Naturally occurring spinach defensins confer tolerance to citrus greening and potato zebra chip diseases
Citrus greening or Huanglongbing (HLB) and potato zebra chip (ZC) are devastating crop diseases worldwide (Mora et al., 2021; Stelinski et al., 2024). The diseases are associated with two related, fastidious (unculturable), phloem-limited bacteria, ‘Candidatus Liberibacter asiaticus’ (CLas) and ‘Ca. Liberibacter solanacearum’ (CLso) that occurs in the United States. They are transmitted by the insect vector Diaphorina citri Kuwayama and Bactericera cockerelli (Sulc.), respectively (Mora et al., 2021).
Defensins are short (~40 to 50 amino acids) basic, cysteine-rich peptides integral to the innate immune system in plants, animals, and insects and possess broad-spectrum inhibitory activity against bacterial and fungal pathogens (Cornet et al., 1995; Velivelli et al., 2018). Here, we evaluated whether overexpressing defensins from spinach in citrus and potato can confer tolerance to ‘Ca. Liberibacter spp.’ diseases.
First, we characterized defensin-encoding genes from spinach (Spinacia oleracea) (Mirkov and Mandadi, 2020; Segura et al., 1998). The spinach defensins (SoAMPs) are evolutionarily closer to Group II defensins of Arabidopsis, rice and Medicago (Figure S1a). They possess the conserved Gamma-thionin/knottin-fold and multiple cysteine residues in the amino acid sequence (Figure S1b), and three characteristic antiparallel β-sheets and an α-helix, stabilized by disulfide bridges in the predicted ternary structure (Figure S1c) (Cornet et al., 1995).
Next, we evaluated the efficacy of spinach defensins spp. using Rhizobium rhizogenes-mediated hairy root transformation (Irigoyen et al., 2020). Transgene expression was driven under the Cauliflower mosaic virus (CaMV) 35S promoter in the ‘Ca. Liberibacter spp.’ infected hairy roots (Figure 1a) (Irigoyen et al., 2020). Both SoAMP1 and SoAMP2 expressing hairy roots showed 71–99% reduction (P ≤ 0.05 or P ≤ 0.01) of ‘Ca. Liberibacter spp.’ compared to negative controls (empty vector) (Figure 1b) (Table S1). Next, stable potato transgenic lines expressing SoAMP1 and SoAMP2 were generated using the Agrobacterium tumefaciens-mediated plant transformation. Two independent transgenic lines and non-transformed (NT) plants (negative controls) were challenged with CLso-carrying potato psyllids in controlled no-choice assays. The non-transformed plants developed characteristic zebra chip-associated shoot chlorosis and yellowing symptoms at 28 days post-infection (Figure 1c). Strikingly, the SoAMP-expressing transgenic plants showed attenuated disease symptoms (Figure 1c), reduced CLso titre (2.1–5.2% for SoAMP1 and 10.3–37.9% for SoAMP2) (Figure 1d), 53–130% greater tuber number (Figure 1e) and lower ZC-associated fried chip discoloration (Figure 1e), when compared to the non-transformed plants.
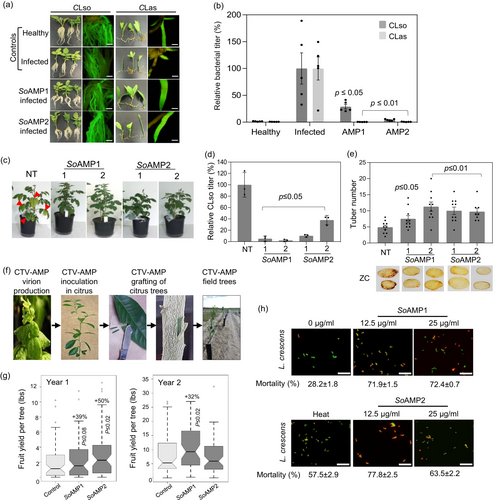
For citrus (var. Hamlin on Carrizo rootstock) evaluation, we utilized a Citrus tristeza virus (CTV) expression vector that is asymptomatic and a well-established transient citrus gene therapy system (El-Mohtar and Dawson, 2014; Folimonov et al., 2007). The SoAMP1 (183 bp) and SoAMP2 (252 bp) genes (Mirkov and Mandadi, 2020) were cloned into a CTV (T36 strain) expression vector between p23/3’ UTR and p13/p20, respectively, followed by Agro-inoculation and grafting to citrus trees as described previously (Figure 1f) (El-Mohtar and Dawson, 2014; Folimonov et al., 2007). The trials were performed in a random-block design (n = 59–60 trees) in Florida fields under a naturally high HLB disease pressure. CTV and SoAMP expression and stability were determined using ELISA and RT-PCR assays (El-Mohtar and Dawson, 2014; Folimonov et al., 2007). Approximately 22% of untreated trees (negative controls) tested positive for CTV, which was expected due to the natural exposure to endemic CTV (Table S2). However, none had any detectable SoAMP expression, indicating no unintended gene transfer between the engineered and endemic CTV strains. Among the SoAMP1 and SoAMP2-treated trees, 55% and 80% tested positive for CTV, respectively. Of them, 71% and 88% showed stable SoAMP1 and SoAMP2 expression, respectively (Table S2). The CLas incidence was 63% and 57% in the SoAMP1 and SoAMP2-treated trees compared to 73% in the untreated trees (Table S2). At harvest, the yield of SoAMP1 and SoAMP2 trees was 40% and 50% greater than that of the untreated trees, respectively (Figure 1g). SoAMP1 trees showed a 32% yield gain in the following year compared to the untreated trees, indicating a potential for multi-year benefits from a single CTV-AMP treatment (Figure 1g).
The mechanism of action of plant defensins against bacteria has not been widely investigated. Previously, a defensin from M. truncatula was shown to induce cell death of Xanthomonas campestris by permeabilization of the plasma membrane (Velivelli et al., 2018). Because ‘Ca. Liberibacter spp.’ are unculturable, a closely related culturable surrogate, Liberibacter crescens, was used to assess the effects of spinach defensins on bacterial membranes. A cytotoxicity/viability assay was used to evaluate membrane permeabilization (Supplementary Methods S1). Briefly, L. crescens cells were incubated for 3 h with different concentrations of SoAMP1 and SoAMP2 (12.5 and 25 μg/mL), followed by a two-colour fluorescent dye staining (DMAO/EthD-III Dye) and fluorescent microscopy to visualize cell permeability and mortality rate. Both SoAMP1 and SoAMP2 induced ~2.5-fold greater cell permeability and mortality compared to untreated cells (negative control) (Figure 1h). In conclusion, based on L. crescens cytotoxicity data, the naturally occurring spinach defensins can inhibit ‘Ca. Liberibacter spp,’ by inducing cell permeability and mortality.
Humans, including sub-populations of infants and children, have a long history of natural exposure to spinach defensins through diet, and there are no reported toxicity or allergenicity concerns. Notably, the US EPA recently ruled that spinach defensins are safe for human consumption when used as a plant-incorporated protectant in citrus and granted a temporary tolerance exemption (EPA, 2021), thus paving the way for their regulatory approval as sustainable products for plant disease management.
Acknowledgements
The manuscript is dedicated to the late T. Erik Mirkov (1959–2018), co-inventor of the spinach defensins. This study was supported by funds from USDA-NIFA (2021-70029-36056; 2025-70029-44033, HATCH TEX0-9621, TEX0-7790), to S.I., C.M., M.I., K.M., and the Southern Gardens Citrus, Texas A&M AgriLife Research IVD Seed Grants (124190-96210), Texas A&M AgriLife IAHA funds to K.M. We thank V. Mora, K. Laughlin, V. Garza, R. Mireles, and M.S. Rajkumar for their technical help.
Competing interests
K.M. is a co-inventor on patents related to spinach defensins [US10,640,784], and W.O.D. is a co-inventor on patents related to the Citrus tristeza virus vector [US86,293,34]. Southern Gardens Citrus, a subsidiary of US Sugar (Clewiston, FL), has exclusive licensing rights, and Silvec Biologics (Gaithersburg, MD) has sub-licensing rights in these technologies for commercialization. Co-author M.S.I. (Southern Gardens Citrus) contributed significantly to the study design and the field trials in Florida. All other authors declare no competing interests.
Author contributions
K.M., W.O.D., C.M. and M.S.I. designed and supervised the experiments. C.S.P., S.I., M.B.D., M.R., D.R., M.M.D., R.B. and C.E.M. performed the experiments. All authors contributed to the data analysis and manuscript preparation.
Open Research
Data availability statement
The data that supports the findings of this study are available in the supplementary material of this article.




