambla et al. (2022) devised single plant selection method for modification of wheat root system in speed breeding programme in hands with phenotyping and marker-assisted selection techniques. The developed wheat lines with modified roots aids in deciphering the role of root system modification to support yield in different environments and soil types.
Lynch, Chimungu, and Brown (2014) proposed that root with ample amount of aerenchyma cells performs well in adverse environmental conditions such as dry and/or low fertility conditions. Nazemi et al. (2016) evidenced that an old Italian durum wheat (Triticum durum Desf.), cultivar ‘Cappelli’, was observed to be with more of aerenchyma-like structures and performed well under marginal environments compared with other cultivars in that study. Even though it reduces metabolic costs, it also has a downside in reducing the radial transport of nutrients in spots of abundance of aerenchyma cells in maize root cortex (Hu et al. 2014; Lynch 2013).
According to genome size-limiting cell size theory, xylem vessel diameter is expected to be limited by the genome size of the crop as that of other cell types. In this context, crops with smaller genome size possess narrow xylem vessel diameter, and crops with larger genome size possess wider xylem vessel diameter. Feng et al. (2022) hypothesized and evidenced by an extensive study on 59 crop families that limiting effect of genome size on xylem vessel diameter holds good under normal environments. Under adverse environments, the environmental pressure shifts the limiting effect of genome size on the xylem vessel diameter. Crops habituated in warmer and moist environments will possess wider xylem diameter and less hydraulic safety as there is no limitation for water supply. Crops habituated in arid and cold environments will possess narrow xylem diameter and high hydraulic safety as they are vulnerable to xylem embolism under limitation of water supply (Choat et al. 2018; Grossiord, Ulrich, and Vilagrosa 2020; Lens et al. 2011). This plasticity enables the angiosperms with smaller genome size to be more successful than gymnosperms with larger genome size in hot and warm climatic conditions of terrestrial ecosystem (Lamy et al. 2014). Hendel et al. (2021) uncovered the genetic basis of ks-related morphological and anatomical seminal root traits using recombinant-inbred lines population, derived from a cross between durum wheat and wild emmer wheat. In addition to this, it evidenced the advantage of low hydraulic conductivity under water-limiting conditions for stable yield. When elite haplotype alleles of the NPF2.12 gene are inactivated, they indirectly promote root growth and enhance nitrogen use efficiency (NUE) by triggering NO signalling under conditions of low nitrate availability (M. N. Siddiqui, Pandey, et al. 2023). Findings of N. Siddiqui, Gabi, et al. (2023) provide valuable insights into the genetic factors influencing root system plasticity, which is essential for enhancing wheat's adaptability to environmental stresses. For the plasticity of total root length, 15 candidate genes spanning across 1A, 1B, 2A, 2B, 4B and 5A chromosomes were reported; 3 candidate genes for plasticity of root average diameter along 7A and 7B chromosomes; and 8 candidate genes for plasticity of number of root tips across 1A, 3A, 4B and 5A were reported. Genomic regions associated with wheat root traits identified through QTL mapping were enlisted in Table 2 and through GWAS were enlisted in Table 3.
| Traits | QTL | Linkage group | Markers | Species | References |
|---|---|---|---|---|---|
| Seminal root angle | QSRA.cgb-1A | 1A | P5522.2–P6934.9 | Triticum aestivum | Liu et al. (2013) |
| QSRA.cgb-2B | 2B | WMC441–WMC344 | |||
| QSRA.cgb-3A | 3A | Xcwm48.1–Xcwm 532 | |||
| QSRA.cgb-7D | 7D | Xgwm44–Xgwm121 | |||
| QRA.qgw-2A | 2A | wPt-3508 | T. aestivum | Christopher et al. (2013) | |
| QRA.qgw-3D | 3D | wPt-731357 | |||
| qRA.qgw-5D | 5D | wPt-731945 | |||
| QRA.qgw-6A | 6A | wPt-5572 | |||
| qRA.qgw-6B.1 | 6B | wPt-734054 | |||
| QRA.qgw-6B.2 | 6B | wPt-730396 | |||
| qSRA-6A | 6A | Triticum durum | Alahmad et al. (2019) | ||
| EPdwRGA-6A | 6A | IWB71119 | T. durum | Alemu et al. (2021) | |
| EPdwRGA-4A | 4A | IWB69385 | |||
| Root length | QMRL.cgb-1B | 1B | CWM140–P8143.282 | T. aestivum | Liu et al. (2013) |
| QMRL.cgb-5D | 5D | Xgwm205.2–Xgwm68 | |||
| QMRL.cgb-7B | 7B | CWM466–P1123.2 | |||
| QTRL.cgb-1B | 1B | P3470.2–P4133.1 | T. aestivum | Liu et al. (2013) | |
| QTRL.cgb-1B | 1B | CWM65–P8222.5 | |||
| QTRL.cgb-3B | 3B | WMC231–Xgwm284 | |||
| QTRL.cgb-3B | 3B | Xgwm644.2–WMC3 | |||
| QTRL.cgb-4B | 4B | Xgwm149–WMC349 | |||
| QTRL.cgb-5D | 5D | Xgwm3–Xgwm43 | |||
| QTRL.cgb-7D | 7D | Xgwm44–Xgwm121 | |||
| QTrl-2A.1 | 2A | wsnp_Ex_c19516_28480622-Xgwm614b | T. aestivum | Kabir et al. (2015) | |
| QTrl-2A.2 | 2A | Xcau529-Excalibur_c12980_2392 | |||
| QTrl-2B | 2B | Xcau285-BS00067828_51 | |||
| QTrl-3A.1 | 3A | Excalibur_c24354_465-Kukri_rep_c102151_697 | |||
| QTrl-3A.2 | 3A | Xwmc695-IAAV5821 | |||
| QTrl-3A.3 | 3A | wsnp_RFL_Contig2699_2402527-BS00056089_51 | |||
| QTrl-3A.4 | 3A | Xbarc1060-Kukri_c43524_106 | |||
| QTrl-4D | 4D | RAC875_c5827_554-wsnp_BF473052D_Ta_2_1 | |||
| QTrl-5A.1 | 5A | wsnp_Ex_rep_c71219_70023450-BobWhite_c14172_113 | |||
| QTrl-5A.2 | 5A | Xksm59-Ku_c102710_1055 | |||
| QTrl-6D | 6D | Xbarc301-Xgdm132 | |||
| QTrl-2B | 2B | Xgwm501-Xcau68 | |||
| QTrl-3B | 3B | Xbarc115-Xwmc291 | |||
| QTrl-4A | 4A | Xcwem34-Xbarc28b | |||
| QTrl-4D | 4D | Xbarc1118-Rht2 | |||
| EPdwTRL-1B | 1B | IWB60732 | T. durum | Alemu et al. (2021) | |
| EPdwTRL-4B | 4B | IWB23476 | |||
| EPdwTRL-5A | 5A | IWA3196 | |||
| 3B | wPt0021 | T. aestivum | Ayalew et al. (2018) | ||
| 4A | wPt4487 | ||||
| 5B | wPt8890 | ||||
| Q.rl.uwa.5AL | 5A | Xbarc1-5A–Xbcd157-5A | T. aestivum | Halder et al. (2023) | |
| QRL.caas-1AL | 1A | AX-89541634–A X-109280493 | T. aestivum | M. Yang, Wang, Hassan, Wu, et al. (2021) | |
| QRL.caas-2BS | 2B | AX-108920782–AX-110463005 | |||
| QRL.caas-7DS | 7D | AX-108952259–AX-111881572 | |||
| QRL.caas-3BS | 3B | AX-86178172–AX-110962448 | |||
| QRL.caas-1BL | 1B | AX-10928674–AX-94446430 | T. aestivum | M. Yang, Wang, Hassan, Li, et al. (2021) | |
| QRL.caas-7AS.1 | 7A | AX-110432090–AX-109345074 | |||
| QRL.caas-6AL | 6A | AX-86165298–AX-109431293 | |||
| QRL.caas-7AL | 7A | AX-109966788–AX-94819074 | |||
| QRL.caas-6BL | 6B | AX-109368729–AX-95094583 | |||
| QRL.caas-7AL | 7A | AX-109966788–AX-94819074 | |||
| QRL.caas-7AS.2 | 7A | AX-109955164–AX-109445593 | |||
| QMrl.sicau-2SY-3D.1 | 3D | AX-109499958–AX-108907550 | T. aestivum | H. Chen et al. (2022) | |
| QMrl.sicau-2SY-3D.2 | 3D | AX-111589572–AX-109260274 | |||
| QMrl.sicau-2SY-3D.3 | 3D | AX-89337262–AX-110042483 | |||
| QMrl.sicau-2SY-7A.1 | 7A | AX-109529523–AX-110402694 | |||
| QMrl.sicau-2SY-7A.2 | 7A | AX-111610630–AX-111511322 | |||
| Seminal root number | QSRN.cgb-2B | 2B | Xgwm429–Xgwm388 | T. aestivum | Liu et al. (2013) |
| QSRN.cgb-3B | 3B | WMC3–P6934.380 | |||
| QSRN.cgb-3D | 3D | Xgwm456–Xgdm8 | |||
| QSRN.cgb-5A | 5A | P2470.2–Xgwm154 | |||
| QSRN.cgb-7A | 7A | P2071.1–Xgwm260 | |||
| qRN.qgw-1B | 1B | wPt-9975 | T. aestivum | Christopher et al. (2013) | |
| qRN.qgw-3A | 3A | wPt-2755 | |||
| qRN1.qgw-3B | 3B | wPt-5261 | |||
| QRN.qgw-4A.1 | 4A | wPt-9251 | |||
| qRN.qgw-4A.2 | 4A | wPt-740561 | |||
| QRN.qgw-6A | 6A | wPt-734004 | |||
| qRN- 5A | 5A | wmc150a | T. aestivum | Hamada et al. (2012) | |
| EPdwTRN-1A.1 | 1A | IWB8696 | T. durum | Alemu et al. (2021) | |
| EPdwTRN-1A.2 | 1A | IWB12589 | |||
| EPdwTRN-1B | 1B | IWB35568 | |||
| EPdwTRN-4A | 4A | IWB21309 | |||
| EPdwTRN-4B.1 | 4B | IWB10265 | |||
| EPdwTRN-4B.2 | 4B | IWB35047 | |||
| EPdwTRN-4B.3 | 4B | IWB66095 | |||
| EPdwTRN-7A | 7A | IWB3767 | |||
| 5A | Excalibur_rep_c68688_103–Kukri_c14944_771 | T. aestivum | Salarpour et al. (2020) | ||
| Root diameter | Q.rdia.uwa.6AL | 6A | Xbarc107-6A–Xmwg934-6A | T. aestivum | Halder et al. (2023) |
| 4B | IACX2314 | T. durum × Triticum dicoccum | Hendel et al. (2021) | ||
| 6A | RFL_Contig3175_1271 | ||||
| QRD.caas-2AL | 2A | AX-109922869–AX-110457187 | T. aestivum | M. Yang, Wang, Hassan, Wu, et al. (2021) | |
| QRD.caas-4AS | 4A | AX-111788010–AX-110061005 | |||
| QRD.caas-5BL | 5B | AX-94815980–AX-111449617 | |||
| QRD.caas-1AL | 1A | AX-94796020–AX-111736411 | |||
| QRD.caas-1DS | 1D | AX-109849862–AX-108727857 | |||
| QRD.caas-4BL | 4B | AX-110367312–AX-110050167 | |||
| QRD.caas-5AL | 5A | AX-109958693–AX-94700681 | |||
| Root dry weight | EPdwRDW-1B | 1B | IWB60732 | T. durum | Alemu et al. (2021) |
| EPdwRDW-3A | 3A | IWB67049 | |||
| EPdwRDW-4A | 4A | IWB21309 | |||
| Q.rm.uwa.6AS | 6A | Xbcd21-6A–Xcmwg652-6A | T. aestivum | Halder et al. (2023) | |
| Q.rm.uwa.7AL | 7A | Xcdo347-7A–Xbarc275-7A | |||
| QRDW.caas-2AS | 2A | AX-110988586–AX-110607196 | T. aestivum | Halder et al. (2023) | |
| QRDW.caas-2BS | 2B | AX-108920782–AX-110463005 | |||
| QRDW.caas-4BL | 4B | AX-94496964–AX-109446017 | |||
| QRDW.caas-4BL | 4B | AX-86179151–AX-109387538 | |||
| QRDW.caas-6BL | 6B | AX-109558906–AX-110028322 | |||
| QRDW.caas-7BL | 7B | AX-95025477–AX-94890497 | |||
| 4A | wsnp_Ex_rep_c66324_64493429–BS00072025_51 | T. aestivum | Salarpour et al. (2020) | ||
| QRDW.caas-4BS | 4B | AX-111068079–AX-111164540 | T. aestivum | M. Yang, Wang, Hassan, Li, et al. (2021) | |
| QRDW.caas-4DS | 4D | AX-109816583–AX-109478820 | |||
| QRDW.caas-3AS | 3A | AX-111507145–AX-109273188 | |||
| QRDW.caas-4BS | 4B | AX-111068079–AX-111164540 | |||
| qRDW-1A | 1A | Xcfd58-Xcfa21581 | T. aestivum | Y. Xu et al. (2023) | |
| qRDW-3A | 3A | Xwmc11-Xgwm369 | |||
| qRDW-4A | 4A | Xbarc52-Xbarc70.1 | |||
| qRDW-5B | 5B | Xbarc4-Xbarc216 | |||
| Axial hydraulic conductance | 2B1 | Tdurum_contig12176_1049 | T. durum × T. dicoccum | Hendel et al. (2021) | |
| 2B2 | Excalibur_c44325_392 | ||||
| 4A1 | Kukri_c4210_480 | ||||
| 4A2 | BobWhite_rep_c66057_98 | ||||
| Xylem area | 1B | Ex_c68416_859 | T. durum × T. dicoccum | Hendel et al. (2021) | |
| 2B | wsnp_RFL_Contig3911_4319047 | ||||
| 3A | wsnp_Ex_rep_c69314_68244036 | ||||
| 3B | BS00024499_51 | ||||
| Central meta xylem diameter | 1B | Excalibur_c51270_185 | T. durum × T. dicoccum | Hendel et al. (2021) | |
| 2B1 | Tdurum_contig11778_144 | ||||
| 2B2 | Ku_c35807_609 | ||||
| 3A | RAC875_c4841_753 | ||||
| 6A | GENE-4052_338 | ||||
| Project root area | QPRA.cgb-3B | 3B | Xgwm644.2–WMC3 | T. aestivum | Liu et al. (2013) |
| QPRA.cgb-4A | 4A | Xgwm601–Xgwm610 | |||
| QPRA.cgb-4B | 4B | Xgwm149–WMC349 | |||
| QPRA.cgb-7D | 7D | Xgwm44–Xgwm121 | |||
| Root surface area | QRSA.cgb-3B | 3B | Xgwm644.2–WMC3 | T. aestivum | Liu et al. (2013) |
| QRSA.cgb-4A | 4A | Xgwm601–Xgwm610 | |||
| QRSA.cgb-4B | 4B | Xgwm149–WMC349 | |||
| QRSA.cgb-7D | 7D | Xgwm44–Xgwm121 |
| Trait | SNP | Linkage group | Position (Mbps) | R2 | Crop | References |
|---|---|---|---|---|---|---|
| Root number | AX-110377484 | 3B | 590.2 | 0.0984 | Synthetic hexaploidy wheat | Khalid et al. (2024) |
| AX-94917006 | 5D | 453.0 | 0.0984 | |||
| AX-158548035 | 6A | 259.2 | 0.0984 | |||
| Chr1B-548183547 | 1B | 548.1 | 0.0675 | |||
| Chr2D-40790142 | 2D | 40.7 | 0.0675 | |||
| Chr3D-596511299 | 3D | 596.5 | 0.0675 | |||
| Chr4D-9656202 | 4D | 9.6 | 0.0675 | |||
| Chr5D-430135466 | 5D | 430.1 | 0.0675 | |||
| Chr7B-416779676 | 7B | 416.7 | 0.0675 | |||
| Chr7B-519025304 | 7B | 519.0 | 0.0675 | |||
| Chr7D-353261268 | 7D | 353.2 | 0.0675 | |||
| Chr7D-577311740 | 7D | 577.3 | 0.0675 | |||
| Chr7D-81036328 | 7D | 81.0 | 0.0675 | |||
| Root angle | Chr1D-436299235 | 1D | 436.2 | 0.0511 | Synthetic hexaploidy wheat | Khalid et al. (2024) |
| Chr2A-248885838 | 2A | 248.8 | 0.0511 | |||
| Chr2A-726250210 | 2A | 726.2 | 0.0511 | |||
| Chr2B-461245654 | 2B | 461.2 | 0.0511 | |||
| Chr3D-26032651 | 3D | 26.0 | 0.0511 | |||
| Chr3D-478052800 | 3D | 478.0 | 0.0511 | |||
| Chr4B-356746086 | 4B | 356.7 | 0.0511 | |||
| Chr6B-429784717 | 6B | 429.7 | 0.0511 | |||
| Chr7B-461920582 | 7B | 461.9 | 0.0511 | |||
| Chr7D-250874793 | 7D | 250.8 | 0.0511 | |||
| Seminal root angle | IWB58749 | 5D | 67 | 10.70 | Hexaploid wheat | Paez-Garcia et al. (2024) |
| IWA623 | 3A | 112 | 6.76 | |||
| IWB3423 | 3B | 62 | 6.63 | |||
| IWB13249 | 2B | 139 | 6.22 | |||
| IWB28562 | 2B | 139 | 6.22 | |||
| IWB65397 | 2B | 140 | 6.17 | |||
| IWB58931 | 3B | 25 | 6.05 | |||
| IWB72241 | 7B | 136 | 5.69 | |||
| IWB69656 | 7B | 140 | 5.47 | |||
| IWB50247 | 5D | 72 | 5.25 | |||
| IWB44344 | 2B | 140 | 5.16 | |||
| IWB44399 | 2B | 157 | 5.14 | |||
| Total root length | AX95222115 | 6A | 425 | 12.15 | Hexaploid wheat | Paez-Garcia et al. (2024) |
| AX94404743 | 6B | 114 | 17.59 | |||
| AX94501214 | 1B | 281 | 31.73 | |||
| AX-490,522,663 | 1A | 0.135 | Hexaploid wheat | N. Siddiqui, Gabi, et al. (2023) | ||
| AX-476,020,090 | 1B | 0.088 | ||||
| AX-473,530,929 | 1B | 0.080 | ||||
| AX-89,768,547 | 2A | 0.098 | ||||
| AX-585,941,635 | 2B | 0.136 | ||||
| AX-20,836,050 | 3A | 0.138 | ||||
| AX-526,932,489 | 4B | 0.138 | ||||
| AX-704,835,640 | 5A | 0.134 | ||||
| AX_109924351 | 1B | 677.9–682.4 | 14.8 | L. Li, Peng, et al. (2019) | ||
| AX_111653240 | 2B | 2.6–4.8 | 14.0 | |||
| AX_111251784 | 2B | 199.5 | 12.2 | |||
| AX_94405934 | 2B | 239.8 | 11.9 | |||
| AX_108756976 | 2B | 568.8 | 15.6 | |||
| AX_110594265 | 2D | 144.3–175.5 | 15.5 | |||
| AX_111505152 | 2D | 285.4 | 10.5 | |||
| AX_109948487 | 3B | 255.7 | 12.1 | |||
| AX_111706221 | 4A | 46.1 | 12.0 | |||
| AX_109410506 | 5B | 607.5 | 13.1 | |||
| AX_108912470 | 7B | 332.3 | 11.9 | |||
| RSA | AX-814,183,606 | 3B | 0.105 | Hexaploid wheat | N. Siddiqui, Gabi, et al. (2023) | |
| AX-814,356,941 | 3B | 0.089 | ||||
| AX-479,202,697 | 5A | 0.119 | ||||
| AX-549,850,407 | 5D | 0.082 | ||||
| No. of root forks | AX-65,417,557 | 2B | 0.081 | Hexaploid wheat | N. Siddiqui, Gabi, et al. (2023) | |
| AX-243,102,306 | 2B | 0.099 | ||||
| Root volume | AX-695,555,707 | 3B | 0.083 | Hexaploid wheat | N. Siddiqui, Gabi, et al. (2023) | |
| AX-814,183,606 | 3B | 0.086 | ||||
| AX-95252696 | 7B | 706.85 | 47.14 | Ramappa et al. (2023) | ||
| AX-95133300 | 4A | 68.69 | 8.96 | |||
| Total root area | AX_110979981 | 1A | 498.5 | 12.8 | Hexaploid | L. Li, Peng, et al. (2019) |
| AX_89723417 | 1B | 466.3 | 12.9 | |||
| AX_109875446 | 1B | 550.7–555.1 | 15.0 | |||
| AX_109924351 | 1B | 675.8–682.4 | 14.8 | |||
| AX_110062330 | 1D | 35.3 | 12.5 | |||
| AX_111653240 | 2B | 2.6–4.8 | 14.0 | |||
| AX_108881774 | 2B | 193.1–199.5 | 12.7 | |||
| AX_94405934 | 2B | 239.8 | 11.9 | |||
| AX_108756976 | 2B | 568.8 | 15.6 | |||
| AX_110977006 | 2B | 793.2 | 9.6 | |||
| AX_111220010 | 2D | 16.7 | 9.3 | |||
| AX_110594265 | 2D | 145.7–175.5 | 15.5 | |||
| AX_111505152 | 2D | 285.4 | 10.5 | |||
| AX_111617187 | 3A | 659.8–660.9 | 12.7 | |||
| AX_109948487 | 3B | 255.7 | 12.1 | |||
| AX_111706221 | 4A | 46.1 | 12.0 | |||
| AX_108830894 | 4B | 1.1 | 12.9 | |||
| AX_109410506 | 5B | 604.1–607.9 | 13.1 | |||
| AX_110914792 | 5B | 654.9 | 12.2 | |||
| AX_110196801 | 5D | 545.7 | 12.0 | |||
| AX_109584945 | 6B | 604.1 | 13.4 | |||
| AX_95684378 | 7B | 186.9 | 13.3 | |||
| AX_108912470 | 7B | 332.3 | 11.9 | |||
| AX_111157278 | 7D | 181.3 | 13.0 | |||
| Average root diameter | AX_110979981 | 1A | 498.5–503.4 | 12.8 | Hexaploid | L. Li, Peng, et al. (2019) |
| AX_89723417 | 1B | 466.3 | 12.9 | |||
| AX_109924351 | 1B | 675.8–682.4 | 14.8 | |||
| AX_110062330 | 1D | 35.3–36.5 | 12.5 | |||
| AX_111107192 | 2A | 43.3–43.5 | 12.4 | |||
| AX_111653240 | 2B | 2.6–4.8 | 14.0 | |||
| AX_108881774 | 2B | 193.1–199.5 | 12.7 | |||
| AX_109897011 | 2B | 238.2–239.8 | 12.3 | |||
| AX_108756976 | 2B | 568.8 | 15.6 | |||
| AX_111220010 | 2D | 16.7 | 9.3 | |||
| AX_110594265 | 2D | 145.7–147.2 | 15.5 | |||
| AX_111505152 | 2D | 285.4 | 10.5 | |||
| AX_111617187 | 3A | 659.8–660.9 | 12.7 | |||
| AX_111706221 | 4A | 46.1 | 12.0 | |||
| AX_108830894 | 4B | 1.1–5.0 | 12.9 | |||
| AX_111047107 | 5B | 438.9 | 13.5 | |||
| AX_109410506 | 5B | 593.4–607.9 | 13.1 | |||
| AX_110914792 | 5B | 654.9 | 12.2 | |||
| AX_110196801 | 5D | 545.7 | 12.0 | |||
| AX_109584945 | 6B | 604.1–604.7 | 13.4 | |||
| AX_95684378 | 7B | 186.9 | 13.3 | |||
| AX_108912470 | 7B | 332.3 | 11.9 | |||
| AX_111157278 | 7D | 181.3 | 13.0 | |||
| Root diameter | AX-94446435 | 3D | 43.02 | 0.00 | Hexaploid wheat | Ramappa et al. (2023) |
| AX-94470331 | 4D | 5.69 | 89.43 | |||
| AX-95234949 | 3A | 56.39 | 0.13 | |||
| AX-94492491 | 7A | 581.85 | 0.27 | |||
| AX-94811606 | 4D | 53.24 | 0.24 | |||
| AX-95227434 | 7D | 312.98 | 0.22 | |||
| AX-94575638 | 6A | 531.10 | 16.82 | |||
| AX-94449793 | 5B | 547.58 | 40.01 | |||
| AX-690,934,270 | 2A | 0.077 | N. Siddiqui, Gabi, et al. (2023) | |||
| AX-31,875,912 | 7A | 0.084 | ||||
| AX-700,388,976 | 7B | 0.077 | ||||
| No. of root tips | AX_108944064 | 1B | 675.8–677.9 | 13.6 | Hexaploid | L. Li, Peng, et al. (2019) |
| AX_111653240 | 2B | 2.6–6.4 | 14.0 | |||
| AX_94405934 | 2B | 239.8 | 11.9 | |||
| AX_108756976 | 2B | 568.8 | 15.6 | |||
| AX_110594265 | 2D | 144.3–150.7 | 15.5 | |||
| AX_108834280 | 2D | 175.9 | 12.4 | |||
| AX_109410506 | 5B | 607.5 | 13.1 | |||
| AX-490,522,663 | 1A | 0.233 | Hexaploid wheat | N. Siddiqui, Gabi, et al. (2023) | ||
| AX-579,774,126 | 2B | 0.233 | ||||
| AX-20,836,050 | 3A | 0.239 | ||||
| AX-527,283,513 | 4B | 0.234 | ||||
| AX-705,374,739 | 5A | 0.233 |
However, the integration of root traits in the breeding programme is a challenging task because of large number of genes involved in root anatomy including aerenchyma; however, many of these genes are highly pleiotropic and can influence number of anatomical traits, which may or may not be positively correlated with each other.
6 Rhizosphere and Its Interaction With Root
The rhizosphere is a fascinating and dynamic zone of soil that surrounds and is influenced by plant roots. This area is teeming with microorganisms, including bacteria, fungi and nematodes, which interact with the plant roots in various ways.
6.1 Interaction With Growing Environment
Many of the anatomical traits of root are assumed to have an adaptive response to production condition as crop plants are generally grown in very diverse ecologies. Three kinds of cells, namely, epidermis, cortex and stele (Coudert et al. 2010; Dolan et al. 1993), contribute towards different root phenotypes (Lux et al. 2004; Taleisnik et al. 1999). The cells constituting xylem vessels and phloem tissue are responsible for transport of water and dissolved minerals (Petricka, Winter, and Benfey 2012). Photosynthetic carbon assimilation (Tanaka, Adachi, and Yamori 2019) is a major factor deciding crop productivity, which is mainly supported by sufficient water and nutrient uptake by roots.
M. N. Siddiqui et al. (2022) evidenced that barley crop grown in different growing conditions such as organic and conventional conditions for 20 years resulted in segregation of root morphology and anatomical traits because of natural selection. Barley crops grown in organic growing conditions have narrow root angel, thin and long roots with increased number of metaxylem vessels whereas crops grown in conventional conditions have broader short roots with wider root angel and fewer metaxylem vessels. These evolutions occurred because of natural selection for the better utilization of available soil resources. Plasticity of root traits will aid in sustainable production under adverse climatic conditions.
Natural selection results in selection of fittest individual which often selects selfish traits but not improves population performance many a times. The aim of breeding programmes is to improve the population performance rather than individual performance (Denison, Kiers, and West 2003; Donald 1968; Weiner 2003). Wheat cultivars with large and shallow root system perform well as individuals, as this aids in better uptake of water and nutrients from the soil. Whereas it overlaps with the neighbouring plants when they are planted in closer spacing as in real field conditions and creates competition among the plants for available resources. This competition due to overproduction of roots has been called a ‘tragedy of commons’ (Gersani et al. 2001; D.-Y. Zhang, Sun, and Jiang 1999), resulting in lower population yield. This shows the presence of trade-off between seminal root growth angle, root number and root length. Crops with narrow root growth angel and deep roots will be beneficial in terms of population performance over the toll of individual fitness. Group selection for narrow and deep roots evidenced yield improvement in the previous breeding programmes (Y. H. Zhu et al. 2019).
6.2 Interaction With Soil Biota
Plants are sessile in nature and their roots have direct contact with soil for anchorage, water and nutrient resources. The huge variation in root anatomy and architecture found in crop plants is the reflection of evolutionary interaction that happened in rhizosphere community structure. Soil has both physical and biological characteristics, and both are not static and changing over the period. Roots below the ground are exposed to variety of organism including bacteria, oomycetes, fungi and nematodes and may have combative or symbiotic interaction with these organisms. It will, therefore, always be interesting to understand how crop root anatomy has been influenced by soil biota and vice versa. Symbiotic biota play an important role for survival and health of plants (Richards and Passioura 1989). Crops are more susceptible to root herbivory compared with shoot herbivory (Zvereva and Kozlov 2012). This shows the prerequisite of mutual and positive interaction among the soil biota and crops. Soil biota have both positive and negative impacts on the plant performance. For example, formation of arbuscular mycorrhizal network around the roots will enhance the water and nutrient uptake efficiency of plants for at least 15 cm beyond the actual root surface (Jansa, Mozafar, and Frossard 2003). Symbiosis formation among roots and arbuscular mycorrhiza will trigger plants' defence system (Pozo and Azcón-Aguilar 2007). It will also help in maintaining the soil aggregate stability (Rillig and Mummey 2006). Association with mycorrhiza aids in improving water uptake under water-limited conditions, by triggering the expression of aquaporins in root tissue (Bárzana et al. 2014).
Rhizosphere microbiome has a potential contribution in altering the nutrient uptake status of crops (Compant et al. 2019). Microbes habiting in rhizosphere aids in nutrient mobilization from nearby regions that are inaccessible to the plant roots and helps in conversion of complex forms of nutrients into simple forms for easy uptake by plants. Crops utilize the macropores available in the soil for the better exploration of the soil resources. Earthworms play an important role in improving the soil macropores and soil aeration, which paves way for the deeper exploration of the soil for water and nutrients. Insects and nematodes are the major role players in rhizosphere region as beneficial and threat to the crops. Crops are more susceptible to root herbivory, which results in lodging and reduced uptake of soil resources. Spatial and temporal habitation of insects and nematodes decides its role as harmful or harmless. For instance, in wheat (Triticum aestivum L.), cyst nematodes are having feeding sites adjacent to the metaxylem vessels. The resistance to cyst nematodes in wheat is by spatial separation of feeding site of nematodes from the vascular system, which prevents the catastrophic destruction of vascular system (Levin et al. 2021). In addition to this, accumulation of defensive compounds in particular cells layers will prevent the vascular system from damage and keeps the mobilization of water and nutrients intact. These defensive compounds restrict the insects and nematodes from reaching the inner layers of stele where the vascular system operates. In essence, the mute question is whether such information can be combined in designing root cell-type architecture to maximize the beneficial interaction with symbiont and minimize the negative impact of pathogens. Even presence of roots along with its symbionts under no till condition helps not only climate resilient crop cultivation but also provide much stronger avenue for nutrient acquisition by optimizing CN ratio.
7 Carbon Sequestration
Humans are releasing huge amount of carbon per capita in various forms compared with previous decades. This had a dramatic impact on the global climate, which we are experiencing nowadays. There are several media available in nature for sequestration of carbon from atmosphere back into ocean, forests and vegetations. Among these, the ocean sequesters one-third of anthropogenic carbon, but it has less net carbon sequestration capacity compared with forests per unit area. Soil has the greater potential to store more carbon and retain for a longer time. Soils can store two times more carbon than atmosphere and three times more than the vegetation it holds (Kell 2011, 2012; Post et al. 1982). One of the major ways of trapping carbon in terrestrial ecosystem is through photosynthesis. Carbon sequestered in roots lasts 2.4 times longer than carbons sequestered in above-ground parts of the plants (Rasse, Rumpel, and Dignac 2005). This shows the importance of sequestration of more carbon in root system in future for balancing the carbon present in atmosphere. Plants with higher root biomass can sequester more carbon into the soil (Kell 2011, 2012; Lynch and Wojciechowski 2015). In wheat, root biomass has been found to be nonconflicting with above-ground biomass (Ranjan et al. 2021) and, therefore, can be exploited as an important means to improve the soil organic carbon content and aids in combating the climate change.
The changing climatic condition, more strongly indicated by rise in temperature or pattern of temperature during various cropping season and total precipitation or pattern of precipitation, not only alters the supply of carbon resource (Kim and Kug 2022) but also affects its allocation of biomass to above- and below-ground plant parts as indicated by enhanced allocation of biomass towards roots during drought and elevated CO2 (Zhou et al. 2022) in contrast to allocation to shoots under the condition of increased supply of nitrogen and water. Zhou et al. (2022) have predicted that climate warming by a mean temperature of 2.5°C will enhance the biomass allocation to roots; however, it will be strongly influenced by mycorrhizal association. To develop a long-term solution to mitigate climate change, a carbon-negative food chain supply system must be developed, which requires modification both in crop management and plant genetics. The effect of most of the management techniques like conservation agriculture (Yadav, Kumar, et al. 2021) for improving the soil health through carbon sequestration is limited to only top profile of soils, which can very easily respire back into the environment. Genetic adaptation to lower N requirements will create deeper, more massive root systems with heavy tillering (Paustian et al. 2016) and thus greater carbon input. Beside genetic change, genotype × management interaction can be effectively adapted in wheat to stabilize wheat yield at higher yield (Yadav, Gaikwad, and Bhattacharyya 2017) by seeding wheat early (October in India) with mild vernalization requiring genotypes, restricting the first irrigation for a longer duration to create strong and deeper root system with higher biomass. This might be very relevant under the condition of central India like situations where the water is limited, temperature is comparatively elevated throughout the cropping season and total duration available under timely seeding crops is around 120–130 days. Many researchers have assumed that increased allocation of root biomass would come at the cost of above-ground allocation. However, this assumption has been challenged by findings that show a positive correlation between root biomass and yield (Ranjan et al. 2023) and no trade-off between root biomass and yield in both maize and soybean (Ordóñez et al. 2020). Root anatomical traits, such as cell wall thickness, play a role in improving the efficiency of carbon sequestration and can be used as selection criteria. Cell wall components such as lignin degrades much slower than cellulose and hemicellulose, which retains the carbon in the soil for a longer time (Berg and McClaugherty 2008; Kögel-Knabner 2002; Yue et al. 2016).
8 Root as Sensor
Root acts as the primary sensory organs that perceives signals about biotic and abiotic stresses from the edaphic environment. It serves as a primary receiver and further initiates the cascade of process and makes the plant ready to cope up with the adverse conditions. Vascular system aids in long-distance transport of signals from root to shoot for optimum performance of crop in accordance with the prevailing environment. Cytokinin synthesized in root are mobilized to shoots for signalling shoot elongation (Ko et al. 2014). Y. Zhang et al. (2021) found that root-synthesized abscisic acids play an important role in preventing water loss by altering guard cell performance. In addition to hormones, root derived metabolites also play a role in long-distance signalling through xylem from root to shoot. Nicotine, an alkaloid biosynthesized in roots of tobacco, is translocated to aerial parts of plants, which plays a key role in defence reaction against herbivory attack (Dawson 1941; Steppuhn et al. 2004). Tropane alkaloids synthesized in roots are translocated to shoots via xylem (Kohnen-Johannsen and Kayser 2019).
9 Phenotyping
Breeding of crops starts a way back in the history of mankind and crossed long roads of improvement in yield and other above-ground traits. Existing climate change conditions imposed a great barrier in further improvement of yield under adverse conditions. This brings out the importance of improving the root traits for the sustainable crop production and food security in future. Evaluating plant root systems presents significant challenges. Firstly, assessing the root system often necessitates destructive methods, leading to partial or complete loss of the roots. Secondly, the characteristics of an ideal root system for optimal crop growth remain elusive, as they vary widely depending on environmental conditions. This variability complicates the identification of universally optimal root traits. There is a popular notion that ‘breeding for optimizing root systems of crop species is too difficult and/or expensive’. These notations arise as there was lacuna in high-throughput phenotyping for root traits in the past decades. Recent research evidenced that few high-throughput phenotyping facilities available nowadays make it feasible for exploring the root traits in detail and can be subjected for crop improvement. Generally, phenotyping of root falls into two categories: (1) direct/destructive methods and (2) indirect/nondestructive methods (Figure 3).
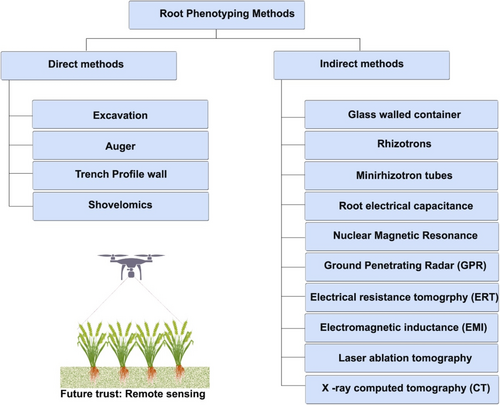
Direct methods involve destructive sampling; therefore, the same plant cannot be observed in different growth stages. It includes excavation, auger, trench profile wall and shovel-omics approaches. Samples obtained through direct method can be used for studying morphological and anatomical features of the roots. Indirect methods involve nondestructive sampling, and the plant can be observed at different growth stages over the entire life span in order to study the root growth dynamics (Rambla et al. 2023).
Rhizotrons and minirhizotrons are structures used for studying the RSA of the crops. Evaluation of large number of genotypes under rhizotrons is difficult and limited by infrastructure. Ground-penetrating radar is a geophysical technique that makes use of difference in electromagnetic properties among soil and roots by employing high-frequency radio waves. This technique has been successfully employed in estimating the growth of storage roots in cassava (Delgado et al. 2017) and bulk root biomass and diameter in winter wheat (Liu et al. 2018). This technique holds good for roots with wider diameters.
Electrical resistance tomography and electromagnetic inductance are the techniques employed for exploring the soils at different depths. These techniques are used for discriminating soil drying profile of different genotypes in field condition based on the apparent conductivity in relation to soil moisture content (Whalley et al. 2017). Magnetic resonance imaging and X-ray computed tomography are techniques used for observing root system in 4D, which includes time series observation as fourth dimension. It also has disadvantages like high cost, radiation damage to plants and limiting availability.
Laser ablation tomography is a novel imaging technique that utilizes pulsed UV laser for high-throughput 3D quantitative and qualitative observation of root anatomical traits (Chimungu, Loades, and Lynch 2015; Galindo-Castañeda, Brown, and Lynch 2018; Hall and Lanba 2019; Saengwilai et al. 2014; Strock et al. 2019). Laser ablation tomography have several variants that use multispectral and hyperspectral imaging for obtaining more information about the root anatomy. Different compounds in roots such as lignin, suberin and xylan can be differentiated by hyperspectral imaging on laser ablation tomography, which employs the autofluorescence of these compounds after UV excitation (Strock et al. 2019). In addition to morphological and anatomical observations, this technique aids in studying the interaction of soil biota with the root system of the crop in field condition, which provides plethora of information about the soil ecosystem. Levin et al. (2021) studied the interaction among cyst nematode and wheat root system using laser ablation tomography technique. The different methods followed for wheat root phenotyping is listed in Table 4.
| Methods | Traits | References |
|---|---|---|
| Germination paper | Seminal length, seminal count, average seminal emergence angle, average seminal tip angle, root tip angle, maximum width, maximum depth and width–depth ratio | Adeleke et al. (2020); Atkinson et al. (2015); Shorinola et al. (2019) |
| Clear pot method | Seminal root number and angle | El Hassouni et al. (2018); Richard et al. (2018) |
| Rhizotrons | Total root length, root length density (root length per surface area of rhizotrons), rooting depth, root system width, root orders and branching angle | Nagel et al. (2012) |
| Tube rhizotrons | Root depth, deep root intensity and deep root appearance | S. Chen et al. (2019) |
| Ground-penetrating radar | Bulk root biomass and diameter | Liu et al. (2018) |
| Laser ablation tomography | Morphological traits (root length, number, diameter and angle), anatomical traits and root interaction with soil biota | Levin et al. (2021) |
In addition to the above methods, machine learning models such as random forest, support vector machine and convolutional neural networks can be effectively employed in high-throughput root phenotyping and handling of huge data generated through image-based phenotyping in crops that showed in Table 5.
| Model | Description | Crops | Citation |
|---|---|---|---|
| RootNet | A deep CNN model for detecting and segmenting plant roots at the pixel level. | Arabidopsis, maize | Yasrab et al. (2020) |
| ChronoRoot | Uses deep segmentation networks for high temporal resolution phenotyping. | Arabidopsis | Gaggion et al. (2021) |
| Random forest | Used for unbiased identification of distinguishing root traits. | Wheat, barley | Gill et al. (2022) |
| SVM | Employed for pairwise genotype classification based on root traits. | Wheat, barley | Gill et al. (2022) |
| DeepRoot | Combines deep learning with 3D imaging for root trait analysis. | Soybean, maize | E. Han et al. (2021) |
| RootPainter | Utilizes machine learning for semi-automated root image analysis. | Arabidopsis, wheat | Smith et al. (2022) |
Remote sensing is the future thrust area for phenotyping roots in high throughput. This can be achieved by finding the above-ground proxy traits that exactly reflect the below-ground root system. Research has shown a direct positive correlation between root and shoot traits in barley (Arifuzzaman et al. 2014). However, it remains unclear whether the shoots promote the production of more nodal roots or if increased rooting positively influences tillering and other shoot attributes. Understanding this relationship is crucial for developing optimal above-ground proxy traits for easy root phenotyping. Attempts were made, and some significant correlation between cooler canopy and efficient root system in wheat under drought (X. Li, Ingvordsen, et al. 2019) and irrigated conditions was found (Lopes and Reynolds 2010; Pinto et al. 2010). Generation of such index by modelling of remote sensing data will aid breeders in screening and handling larger number of breeding population for root trait improvement. Remote sensing has been used to estimate soil water availability, relative root depth and root/shoot ratio at the genotype level. This technique is in infant stage and needs further studies for using these techniques in field level for usual practice.
10 International Groups in Unearthing the Hidden Half
The International Society of Root Research (ISRR) aims to promote cooperation and communication between root researchers around the world. They bring out the knowledge about root system and their contribution towards the better crop. The International Plant Phenotyping Network (IPPN) is a network of plant phenotyping facilities and experts that provides access to phenotyping platforms, data standards and analysis tools, training and education and outreach activities. The Root Phenomics Research Consortium (RPRC) is a collaborative initiative of researchers from different countries and disciplines who share a common interest in advancing root phenomics research. These international groups bring collaboration among the scientist all over the world who are interested in root research and pave a way for deep knowledge and understanding about the root system.
11 Prospects
Roots are hidden half of the crop which integrate growth, water transport, sensing and signalling. Root hydraulic architecture, a trait that has not been well explored but is very important for optimize functioning of plant under variable environment. Bottlenecks in conventional phenotyping restrict the improvement of root anatomical traits. Development of high-throughput phenotyping techniques such as laser ablation tomography, remote sensing–based phenotyping techniques and machine learning–based data analysis gives trust for remarkable utilization of root traits for crop improvement. On the other hand, with the advances in plant molecular genetics, we can now directly study the genes or genomic regions related to the root traits. GWAS is a powerful approach to identify genes that control a trait of interest. Genomic regions associated with low axial hydraulic conductance in durum wheat, which provide a yield advantage under drought conditions, have been identified and can now be applied to breeding programs (Hendel et al. 2021). The marker trait associations related to root traits reported so far can be employed in marker-assisted crop improvement after due functional validation of the markers. In addition, by using stochastic models and AI methods that are more accurate at the field and farm system level, breeders can test thousands of possible gene combinations in simulations before making the actual crosses and evaluating them in the field. Wheat is a global crop that supports food security worldwide, so we recommend using these methods to improve our knowledge on above- and below-ground trait combinations and to use them efficiently in crop breeding programmes.
12 Conclusion
Uncertain climatic condition and dwindling natural resources create the necessity for climate-smart crop. Beside weather variables, soil environment is point of interaction of plant through root. Soil environment is decided by not only its physical characteristics and also by continuous interaction with soil biota and crop plants and is highly dynamic. Roots are the primary organs that sense the soil environmental changes and adapt to the changing conditions. As the wheat production environment and the impact of climate change are not going to be uniform throughout the world, no single ideotype proposed is assured to perform well under all the environments. However, certain root morphological and anatomical traits like thicker and deeper roots along with proper hydraulic safety have the potential to add value in plant functioning both under water-deficit and optimum conditions. Ideotypes designed for different environments will add to root system diversity and thus can be helpful in coping up with uncertain environment condition. In hands with RSA, root hydraulics plays a role in resource uptake capability. Optimization of hydraulic efficiency of vascular system is the key step in guaranteeing that plants may be climate smart. Recent advances in high-throughput phenotyping and genotyping techniques can help in identifying the genomic regions associated with some key root traits. Integration of these knowledge in development of user-friendly molecular markers can speed up marker-assisted breeding programmes for the improvement of root traits, which will reduce the difficulties in phenotyping of larger number of lines. Filling the gaps of plant–microbe interaction through better understanding of transcriptional regulation shaping root cell type can also help us in combating root-borne diseases and exploiting symbiotic relationship with desirable microbes for better plant functioning. Because improving root traits through breeding requires transdisciplinary approach, we need to collaborate across different disciplines, avoid dogma and tribalism and therefore encourage research that integrates the complexity of real-world systems to address the challenges of the feeding increased population in sustainable way.
Author Contributions
Rajamani Nirmalaruban: conceptualization, writing – original draft. Rajbir Yadav: writing – review and editing. Sugumar S.: writing – review and editing. Alekya Meda: writing – review and editing. Prashanth Babu: writing – review and editing. Manjeet Kumar: writing – review and editing. Kiran B. Gaikwad: writing – review and editing. Naresh Kumar Bainsla: writing – review and editing. Shiv Kumar Singh: writing – review and editing. Suvitha R.: writing – review and editing. Mehdi Rahimi: writing – review and editing.
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data of this study are within the paper.




