A split ribozyme system for in vivo plant RNA imaging and genetic engineering
Notice: This manuscript has been authored by UT-Battelle, LLC under Contract No. DE-AC05-00OR22725 with the U.S. Department of Energy. The United States Government retains and the publisher, by accepting the article for publication, acknowledges that the United States Government retains a non-exclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this manuscript or allow others to do so, for United States Government purposes. The Department of Energy will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan).
Summary
RNA plays a central role in plants, governing various cellular and physiological processes. Monitoring its dynamic abundance provides a discerning understanding of molecular mechanisms underlying plant responses to internal (developmental) and external (environmental) stimuli, paving the way for advances in plant biotechnology to engineer crops with improved resilience, quality and productivity. In general, traditional methods for analysis of RNA abundance in plants require destructive, labour-intensive and time-consuming assays. To overcome these limitations, we developed a transformative innovation for in vivo RNA imaging in plants. Specifically, we established a synthetic split ribozyme system that converts various RNA signals to orthogonal protein outputs, enabling in vivo visualisation of various RNA signals in plants. We demonstrated the utility of this system in transient expression experiments (i.e., leaf infiltration in Nicotiana benthamiana) to detect RNAs derived from transgenes and tobacco rattle virus, respectively. Also, we successfully engineered a split ribozyme-based biosensor in Arabidopsis thaliana for in vivo visualisation of endogenous gene expression at the cellular level, demonstrating the feasibility of multi-scale (e.g., cellular and tissue level) RNA imaging in plants. Furthermore, we developed a platform for easy incorporation of different protein outputs, allowing for flexible choice of reporters to optimise the detection of target RNAs.
Introduction
RNA plays a critical role in plant cellular activities and phenotypes by serving as messengers (i.e., mRNAs), which facilitate translation of genetic information into proteins, or as modulators (e.g., non-coding RNAs) to regulate gene expression (Großkinsky et al., 2015; Ryu et al., 2019; Yang et al., 2020). Technologies are able to capture spatial–temporal dynamics of RNA molecules could be leveraged to unravel the molecular basis of complex phenotypes in plants or facilitate early detection of transcription of plant stress, developmental changes and/or ectopic expression of transgenes. Current technologies for RNA analyses, such as quantitative reverse transcription polymerase chain reaction (qRT-PCR), in situ hybridisation and transcriptome-sequencing (Bleckmann and Dresselhaus, 2016; Islam et al., 2024; Martin et al., 2013; Zhao et al., 2021) require destructive, labour-intensive and time-consuming protocols. Recent advancements in biosensors offer an alternative, non-destructive approach for measuring cellular or molecular activities (Liu et al., 2022). For example, RNA labelling technologies using aptamers have been applied for building biosensors for detection of gene expression (Alamos et al., 2021; Bai et al., 2020). However, these technologies require modification of input RNA signals and are thus not feasible for monitoring temporal and spatial patterns of native RNA signals in plants. Therefore, there is a pressing need for innovations in biosensors to enable in vivo detection of RNA molecules within plants.
In addition, engineered plant biosystems have exciting potential in developing solutions to many global challenges, including food security, sustainability, environmental remediation, etc. (Liang et al., 2011; Yang et al., 2020). Our ability to design and build synthetic plant biosystems is a step change to meet the ever-increasing human needs (Liang et al., 2011; Yang et al., 2020). As such, tremendous efforts have been focused on the development of genome engineering tools, synthetic genetic circuits and gene delivery technologies for understanding and reprogramming plant systems (Brophy et al., 2022; Čermák et al., 2017; Liu et al., 2024; Yuan et al., 2023). In vivo RNA imaging would be very powerful technology for testing the target gene expression in engineered plants. Hence, a synthetic RNA signal transducer is in urgent need for monitoring RNA signals in genetically engineered plants.
It was recently demonstrated that transcriptional signals could be converted into orthogonal protein outputs in mammalian and microbial cells using a synthetically split ribozyme system (Gambill et al., 2023; Hasegawa et al., 2006; Selinidis et al., 2024). These split ribozyme systems use a self-splicing ribozyme derived from the Tetrahymena group I intron. To enable biosensing, DNA sequence encoding the Tetrahymena ribozyme is split into two inactive fragments, with each fused to a partial fragment of a reporter gene (e.g., a gene encoding a fluorescent protein) on one end and a guide RNA (gRNA) on the other end. The two gRNAs, which are attached to the two partial ribozyme fragments, are designed to specifically base pair with the two neighbouring regions, respectively, of an RNA target of interest. Upon DNA-to-mRNA transcription, the two partial ribozyme fragments are brought together by the binding of gRNAs to the RNA target, resulting in the self-splicing of the ribozyme fragments and consequently the reassembly of the two partial mRNA fragments of the reporter gene into a full-length transcript, which can be converted to a visible output (e.g., a fluorescent protein) via mRNA-to-protein translation. The split ribozyme reassembly and subsequent splicing occur exclusively in the presence of a target RNA, ensuring specificity in detection. Moreover, because the system is linked to an orthogonal output, a single binding event between the target RNA and the split ribozyme system can initiate the translation of various functional proteins, acting as an RNA signal transducer. However, to our knowledge, the splicing activity of the ribozyme and split ribozyme-based RNA imaging technology have only been applied in single-cell level and have not been applied in plant systems.
Here, we demonstrated the ribozyme-mediated transcript splicing functioned efficiently in Nicotiana benthamiana using Superfolder GFP (sfGFP) as a reporter. Furthermore, we successfully used split ribozyme system to detect various types of transcripts in plants using both transient expression in N. benthamiana leaves and stable transformation in Arabidopsis thaliana. To allow for facile conversion of target RNA signals to protein outputs without the need to redesign the ribozyme, we connected the current split ribozyme platform with other protein outputs via the combination of ribozyme splicing with a 2A protein cleavage mechanism. This breakthrough not only provided a biosensor enabling real-time monitoring of RNA within plant cells but also offered a platform allowing RNA controllable plant cellular programming.
Results
Evaluating ribozyme splicing activity in plants
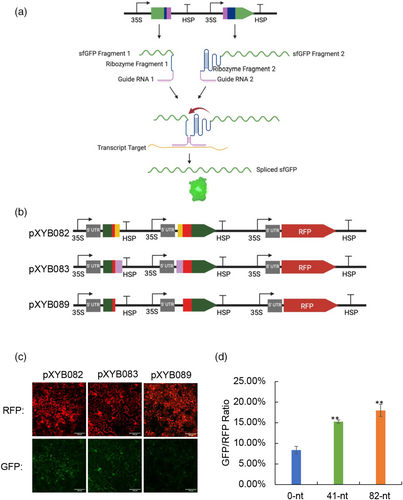
To evaluate ribozyme splicing activity in plants, we used a fluorescence-based splicing assay originally developed in Escherichia coli (Gambill et al., 2023). Specifically, the self-splicing Tetrahymena thermophila ribozyme was inserted into the coding sequence (CDS) of sfGFP, interrupting the translation of the functional protein (Gambill et al., 2023) (Figure 1a). A functional sfGFP transcript can only be translated when the ribozyme excises itself from the surrounding exons and joins the split segments together (Gambill et al., 2023) (Figure 1a). We used the 35S promoter to drive expression of sfGFP inserted with T. thermophila ribozyme with and without an RNA stable structure hairpin 14 (HP14) (Carrier and Keasling, 1999) (Figure 1b,c). Our results demonstrated that the ribozyme splicing activity functions efficiently for splicing of the sfGFP transcript in N. benthamiana, as visualised under a fluorescence microscope/stereoscope (Figure 1d,e and Figure S1). We found that removing the HP14 hairpin in the original design used in E. coli (Gambill et al., 2023) produced weaker sfGFP fluorescence output of the system in N. benthamiana compared with the design containing the HP14 (Figure 1d vs 1e), indicating that this element can enhance the ribozyme splicing activity in plants. Western blot analysis confirmed that the sfGFP transcript fragments flanking the ribozyme were able to reassemble through ribozyme-mediated splicing and be translated into full-length sfGFP (Figure 1f). The intensity of anti-GFP band from the reassembled sfGFP (35S::R_sfGFP) is weaker compared with the positive control (35S::sfGFP) (Figure 1f), indicating that the amount of reconstituted sfGFP protein from ribozyme-mediated splicing is less than that of the positive control (35S::sfGFP).

Engineering a split ribozyme system that converts RNA signals to a visible reporter
Next, we aimed to convert RNA signals into orthogonal protein outputs in plants. The core concept of the split ribozyme system is to attach gRNA sequences to each ribozyme fragment for complementation. These gRNA sequences were engineered to base pair with a transcript and thereby, in the presence of the RNA target, the ribozyme fragments are brought together, enabling splicing to occur (Figure 2a). We tested the split site at nucleotide 15 validated in E. coli (Gambill et al., 2023) in N. benthamiana using Agrobacterium-mediated leaf infiltration. Specifically, we constructed two plasmid vectors (pXYB082, pXYB083), each carrying three expression cassettes. One cassette encodes the sequence of a red fluorescence protein (RFP), while the other two cassettes contain a fragment of sfGFP fused to a fragment of the Tetrahymena ribozyme linked to a gRNA targeting the RFP CDS (Figure 2b). The two plasmid vectors are identical with the exception of gRNAs of differing lengths, 41-nt and 82-nt in pXYB082 and pXYB083, respectively. After leaf infiltration, green sfGFP fluorescence signal was detected in the N. benthamiana leaf tissue infiltrated with pXYB082 (containing a 41-nt gRNA) and pXYB083 (containing an 82-nt gRNA) (Figure 2c and Figure S2a). Because low “off” state fluorescence from the split ribozyme system was consistently detected in E. coli, we then evaluated the potential background noise of split ribozyme system in plant using the plasmid vector (pXYB089) without any gRNAs. Weak sfGFP signal was also detected in the leaf tissue infiltrated with the negative control plasmid vector (pXYB089), but the GFP signal is significantly lower compared with split-ribozyme systems with gRNAs (Figure 2c,d), indicating that the split ribozyme-based biosensor can be used to detect RNAs in plant cells.
To investigate if the increased gRNA length could enhance the florescent signal from the split-ribozyme system, we tested three more gRNAs with lengths of 123-nt (in pXYB084), 164-nt (in pXYB085) and 325-nt (in pXYB086), respectively. Surprisingly, unlike the results seen in E. coli (Gambill et al., 2023), we did not observe an increase in the sfGFP fluorescence signal produced from split ribozyme system with increased gRNA length (Figure S2b).
Using the split ribozyme-based biosensor system to detect different RNA signals in plants
We then tested the split ribozyme-based biosensor system targeting an Arabidopsis thaliana gene, PLT1 (AT3G20840), which encodes an AP2 transcription factor expressed in the root quiescent center (Nawy et al., 2005) using N. benthamiana leaf infiltration. We cloned the AT3G20840 CDS under a strong promoter 35S (pXYB151). We also created a biosensor vector with 82-nt gRNAs targeting the AT3G20840 CDS (pXYB147) (Figure 3a). Green sfGFP signal was detected in the N. benthamiana leaf tissue co-infiltrated with pXYB147 and pXYB151, whereas sfGFP signal was not detected in the leaf tissue infiltrated with pXYB147 or pXYB151 alone (Figure 3b and Figure S3a). Furthermore, we used the split ribozyme-based biosensor to detect infection by tobacco rattle virus (TRV). We engineered two biosensor vectors (pXYB155 and pXYB156) with 82-nt gRNAs targeting different regions of the TRV RNA sequence (Figure 3a). sfGFP signals were detected in the N. benthamiana leaf tissues co-infiltrated with TRV and one of the two biosensor constructs (pXYB155 and pXYB156). No sfGFP signal was detected in the leaf tissues infiltrated with TRV alone or the biosensor (pXYB155 or pXYB156) alone (Figure 3c and Figure S3b). These results indicate that the split ribozyme-based biosensor system can be used in vivo to detect RNAs derived from diverse sources using transient expression approaches.
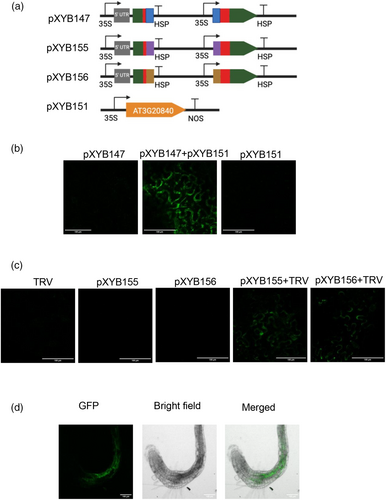
Based on the success of utilising the split ribozyme-based biosensor system to detect RNAs through transient expression in N. benthamiana leaves, we engineered the biosensor construct pXYB147 (Figure 3a) with 82-nt gRNAs targeting the AT3G20840 CDS into A. thaliana using Agrobacterium-mediated stable transformation. The sfGFP signal was detected in the roots of three T1 transgenic Arabidopsis plants (Figure 3d and Figure S4), indicating that the split ribozyme-based biosensor system can also be used to detect endogenous gene expression at the cellular level in plants with high specificity.
Engineering a split ribozyme-based platform for orthogonal protein outputs
RNA detection using the split ribozyme-based biosensor system with sfGFP as a reporter requires the use of a fluorescence microscope, which is technically demanding and time consuming and does not allow detection in live tissues. To address this limitation, we replaced sfGFP with a GFP-like protein (eYGFPuv), which was recently utilised for in vivo visualisation of transgene expression under a UV flashlight (Yuan et al., 2021). First, we tested if ribozyme-mediated splicing can reassemble two partial fragments of the eYGFPuv transcripts in plant cells using N. benthamiana leaf infiltration. Ribozyme-mediated cleavage at the 5′ splice site depends on formation of a base-paired helix P1 between the Internal Guide Sequence (IGS) and sequences adjacent the splice site, and the presence of a U·G “wobble” base-pair positioned 4–6 residues from the base of this helix defines the susceptible phosphodiester bond (Köhler et al., 1999). Therefore, the ribozyme must be inserted immediately downstream of an uracil where the IGS should start with a guanine and the remaining IGS sequence is in reverse complementation to the first 5 base pairs of the P1 helix (Köhler et al., 1999). Following these principles, we tested different insertion sites of eYGFPuv CDS and designed alternate IGS for different target sites. We discovered that the eYGFPuv with the ribozyme inserted after the last amino acid of Y72 showed a strong fluorescence signal under a UV flashlight (Figure 4a,b). However, only one of six replicates showed desirable eYGFPuv signal, indicating correct splicing efficiency was low. To enhance the ribozyme splicing efficiency, we modified the IGS of the original ribozyme design, known to pair with the P1 helix and tested different RNA stable motif in eukaryotes (Figure S5). However, none of those optimisations efficiently enhanced the ribozyme splicing activity in eYGFPuv (Figure S5). These results suggest that ribozyme splicing efficiency is different under alternate transcript contexts in plants. Variable ribozyme-mediated splicing efficiency was also observed in mammalian cells (Hasegawa et al., 2004; Rogers et al., 2002). Hence, a modular platform allowing for simple exchange of the output protein would be helpful for avoiding the inconsistent ribozyme splicing activity in different context.
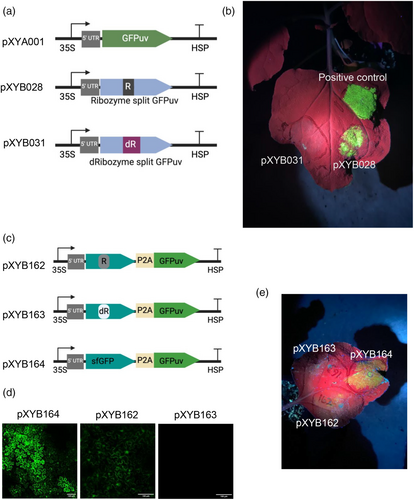
To bypass the need to redesign ribozyme insertion sites for different protein outputs (e.g., eYGFPuv), we created a construct (pXYB162) with eYGFPuv attached via the 2A self-cleavage peptide P2A to sfGFP (Figure 4c), which was optimised for ribozyme-mediated splicing. Because the ribozyme contains stop codons in-frame with sfGFP and eYGFPuv, the production of these two functional proteins requires self-splicing of the ribozyme. The sfGFP signal was detected in the N. benthamiana leaf tissue infiltrated with the construct pXYB162 (containing two sfGFP fragments flanking the ribozyme) although the signal was weaker than that in leaf tissue infiltrated with the positive control construct pXYB164 (containing intact sfGFP); no sfGFP signal was detected in the leaf tissue infiltrated with the negative control construct pXYB163 (containing two sfGFP fragments flanking a catalytically dead G264A mutant ribozyme with IGS and PI loop removed) (Figure 4d and Figure S6). This result indicates that the eYGFPuv attachment did not affect the reassembly of sfGFP fragments through ribozyme-mediated transcript splicing.
While the eYGFPuv fluorescence signal detected in N. benthamiana leaf tissue infiltrated with pXYB162 was much weaker than that in leaf tissue infiltrated with the positive control construct pXYB164, we do demonstrate that ribozyme-mediate splicing and 2A self-cleavage peptide can be combined and still produce functional proteins (Figure 4e).
Discussion
Plant genome engineering has been widely used for understanding plant biology and improving plant performance. For examples, using CRISPR-based genome engineering tools, functional genomics research has been achieved in different plant species (Liu et al., 2019, 2020) and novel synthetic genetic circuits have been developed by synthetic transcriptional regulators for predictably altering root structure (Brophy et al., 2022). Hence, there is broad interest in developing new synthetic genetic engineering tools to monitor plant biosystems. Currently, with the advance of single-cell RNA-seq (scRNA-seq) technologies, more and more RNA signals coupling with cellular dynamics have been revealed (Islam et al., 2024). Therefore, there is a strong motivation for developing an RNA transducer able to convert RNA signals into different orthogonal protein output for RNA imaging and genetic engineering at cellular level in plants.
Currently, RNA imaging technology in plants mainly relied on the aptamer RNA-labelling techniques (Alamos et al., 2021; Bai et al., 2020). Those techniques always require modification of input RNA signals and thereby limit their application for monitoring the expression patterns of endogenous genes or invasion RNA signals. In this study, we have successfully demonstrated that the split ribozyme system can convert RNA signals into visible reporter in plants through both transient expression and stable transformation. By attaching gRNA sequences targeting RNA inputs to each ribozyme fragment, this system allows detection of different RNA signals without modifying the RNA inputs in plants.
Regarding the gRNA design, unlike the results seen in E. coli (Gambill et al., 2023), there is no increase in the sfGFP fluorescence signal produced from split ribozyme system with increased gRNA length in plant system. This indicates that the endogenous RNA processing machinery in eukaryotes might interfere with ribozyme system and hereby cause the different performance from microbial system. Further experiments are needed for optimisingoptimizing this split ribozyme system by testing different gRNAs design.
Previously, only the hammerhead ribozyme has been applied for multiple gRNAs production for genome editing in plants (Čermák et al., 2017). Here, we demonstrated that the ribozyme derived from the Tetrahymena group I intron function efficiently in plants, which will expand the application of ribozyme in plant synthetic biology. Our results indicated that RNA stable motifs can enhance the ribozyme's self-splicing activity in plants. Ribozyme's self-splicing efficiency determines the sensitivity of this biosensor. With low splicing efficiency, moderately or weakly expressed genes' RNAs may not be able to be detected by the split ribozyme-based biosensor. Although our results have demonstrated that the split ribozyme-based biosensor can detect native gene expression in Arabidopsis, further experiments are needed to evaluate if this biosensor system can detect the RNAs of moderately or weakly expressed genes in plants. In addition, we also observed various splicing efficiency of ribozyme when we inserted the ribozyme into different protein CDSs, indicating that ribozyme reaction efficiency is not stable in complex intracellular environment of plant cells. Similar phenomenon has been observed in mammalian cells (Hasegawa et al., 2004). Hence, it might be necessary to identify improved trans-splicing ribozyme variants via a systematic search from a library of ribozymes in the context of living plant cells.
Furthermore, we engineered a modular platform to convert RNA signals to different protein outputs via combining the 2A peptide self-cleavage activity with the ribozyme-mediated transcript splicing activity. Because our design of “Ribozyme_sfGFP-2A-protein” can produce functional proteins, this configuration could potentially be used for designing new split ribozyme-based systems with any functional protein as an output for RNA-based genome engineering at cellular level in plants, although further work is necessary to optimise the Ribozyme_sfGFP-2A-protein configuration for optimising the expression of the second functional protein after 2A splicing.
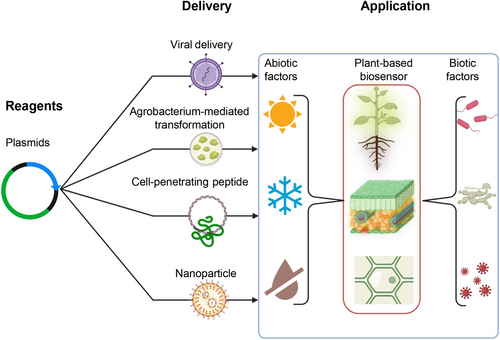
We named this biosensor system “Plant RNA Vision”, which is expected to have broad applications in plant biology and plant biotechnology, such as facilitating the experimental validation of tissue/cell-type specific gene expression predicted by single-cell transcriptome-sequencing (Islam et al., 2024). For example, in this research, we demonstrated that “Plant RNA Vision” could be used to image the expression of a tissue/cell-type specific gene in Arabidopsis using Agrobacterium-mediated transformation (Figure 3d). Incorporating with different delivery methods (e.g., DNA delivery mediated by viruses, agrobacteria, cell-penetrating peptides or nanoparticles), this system can be developed into tool kits for monitoring plant RNA signals responding to internal and external stimuli, as illustrated in Figure 5. Besides monitoring plant gene expression, this biosensor technology can be used to directly detect RNAs of non-plant origin, such as plant RNA viruses, as demonstrated in Figure 3c. Furthermore, the “Plant RNA Vision” technology can be potentially integrated into the modern imaging-based plant phenotyping system to achieve simultaneous in vivo visualisationsvisualization of molecular (e.g., RNA abundance) and physiological traits (e.g., plant growth, photosynthesis, stress responses) in the greenhouse and field, filling a technical gap between lab-based molecular biology analysis and greenhouse/field-based plant phenotyping.
In conclusion, we successfully established a synthetic RNA signals transducer in plants using split ribozyme system, which holds great potential for building RNA-mediated tunable genetic circuits to establish new capabilities for plant synthetic biology and plant phenomics research.
Plant materials and methods
Plant materials
Arabidopsis wild-type Col-0 plants and Nicotiana benthamiana (GenBank: PRJNA170566) plants were grown in the soil in the growth chamber at 21 °C under 100 μmol m−2 s−1 white light with 12-h light/12-h dark photoperiod.
Plasmids construction
All the DNA constructs used in this study are listed in Table S1, which were created via Gibson assembly using NEBuilder® HiFi DNA Assembly master mix (NEB, Cat. No. E2621) or Golden Gate assembly using the Modular Vector Library cloning Kit from Dr. Dan Voytas' lab at the University of Minnesota (Čermák et al., 2017). Sequence information for the DNA constructs was provided in Tables S2–S5. gRNA sequences design was illustrated in Figure S7. Sequences were synthesised by TWIST Bioscience (South San Francisco, United States).
Tobacco leaf infiltration
The Agrobacterium tumefaciens strain GV3101 harbouring the plasmid of interest was infiltrated into the leaves of five- to six-week-old N. benthamiana plants using a 1 mL syringe without a needle as described previously (Li, 2011; Yuan et al., 2022). Briefly, cultures containing the vector of interest were grown in lysogeny broth (LB) medium for 36–48 h and resuspended in agroinfiltration buffer (10 mM MES, pH 5.6, 10 mM MgCl2 and 200 μM acetosyringone) to an optical density of 0.5 at 600 nm (OD600). Bacterial suspensions were incubated for 2–4 h at room temperature and then mixed for co-expression experiments. Agroinfiltrations were carried out through the abaxial surface of the three youngest fully expanded leaves of each plant with a 1-mL needle-free syringe. At least two independent experiments were conducted for each treatment. Each treatment includes six replicates.
Stable transformation in Arabidopsis thaliana
The Agrobacterium strain ‘GV3101’ was used for the transformation of A. thaliana wild type ‘Col-0’ via the floral dip method with modification as described previously (Yuan et al., 2021). T1 seeds co-transformed with two constructs were selected on K1 medium (1 L; 2.154 g MS, 100 mg myo-inositol, 0.5 g MES, 10 g sucrose, 8 g agar if plates; based on the recommendation of ABRC (Arabidopsis Biological Resource Center) (https://abrc.osu.edu/seed-handling) for Arabidopsis seed germination) with 50 mg/L Kanamycin and 25 mg/L Hygromycin. Seeds were put under dark at 4 °C for 2 days and then germinated for 6–8 days under 100–150 μmol m−2 s−1 fluorescent warm white light, 12 h light/12 h dark, 20 °C and 70% humidity. Then, seedlings were collected for GFP visualisation.
Fluorescence measurements
The fluorescence of sfGFP and RFP was visualised and imaged using a Zeiss LSM 710 confocal microscope and images were analysed with the images were analysed with Fiji software (Schindelin et al., 2012). GFPuv signal was visualised using LIGHTFE UV302D (365 nm) (Yuan et al., 2021).
Statistical analysis
The sample average and standard deviation (SD) were calculated from three replicates of each treatment. Significance in all experiments was assessed using a T-Test with an alpha of 0.05. In all cases, resulting P-values of <0.05 for fluorescence intensity of experimental treatment compared to negative control were considered significantly different.
Western blot
Leaf discs from infiltrated tobacco leaves were harvested and ground into a fine powder in liquid nitrogen. Total proteins were then extracted by adding 2× Laemmli sample buffer (BioRad) and boiling for 10 min. Cell debris was removed by centrifugation at 16 000 g for 10 min at 4 °C. The supernatant was resolved on a 4%–20% SDS–PAGE gel (BioRad) and transferred to polyvinylidene fluoride membranes (Millipore). Anti-GFP polyclonal antibodies (Invitrogen) were used as primary antibodies at a 1:2000 (v/v) dilution. Immunoblots were detected using peroxidase-conjugated anti-rabbit IgG (Sigma-Aldrich) at a 1:7500 (v/v) dilution and ECL substrate (Thermo Scientific) with the BioRad ChemiDoc gel documentation system.
Acknowledgements
The writing of this manuscript was supported by the U.S. Department of Energy, as part of the Secure Ecosystem Engineering and Design (SEED) Scientific Focus Area. This material is also based upon work supported by the Center for Bioenergy Innovation (CBI), U.S. Department of Energy, Office of Science, Biological and Environmental Research Program under Award Number ERKP886. Oak Ridge National Laboratory is managed by UT-Battelle, LLC for the U.S. Department of Energy under Contract Number DE-AC05-00OR22725.
Author contributions
X.Y. conceived, designed and supervised the project and reviewed and edited the manuscript. G.A.T. supervised the project and reviewed and edited the manuscript. Y.L. conceived, designed and performed experiments, analysed the data and wrote the manuscript. R.R., M.T.I. and R.A. performed the experiments in Arabidopsis transformation. T.Y. performed the western blot experiments. B.A.B. helped with confocal data analysis. I.D.V. and C.E. provided information for initiating the project and offered counselling service for some of the experiments. P.E.A and J.C. reviewed and edited the manuscript. All authors read and contributed to the content, edited or reviewed it.
Conflicts of interest
The authors declare no competing interests.
Open Research
Data availability statement
The plasmids generated in this study will be available at Addgene (https://www.addgene.org/).




