Improving hybrid rice parental lines for blast resistance by introgression of broad-spectrum resistance genes Pi54 and Pi9 by marker-assisted selection
Abstract
Hybrid rice technology offers a great promise to produce 15% to 20% more yield than pure line varieties. The success of hybrid rice hinges on developing superior parental lines. To improve the blast resistance of hybrid rice parental line RP5933-1-19-2R, crosses were made with donors of two major blast resistance genes namely, Pi54 (Tetep) and Pi9 (IR71033–121-15) and the resulting F1s were confirmed for their hybridity by using Pi54MAS and NMSMPi9-1 genic markers. The confirmed F1s were intercrossed to obtain ICF1s and selected positive plants by markers were backcrossed to the recurrent parent, as well as selfed for advancing further to BC1F3 and ICF4 generations. The segregating plants were phenotyped for blast resistance at Uniform Blast Nursery. The identified complete restorers namely, RP 6619-1, RP 6616-26, RP 6619-3 and RP 6619-11 with Pi9 and Pi54 genes would serve as donors for broad spectrum blast resistance. This could ultimately lead to the development of new rice hybrids with improved resistance to blast disease, which is crucial for sustainable rice production and food security.
1 INTRODUCTION
Rice is major staple food crop for more than half of the world's population. India leads the world in rice cultivation with an area of 45.7 million hectares and is second in production with 124.36 million tonnes during the year 2020–2021 (Indiastat, 2020–2021). It is estimated that 70% to 100% increase in the cereal food supply is required by 2050 to feed the predicted world population of 9.8 billion people (Godfray et al., 2010). Hybrid rice technology has great potential to enhance rice productivity further by 15% to 20%. However, multiple biotic and abiotic factors impact hybrid rice production. Among them, rice blast disease caused by Magnaporthe oryzae is a serious limiting factor and also most devastating rice disease in the world (Prasad et al., 2009; Zeigler et al., 1994) with a potential threat to global rice production and food security (Ashkani et al., 2015; Wang et al., 2015).
The exploitation of host plant resistance is well demonstrated approach for combating rice blast disease. Around 130 major genes for blast resistance have been identified so far, and 30 of them have been molecularly cloned (Yin et al., 2021). The major dominant blast-resistant gene Pi54 was mapped on the long arm of chromosome 11 in the Vietnamese genotype, Tetep, which exhibits broad-spectrum resistance against geographically diverse isolates of M. oryzae (Rai et al., 2011). The closely linked markers to Pi54 gene (Sharma et al., 2005) as well as functional markers (Ramkumar et al., 2011) are available for marker-assisted breeding. Another major blast-resistant gene Pi9 confers broad-spectrum resistance to diverse M. oryzae isolates (Khanna et al., 2015). It was originally derived from the wild rice, Oryza minuta, a tetraploid species with BBCC genomes and it was mapped on chromosome 6 and has proven to be efficient in Indian conditions as well (Qu et al., 2006). Several rice breeders have utilized the Pi54 gene and successfully introduced into rice varieties and hybrid rice parental lines with the help of markers (Ellur et al., 2016; Hari et al., 2013). The Pi9 gene is very less exploited in developing blast-resistant cultivars (Tian et al., 2016). Breeding for blast-resistant rice hybrids requires at least one parental line to be blast resistant. The present study aimed to improve the blast resistance of a high-yielding restorer line, RP5933-1-19-2R by deploying marker-assisted selection combined with stringent phenotypic selection to integrate two broad-spectrum blast resistance genes Pi54 and Pi9. The improved lines were utilized in developing three-line experimental rice hybrids by crossing with WA-CMS lines, to identify potential blast-resistant restorers for three-line hybrid rice breeding.
2 MATERIALS AND METHODS
The high yielding rice (Oryza sativa L.) restorer line RP5933-1-19-2R (IET 25352) possessing major fertility restorer gene Rf4, was used as a recurrent parent. It is a medium duration, high yielding restorer with short bold grains. The Vietnamese rice variety Tetep, highly resistant to blast was used as a donor parent for Pi54. The introgression line IR71033-121-15 derived from Oryza minuta through embryo rescue technique developed by IRRI, Philippines (Rahman et al., 2011) has been received and maintained at ICAR-IIRR, Hyderabad as a differential source for Pi9 was used as another donor. The HR12 genotype was utilized as a susceptible check and all seed materials were provided by ICAR-Indian Institute of Rice Research (IIRR), Hyderabad.
2.1 Marker-assisted breeding for developing blast resistant lines
The marker-assisted selection was deployed to introgress two key blast resistant genes (Pi54 and Pi9) into a restorer RP5933-1-19-2R. This restorer was crossed as a female parent with the donors namely, Tetep and IR71033–121-15 in two independent crosses i.e., RP5933-1-19-2R × Tetep and RP5933-1-19-2R × IR71033–121-15. The F1s obtained from both crosses were confirmed for their true hybridity using markers. In order to bring both the target genes into a single genotype, the confirmed positive F1s were intermated with one another. The resulting F1s positive for both the genes (Pi54 and Pi9) were backcrossed with the recurrent parent to produce BC1F1s. Concurrently, positive intercross F1s were also selfed to raise the ICF2s. In the BC1F1 generation, foreground selection with genic markers coupled with phenotypic selection followed by background selection to estimate the recurrent parent genome (RPG) recovery was carried out. The selected plants with the target genes and maximum RPG recovery were selfed to produce BC1F2 and advanced to BC1F3 generation. Similarly, the intercross F2 plants containing target genes namely, Pi54 and Pi9 in homozygous status were selfed for two generations and forwarded to ICF4 by following the marker-assisted foreground and stringent phenotypic selection. Both BC1F2 and intercross F2 populations were evaluated for resistance against blast disease in the Uniform Blast Nursery (UBN). The blast incidence was recorded as per SES (IRRI, 2013). The entire breeding scheme is represented in Figure 1.
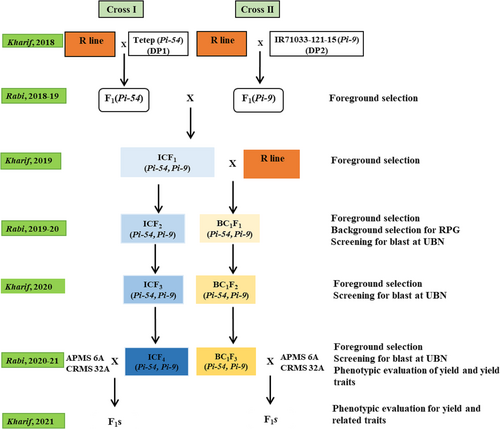
2.2 DNA extraction and PCR amplification
Leaves from 45 days old seedlings were collected and genomic DNA was isolated using the standard CTAB procedure (Zheng et al., 1995). The PCR reaction mixture contained 1 U Taq DNA polymerase, 5 pmol of each forward and reverse primers, 10X PCR buffer (10 mM Tris–HCl pH 8.3, 50 mM KCl), 1.5 mM MgCl2, .2 mM each dNTPs, and 40 ng template DNA in a final volume of 10 μl. The PCR amplification was carried out in a thermal cycler with standard thermal regimes specific to each SSR marker. The amplicons were run on 3% agarose gel and the DNA profile was documented using Alpha Imager 1200.
2.3 Molecular screening for blast resistance and fertility restoration
A codominant marker Pi54-MAS, specific to the Pi54 gene (Ramkumar et al., 2011), and Pi9 gene-specific SSR marker, NMSMPi9–1 (Kumar et al., 2017), were deployed for foreground selection to identify the positive plants with Pi54 and Pi9 in all generations. The SSR marker RM 6100 linked to the major fertility restorer gene Rf4 located on chromosome 10 was utilized in molecular screening to identify restorers (Table 1).
2.4 Marker assisted background selection
2.5 Evaluation for blast resistance in uniform blast nursery
All the gene introgressed lines, as well as the susceptible parent, RP5933-1-19-2R and donors were tested for blast resistance at uniform blast nursery (UBN), at ICAR-IIRR, Hyderabad. Each test entry was sown in a 50 cm long, 10 cm apart row with susceptible HR 12 entry after every five test entries. To guarantee homogeneous disease transmission, the entire nursery was planted with susceptible variety, HR 12 on all sides. At the fourth-leaf stage, a hand-operated atomizer was used to spray a spore suspension of Pyricularia oryzae isolate PO IIRR-31 at a concentration of 1 × 10−5 conidia/ml. The PO IIRR-31 is a highly virulent culture isolated from rice blast samples collected from farmer's field by following standard tissue isolation procedure (Tuite, 1969) and maintained by Plant Pathology section, ICAR-IIRR, Hyderabad. The pathogen infection and disease dissemination were increased by water fogging by sprinkler irrigation and also by covering nursery beds with polythene sheets during the night. After 15 days of inoculation, disease reaction was scored on a 0–9 scale as per SES, IRRI, 2013.
2.6 Assessment of agronomic performance of improved lines for blast resistance
The selected ICF4 and BC1F3 lines carrying both target genes, together with their recipient and donor parents, were planted in a randomized block design (RBD) with three replications in order to evaluate various agro-morphological parameters at ICAR-IIRR, Hyderabad during Kharif 2021. The phenotypic observations like days to 50% flowering, plant height (cm), number of productive tillers per plant, panicle length (cm), grain yield per plant (g), and 1000-grain weight (g) were recorded on five randomly chosen plants from each entry in three replications. By using Microsoft office excel, the data was statistically analysed. The selected superior lines were advanced to the next generations by selfing and seed production was done. The best few lines were nominated to AICRIP disease screening nursery (DSN) for multi-location testing during Kharif 2022.
2.7 Development of blast resistance three-line experimental rice hybrids
The selected desirable phenotypic blast resistance lines were crossed with WA-CMS lines namely, APMS6A and CRMS32A for developing experimental rice hybrids during Kharif 2021. The F1 hybrids were evaluated during Rabi 2021–2022 along with commercial hybrid checks for fertility restoration, spikelet fertility (%) and grain yield heterosis to identify potential blast resistance restorers for three-line hybrid rice breeding.
3 RESULTS
3.1 Marker-assisted introgression of Pi54 and Pi9 into the restorer line, RP5933-1-19-2R
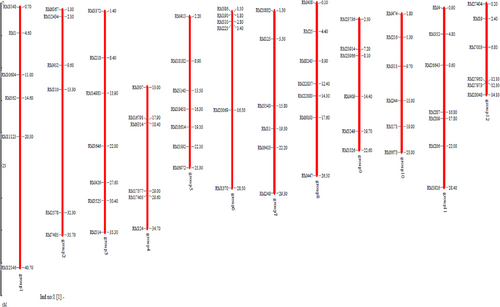
The true F1 heterozygous plants derived from crosses namely, RP5933-1-19-2R × Tetep and RP5933-1-19-2R × IR71033–121-15 were identified for the presence of Pi54 and Pi9 using Pi54-MAS and NMSMPi9–1 markers, respectively. The positive F1 plants harbouring target genes were crossed to generate intercross F1s (ICF1). A total of 16 plants out of 46 ICF1s were positive for both Pi54 and Pi9 showing heterozygosity and were backcrossed with RP parent, to obtain BC1F1 seeds and simultaneously selfed, to produce ICF2s. All the BC1F1 plants were subjected to foreground selection for blast resistance genes using gene-specific markers. Out of 114 plants screened, 22 BC1F1 plants were heterozygous for both Pi54 and Pi9 genes (Figure 2). Based on phenotypic similarities with RP5933-1-19-2R and other relevant agro-morphological characters, 10 best BC1F1 plants were selected for background selection (Table 2). To determine the recurrent parent genome composition in selected BC1F1 plants, the identified 76 polymorphic SSR markers between parents were utilized in background analysis (Figure 3). The RPG recovered in the BC1F1 ranged from 59.2% to 85.9%. Three plants with the highest recurrent parent genome recovery (RPG) namely, BC1F1-67 (85.9%), BC1F1-113 (82.7%) and BC1F1–50 (82.3%) were selfed to produce BC1F2 seeds (Table 2). Around 150 BC1F2 plants were raised, by foreground selection, only 23 plants harbouring the two target genes (Pi54 and Pi9) in the homozygous state were identified and advanced to BC1F3 generation.
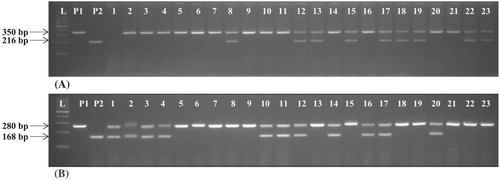
| Plant identity | Gene status | RPG (%) | Grain yield (g) |
|---|---|---|---|
| BC1F1-3 | Pi54 + Pi9 | 59.2 | 30.85 |
| BC1F1-10 | Pi54 + Pi9 | 73.3 | 34.52 |
| BC1F1-12 | Pi54 + Pi9 | 68 | 33.85 |
| BC1F1-17 | Pi54 + Pi9 | 66.6 | 32.3 |
| BC1F1-39 | Pi54 + Pi9 | 65.1 | 32.5 |
| BC1F1-50 | Pi54 + Pi9 | 82.3 | 38.74 |
| BC1F1-67 | Pi54 + Pi9 | 85.9 | 30.15 |
| BC1F1-71 | Pi54 + Pi9 | 73.6 | 42.26 |
| BC1F1-100 | Pi54 + Pi9 | 71.6 | 45.65 |
| BC1F1-113 | Pi54 + Pi9 | 82.7 | 33.43 |
- Abbreviation: RPG, recurrent parent genome.
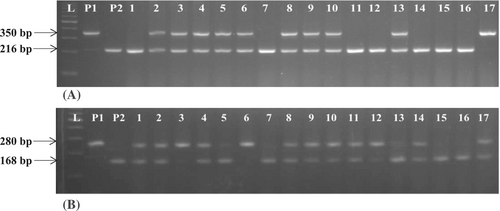
Simultaneously 138 ICF2 were raised and subjected to molecular analysis for the presence of Pi54 and Pi9 genes. Out of 138 ICF2 plants subjected to MAS, 34 plants were identified to be homozygous for both the Pi54 and Pi9 genes (Figure 4); of these, 22 plants were observed to be homozygous for major fertility restorer gene Rf4. The selected 22 plants were selfed and advanced to ICF3 generation. Of these, 14 best lines were chosen based on desirable phenotypic characters such as, plant height, panicle length, number of productive tillers, culm strength and advanced to the next generation. Along with the backcross derived lines carrying two genes, plants with either of the genes, that is, BC1F1 with only Pi54 gene or BC1F1 with only Pi9 gene, similar to recurrent parent were also advanced up to BC1F3 generation by phenotypic selection for yield-attributing characters.
3.2 Evaluation of improved restorer lines against blast disease
The donor parents namely, Tetep and Oryza minuta derived line IR71033-121-15 showed higher level of resistance for rice blast with a score of ‘1’ while the restorer line, RP5933-1-19-2-R, which is susceptible to blast disease with the lesions score of ‘8’. With a score of ‘9’, the check HR 12 demonstrated a strong susceptible reaction. All the selected BC1F3 lines with one or two genes, ICF4 lines with two blast resistance genes displayed higher level of resistance with score ranging from 1–2 (Tables 3 and 4 and Figure 5).
| S. no | Entry | Gene status | Blast resistance score | Disease reaction |
|---|---|---|---|---|
| 1 | RP 6619-1 | Pi54Pi54, Pi9Pi9 | 1 | R |
| 2 | RP 6619-2 | Pi54Pi54, Pi9Pi9 | 2 | R |
| 3 | RP 6619-3 | Pi54Pi54, Pi9Pi9 | 2 | R |
| 4 | RP 6619-4 | Pi54Pi54, Pi9Pi9 | 1 | R |
| 5 | RP 6619-5 | Pi54Pi54, Pi9Pi9 | 2 | R |
| 6 | RP 6619-6 | Pi54Pi54, Pi9Pi9 | 3 | R |
| 7 | RP 6619-7 | Pi54Pi54, Pi9Pi9 | 2 | R |
| 8 | RP 6619-8 | Pi54Pi54, Pi9Pi9 | 1 | R |
| 9 | RP 6619-9 | Pi54Pi54, Pi9Pi9 | 2 | R |
| 10 | RP 6619-10 | Pi54Pi54, Pi9Pi9 | 2 | R |
| 11 | RP 6619-11 | Pi54Pi54, Pi9Pi9 | 2 | R |
| 12 | RP 6619-12 | Pi54Pi54, Pi9Pi9 | 2 | R |
| 13 | RP 6619-13 | Pi54Pi54, Pi9Pi9 | 1 | R |
| 14 | RP 6619-14 | Pi54Pi54, Pi9Pi9 | 1 | R |
| 15 | RP-5933-1-19-2 R | – | 6 | S |
| 16 | Tetep | Pi54 | 1 | R |
| 17 | IR71033-121-15 | Pi9 | 1 | R |
| 18 |
HR-12 (Susceptible check) |
– | 9 | HS |
- Abbreviations: HS, highly susceptible; R, resistant; S, susceptible.
| S. no | Entry | Gene status | Blast resistance score | Disease reaction |
|---|---|---|---|---|
| 1 | RP 6616-26 | Pi54 + Pi9 | 1 | R |
| 2 | RP 6616-46 | Pi54 + Pi9 | 1 | R |
| 3 | RP 6616-51 | Pi54 + Pi9 | 2 | R |
| 4 | RP 6616-67 | Pi54 + Pi9 | 1 | R |
| 5 | RP 6616-73 | Pi54 + Pi9 | 1 | R |
| 6 | RP 6616-75 | Pi54 + Pi9 | 1 | R |
| 7 | RP 6616-80 | Pi54 + Pi9 | 2 | R |
| 8 | RP 6616-83 | Pi54 + Pi9 | 1 | R |
| 9 | RP 6617-23 | Pi9 | 2 | R |
| 10 | RP 6617-29 | Pi9 | 2 | R |
| 11 | RP 6617-30 | Pi9 | 2 | R |
| 12 | RP 6617-31 | Pi9 | 2 | R |
| 13 | RP 6617-38 | Pi9 | 1 | R |
| 14 | RP 6617-75 | Pi9 | 2 | R |
| 15 | RP 6618-27 | Pi54 | 1 | R |
| 16 | RP 6618-29 | Pi54 | 2 | R |
| 17 | RP 6618-36 | Pi54 | 2 | R |
| 18 | RP 6618-44 | Pi54 | 2 | R |
| 19 | RP 6618-45 | Pi54 | 2 | R |
| 20 | RP 5933-1-19-2 R | – | 6 | S |
| 21 | Tetep | Pi54 | 1 | R |
| 22 | IR71033-121-15 | Pi9 | 1 | R |
| 23 |
HR-12 (Susceptible check) |
– | 9 | HS |
- Abbreviations: HS, highly susceptible; R, resistant; S, susceptible.

3.3 Agro-morphological performance of improved blast resistant lines
The phenotypic performance of the improved ICF4 and BC1F3 lines bearing genes for blast resistance are presented in Tables 5–8. The grain yield of improved lines ranged between 28.3–32.4 g per plant, the improved BC1F3 lines possessing both Pi54 and Pi9 genes were recorded equivalent or higher yield than the recurrent parent RP5933-1-19-2R (3.4 g per plant) (Table 5). The thousand- grain weight, ranged between 18.5 and 22.8 g indicating lines possessing desirable medium slender or fine grain type. All the selected lines showed a good number of productive tillers per plant, the mean value varied between 1.4–14.8, while panicle length ranged between 24.5 to 26.9 cm. The improved lines namely, RP6616-26 (31.3 g), RP6616-51 (3.9 g) and RP6616-80 (32.4 g) recorded higher yield than restorer line, RP5933-1-19-2R, in combination with reduced days to flowering duration (Table 5). The line RP6616–80 had a longer panicle (26.9 cm) with more filled grains than the recurrent parent (25.3 cm). The BC1F3 lines possessing either Pi54 or Pi9 gene and their phenotypic observations are presented in Tables 7 and 8. The selected ICF4 lines possessing both Pi54 and Pi9 genes exhibited comparative performance to the recurrent parent (Table 6).
| Entry | DFF (days) | PH (cm) | PT | PL (cm) | FG/P | 1000 SW (g) | GY/P (g) |
|---|---|---|---|---|---|---|---|
| RP 6616-26 | 97.7 ± 0.9 | 104.4 ± 0.4 | 12.1 ± 0.6 | 25.3 ± 0.29 | 136.4 ± 1.9 | 20.5 ± 0.3 | 31.3 ± 0.5 |
| RP 6616-46 | 100.0 ± 1.0 | 98.3 ± 1.2 | 12.2 ± 0.5 | 26.5 ± 0.33 | 139.3 ± 2.0 | 21.4 ± 0.5 | 29.4 ± 1.1 |
| RP 6616-51 | 99.3 ± 1.2 | 97.8 ± 0.4 | 14.8 ± 0.4 | 25.1 ± 0.29 | 130.1 ± 1.5 | 20.1 ± 0.6 | 30.9 ± 0.7 |
| RP 6616-67 | 100.7 ± 1.2 | 95.3 ± 1.0 | 11.1 ± 0.6 | 26.6 ± 0.38 | 123.2 ± 1.0 | 22.0 ± 0.7 | 28.3 ± 0.9 |
| RP 6616-73 | 104.1 ± 0.6 | 108.9 ± 1.0 | 10.4 ± 0.3 | 24.5 ± 0.39 | 145.0 ± 2.7 | 19.6 ± 0.5 | 29.4 ± 0.5 |
| RP 6616-75 | 103.0 ± 1.2 | 106.0 ± 0.9 | 13.1 ± 0.4 | 26.3 ± 0.33 | 157.5 ± 1.9 | 20.4 ± 0.3 | 30.3 ± 0.4 |
| RP 6616-80 | 93.7 ± 1.2 | 99.2 ± 1.2 | 12.3 ± 0.4 | 26.9 ± 0.30 | 155.4 ± 4.4 | 20.1 ± 0.4 | 32.4 ± 0.3 |
| RP 6616-83 | 101.5 ± 1.0 | 92.1 ± 0.6 | 12.0 ± 0.6 | 25.6 ± 0.22 | 145.3 ± 1.5 | 18.6 ± 0.3 | 28.9 ± 0.8 |
| RP 5933-1-19-2 R | 104.0 ± 0.6 | 93.3 ± 0.4 | 13.8 ± 0.1 | 25.3 ± 0.17 | 154.5 ± 2.5 | 19.6 ± 0.5 | 30.4 ± 0.4 |
- Abbreviations: DFF, days to 50% flowering; FG/P, no. of filled grains per panicle; GY/P, grain yield per plant; PH, plant height; PL, panicle length; PT, no. of productive tillers; 1000SW, 1000- seed weight; ±, standard error.
| Entry | DFF (days) | PH (cm) | PT | PL (cm) | FG/P | 1000 SW (g) | GY/P (g) |
|---|---|---|---|---|---|---|---|
| RP 6619-1 | 104.0 ± 0.6 | 106.7 ± 0.3 | 10.3 ± 0.51 | 24.7 ± 0.33 | 145.7 ± 2.19 | 21.8 ± 0.24 | 27.6 ± 0.59 |
| RP 6619-2 | 101.7 ± 0.3 | 109.3 ± 0.7 | 11.8 ± 0.62 | 25.4 ± 0.30 | 151.3 ± 2.03 | 18.1 ± 0.12 | 29.7 ± 0.22 |
| RP 6619-3 | 101.0 ± 1.2 | 107.7 ± 0.3 | 11.1 ± 0.46 | 25.0 ± 0.12 | 134.0 ± 1.53 | 19.9 ± 0.26 | 30.9 ± 0.18 |
| RP 6619-4 | 103.0 ± 0.6 | 104.0 ± 0.6 | 10.3 ± 0.33 | 25.4 ± 0.23 | 130.0 ± 1.53 | 18.8 ± 0.09 | 29.6 ± 0.32 |
| RP 6619-5 | 106.0 ± 0.6 | 88.0 ± 0.6 | 11.8 ± 0.62 | 23.3 ± 0.47 | 122.0 ± 2.08 | 16.8 ± 0.24 | 27.6 ± 0.59 |
| RP 6619-6 | 101.7 ± 0.3 | 84.3 ± 0.3 | 12.4 ± 0.32 | 25.1 ± 0.38 | 84.3 ± 1.86 | 21.4 ± 0.29 | 26.3 ± 0.58 |
| RP 6619-7 | 100.7 ± 0.9 | 99.7 ± 0.7 | 11.3 ± 0.51 | 25.0 ± 0.29 | 107.0 ± 2.65 | 19.9 ± 0.15 | 26.9 ± 0.33 |
| RP 6619-8 | 99.3 ± 0.9 | 100.7 ± 0.3 | 10.2 ± 0.42 | 23.6 ± 0.19 | 98.3 ± 1.05 | 21.6 ± 0.17 | 23.2 ± 0.43 |
| RP 6619-9 | 93.7 ± 0.7 | 102.7 ± 0.7 | 9.2 ± 0.49 | 24.8 ± 0.12 | 133.5 ± 2.18 | 19.8 ± 0.12 | 25.8 ± 0.39 |
| RP 6619-10 | 102.7 ± 0.3 | 110.7 ± 0.3 | 9.3 ± 0.40 | 24.3 ± 0.17 | 144.3 ± 2.23 | 21.9 ± 0.06 | 25.7 ± 0.38 |
| RP 6619-11 | 100.3 ± 0.9 | 113.3 ± 0.9 | 10.1 ± 0.49 | 25.3 ± 0.15 | 127.6 ± 2.81 | 20.3 ± 0.20 | 26.4 ± 0.29 |
| RP 6619-12 | 97.7 ± 0.7 | 97.0 ± 0.6 | 9.4 ± 0.49 | 26.3 ± 0.33 | 116.0 ± 1.86 | 18.1 ± 0.06 | 26.7 ± 0.34 |
| RP 6619-13 | 96.7 ± 0.3 | 112.0 ± 0.6 | 12.2 ± 0.42 | 25.7 ± 0.18 | 127.6 ± 1.89 | 21.3 ± 0.21 | 24.9 ± 0.58 |
| RP 6619-14 | 97.0 ± 0.6 | 92.7 ± 0.9 | 13.0 ± 0.58 | 24.2 ± 0.17 | 115.3 ± 1.45 | 20.9 ± 0.20 | 30.6 ± 0.20 |
- Abbreviations: DFF, days to 50% flowering; FG/P, no. of filled grains per panicle; GY/P, grain yield per plant; PH, plant height; PL, panicle length; PT, no. of productive tillers; 1000SW, 1000- seed weight; ±, standard error.
| Entry | DFF (days) | PH (cm) | PT | PL (cm) | FG/P | 1000 SW (g) | GY/P (g) |
|---|---|---|---|---|---|---|---|
| RP 6618-27 | 101.4 ± 1.2 | 103.0 ± 0.8 | 11.4 ± 0.5 | 26.5 ± 0.3 | 118.4 ± 2.9 | 22.2 ± 0.5 | 31.2 ± 0.8 |
| RP 6618-29 | 99.6 ± 1.0 | 102.3 ± 1.0 | 12.1 ± 0.8 | 26.8 ± 0.5 | 137.0 ± 1.8 | 20.4 ± 0.5 | 30.3 ± 0.4 |
| RP 6618-36 | 103.5 ± 0.4 | 104.9 ± 0.7 | 11.3 ± 0.5 | 24.6 ± 0.4 | 132.2 ± 1.5 | 19.6 ± 0.4 | 28.4 ± 0.9 |
| RP 6618-44 | 101.7 ± 1.1 | 105.6 ± 0.6 | 10.0 ± 0.5 | 26.3 ± 0.5 | 123.0 ± 1.1 | 20.5 ± 0.4 | 33.0 ± 0.2 |
| RP 6618-45 | 97.2 ± 0.6 | 109.4 ± 0.5 | 10.6 ± 0.7 | 27.0 ± 0.2 | 107.6 ± 2.2 | 18.7 ± 0.3 | 25.3 ± 0.4 |
- Abbreviations: DFF, days to 50% flowering; FG/P, no. of filled grains per panicle; GY/P, grain yield per plant; PH, plant height; PL, panicle length; PT, no. of productive tillers; 1000SW, 1000- seed weight; ±, standard error.
| Entry | DFF (days) | PH (cm) | PT | PL (cm) | FG/P | 1000 SW (g) | GY/P (g) |
|---|---|---|---|---|---|---|---|
| RP 6617-23 | 99.3 ± 0.7 | 101.7 ± 0.9 | 11.8 ± 0.5 | 25.9 ± 0.1 | 132.5 ± 1.2 | 21.2 ± 0.3 | 30.1 ± 0.6 |
| RP 6617-29 | 94.2 ± 0.4 | 98.4 ± 1.0 | 12.1 ± 0.6 | 25.2 ± 0.2 | 131.7 ± 1.3 | 18.8 ± 0.5 | 28.4 ± 0.9 |
| RP 6617-30 | 103.2 ± 0.6 | 97.8 ± 0.8 | 9.3 ± 0.4 | 26.2 ± 0.3 | 125.6 ± 1.3 | 16.6 ± 0.4 | 26.0 ± 0.1 |
| RP 6617-31 | 100.7 ± 1.3 | 103.3 ± 1.0 | 9.6 ± 0.4 | 25.2 ± 0.3 | 141.3 ± 1.2 | 19.5 ± 0.4 | 30.0 ± 0.4 |
| RP 6617-38 | 98.1 ± 0.8 | 107.7 ± 0.7 | 9.2 ± 0.5 | 25.5 ± 0.4 | 124.0 ± 1.0 | 17.8 ± 0.8 | 25.8 ± 0.6 |
| RP 6617-75 | 96.3 ± 0.5 | 106 ± 0.7 | 12.3 ± 0.4 | 26.1 ± 0.4 | 140.2 ± 1.8 | 20.0 ± 0.6 | 28.2 ± 1.0 |
- Abbreviations: DFF, days to 50% flowering; FG/P-No. of filled grains per panicle; GY/P, grain yield per plant; PH, plant height; PL, panicle length; PT, no. of productive tillers; 1000SW, 1000- seed weight; ±, standard error.
3.4 Agronomic performance of experimental rice hybrids
The selected best plants from BC1F3 and ICF4 families possessing major fertility restorer gene Rf4 along with the two blast resistance genes with acceptable phenotypic superiority were crossed with two CMS lines namely, APMS6A and CRMS6A to produce F1 hybrids. The hybrids obtained were evaluated for their yield, and yield component traits (Table 9). It is observed that most of the hybrids grain yield heterosis deviated from the original parent combinations. Few hybrid combinations significantly recorded higher grain yield heterosis than the parental combinations namely, APMS6A × RP5933-1-19-2R and CRMS6A × RP5933-1-19-2R and commercial check hybrids (Table 9). These experimental hybrids were produced and evaluated to assess the fertility restoration status of improved lines.
| F1/hybrids | DFF (days) | PH (cm) | PT | PL (cm) | SPF (%) | GY/P (g) |
|---|---|---|---|---|---|---|
| APMS 6AX RP 6616-26 | 103 | 106 ± 0.7 | 11 ± 0.8 | 25.3 ± 0.5 | 86 | 29.5 ± 0.6 |
| APMS X RP 6616-73 | 109 | 110 ± 0.9 | 12 ± 0.6 | 21.7 ± 0.5 | 88 | 22.0 ± 3.2 |
| APMS 6A x RP 6616-75 | 108 | 113 ± 1.0 | 10 ± 1.4 | 20.3 ± 0.3 | 76 | 18.8 ± 3.7 |
| APMS 6A x RP 6619-1 | 110 | 106 ± 0.4 | 11 ± 1.2 | 21.7 ± 0.6 | 92 | 36.0 ± 0.5 |
| APMS 6A x RP 6619-2 | 105 | 114 ± 0.6 | 11 ± 0.6 | 27.7 ± 0.3 | 89 | 24.1 ± 2.5 |
| APMS 6A x RP 6619-3 | 104 | 112 ± 0.3 | 10 ± 0.4 | 20.3 ± 0.8 | 88 | 23.0 ± 5.2 |
| APMS 6A x RP 6619-4 | 107 | 110 ± 0.4 | 14 ± 1.7 | 21.0 ± 0.5 | 94 | 24.1 ± 0.7 |
| APMS 6A x RP 6619-10 | 110 | 112 ± 0.6 | 11 ± 0.8 | 22.3 ± 0.3 | 87 | 23.5 ± 0.7 |
| APMS 6A x RP 6619-11 | 100 | 112 ± 1.0 | 18 ± 0.6 | 21.0 ± 0.5 | 88 | 21.9 ± 1.5 |
| APMS 6A x RP 6619-13 | 98 | 114 ± 1.2 | 10 ± 1.6 | 25.0 ± 0.5 | 75 | 21.3 ± 0.8 |
| CRMS 32A x RP 6616-26 | 110 | 108 ± 0.3 | 10 ± 2.0 | 22.3 ± 1.1 | 82 | 27.3 ± 1.2 |
| CRMS 32A x RP 6616-75 | 112 | 109 ± 0.4 | 12 ± 0.8 | 22.7 ± 0.8 | 84 | 18.3 ± 0.8 |
| CRMS 6A x RP 6619-1 | 113 | 108 ± 0.7 | 9 ± 0.6 | 21.0 ± 0.5 | 78 | 19.5 ± 3.5 |
| CRMS 6A x RP 6619-2 | 110 | 101 ± 0.5 | 11 ± 0.7 | 22.0 ± 0.5 | 79 | 24.9 ± 2.0 |
| CRMS 6A x RP 6619-3 | 114 | 106 ± 0.3 | 14 ± 0.8 | 23.0 ± 0.6 | 91 | 28.1 ± 1.5 |
| CRMS 6A x RP 6619-4 | 116 | 100 ± 0.3 | 12 ± 0.4 | 22.7 ± 0.8 | 86 | 26.0 ± 2.5 |
| CRMS 6A x RP 6619-10 | 108 | 104 ± 1.0 | 10 ± 1.4 | 20.7 ± 0.3 | 88 | 21.0 ± 2.0 |
| CRMS 6A x RP 6619-11 | 102 | 108 ± 0.6 | 10 ± 0.8 | 25.0 ± 1.5 | 84 | 29.4 ± 1.5 |
| CRMS 6A x RP 6619-13 | 102 | 105 ± 0.6 | 12 ± 1.0 | 22.7 ± 0.8 | 75 | 22.3 ± 1.5 |
| APMS 6A x RP 5933-1-19-2 R | 104 | 107 ± 0.3 | 13 ± 0.6 | 23.7 ± 0.6 | 90 | 27.8 ± 2.5 |
| CRMS 32 A x RP 5933-1-19-2 R | 110 | 105 ± 0.4 | 11 ± 0.8 | 20.3 ± 0.3 | 91 | 30.3 ± 2.5 |
| HRI 174 (Medium duration) | 101 | 103 ± 2.0 | 14 ± 1.2 | 22.3 ± 0.5 | 96 | 30.7 ± 0.5 |
| 27P63 (Medium slender grain type) | 99 | 105 ± 2.0 | 16 ± 1.7 | 20 ± 0.7 | 96 | 19.5 ± 0.8 |
| DRRH-3 (Medium slender grain type) | 108 | 104 ± 1.7 | 10 ± 1.5 | 23 ± 0.8 | 94 | 22.0 ± 0.5 |
- Abbreviations: DFF, days to 50% flowering; GY/P, grain yield per plant; PH, plant height; PL, panicle length; PT, no. of productive tillers; SPF, spikelet fertility percentage; ±, standard error.
4 DISCUSSION
Rice crop plays an important role in global food security and no other crop can substitute rice, because it is a staple food for more than half of the world's population. Hybrid rice technology offers a great promise to produce 15 to 20% more yield in comparison to pure line varieties. The success of hybrid rice technology has been very well demonstrated by China. In India, a total 133 rice hybrids have been released for commercial cultivation. Among these, 40 hybrids have been released by the public sector, while the remaining 93 were developed by the private sector. During 2021, hybrid rice was cultivated in an area of 3.5 m ha and 80% of the total hybrid rice area is in the states of Uttar Pradesh, Jharkhand, Chhattisgarh, Madhya Pradesh, Odisha and Haryana (ICAR-IIRR, 2022). The adoption of hybrid rice technology has been slower than expected in the country due to certain challenges like moderate levels of heterosis, susceptibility of the hybrids to biotic and abiotic stresses, grain quality issues and higher seed cost of rice hybrids. Another concern is the lack of parental lines with specific desirable traits for developing heterotic rice hybrids (Ponnuswamy et al., 2020). Hence, parental line improvement must be strengthened to develop highly heterotic rice hybrids.
Among various biotic stresses, rice blast is the most devastating disease and can cause yield loss up to 100% (Khush, 2005). The deployment of host plant resistance is an effective strategy for combating rice blast disease. Most of the time resistance offered by a single R gene is not durable and often failed due to genetic diversity and pathogenic variability of M. oryzae (Li et al., 2017, 2019). The presence of minimum two R genes is necessary to obtain durable and broad-spectrum resistance, as it is extremely unlikely for a given race to overcome the combination of genes at a time (Ying et al., 2022). The dominant, broad-spectrum blast resistance gene Pi54 is well known to be very effective against the majority of isolates across India (Abhilash Kumar et al., 2016; Thakur et al., 2015). The Pi9 gene showed broad spectrum resistance against diverse Magnaporthe grisea isolates in eastern (Imam et al., 2014; Variar et al., 2009) and northern regions of India (Khanna et al., 2015). Zhou et al. (2020) reported that Pi9 gene confers enhanced resistance to leaf or neck blast. While there have been many rice varieties fortified with blast resistance genes using MAS/MABB approach, very limited reports specifically focused on using MAS to improve blast resistance in hybrid rice parental lines. Singh et al. (2023) transferred Pi2 gene into an elite maintainer line DRR 9B, while Abhilash Kumar et al. (2016) enhanced blast resistance of restorer line RPHR-1005, whereas Singh et al. (2012) incorporated Piz-5 and Pi54 into PRR78, a restorer line of aromatic rice hybrid Pusa RH10. Our target was to introduce broad-spectrum blast resistance genes namely, Pi54 and Pi9 into the restorer to intensify the spectrum and durability of blast resistance for developing three-line heterotic rice hybrids.
Marker-assisted backcross breeding (MABB) has become a favoured approach for transferring blast resistance genes in rice breeding due to the availability of robust linked/genic markers for most of the Pi loci. Pi54MAS marker specific to Pi54 and NMSMPi9-1 marker specific to Pi9 gene (Kumar et al., 2017; Ramkumar et al., 2011) were deployed for foreground selection. Through back ground analysis utilizing 76 polymorphic markers the recurrent parent genome recovered ranged from 59.2% to 85.9% at BC1F1 generation (Table 2). Theoretically, at BC1, it is expected that the recurrent parent genome would be 75%, here the ICF1 plants were backcrossed with recurrent parent, hence there is a possibility that ICF1 plant will have 75% of recurrent parent genome and followed by one backcrossing, there is a possibility of 87.5% of recurrent parent genome at BC1F1 generation. In BC1 generation, the proportion of recurrent parent (RP) genome in the selected plants would be distributed within a mean of 75% and in the same population, individual plants may contain 85% of the RP genome (Frisch et al., 1999).
The improved lines possessing both Pi54 and Pi9 genes exhibited higher level resistance (Tables 3 and 4 and Figure 5). To test the fertility restoration status of improved lines, molecular screening for the presence of Rf4 gene as well as test crossing with CMS lines were undertaken. The results were satisfying, that improved lines with blast resistance could restore the fertility of WA-CMS lines with comparable grain yield heterosis as that of recurrent parent. The promising heterotic combinations identified in the study will be subjected to large scale seed production and evaluated against blast disease in the hot spots for both leaf and neck blast before nominating for multi- location testing in India for releasing it for commercial cultivation. The identified complete restorers namely, RP 6619-1, RP 6616-26, RP 6619-3 and RP 6619-11 possessing both Pi9 and Pi54 will be very useful for hybrid rice breeding and serve as valuable donors of broad spectrum blast resistance. Overall, the introgressed lines stand a better chance of surviving under blast disease epidemics and have the potential to be adopted by farmers in India.
5 CONCLUSION
For the first time, it has been attempted to introduce both Pi54 and Pi9 into a high-yielding hybrid rice parental line, resulting in broad spectrum blast resistance lines. These newly developed lines are expected to be extremely useful in resistance breeding, as well as in three-line hybrid breeding, which could contribute to sustainable rice production and food security.
AUTHOR CONTRIBUTIONS
PR conceptualized the project, project management, planning, execution, finalizing manuscript and submission. TS, GS and EB were involved in conducting experiments. TS recorded data, analysis and prepared a manuscript. MSP is involved in blast screening and evaluation. VH, KNY, ASHP and RMS were involved in critical inputs and preparation of the research manuscript.
ACKNOWLEDGEMENTS
The first author expresses her sincere gratitude to ICAR-Indian Institute of Rice Research, Hyderabad for providing the required facilities for conducting research and to PJTSAU, Hyderabad for her PhD fellowship. All the authors are thankful to ICAR-IIRR, Hyderabad, for the necessary support to conduct the research work successfully.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
All the data presented in the manuscript are available.




