CRISPR/Cas9-mediated disruption of DA1 enhances both biomass yield and quality in poplar
Biofuels have become important renewable energy alternative to fossil fuels in modern days. The non-food woody lignocellulose biomass drawn more attention to produce biofuel since it is not against the world's food security. Among which, poplar serves as an ideal woody biomass because of its fast growth and inherent secondary cell wall characteristics (Cho et al., 2018). Lignocellulose (secondary cell wall) is mainly composed of lignin, cellulose and hemicellulose. Among which, lignin imparts most significant recalcitrance in deconstruction of the wall materials for saccharification process. Lignin is consist of three monolignols, p-hydroxyphenyl (H), guaiacyl (G) and syringyl (S) units, and the relative abundance of H-, G- and S-units, especially the S/G ratio have been proved to be tightly associated with biomass recalcitrance (Zhang et al., 2020). Moreover, lignin-modified plants often exhibit irregular growth which consequently affect biomass production, thus limiting the benefits of lignin modification (Gui et al., 2020). Therefore, searching for the lignin-modified poplar genetic materials with improved both the quantity and quality of wood is critical for the utilization of woody lignocellulosic biomass in the production of biofuel.
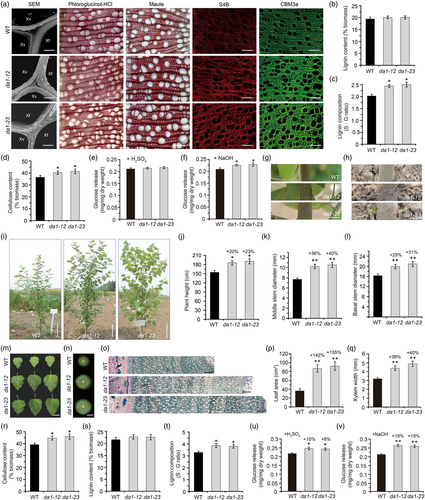
Previous studies revealed that DA1 gene family play a significant role in regulating organ and grain yields across various plant species, including maize, wheat and rice (Gao et al., 2021; Liu et al., 2020; Xie et al., 2018). In our previous study, we identified PagDA1 in poplar as a suppressor of vascular cambial development and wood formation (Tang et al., 2022). In this study, we further demonstrate da1 mutant created by CRISPR/Cas9 technology possesses great practical application in producing more and better wood biomass. We firstly found that da1 poplars exhibited significantly increased biomass compared with WT in greenhouse conditions, such as plant height, stem diameter and fresh weight (Figure S1a–d). Next, we investigated whether DA1 could impact the secondary wall deposition during wood formation. The expression patterns of PagDA1 genes and RNA in situ hybridization analysis revealed that both PagDA1a and PagDA1b showed high expression level in secondary xylem cell (Figure S2), indicating a potential role of PagDA1 in regulating secondary wall formation. Therefore, we analysed the secondary wall components and composition. While no significant difference in xylem fibre thickness between da1 and WT plants, a slight increase of vessel thickness was observed in da1-23 compared to the WT (Figure 1a; Figure S1e,f). We next stained lignin in cross sections of the basal stem xylem with phloroglucinol and it exhibited no significant difference between da1 and WT plants (Figure 1a), which was consistent with the lignin content detected (Figure 1b). We further evaluated lignin composition by analysing the lignin monomer content and found the abundance of S-lignin increased in both da1 lines through Maule staining (Figure 1a). Consistently, as measured by thioacidolysis and GC–MS, the S/G ratio was increased remarkably in da1 plants compared with WT (Figure 1c; Table S1). Furthermore, cellulose content detection revealed a marked 15% increase in da1 plants compared to WT (Figure 1d). The subsequent pontamine fast scarlet 4B (S4B) staining and 3 carbohydrate-binding module (CBM3a) immunolabelling assays further verified both cellulose and crystallized cellulose content exhibited an increase in da1 plants (Figure 1a). Increased S/G lignin ratio normally affects saccharification efficiency from poplar lignocellulosic biomass. We therefore investigated the saccharification efficiency in da1 plants. While no significant change of glucose release by H2SO4 treatment, an obviously increased glucose release was detected in da1 compared to WT plants by NaOH treatment (Figure 1e,f). Together, these results suggested da1 poplars grown in greenhouse exhibited both enhanced biomass yield and wood quality through modification of lignin composition.
We further performed transcriptome sequencing in the comparisons of da1 versus WT. The two da1 lines exhibited 756 differentially expressed genes (DEGs) relative to WT (Figure S3). These DEGs are related to various pathways, including cell wall biogenesis, cell division and secondary metabolites (Figure S3a). The expression of genes related to lignin biosynthesis, hemicellulose and pectin modification exhibited alterations in da1 compared to WT. Moreover, changes were observed in the expression of transcription factors regulating secondary cell wall and hormone biosynthesis genes (Figure S3b). Subsequently, we used qRT-PCR to validate the RNA-seq results. The expression of lignin biosynthesis genes were up- and down-regulated. Notably, the expression of S-unit lignin biosynthesis related genes F5H1 and COMT2 were increased in da1 compared with WT (Wu et al., 2019), which was in consistent with the enhanced content of S-lignin in da1 plants (Figure S4; Table S1). Consistent with the change in cellulose content, there was a substantial up-regulation in the expression of cellulose biosynthesis-related genes CESA7 and CESA8 (Figure S4). These results implied that transcriptomic changes in da1 mutants were consistent with the changes in secondary cell wall composition.
We also demonstrated a further improvement in biomass yield and quality of da1 poplars grown in the field than in the greenhouse. Compared to the WT, two da1 lines cultivated in the field for 1 year showed remarkable enhancement in plant height, stem diameter and leaf size, with increase of approximate 20% in height and >25% in both middle and basal stem diameters (Figure 1g–m). Moreover, the xylem formation of da1 lines displayed significantly increased (35%) compared to the WT (Figure 1n–q). These observations indicated da1 poplars exhibited significantly increase of biomass yield in field grown conditions. We further assessed the alterations of cell wall components and lignin composition as well as the saccharification efficiency of poplar plants grown in the field. These cell wall-related parameters were all in consistent with those grown in the greenhouse, and the glucose release of da1 lines cell wall residues (CWR) was significantly increased by approximately 10% when pretreated with H2SO4 or 15% by NaOH compared to WT (Figure 1r–v). In summary, our study revealed that knock out of DA1 genes in poplar can not only remarkably promote wood formation, but also significantly increase the content of cellulose and the S/G lignin ratio, thus ultimately enhance the saccharification efficiency. We also demonstrated a further improvement in biomass yield and quality of da1 poplars grown in the field than in the greenhouse. Therefore, this work imply a great potential of PagDA1 as a valuable target for the molecular breeding to improve both biomass yield and quality poplar, and the exact mechanism of poplar DA1 regulating plant biomass need to further investigated in the future.
Acknowledgements
We thank Prof. Yaoguang Liu (South China Agricultural University) for providing pYLCRISPR/Cas9 vectors. This work was supported by the National Key Research and Development Program of China (2021YFD2200205) and the National Natural Science Foundation of China (32322058, 32071725 and 32471904).
Conflict of interest
The authors have declared no conflict of interest.
Author contributions
Xianfeng Tang and Gongke Zhou designed the experiments. Xianfeng Tang, Yi Wang, Junhang Fu, Xiaofei Li and Wei Wang performed the experiments. Xianfeng Tang, Yi Wang, Dian Wang, Congpeng Wang, Mengzhu Lu and Shaofeng Li analysed the data. Dian Wang and Xianfeng Tang wrote the manuscript. All authors discussed the results and commented on the manuscript.
Open Research
Data availability statement
The data that supports the findings of this study are available in the supplementary material of this article.




