BR signalling haplotypes contribute to indica–japonica differentiation for grain yield and quality in rice
Summary
The functional difference of natural variations in conserved BR signalling genes and the genetic basis of rice indica–japonica differentiation are important yet unknown. Here, we discovered natural variations of the four key components (OsBRI1, OsBAK1, OsGSK3 and OsBZR1) in BR signalling pathway by GWAS using an indicator of indica–japonica differentiation in rice. Two major BR signalling haplotypes (BSHs), caused by co-selected variations of the four genetically unlinked genes, were identified to be highly differentiated between rice subspecies. The genetic contributions of BSHs to grain yield and quality were much higher than that of each component. Introducing alleles of japonica into indica employing substitution lines of OsBAK1, complementation lines of OsGSK3 and genetic populations of OsBRI1/OsBAK1/OsGSK3 confirmed their functional differences between two subspecies. The BSH differentiation led to weaker interaction between OsBRI1 and OsBAK1, stronger autophosphorylation and kinase activity of OsGSK3, less RNA/proteins and stronger phosphorylation of OsBZR1, and weaker BR sensitivity in indica than japonica rice, and regular expression trends of BR-response genes between subspecies, and then synergistically enhanced yield and superior quality of indica. Our results demonstrate that BSHs contribute to rice inter-subspecies diversity, and will provide proof-of-concept breeding strategy and useful targets in crops.
Introduction
Natural variation of plants and animals is the raw material of their domestication, subsequent improvement and a vital resource underpinning the world's food supply (Stange et al., 2021; Wallace et al., 2018). In rice, grain yield is determined by grain size/shape, grain number and plant architecture (plant height, tiller or panicle number, panicle size, leaf angle and leaf morphology), while grain quality encompasses appearance quality traits (grain shape and chalkiness), cooking and eating quality traits (amylose, protein and oil content, gel consistency, gelatinization temperature by alkali spreading value), milling quality (head rice rate, appearance quality traits), nutrition quality (amylose, protein and oil content, minerals, unsaturated fatty acids, vitamins and anthocyanins) and hygiene quality (heavy metals and allergens) (Fitzgerald et al., 2009; Jakobson and Jarosz, 2020; Siebenmorgen et al., 2013; Wang et al., 2018; Wang and Li, 2008; Wei et al., 2021; Wing et al., 2018; Xing and Zhang, 2010; Zhao et al., 2022; Zhou et al., 2020), and they are agronomical and economical traits controlled by multiple quantitative trait loci (QTL). In addition, many signalling pathways are involved in regulating these yield and quality traits (Chen et al., 2022; Fan and Li, 2019; Fitzgerald et al., 2009; Li et al., 2019; Siebenmorgen et al., 2013; Wang et al., 2018; Wang and Han, 2022; Wang and Li, 2008; Wei et al., 2021; Wing et al., 2018; Xing and Zhang, 2010; Zhao et al., 2022; Zhou et al., 2020). However, most of these genes were identified by mutants in a single background, hardly applied into molecular breeding programmes (Chen et al., 2022; Wang and Han, 2022). QTL genes with different allelic effects on a single trait can be easily selected for molecular breeding (Chen et al., 2022; Costanzo et al., 2019; Liang et al., 2021; Wang and Han, 2022). Thus, the identification of the causal genes underlying QTL is central in crop molecular breeding (Costanzo et al., 2019; Liang et al., 2021).
During rice domestication, Asian-cultivated rice has two major subspecies, xian/indica and geng/japonica, which have diverged in morphological characteristics, yield traits and grain quality as described above and stress resistance (Cui et al., 2022; Huang et al., 2012; Kovach et al., 2007; Wang et al., 2018, 2022). Each of these yield and quality traits may serve as an indicator for QTL genetic linkage analyses or genome-wide association studies (GWAS) of indica/japonica differentiation (Huang and Han, 2014; Zhang et al., 2021). The genetic basis of indica–japonica differentiation is crucial for rice domestication and exploitation of inter-subspecific heterosis (Cui et al., 2022; Huang et al., 2012; Kovach et al., 2007; Wang et al., 2018, 2022). However, the molecular genetic basis of rice indica–japonica differentiation for these traits is largely unknown, though there are some reports about that.
Brassinosteroids (BRs) are steroid hormones that control various plant developmental and physiological processes, and regulate multiple yield traits in crops (Nolan et al., 2020; Tong and Chu, 2018). The BR signalling pathway has been extensively studied, and most components of this pathway are greatly conserved in Arabidopsis and rice (Divi and Krishna, 2009; Liu et al., 2021; Yang et al., 2011). In brief, in the absence of BRs, the BR receptor BR INSENSITIVE 1 (BRI1) remains in an inactive state causing by the interaction with the inhibitory protein BRI1 KINASE INHIBITOR1 (BKI1) (Wang et al., 2001; Wang and Chory, 2006). In addition, BR INSENSITIVE 2 (BIN2) phosphorylates BRASSINAZOLE RESISTANT 1 (BZR1)/BRI1-EMS SUPPRESSOR 1 (BES1), to destabilize its protein (He et al., 2002), inhibit the DNA-binding activity and induce the cytoplasmic localization of BZR1/BES1 by modulating its interaction with the 14-3-3 proteins (Gampala et al., 2007). BZR1 is a positive regulator of the BR signalling pathway, and a transcriptional repressor of BR biosynthetic genes for the feedback regulation (He et al., 2005; Wang et al., 2002). When BRs are present, they are perceived by the receptors BRI1 and BRI1-ASSOCIATED KINASE 1 (BAK1) (He et al., 2013; Li et al., 2002), and the BR binding activates the BRI1 kinase, by inducing BRI1's dissociation with BKI1 and full activation of BRI1 through trans-phosphorylation between the cytoplasmic kinase domains of BRI1 and BAK1 (He et al., 2013; Li et al., 2002; Wang and Chory, 2006). The activated receptors activate several substrates such as cytoplasmic BR-SIGNALLING KINASEs (BSKs), CONSTITUTIVE DIFFERENTIAL GROWTH 1 (CDG1) kinase and the BRI1-SUPPRESSOR 1 (BSU1) phosphatase, which repress the kinase activity of BIN2/ GLYCOGEN SYNTHASE KINASES 3 (GSK3)-LIKE KINASEs (GSKs), a central negative regulator of the BR signalling pathway, to release its repression on the master co-activator BZR1, which regulates BR-responsive genes (Gampala et al., 2007; Kim et al., 2011; Tang et al., 2008). OsBRI1, OsBAK1, OsGSK2 and OsBZR1 are orthologous to BRI1, BAK1, BIN2/GSK2 and BZR1 in Arabidopsis, respectively, and are the four major components of the BR signalling pathway in rice (Tong and Chu, 2018). Various BR signalling genes, such as OsBRI1, OsBAK1, OsGSKs, OsBZR1, DLT, OsLIC, OsWRKY53, OsGRF4, OsmiR396d, GL2, RLA1/SMOS1 and OsREM4.1, control yield traits in rice (Che et al., 2015; Gui et al., 2016; Li et al., 2019; Qiao et al., 2017; Tang et al., 2018; Tian et al., 2021; Tong et al., 2009; Tong and Chu, 2018; Yang et al., 2011; Zhang et al., 2012). BR biosynthetic genes in rice, such as BRD1 (Hong et al., 2002), BRD2 (Hong et al., 2005), DWARF2 (D2) (Hong et al., 2003), OsDWARF4 (Sakamoto et al., 2006) and D11 (Wu et al., 2016; Zhou et al., 2017), have been isolated by identifying BR-deficient mutants. Different allelic mutants of the same gene involved in BR biosynthesis exhibit various yield potentials (Wu et al., 2016; Zhou et al., 2017). Although the basic functions of the major BR signalling components have been identified by mutants, their application in molecular breeding has been insufficient due to their extreme phenotypes (Divi and Krishna, 2009; Tong and Chu, 2018) and little is known about the natural variation of these genes and the contribution of BR signalling to grain quality.
Here, by GWAS using an indicator of indica–japonica differentiation, we identify the natural variations of the four key BR signalling components in rice, propose a new concept of BSHs and discover their collaborative genetic contributions to grain yield, quality and inter-subspecies differentiation. We further reveal that the BR signalling and sensitivity are generally weaker in indica than in japonica, which are collaboratively caused by BSHs, explaining why there is a generally higher grain yield in indica than in japonica. We also confirm that BR signalling components affect many grain quality traits in rice. Our findings shed new light on the understanding of BR signalling diversity, and provide the novel proof-of-concept breeding strategy and targets for crop genetic improvement.
Results
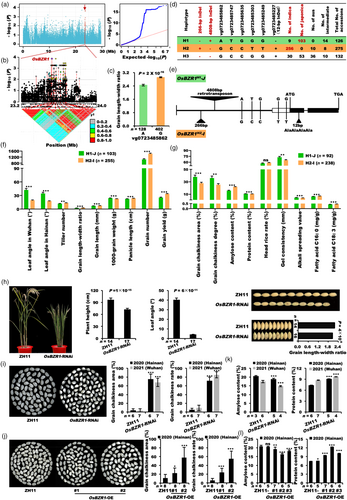
Two major haplotypes of OsBRI1 and OsBAK1 contribute to indica–japonica differentiation for grain yield and quality
To reveal the genetic basis of indica–japonica differentiation in rice, a diverse and worldwide rice mini-core collection of 533 accessions were used for GWAS analysis by a linear mixed model (LMM) (Figure S1a,b and Table S1), with grain shape (grain length–width ratio) as an indicator of rice inter-subspecies differentiation. Several GWAS loci for grain length–width ratio were identified. In the two major loci on chromosomes 1 and 8 (Figure 1a), two peak point genes, LOC_Os01g52050 encoding a BR receptor OsBRI1 and LOC_Os08g07760 encoding a BR co-receptor OsBAK1, were identified by linkage disequilibrium (LD) block and other analyses (Figure 1b–g). In 1-mm panicles of the 533 accessions and young panicles at different stages of Minghui 63 (MH63) and Zhenshan 97 (ZS97), both OsBRI1 and OsBAK1 had higher expression level than that of BR biosynthesis gene D11 (Figure S2a,b), which is particularly and highly expressed in young panicles (Wu et al., 2016), indicating that OsBRI1 and OsBAK1 are highly expressed genes in young panicles and may play a role in controlling grain size. The two major haplotypes genotyped by the leading SNPs of the two above genes had significantly differences between grain length–width ratio in the mini-core collection (Figure 1c). Next, we identified all the natural variations in the promoter and coding sequence (CDS) regions of these two genes in the 533 accessions (Zhao et al., 2015, 2021) (Table S2). In total, there were 47 and 121 natural variations for OsBRI1 and OsBAK1, respectively. Interestingly, most genotypes of these natural variations co-segregated from each other in both OsBRI1 and OsBAK1 (Table S2), although they were located on different chromosomes. Thus, we combined all the 13 representative variations of OsBRI1 with 11 representative variations of OsBAK1 together for further haplotype analysis (Figure 1d). These variations in both OsBRI1 and OsBAK1 were grouped into five haplotypes, H1–H5 (Figure 1d). More interestingly, two major haplotypes, H1 and H4 are absolutely predominant in japonica (99.1%) and indica (97.9%) rice, which were named as OsBRI1-OsBAK1H1-J and OsBRI1-OsBAK1H4-I, respectively (Figure 1d,e). In the above natural variations, two SNPs cause two nonsynonymous mutations in the LRR (leucine-rich repeat) region of OsBRI1, and two SNPs cause one nonsynonymous mutation in the signalling peptide region of OsBAK1 (Figure 1e). Compared with OsBRI1-OsBAK1H4-I, OsBRI1-OsBAK1H1-J had larger leaf angle, fewer tiller number, much smaller grain length–width ratio, shorter panicles, fewer grain number per plant and lower grain yield per plant (Figure 1f). Besides, compared with OsBRI1-OsBAK1H4-I, OsBRI1-OsBAK1H1-J had higher grain chalkiness area and degree, consistent with the finding that OsBAK1 could be identified by GWAS for grain chalkiness in 5-year field trials (unpublished data from our laboratory) (Figure S3), lower amylose content and head rice rate, higher protein content, gel consistency and alkali spreading value, and less fatty acid C16: 0 and C18: 3 (Figure 1g). These results show that the two major haplotypes caused by co-segregated natural variations of OsBRI1 and OsBAK1 contribute significantly to indica–japonica differentiation for grain yield and quality traits in rice, highly consistent with the common sense of these typical differentiation features between indica and japonica rice.
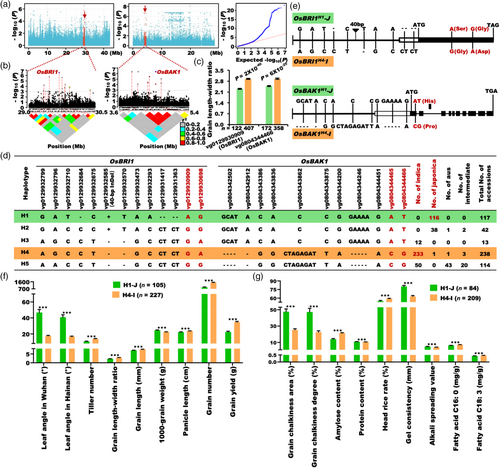
To investigate the function of OsBRI1 and OsBAK1, we generated their knockout mutants by the CRISPR-Cas9 system under the background of a japonica variety Zhonghua 11 (ZH11), which has strong function alleles of both genes (see bellows). Three independent osbri1-CR lines with different mutations were generated (Figure S4a). However, the homozygous mutant seedlings of OsBRI1 could only be isolated from heterozygous lines and had extremely severe phenotypes, such as severe dwarfism, abnormal leaf blade morphology and dark-green leaves (Figure S4b), resulting in lethality at the early seedling stage. We did not obtain any seed with the homozygous frameshift genotype of OsBRI1. Therefore, the OsBRI1-RNAi line in ZH11 was used to characterize its function. Compared with the wild type, the OsBRI1-RNAi transgenic line showed lower plant height, erect leaves, shorter grain length and length–width ratio (Figure 2a), higher grain chalkiness area and rate (Figure 2b), lower amylose content and higher protein content (Figure 2c).
To confirm whether there are functional differences between OsBAK1H1-J and OsBAK1H4-I, we screened the interconnected Chromosome Segment Substitution Lines (CSSLs) with indica variety 9311 as recurrent parent and japonica variety Nipponbare (NIP) as donor parent (Zhang et al., 2022), and found that 9311 introduced by the endogenous OsBAK1 from NIP, which named 9311(OsBAK1NIP), had lower plant height, larger leaf angle, longer grains, smaller panicle length, lower grain number and yield per plant (Figures 2d,e and S4c), and lower grain chalkiness area and rate than those of 9311(OsBAK19311) (Figure 2f). In addition, we generated three independent homozygous knockout lines to investigate the function of OsBAK1 in ZH11 (Figure S4d). They all displayed a typical BR loss-of-function phenotype, including lower height, smaller leaf angle, shorter panicle, smaller grain length–width ratio and length without changes in grain width (Figures 2g–i and S4e–g), higher grain chalkiness rate and area, lower amylose content and higher protein content than those of the wild type (Figure 2j,k). These yield and quality trends of the RNAi lines or knockout mutants of OsBRI1 and OsBAK1 were consistent with those of the rice mini-core collection (Figure 1f,g). Taken together, OsBRI1 and OsBAK1 are the important loci for indica–japonica differentiation in grain yield and quality traits in rice.
Contribution of natural variations in OsGSK3 to rice inter-subspecies diversity
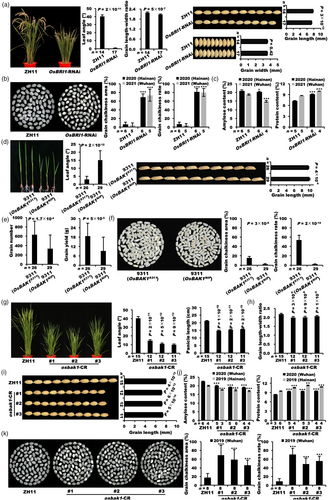
Besides OsBRI1 and OsBAK1, we also identified a peak point on chromosome 2 by GWAS analysis using grain length–width ratio as an indicator of indica–japonica differentiation (Figure 3a). Coincidentally, we found a gene LOC_Os02g14130, encoding a GSK3/SHAGGY-like kinase, located in the peak point region and also supported by LD block analysis (Figure 3b). LOC_Os02g14130 was annotated as OsGSK3, a homologue of BIN2 in Arabidopsis. There was significant difference in grain length–width ratio between the two genotypes of the peak SNP in OsGSK3, indicating that natural variations of OsGSK3 contribute to grain length–width ratio variation (Figure 3c). Four haplotypes were identified by 13 representative variations out of all 87 natural variations in OsGSK3 in the rice mini-core collection (Figure 3d and Table S3). H1 and H3 are two haplotypes of OsGSK3, which are predominant in japonica (110 out of 114) and indica (269 out of 287) subspecies, respectively, accounting for the majority of 533 accessions (Figure 3d). Aligning the sequence between OsGSK3H1-J and OsGSK3H3-I, we found that representative variations included seven InDels and one SNP in the promoter, a 26-bp InDel in the intron, and three SNPs causing nonsynonymous mutations in the exons (Figure 3e and Table S3). The SNP vg0207719663, which disturbs the stop codon of OsGSK3, and another SNP vg0207720567, located in the OsGSK3 kinase domain, may be important variants (Figure 3d,e).
Then, we analysed grain yield and quality traits of the two major haplotypes in the rice mini-core collection (Figure 3f,g). Like the phenotypes of the two major haplotypes of OsBRI1 and OsBAK1, compared with OsGSK3H1-J, OsGSK3H3-I had smaller leaf angle, increased tiller number, much larger grain length–width ratio (with longer and slender grains), often longer panicles, increased grain number and yield per plant (Figure 3f), lower grain chalkiness area and degree, higher amylose content, less protein content, higher head rice rate, lower gel consistency and alkali spreading value, and higher fatty acid C16: 0 and C18: 3 (Figure 3g). These results are highly consistent with the typical agronomic features between indica and japonica subspecies, showing that the two major haplotypes of OsGSK3 prominently contribute to rice inter-subspecies diversity for grain yield and quality traits.
We generated gene-edited plants of OsGSK3 in ZH11 using the CRISPR-Cas9 system, and obtained four independent homozygous frameshift lines (osgsk3-CR) (Figure S5a). Compared with the wild type, the osgsk3-CR mutants displayed larger leaf angle, grain size (longer grain length, but no significant difference in grain width), panicle length (Figures 3h and S5b,c) and higher grain chalkiness area and rate (Figure 3i). Besides the knock out mutants, we generated the complementation materials of OsGSK3 by transforming the allele from the japonica ZH11 into the indica MH63, but actually increased the RNA and proteins levels of OsGSK3 to obtain a material similar to overexpression lines (Figures 3j and S6a,b). In the three independent complementation lines (OsGSK3-C), the plant architecture was more upright, the plant height and grain length–width ratio especially grain length were significantly smaller than those of MH63 (Figures 3j and S6c,d), indicating that more OsGSK3 negatively regulates grain length and plant architecture. Taken together, OsGSK3 is an important locus for indica–japonica differentiation in grain yield and quality traits in rice.
Two major haplotypes of OsBZR1 contribute greatly to indica–japonica differentiation
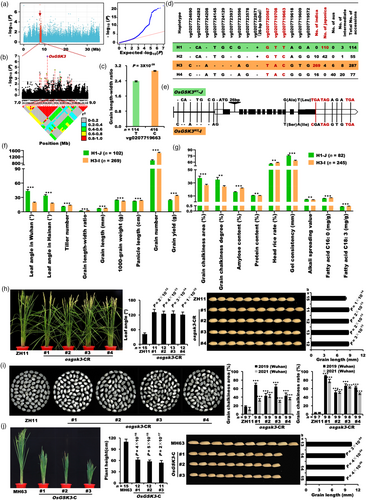
On chromosome 7, a peak resided in the region spanning 23.2–24.0 Mb was detected by the GWAS, and supported by LD block analysis (Figure 4a,b). In this region, we focused on LOC_Os07g39220, a gene encoding OsBZR1. The two haplotypes of the peak SNP vg0723485862 of OsBZR1 showed a distinct difference in grain length–width ratio (Figure 4c). Combining all 32 variations of OsBZR1 in the database with our genotypic identification (Table S4; Figure S7), we detected eight major representative variations of OsBZR1 (Figure 4d). The eight variations could be classified into three haplotypes (H1–H3), including 103 japonica accessions out of 126 accessions in H1, which was named as OsBZR1H1-J, and 256 indica accessions out of 275 accessions in H2, which was named as OsBZR1H2-I (Figures 4d,e and S7). For OsBZR1H2-I, there was a 12-bp InDel in the first exon leading to the insertion of four amino acids, two big InDels (4808 bp and 266 bp) adjacent to each other and five SNPs in the promoter region (Figure 4d,e; Figure S7). The 4808-bp insertion encoded a retrotransposon protein. Compared with OsBZR1H1-J, OsBZR1H2-I increased panicle length, grain length, grain length–width ratio, tiller number, grain number per plant and yield per plant, but decreased leaf angle and 1000-grain weight in 533 accessions (Figure 4f). In addition, OsBZR1H2-I had lower grain chalkiness area and degree, consistent with the result that OsBZR1 could be identified by grain-chalkiness GWAS from 5-year field trials (unpublished data from our lab) (Figure S8), lower protein content, gel consistency and alkali spreading value, but higher amylose content, fatty acid C16: 0 and C18: 3 than those of OsBZR1H1-J (Figure 4g). These results were highly consistent with the typical separation features between two major subspecies, suggesting that the two major haplotypes of OsBZR1 contribute greatly to indica–japonica differentiation in rice.
Single mutant, RNAi and overexpression (OE) lines of OsBZR1 in japonica ZH11 were generated to study its function. The single mutant of OsBZR1 (osbzr1-CR) did not exhibit significant phenotypic change compared to the wild type (Liu et al., 2021), due to functional redundancy of its homologous genes. The OsBZR1-RNAi lines showed typical BR insensitive phenotypes, including dwarfism and reduced leaf angle (Figure 4h), and reduced grain length–width ratio, mainly due to the decrease of grain length (Figure 4h). In contrast, OsBZR1 or Osbzr1-D (a gain-of-function mutation of OsBZR1) OE lines increased grain length–width ratio and grain length (Figure S9). Unexpectedly, both OsBZR1-RNAi and -OE lines showed increased grain chalkiness area and rate (Figure 4i,j), increased protein content with decreased amylose content (Figure 4k,l) than those of wild type, indicating that the balance of BR signalling intensity is important for maintaining grain quality traits in rice. Taken together, OsBZR1 is an important locus for grain yield and quality traits in rice.
BR signalling haplotypes (BSHs) synergistically contribute greatly to inter-subspecies differentiation, grain yield and quality
Coincidentally, the expression levels of OsBRI1, OsBAK1, OsGSK3 and OsBZR1 were highly correlated with each other in 1-mm panicles of the mini-core collections (Figure 5a), indicating that they may function in the same way in the 533 accessions. We then picked out the functional variations of these four genes, and interestingly, we found that most of their genotypes were highly co-segregated or selected in the rice mini-core collection, although they were located on four different chromosomes (Figure 5b; Table S5). Therefore, we combined these four genes together for further haplotype analysis (Figure 5b). As a result, seven BR signalling haplotypes (BSHs) were identified and named BSH1–7 (Figure 5b; Table S5), among which BSH2 and BSH6 were the two major BSHs, accounting for the majority of 533 accessions, predominant in japonica (99.1%, BSH2-J) and indica (96.7%, BSH6-I) subspecies, respectively (Figure 5b). Furthermore, we performed Tajima's D, Fst as well as Pi analyses using a natural population with 354 indica and 175 japonica accessions (Figures 5c and S10). The Fst estimation indicates that OsBRI1, OsBAK1 and OsGSK3 show high genetic differentiation between indica and japonica subspecies (Figure S10a). The Pi and TajimaD estimates indicate the three genes are in the low nucleotide diversity regions (Figures 5c and S10b). These results suggested they have undergone strong selection pressures where particular alleles are advantageous. In contrast, OsBZR1, which shows high genetic differentiation (Figure S10a) and high nucleotide diversity (Figures 5c and S10b), suggesting that it may be subject to stabilizing selection or adaptive evolutionary pressures, reflecting its role in providing beneficial traits that enhance adaptability in response to environmental challenges. In addition, the higher nucleotide diversity values of all the four genes in japonica compared to indica suggest indica rice had experienced stronger selection than japonica rice (Figures 5c and S10b). Together, these observations suggest that OsBRI1, OsBAK1 and OsGSK3 represent critical adaptive targets under strong selection, while OsBZR1 is a locus of high evolutionary potential and adaptability. This differential pattern of selection highlights the distinct evolutionary processes shaping these genes, emphasizing their roles in the populations' response to environmental pressures.
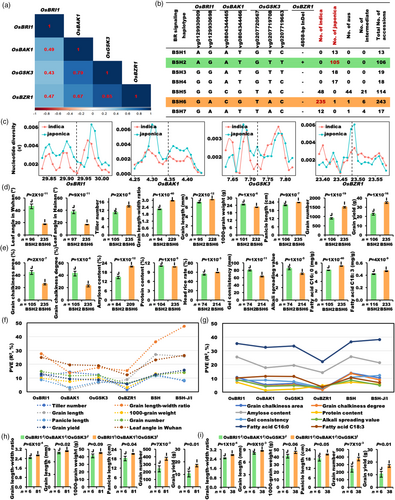
To further explore the functional differences between BSH2-J and BSH6-I, the two major BSHs, multiple grain yield and quality traits were analysed using the mini-core collection (Figure 5d,e). Compared with BSH2-J, BSH6-I had compact leaf angle in Wuhan (−61.9%) and Hainan (−54.9%), enhanced tiller number (+28.6%), grain length–width ratio (+34.1%), grain length (+8.7%), panicle length (+9.1%), grain number per plant (+61.5%) and increased grain yield per plant (+54.8%) (Figure 5d), and had decreased grain chalkiness area (−42.3%) and degree (−46.8%), lower grain protein content (−2.4%), gel consistency (−20.3%), alkali spreading value (−15.9%) and enhanced grain amylose content (+49%), head rice rate (+7.7%), fatty acid C16: 0 (+14.3%) and C18: 3 (+11.5%) in 533 accessions (Figure 5e). These results were highly consistent with the understanding of the typical differentiation traits between indica and japonica rice, and further indicated that the two major BSHs have important effects on the inter-subspecies differentiation, grain yield and quality traits in rice.
The genetic contributions of the four genes and BSHs to major grain yield and quality traits in the rice mini-core collection were analysed by Analysis of Variance (ANOVA) with genotypes of the functional variations of these genes and phenotypes of 533 accessions (Figure 5f,g and Table S6). OsBRI1, OsBAK1, OsGSK3, OsBZR1, all BSHs and two major BSHs (BSH2-J and BSH6-I) explained 13.7%–47.6% variation in grain length–width ratio, 1.5%–27% variation in grain length, 3.1%–13.9% variation in 1000-grain weight, 12.2%–26.4% variation in leaf angle in Wuhan, 12.4%–29.9% variation in leaf angle in Hainan, 1.5%–14.2% variation in tiller number, 3%–11.6% variation in panicle length, 5.9%–14.8% variation in grain number per plant and 5.7%–15.7% variation in grain yield per plant in the rice mini-core collection (Figure 5f). For grain quality traits, they explained 2.4%–10.7% variation in grain chalkiness area, 1.8%–13.6% variation in grain chalkiness degree, 14.2%–25.8% variation in amylose content, 1.3%–7.2% variation in protein content, 3.2%–12.2% variation in gel consistency, 3.8%–9.2% variation in alkali spreading value, 22.2%–38.2% variation in fatty acid C16:0 and 3.8%–11.5% variation in fatty acid C18:3 in the rice mini-core collection (Figure 5g). Notably, the genetic contribution of BSHs to these grain yield and quality traits was much higher than that of each single component in BR signalling pathway (Figure 5f,g).
To confirm the correlation between BR signalling component variations and phenotypes, we generated an F2 genetic segregation population of OsBRI1/OsBAK1/OsGSK3 by crossing the japonica subspecies NIP with the indica subspecies C418 (Figures 5h,i and S11). In the F2 population of 415 individuals, OsBRI1, OsBAK1 and OsGSK3 separated freely due to different locations on three chromosomes. Two separate alleles of OsGSK3 alone isolated from two parents had significant effects on grain length–width ratio, grain length, 1000-grain weight, panicle length, grain number and yield per plant (Figure S11a), and the trends were the same as that in 533 accessions (Figure 5d). When analysing OsBRI1, OsBAK1 and OsGSK3 as a whole, OsBRI1J/OsBAK1J/OsGSK3J from NIP and OsBRI1I/OsBAK1I/OsGSK3I from C418 could separate these yield traits as significantly as OsGSK3 alone (Figure 5h). In addition, since OsBRI1 and OsBAK1 act upstream of BR signalling pathway (He et al., 2013; Li et al., 2002), and OsGSK3 is their downstream (Kim et al., 2011), we analysed OsGSK3 under the OsBRI1-OsBAK1 modules from NIP and C418 parents, respectively. In the OsBRI1J/OsBAK1J background (both OsBRI1 and OsBAK1 from NIP), compared with OsGSK3J, OsGSK3I enhanced grain length–width ratio (+11.4%), grain length (+7.5%), 1000-grain weight (+11.9%), panicle length (+14.9%), grain number per plant (+56.4%) and increased grain yield per plant (+64.2%) in the F2 genetic population (Figure 5i). However, in the OsBRI1I/OsBAK1I background (both OsBRI1 and OsBAK1 from C418), compared with OsGSK3J, the OsGSK3I only had no or little effects on these yield traits in the F2 genetic population (Figure S11b), indicating that OsGSK3 had different functions in japonica and indica subspecies. Furthermore, in the F3 genetic population derived from five independent F2 homozygous lines of OsBRI1J/OsBAK1J/OsGSK3J and OsBRI1I/OsBAK1I/OsGSK3I, all yield traits of indica genotypes were much higher than those of japonica genotypes, which was consistent with the F2 genetic population (Figure S11c).
These results demonstrated that the BSHs have undergone directional selection and synergistically contribute to the indica–japonica differentiation in grain yield and quality traits.
The BR signalling in japonica rice is generally stronger than indica rice
To find out the reason for the synergistic effect of BSHs on indica–japonica differentiation for grain yield and quality, we conducted a number of molecular and physiological experiments at six different levels (Figure 6). First, since the interaction between the receptor BRI1 and the co-receptor BAK1 is very important in the BR signalling pathway (Li et al., 2002), and there are several non-synonymous variations in the key structural motifs of both OsBRI1 and OsBAK1 between the two major BSHs, BSH2-J and BSH6-I (Figures 1e and 5b), we performed split luciferase complementation assay (Split-LUC) in the same tobacco leaf to investigate the strength of their interaction intensity (Figure 6a). OsBRI1BSH2-J and OsBRI1BSH6-I showed no difference in interaction with OsBAK1BSH2-J, OsBAK1BSH6-I, OsBRI1BSH2-J or OsBRI1BSH6-I (Figure 6a), suggesting that the two variations in the LRR domain of OsBRI1 may not be the cause of the difference in interaction intensity between OsBRI1 and OsBAK1. But the interaction intensity of OsBRI1BSH2-J and OsBAK1BSH2-J from japonica rice was obviously higher than that of OsBRI1BSH6-I and OsBAK1BSH6-I from indica rice (Figure 6a). The interaction intensity between OsBAK1BSH2-J and OsBRI1BSH6-I was also higher than that between OsBAK1BSH6-I and OsBRI1BSH2-J (Figure 6a). The results confirmed a stronger interaction between the two receptors OsBRI1 and OsBAK1 in japonica than in indica rice, indicating a stronger BR signalling in japonica than in indica rice. We considered whether the subcellular distributions corresponding to the two haplotypes of OsBAK1 was changed, but there seemed no remarkable difference in subcellular localization of the two haplotypes of OsBAK1 in tobacco leave and rice protoplast cells: They were both located primarily in the cell membrane (Figures 6b and S12a). However, western blot showed that the protein level of OsBAK1BSH2-J-GFP (ratio of GFP band value to control) was much higher than that of OsBAK1BSH6-I-GFP (Figures 6b and S12b), indicating that the protein level of OsBAK1 increased in BSH2-J compared to BSH6-I. In addition, to investigate the role of OsBRI1 and OsBAK1 promoters, and identify whether the difference in OsBAK1 protein levels was caused by the difference in RNA level, we compared the expression levels of OsBRI1 and OsBAK1 in BSH2-J and BSH6-I, but no significant difference was found in the OsBRI1's expression levels between the two haplotypes (Figure S13a), suggesting that the variations of OsBRI1 promoter are not associated with the natural variation of grain length–width ratio. Whereas the expression of OsBAK1 in BSH2-J was lower than that in BSH6-I in the 1-mm panicles (Figure S13b and Table S7), consistent with the lower expression level of OsBAK1BSH2-J than that of OsBAK1BSH6-I in dual-luciferase assay (Figure S13c), indicating that the difference of OsBAK1 protein levels between the two subspecies is not caused by promoter variations, but by two natural variations in the signal peptide sequence of OsBAK1, due to some unknown reasons.
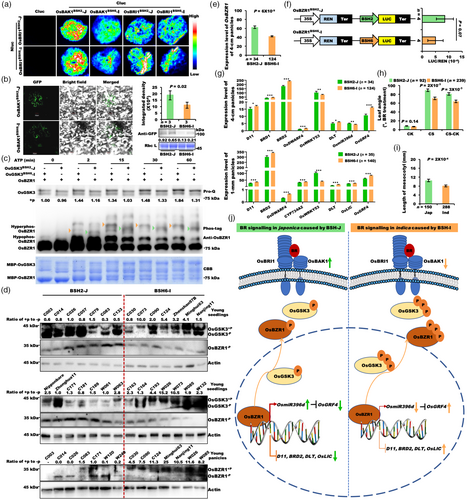
Second, to identify stop codon variations of OsGSK3 between BSH2-J and BSH6-I, we expressed and purified four forms of OsGSK3 with different stop codons derived from indica and japonica alleles, respectively (Figure S14a). The results of sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) showed that the shift rate of OsGSK3 in the first two indica forms was slower than that in the latter two japonica forms (Figure S14b). These results suggest that the protein size of OsGSK3BSH6-I is larger than that of OsGSK3BSH2-J, due to the loss of the first stop codon of the BSH6-I haplotype compared to the BSH2-J haplotype caused by the SNP vg0207719663. Considering two nonsynonymous mutations in the kinase domain of OsGSK3, we compared the kinase activity of OsGSK3BSH2-J and OsGSK3BSH6-I proteins. In vitro kinase assays revealed that the autophosphorylation level of OsGSK3BSH6-I was significantly higher than that of OsGSK3BSH2-J (Figure 6c, top). To determine whether these two haplotypes affect the activity of OsGSK3, we selected OsBZR1 as the primary substrate for OsGSK3 to evaluate its kinase activity (He et al., 2002). We found that OsBZR1 could be phosphorylated by both OsGSK3BSH2-J and OsGSK3BSH6-I. However, the shift rate and amount of OsBZR1 phosphorylated by OsGSK3BSH6-I were significantly slower and much higher than those phosphorylated by OsGSK3BSH2-J, indicating that the kinase activity of OsGSK3 in indica rice is much higher than in japonica rice (Figure 6c, middle). The results suggested that these three nonsynonymous mutations represent the functional variations of OsGSK3 between BSH2-J and BSH6-I, and they result in weaker kinase activity of OsGSK3 and weaker phosphorylation level of OsBZR1 in japonica rice, further suggesting that a stronger BR signalling in japonica than in indica rice due to the negative role of OsGSK3 in BR signalling.
Third, to further investigate whether the phosphorylations of OsGSK3 and OsBZR1 are associated with indica–japonica differentiation in rice, we randomly selected 14 lines from BSH2-J and BSH6-I accessions in the mini-core collection and extracted total protein extracts from their seedlings. Two polyclonal antibodies against OsBZR1 and OsGSK3 were tested by total protein extracts from OsBZR1-OE and osgsk3-CR seedlings, respectively (Figure S15a,b). We found much stronger bands in the OsBZR1-OE lines and much weaker bands in the osgsk3-CR lines than the wild type (ZH11) (Figure S15a,b), indicating that polyclonal antibodies against OsBZR1 and OsGSK3 can be used to detect endogenous levels of OsBZR1 and OsGSK3, respectively. Results of phos-tag showed the much higher phosphorylation level of the OsGSK3 protein in indica rice than in japonica rice (P = 0.004, n = 14 for each haplotype) (Figure 6d, upper and middle), consistent with the result of in vitro kinase assays that the autophosphorylation level of OsGSK3BSH6-I was significantly higher than that of OsGSK3BSH2-J (Figure 6c). However, the phosphorylation state of the OsBZR1 protein, the non-functional form of OsBZR1 bound by 14-3-3 protein and retained in cytoplasm (Bai et al., 2007; Gampala et al., 2007), was hardly detected in both BSH2-J and BSH6-I 10-day young leaves (Figure 6d, upper and middle), which might be the fact that the accumulation of phosphorylated OsBZR1 in young leaves is much lower than in mature tissues (Tong et al., 2012). But the protein level of the non-phosphorylated and functional OsBZR1 in japonica rice was much higher than in indica rice (Figure 6d, upper and middle), showing that the BR signalling in japonica is generally stronger than in indica rice. To detect the phosphorylation status of endogenous OsBZR1 in mature tissues, we randomly selected seven materials from BSH2-J and BSH6-I accessions and prepared total protein extracts from their 3-cm panicles. The results showed that the phosphorylation level of OsBZR1 protein in indica rice was much higher than in japonica rice (P = 0.002, n = 7 for each haplotype) (Figure 6d, bottom), consistent with the results of in vitro kinase assays in which the shift of OsBZR1 phosphorylated by OsGSK3BSH6-I was significantly slower and much more than that by OsGSK3BSH2-J (Figure 6c). These results further confirmed the conclusion that the BR signalling of indica rice was generally weaker than that of japonica rice. Additionally, the RNA expression of OsGSK3BSH2-J was much lower than that of OsGSK3BSH6-I in 1-mm panicles (Figure S16a and Table S7), which was consistent with the fact that the expression level of OsGSK3 in all tissues of japonica rice NIP was lower than that of two indica rice ZS97 and MH63 (Figure S16b,c), further indicating the stronger BR signalling in japonica than in indica rice.
Fourth, considering the two big InDels in OsBZR1 promoter, we analysed the expression levels of OsBZR1 between BSH2-J and BSH6-I in the mini-core collection of 533 accessions (Table S7). In 4-cm panicles, the expression level of OsBZR1BSH2-J was much higher than that of OsBZR1BSH6-I (Figure 6e), which was consistent with the higher expression level of OsBZR1 in the japonica variety NIP than that in the two indica varieties ZS97 and MH63 in all tissues (Figure S17), indicating a stronger BR signalling in japonica rice than that in indica rice. Then we analysed two promoter fragments of OsBZR1 from BSH2-J and BSH6-I. Compared with BSH6-I, BSH2-J promoter fragment had more AP2-bound cis-elements and less WRKY-bound cis-elements (Table S8). To investigate whether the variations in the promoter region changed the expression level of OsBZR1, we further performed transient assays of these two promoter fragments in the same tobacco leaves (Figure 6f). Compared with the activity of the OsBZR1BSH2-J promoter fragment, the activity of the OsBZR1BSH6-I promoter fragment was greatly reduced (Figure 6f), further suggesting that the BR signalling of BSH2-J is stronger than that of BSH6-I.
Fifth, the expression levels of OsBZR1 target genes between BSH2-J and BSH6-I were compared using RNA-seq data from both 1-mm and 4-cm panicles of the rice mini-core collection (Table S7). The expression levels of the genes (D11, BRD1, BRD2, OsDWARF4, CYP734A2, DLT and OsLIC) negatively regulated by OsBZR1 (Tong et al., 2012; Wang et al., 2002; Zhang et al., 2012) in BSH2-J were significantly lower than those in BSH6-I (Figure 6g). Besides, the expression of OsmiR396d positively regulated by OsBZR1 was increased in BSH2-J, while the expression of OsmiR396d target gene OsGRF4 (Tang et al., 2018) was decreased in BSH2-J (Figure 6g). The regular expression trend of these BR signalling pathway output genes between indica and japonica rice was consistent with the phosphorylation trend of OsBZR1 and OsGSK3, as shown in Figure 6c,d.
Sixth, seedlings of 533 accessions were treated with BR to measure the BR sensitivity between BSH2-J and BSH6-I (Figure 6h). Compared with BSH6-I, BSH2-J had an increased BR sensitivity (+20.9%) in 533 accessions (Figure 6h). In addition, the mesocotyl, also controlled by BR signalling (Sun et al., 2018), was longer in japonica rice than in indica rice (Figure 6i). These results showed that the two major BSHs differentially regulate BR response genes and BR sensitivity in rice.
In brief, the above six results verified the weaker interaction between OsBRI1 and OsBAK1, stronger autophosphorylation and kinase activity of OsGSK3, less RNA/proteins and stronger phosphorylation of OsBZR1, and weaker BR sensitivity in indica rice than those in japonica rice, the regular expression trends of BR-response genes and the regular BR sensitivity between these two subspecies, and confirmed that the BR signalling in japonica is stronger than that in indica rice and why BSHs contribute greatly to indica–japonica differentiation.
Discussion
Based on our findings, we proposed a model to illustrate how BR signalling pathway differentiates between japonica and indica subspecies (Figure 6j). In japonica rice, higher OsBAK1 protein level due to its natural variations leads to stronger interaction between OsBRI1 and OsBAK1, decreased autophosphorylation level, kinase activity and expression level of OsGSK3 result in less phosphorylation of OsBZR1, coupled with increased expression of OsBZR1 RNA, enhanced BR signalling in japonica rice, resulting in increased functional OsBZR1 protein with lower phosphorylation level, further causing the regular up- or down-regulated expression trends of BR-response genes (Figure 6j, Left). Conversely, lower OsBAK1 protein level, higher kinase activity of OsGSK3 coupled with higher OsBZR1 phosphorylation and lower OsBZR1 RNA reduced BR signalling in indica subspecies (Figure 6j, Right).
The BR sensitivity of BSH2-J is obviously stronger than BSH6-I (Figure 6h), consistent with that the leaf angle of BSH2-J was notably higher than BSH6-I (Figure 5d). The BR sensitivity differences in our study seem to be opposite to a previous research on OsBSK2, that is, japonica rice showed lower BR sensitivity than indica rice (Yin et al., 2022). In fact, we analysed three important BR-related response traits for the two major haplotypes C and T of OsBSK2 used in the previous research (Yin et al., 2022) (Figure S18a,b). Leaf angle, BR sensitivity after BR treatment and mesocotyl length of OsBSK2 C genotypes (mainly japonica rice) were greater than those of the T genotypes (mainly indica rice) (Figure S18b), which was highly consistent with the differentiation trend of japonica and indica rice in our study but inconsistent with the previous one (Yin et al., 2022) (Figures 5d and 6h,i). The inconsistent results of the two studies may be due to the statistical number of japonica and indica accessions (135 and 259 in our study, respectively, compared to <80 in the previous study) or the use of different accessions for phenotypic data. Furthermore, since our results also support our main conclusion of the higher BR signalling in japonica than indica, which was confirmed by many biochemical and genetic evidences (Figure 6), we believe that japonica rice has a significantly stronger BR sensitivity than indica rice. Finally, the enhanced BR signalling of BSH2-J resulted in corresponding expression changes of many downstream output genes targeted by OsBZR1 (Figure 6g), thereby regulating many grain yield and quality traits in rice (Figure 5d,e). Thus, these two major BSHs synergistically modulate the intensity difference of BR signalling between japonica and indica rice.
The mechanism of indica–japonica differentiation is a very attractive issue (Jiang et al., 2022; Kovach et al., 2007; Wang and Han, 2022). By conducting GWAS using rice grain length–width ratio as an indicator of indica–japonica differentiation, we discovered the natural genetic variation in the four key components of BR signalling pathway underlying inter-subspecies diversity in rice (Figures 1-6). Most functional variations of these four genes co-segregated in the rice mini-core collection and were divided into two major BSHs, showing a very strong indica–japonica differentiation (Figure 5b). The genetic contributions of BSHs to these grain yield and quality traits were much higher than those of a single component in BR signalling pathway in the mini-core collection (Figure 5f,g), further suggesting the collaborative contribution of natural variations in BR signalling pathway to the genetic basis of indica-japonica differentiation in rice. A study showed that the reduction percentage of grain size and number for OsBAK1 knockout lines in the indica background was obviously bigger than that in the japonica background (Yuan et al., 2017). Our studies showed that the yield and grain-quality traits of BSH in indica rice with weak BR signalling were much higher and better than those of japonica rice, respectively (Figure 5d,e), which is supported by the previous important studies that reducing BR signalling enhances grain yield in semi-dwarf wheat (Song et al., 2023) and inhibiting tissue-specific BR enhances rice panicle branching and grain yield (Zhang et al., 2024). Our results also provided molecular and biochemical evidences for functional differences between OsBAK1 mutants in indica and japonica rice (Yuan et al., 2017). Thus, the two rice subspecies utilize divergent BR signalling pathways that contribute momentously to the co-divergence of multiple yield and quality traits. Two major BSHs co-diverged with indica–japonica differentiation over a large time scale of rice domestication, which may be the result of the long-term adaptation of rice to different ecological and geographical environments and the accumulation of divergent selection of natural variations in distinct breeding programmes. However, for few rice accessions of other BSHs, deviations from the strict co-divergence of BSHs between two subspecies exist (Figures 5b,c and S10), indicating that additional evolutionary processes such as cross-subspecies transmission or gene flows are at work.
We are confused about why BSH6-I with a weak BR signalling results in large grains in indica rice, while BSH2-J with a strong BR signalling results in small grains in japonica rice (Figure 5d). We guessed whether a major gene that could help to explain this phenomenon. Interestingly, OsSPL13 has been reported to have a big effect on grain length differentiation in temperate japonica and tropical japonica rice, and it was found that the large-grain allele of OsSPL13 in tropical japonica rice was introgressed from indica rice (Si et al., 2016). Then, in the large-grain allele background of OsSPL13, the effect of functional variations of the four key components in BR signalling pathway on grain length was analysed (Table S9). Functional variations of OsBAK1 (vg0804344465, vg0804344466) and OsGSK3 (vg0207720567, vg0207719708 and vg0207719663) could significantly distinguish grain length in the mini-core collection, of which haplotypes mainly from japonica rice had longer grain length than haplotypes mainly from indica rice under the same large-grain allele background of OsSPL13 (Figure S19). These results were consistent with the fact that longer grain-length phenotypes of introgression line of the native OsBAK1 from japonica into indica rice and OsGSK3 knockout lines, and smaller grain length of OsBAK1 knockout lines in ZH11 background (Figures 2d,i and 3h).
Although many components of the BR signalling pathway have been identified by screening mutants or interacting proteins (Gampala et al., 2007; He et al., 2002; Wang et al., 2001), their application in molecular breeding is limited, and their natural variations in regulating grain yield and quality traits remain unknown. Here, we provided several evidences to support the conclusion that many natural variations of OsBRI1, OsBAK1, OsGSK3 and OsBZR1 control grain length–width ratio, leaf angle, plant and panicle architecture, grain yield per plant, grain chalkiness, amylose and protein content, gel consistency and fatty acid content in rice (Figures 1-4). Therefore, we proposed a new concept, BSHs (Figure 5b), which combines the haplotypes of these genes together to form a signalling module with diverse forms and functions, laying the genetic basis for indica–japonica differentiation. Two major BSHs are caused by co-segregated or co-selected natural variations of the four genetically unlinked genes, and their differentiation between the two rice subspecies resulted in improved grain yield and quality traits in indica rice (Figure 5d,e). The introgression line of OsBAK1 from the japonica NIP into the indica 9311 (Figures 2d–f and S4c) and the two genetic segregation populations of OsBRI1/OsBAK1/OsGSK3 by crossing the japonica NIP with the indica C418 (Figures 5h,i and S11) further confirmed the functional difference between the two major BSHs in grain yield and quality traits. There is a generally weaker BR signalling in indica rice than that in japonica rice (Figure 6h,i), which is collaboratively caused by natural variations in the four genes. That is why the yield of indica rice is generally higher than that of japonica rice (Figure 5d). Moreover, the indica BSH has lower grain chalkiness, higher head rice rate and fatty acid, thus promoting better grain quality of indica rice (Figure 5e), further indicating that the indica BSH is a more favourable haplotype for improving quality traits in rice. It is better to construct the introgression lines of the indica BSH with these four genes into an elite japonica variety by backcrossing, and construct the transgenic lines of the indica BSH into a japonica background with these four genes knocked out by CRISPR to further confirm our conclusions and to breed high-yield and superior-quality japonica rice. Besides, all the transgenic lines with too much or too less BR signalling have enhanced grain chalkiness and protein content while decreased amylose content, indicating that BR signalling intensity affects grain quality, and appropriate BR signalling intensity is more important for achieving superior quality rice (Figures 2b,c,f,k, 3i and 4i–l). Coincidentally, moderate inhibition of BR signalling, such as the very weak mutant osdwarf4-1 due to its redundancy (Sakamoto et al., 2006), and weaker BR-deficient transgenic rice through partial inhibition of OsBRI1 expression (Morinaka et al., 2006), finally enhanced grain yield, while too much or too less BR signalling intensity, due to trades-offs among various traits, is not beneficial to increase grain yield (Wang et al., 2024). Therefore, effective manipulation of BR signalling intensity to leverage its positive benefits while mitigating adverse effects holds the prospect of significantly enhancing yield and quality. In conclusion, our study may help hybrid rice breeders to design different BR signalling intensity by shuffling or combining different alleles of the four genes in utilizing the super-heterosis of indica–japonica hybridization, and provide a novel strategy for resolving grain yield or quality problems in inter-subspecies breeding programmes.
Methods
GWAS using an indicator of indica–japonica differentiation
For the GWAS, a sequenced diverse panel of the rice mini-core collection consisting of 533 accessions was used, including 215 accessions from China's core collection of Oryza sativa L. and 318 accessions from other 58 countries worldwide (Table S1). A total of 4 131 700 SNPs with MAF (minor allele frequency) > 5% were retained for GWAS analysis. GWAS was performed by the factored spectrally transformed linear mixed model (FaST-LMM) (Lippert et al., 2011). The Manhattan plots were mapped by the qqman package. Population structure was evaluated by ADMIXTURE (Alexander et al., 2009). The number of K was plotted against the cross-validation (CV) value to determine the most suited value of K. To avoid the false positive, we applied the –tfileSim and –covar parameters of factored spectrally transformed linear mixed model (FaST-LMM) (Lippert et al., 2011) to control the kinship and population structure. The threshold for genome-wide significance was evaluated with Bonferroni correction (corrected P = 0.05/n, n is the effective number of independent SNPs across the genome). The n was estimated by pruning the SNP dataset using PLINK (version 1.9). We set 1.37 × 10−6 as the genome-wide threshold using grain length–width ratio (grain shape) as an indicator of indica–japonica differentiation. An R package LDheatmap was used for linkage-disequilibrium block analysis.
Natural variation and haplotype analysis
Sequence variations of OsBRI1, OsBAK1, OsGSK3 and OsBZR1 from 533 rice accessions were downloaded from the RiceVarMap2.0 website (http://ricevarmap.ncpgr.cn) (Zhao et al., 2015, 2021). Sequence variations (MAF > 10%) in the 2-kb promoter region, the coding region and the 0.5-kb downstream region were selected and used to perform haplotype analyses. Because the numerous variations are hard to analyse and many of them co-segregated with each other, we picked out some of them that can represent the genotype of many other variations and are located at key positions of the analysed gene, and named them as representative variations of the gene. All the natural variations, representative variations and haplotypes of the four genes in the 533 rice accessions were listed in Tables S2–S4 and S9.
Field planting and design, and measurement of yield, BR sensitivity and grain quality
All the materials were grown in normal seasons at the Experimental Stations of Huazhong Agricultural University, Wuhan and Lingshui, China. The planting density was 16.5 cm between plants in a row, and the rows were 26 cm apart. Field management, including fertilizer application, irrigation and pest control, followed normal agricultural practices. Harvested rice grains were air-dried and stored at room temperature for at least 1 month before measuring grain yield and quality traits. Each accession was planted in two replicates with 24 plants (two rows of 12 plants) per replicate.
Ten randomly chosen and fully filled grains from each plant were lined up length- and width-wise to measure grain length and width with a Vernier calliper, respectively. Grain weight was calculated based on 200 grains and was converted to 1000-grain weight. Plant height, panicle length and leaf angle were measured with a ruler and protractor in the field, respectively. The tiller number was counted in the field. The phenotype data of grain length-width ratio, grain length, grain width, 1000-grain weight, tiller number, panicle length, grain number per plant (number of total flowers × panicle seed setting rate) and grain yield per plant of the 533 accessions can be obtained from our RiceVarMap2.0 website (http://ricevarmap.ncpgr.cn) (Zhao et al., 2015, 2021). The leaf angle of Wuhan and Hainan of the 533 accessions was measured by Dong et al.(2018). The length of mesocotyl of the 533 accessions was obtained from Sun et al. (2018).
For the BR sensitivity assays of the 533 accessions, the first intact leaf with partial leaf blade and sheath after germination was excised from at least twenty 1-week-old etiolated seedlings of every accession, inserted vertically into a 100 nM CS or control solution and incubated in the dark for 72 h (Sun et al., 2015). The BR sensitivity of every accession is equal to CS treated minus CK.
Grain chalkiness area and rate, and head rice rate were assessed visually as previously described (Li et al., 2014). The percentage of chalky grains in total dehulled grains was used as the measurement of grain chalkiness rate. Head rice rate was the percentage of milled rice grains that remain intact. The amylose content of polished flour ground by milled rice and the protein content of dry dehulled grains were detected using an XDS Near-Infrared Rapid Content Analyzer (FOSS, Denmark), with near-infrared reflectance spectroscopy as previously described (Chen et al., 2018; Perbandt et al., 2010). The gel consistency and alkali spreading value were measured following the methods as previously described (Wang et al., 2007). In gel consistency measurement assay, about 100 mg of rice flour wetted with 0.2 mL 95% ethanol containing 0.025% (w/v) bromthymol blue was added into the tube with 2 mL 0.2 N NaOH, mixed and boiled in water bath for 8 min, cooled to room temperature for 5 min and in an ice-water bath for 20 min, and then laid down horizontally for 1 h later to measure the gel length. For measurement of alkali spreading value, six intact milled rice were needed to incubate with 10 mL of 1.7% KOH at 30 °C for 23 h and measuring the degree of spreading was on a 7-point scale. For measurement of fatty acids, 4.5 mL of sulfuric acid:methanol solution (volume 5:100) and 1.07 mg of margaric acid (C17:0) dissolved in chloroform were added into 0.2 g milled rice flour sample, mixed in a 85 °C water bath for 2 h, cooled to room temperature and the fatty acid methyl esters were extracted with 2 mL n-hexane and analysed by GC-MS (Zhou et al., 2021). The protein content and fatty acid of the 533 accessions were measured by Chen et al. (Perbandt et al., 2010) and Zhou et al (2021), respectively.
All detailed grain yield and quality trait values of the mini-core collection are listed in Table S1.
Detection of 4808-bp and 266-bp InDels in the promoter of OsBZR1
The two big 4808-bp and 266-bp InDels were found when comparing the sequences of the indica variety MH63 and the japonica variety NIP. The young leaves of 533 accessions at 2-week-old seedlings were harvested and extracted with TPS buffer (0.1 M Tris–HCl, 0.01 M EDTA and 1 M KCl). We identified the genotypes of the 533 accessions via PCR using genomic primers. OsBZR1 (F1 + R1) and OsBZR1 (F2 + R1) were used to detect the 4808-bp InDel, OsBZR1 (F1 + R2) was used to detect the 266-bp InDel and the PCR products were separated by 2% agarose gel (Figure S7). All primers for genotyping are listed in Table S10.
Vector construction and plant transformation of transgenic plants
The sgRNA-Cas9 vector was provided by Prof. Yunde Zhao (Gao and Zhao, 2014). For the construction of the CRISPR vectors of OsBRI1, OsBAK1, OsGSK3, 23 nt sgRNA sequences specific to each target gene were designed, and their off-target possibility was detected at the off-target website (http://skl.scau.edu.cn/offtarget). For the construction of the complementation vector for OsGSK3, a 5.825-kb genomic region, including putative promoter and the complete open reading frame of OsGSK3 was amplified from ZH11 and cloned into the vector pCAMBIA1300. For the construction of the overexpression vectors, the coding sequences of OsBZR1 and Osbzr1-D were amplified from ZH11 and cloned into the vector pCAMBIA1301U driven by a maize ubiquitin promoter and fused with a 3 × FLAG tag at the C terminal, respectively. All the obtained constructs were confirmed by sequencing analysis and the CRISPR vectors and overexpression vectors were transformed into the ZH11 callus mediated by Agrobacterium strain EHA105 (Zhang et al., 2021). All primers for the vector construction are listed in Table S10.
RNA extraction and qRT-PCR
For expression analysis, total RNA was extracted from 1-week-old seedlings of MH63, OsGSK3-C plants, and 2-week-old seedlings leaves of ZH11, OsBZR1-OE (−), OsBZR1-OE (+) and osbzr1-D-OE plants, using a TRIzol RNA extraction kit (Invitrogen). Reverse transcription of total RNA used the M-MLV reverse transcriptase (Invitrogen). Real-time PCR reactions were performed with FastStart Universal SYBR® Green Master (Roche). The reaction components were mixed and the qRT-PCR reaction program using the standard protocol of the QuantStudio™ 6 Flex System. The Ubiquitin gene (LOC_Os03g13170) was used to normalize the data. At least two biological replicates were performed for each line. The primers used for qRT-PCR are listed in Table S10.
Genotyping the mutant alleles of OsBRI1 in osbri1-CR transgenic lines
We identified the genotypes of OsBRI1 in osbri1-CR transgenic lines via PCR using the genomic primers OsBRI1-PAGE-F and OsBRI1-PAGE-R. PCR products were separated by 6% PAGE gel. All primers for genotyping are listed in Table S10.
Detection of selection signatures
To detect regions with significant signatures of selective sweep, three methods (FST, nucleotide diversity (π/Pi) and Tajima's D) were used. We obtained the genetic variations in the 200-kb region around these genes in the RiceVarMapv2.0 database (including 295 indica and 156 japonica accessions), and converted them into variant call format (VCF) files, and then analysed their nucleotide diversity using VCFtools (0.1.16). Sliding window size of 100 kb and a step size of 10 kb was performed across the genome.
Split-LUC assay
Full-length cDNA fragments of OsBRI1BSH2-J and OsBAK1BSH2-J, OsBRI1BSH6-I and OsBAK1BSH6-I were amplified using total cDNA from ZH11 (japonica, J) and MH63 (indica, I) as the template, respectively. All the fragments were inserted into pCAMBIA1300-35S-cLUC-RBS and pCAMBIA1300-35S-HA-nLUC-RBS vectors (Chen et al., 2008), which were provided by Prof. Jianmin Zhou (Chinese Academy of Sciences). Two vectors containing the proteins for testing various protein–protein interactions were co-transfected into the same 3-week-old tobacco (Nicotiana benthamiana) leaves mediated by the Agrobacterium strain EHA105 with the same concentration of bacterium (OD = 1.0). After 2 days, LUC signals were captured by cooled CCD-imaging apparatus (Tanon 5200) with 1 mM luciferin (Promega, E1605). Each assay was repeated at least three times. Primers used in this assay are listed in Table S10.
Subcellular localization
Full-length cDNA fragments of OsBAK1BSH2-J and OsBAK1BSH6-I were cloned into pCAMBIA1300 vector fused with GFP at the C terminal, which was provided by Prof. Lizhong Xiong (Huazhong Agricultural University). Then, pCAMBIA1300-OsBAK1BSH2-J-GFP (OsBAK1BSH2-J-GFP) and pCAMBIA1300-OsBAK1BSH6-I-GFP (OsBAK1BSH6-I-GFP) were transiently expressed in the same 3-week-old tobacco leaves mediated by the Agrobacterium strain EHA105 with the same concentration of bacterium (OD = 1.0) or were transformed into rice protoplast cells with the same amounts of plasmids. The fluorescence signal was observed in the same fluorescence parameters with confocal laser scanning microscopy (Olympus FV1200) at 2 days after transformation in tobacco leaves or 16 h after transformation in rice protoplast cells. The GFP fluorescence intensity was quantified using the software ImageJ. Primers used in this assay are listed in Table S10.
Western blot
To compare the protein level of OsGSK3 between MH63 and OsGSK3-C lines, 2-week-old seedlings were prepared and saved in a –80 °C refrigerator. To compare the protein level of OsBAK1 between OsBAK1BSH2-J-GFP and OsBAK1BSH6-I-GFP fusion proteins in the same tobacco leaf epidermal cells, the same size of tobacco leaves containing OsBAK1BSH2-J-GFP or OsBAK1BSH6-I-GFP fusion proteins were prepared and saved in a –80 °C refrigerator. To compare the protein level and phosphorylation status of OsGSK3 and OsBZR1 between BSH2-J and BSH6-I, the 10-days young leaves and 3-cm panicles from these two rice subspecies were prepared and saved into −80 °C refrigerator. To extract proteins, samples above were ground into powder in liquid nitrogen, and a protein extraction buffer containing 150 mM NaCl, 50 mM Tris–HCl, PH 7.5, 50% glycerol, 2% Triton X-100, 1 mM EDTA and two tablets EDTA-free Protease Inhibitor Cocktail (Roche) every 100 mL was added into the powder. Samples were boiled with 5 × SDS loading buffer at 95 °C for 10 min and then centrifuged. The supernatants were detected by 12% SDS-PAGE gel. Western blot analysis was performed according to a general procedure. The OsBAK1BSH2-J-GFP and OsBAK1BSH6-I-GFP fusion proteins were detected by commercial GFP mouse antibody (ABclonal, AE012), the phosphorylation signal of OsBZR1 was separated by 12% SDS-PAGE gel and detected by the commercial anti-OsBZR1 (BPI, AbP80051-A-SE), the phosphorylation signals of OsGSK3, including OsGSK3BSH2-J and OsGSK3BSH6-I were separated by phos-tag gel and detected by the OsGSK3 rabbit antibody (provided by Prof. Ji Huang from Nanjing Agricultural University) and the Actin detected by the commercial anti-Actin (ABclonal, AC009) was used as the internal control. The relative protein abundance of OsBZR1 and OsGSK3 was calculated as IODOsBZR1/IODOsActin and IODOsBZR1/IODOsActin, respectively. The phosphorylation signal of OsBZR1 and OsGSK3 were calculated as IODOsBZR1+p/IODOsBZR1-p and IODOsGSK3+p/IODOsGSK3-p, respectively. The IOD (integrated optical density) values of OsBZR1, OsGSK3 and Actin were quantified using the software ImageJ.
Assays of relative promoter activity
Transient assays in tobacco leaves were used to test the effects on gene expression of all the variations in the promoter region of OsBAK1 or OsBZR1. Briefly, the 2-kb promoter fragments upstream of the translation start site of OsBAK1 or OsBZR1 were amplified from ZH11 and MH63 genomes with specific primers and then cloned into the multiple-cloning site of pGreenII 0800-LUC (Li et al., 2014), respectively. Then, pGreenII 0800-LUC containing the two different promoter fragments were transiently expressed in the same 3-week-old tobacco leaves mediated by the Agrobacterium strain EHA105 for 2 days at 28 °C. Then the same size of the transformed tobacco leaves containing the two different promoter fragments were prepared, ground into powder in liquid nitrogen and lysed in 50 μL passive lysis buffer. Firefly and Renilla luciferase were measured using a dual-luciferase assay kit (Promega, E1910) and a TECAN Infinite M200 microplate reader. In the same construct, the Renilla luciferase gene driven by CaMV 35S promoter was used as an internal transformation control. The ratio of LUC to REN activity (LUC/REN) was used to define relative promoter activity. Three biological replicates were conducted for each construct. Primers used for these vectors are listed in Table S10.
Purification of recombinant proteins
The coding sequences of OsGSK3BSH2-J (F + R2/3) and OsGSK3BSH6-I (F + R1/3) were amplified from ZH11 and MH63 total cDNA, respectively. Then, these fragments were sub-cloned into the pMAL-cRI-HAE vector (fused with MBP tag at the N-terminus) and expressed in BL21 (DE3), separately. Proteins were induced in LB medium overnight at 16 °C with 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) and purified by Amylose resin (NEW ENGLAND Biolabs, E8021S). The different forms of OsGSK3 were detected by 12% SDS-PAGE gel. The MBP-OsBZR1 protein, which was provided by Dr. Tao Wang, was also purified by the above method. Primers used for these vectors are listed in Table S10.
In vitro kinase assay
The kinase assay used 0.5 μg MBP-OsBZR1 and 0.3 μg MBP-OsGSK3BSH2-J or MBP-OsGSK3BSH6-I with a mixture containing 20 mM Tris-HCl, PH 7.5, 1 mM DTT, 10 mM MgCl2 and 100 μM ATP at 37 °C for 0, 2, 15, 30, 60 min, respectively. The kinase reactions were terminated by adding the SDS loading buffer and boiling at 75 °C for 10 min. The phosphorylation signal of OsGSK3BSH2-J and OsGSK3BSH6-I were detected by Pro-Q Diamond phosphoprotein gel stain (Molecular Probes, P33300) and captured by the Biorad ChemiDocMP Imaging system. The detailed experimental procedure of Pro-Q Diamond phosphoprotein gel stain was based on the manufacturer's instructions. The phosphorylation states of MBP-OsBZR1 were separated in an 8% (w/v) SDS-PAGE gel containing 100 mM phos-tag and 200 mM MnCl2. After electrophoresis, the gel was washed three times with 2 mM EDTA for 10 min each time, and then washed two times with transfer buffer (50 mM Tris and 40 mM Glycine) before being transferred to a polyvinylidene fluoride membrane. The MBP-OsBZR1 was detected by the OsBZR1 rabbit antibody (ABclonal, A16014). Protein loading amounts were displayed by CBB.
Acknowledgements
This study is supported by Biological Breeding-National Science and Technology Major Project (2023ZD0406902, 2023ZD0407301, 2022ZD0400401), National Natural Science Foundation of China (U22A20470, 32300502, 32301836), the National Key Research and Development Program of China (2021YFF1000202, 2022YFD1200103), Ten thousand Talents Programs, the Major Project of Hubei Hongshan Laboratory (2022hszd025, 2021hszd005), the Fundamental Research Funds for the Central Universities (2662023PY002) and the earmarked fund for the China Agriculture Research System (CARS-01-01) of China. We thank Changyin Wu and Weibo Xie (Huazhong Agricultural University) for kindly providing the RNA-seq data of OsBRI1, OsBAK1, OsGSK3, OsBZR1 and major genes regulated by OsBZR1 among the 533 rice accessions at 1-mm young panicle stage. We thank Lei Wang (Huazhong Agricultural University) for kindly providing the OsBRI1-RNAi and OsBZR1-RNAi transgenic lines; Tao Wang for kindly providing the MBP-OsBZR1 vector; Xuelu Wang and Shiyong Sun (Henan University) for providing the BR sensitivity data of the 533 accessions; and Ji Huang from Nanjing Agricultural University for kindly providing the OsGSK3 rabbit antibody.
Author contributions
X.Y.Y. constructed the genetic materials and collected the phenotypes of the transgenic lines, analysed the transcriptional data, collected the biochemical data, wrote and prepared the original draft. J.C.Z. performed the GWAS and evolution analysis. C.Y.Z. constructed part of genetic materials. P.K.X. and Y.H.L. collected part of grain quality traits of the 533 accessions. S.B.Y. provided the CSSLs of OsBAK1 from NIP in the 9311 background. Y.B.L. designed, conceptualized and supervised the research work, and wrote the manuscript.
Conflict of interest
All the authors declare no competing interests.
Code availability
Sequence data from this article can be found in the rice genome annotation project website (http://rice.uga.edu/cgi-bin/gbrowse/rice) and microRNA database (https://www.mirbase.org) under the following accession numbers: OsBRI1 (LOC_Os01g52050), OsBAK1 (LOC_Os08g07760), OsGSK3 (LOC_Os02g14130), OsBZR1 (LOC_Os07g39220), D11 (LOC_Os04g39430), BRD1 (LOC_Os03g40540), BRD2 (LOC_Os10g25780), OsDWARF4 (LOC_Os03g12260), CYP734A2 (LOC_Os02g11020), OsWRKY53 (LOC_Os05g27730), DLT (LOC_Os06g03710), OsLIC (LOC_Os06g49080), OsmiR396d (osa-MIR396d), OsGRF4 (LOC_Os02g47280) and OsBSK2 (LOC_Os10g42110).
Open Research
Data availability
The data that support the findings of this study are available from the corresponding author upon request. Source data are provided in this paper.




