Simultaneous editing of three homoeologues of TaCIPK14 confers broad-spectrum resistance to stripe rust in wheat
Summary
Wheat stripe rust caused by the fungus Puccinia striiformis f. sp. tritici (Pst) is one of the most destructive wheat diseases resulting in significant losses to wheat production worldwide. The development of disease-resistant varieties is the most economical and effective measure to control diseases. Altering the susceptibility genes that promote pathogen compatibility via CRISPR/Cas9-mediated gene editing technology has become a new strategy for developing disease-resistant wheat varieties. Calcineurin B-like protein (CBL)-interacting protein kinases (CIPKs) has been demonstrated to be involved in defence responses during plant-pathogen interactions. However, whether wheat CIPK functions as susceptibility factor is still unclear. Here, we isolated a CIPK homoeologue gene TaCIPK14 from wheat. Knockdown of TaCIPK14 significantly increased wheat resistance to Pst, whereas overexpression of TaCIPK14 resulted in enhanced wheat susceptibility to Pst by decreasing different aspects of the defence response, including accumulation of ROS and expression of pathogenesis-relative genes. We generated wheat Tacipk14 mutant plants by simultaneous modification of the three homoeologues of wheat TaCIPK14 via CRISPR/Cas9 technology. The Tacipk14 mutant lines expressed race-nonspecific (RNS) broad-spectrum resistance (BSR) to Pst. Moreover, no significant difference was found in agronomic yield traits between Tacipk14 mutant plants and Fielder control plants under greenhouse and field conditions. These results demonstrate that TaCIPK14 acts as an important susceptibility factor in wheat response to Pst, and knockout of TaCIPK14 represents a powerful strategy for generating new disease-resistant wheat varieties with BSR to Pst.
Introduction
Bread wheat (Triticum aestivum L.) is the third-largest cultivated crop species worldwide after maize and rice and is the second-largest consumer food crop after rice (Acevedo-Garcia et al., 2017). Stripe rust caused by Pst is the most important fungal disease of wheat, and the prevalence of stripe rust causes yield losses of up to 50% (Bhardwaj et al., 2019; Herrera-Foessel et al., 2006). Recently, the rapid evolutionary dynamics of pathogens and changes in climate have favoured the development of stripe rust, resulting in more frequent and severe epidemics (Savadi et al., 2018). Currently, approaches to manage these diseases rely on cultivar resistance coupled with fungicide application (Savadi et al., 2018). Development of disease-resistant cultivars is the more cost-effective and environmentally sound strategy for limiting losses due to diseases. However, rust-resistant wheat varieties become susceptible within a short span of about 5–6 years due to the rapid evolution of new pathotypes of Pst, yet more than 10 years are required to develop and release a wheat variety by conventional breeding methods (Beddow et al., 2015). Thus, breeding of resistant wheat varieties via conventional approaches is a challenge for sustainable prevention and control of stripe rust. Therefore, new feasible methods must be discovered to breed for durable and BSR in crop plants at a faster pace.
Plant susceptibility (S) genes encode proteins that facilitate pathogen infection and support compatible plant-pathogen interactions (Garcia-Ruiz et al., 2021). Loss of S gene function may disturb the compatibility between host and pathogens, thereby providing durable and BSR (Garcia-Ruiz et al., 2021; van Schie and Takken, 2014). CRISPR/Cas9 gene editing system is very efficient, fast, easy, and cheap technique for generating targeted gene mutations (Ahmad et al., 2020; Cong et al., 2013). Recently, it has been shown that genetic manipulation of S genes (e.g., Mlo, EDR1, and DMR6) via CRISPR/Cas9 technology can confer disease resistance in a variety of economically important crops (Nekrasov et al., 2017; Wang et al., 2014; Yin and Qiu, 2019; Zaidi et al., 2018; Zhang et al., 2017). Therefore, mutation of S genes via CRISPR/Cas9 technology will be a new potential wheat breeding approach for durable and BSR to wheat rust fungus.
Calcium (Ca2+) is an important second messenger in plant cells. The cytoplasmic Ca2+ concentration in plant cells changes rapidly and dynamically in response to various endogenous or environmental factors, including biotic stress (Aldon et al., 2018; Zhang et al., 2014). The changes in the concentration of Ca2+ are sensed as calcium signal by Ca2+ binding proteins, including CBLs, which transmit or decode the calcium signal to the specific physiological response (Seybold et al., 2014). In plants, CBLs contain the helix E-loop–helix F (EF-hand) motif as the Ca2+ binding domain, and CBLs specifically interact with CIPKs to transmit calcium signals (Tang et al., 2020). Following sequencing of plant genomes, CBLs and CIPKs have been identified in many species throughout the plant kingdom, for example, 10 CBLs and 26 CIPKs have been identified in Arabidopsis thaliana, 10 CBLs and 34 CIPKs in rice, and 24 CBLs and 79 CIPKs in wheat (Ma et al., 2020). In response to different endogenous or environmental factors, different CBLs interact specifically with different CIPKs, resulting in various CBL/CIPK module combinations that trigger various specific signal transmission processes (Ma et al., 2020; Tang et al., 2020). At present, most CBL-CIPK components involved in various abiotic stresses have been intensively studied. However, the function of CBL-CIPK in biotic stress responses is still under-investigated. Recent evidence has shown that tomato CBL10-CIPK6 complex, rice OsCIPK14/15, rice OsCIPK23, rice OsCIPK31, wheat TaCBL4–TaCIPK5 complex, and wheat TaCIPK10 function as positive regulators in plant responses to biotic stress (de la Torre et al., 2013; Kurusu et al., 2010; Lin et al., 2021; Liu et al., 2018, 2019; Shi et al., 2018). In addition, AtCIPK6 and AtCIPK14 were reported as negative regulators in defence response to Pseudomonas syringae in Arabidopsis (Ma et al., 2021; Sardar et al., 2017). However, whether CBL-CIPK components from wheat function as negative regulators (or so-called susceptibility (S) factors) of disease resistance is still unclear.
Previous studies have found that the ectopic expression of wheat TaCIPK14 enhances salt and cold tolerance in tobacco (Deng et al., 2013). Phylogenetic analyses indicate that TaCIPK14 and TaCIPK15 are the orthologues of OsCIPK14/15 (Sun et al., 2015). In rice, OsCIPK14/15 positively regulates microbe-associated molecular pattern (MAMP)-induced defence responses (Kurusu et al., 2010). In wheat, transcript levels of TaCIPK14 and TaCIPK15 were affected by treatment with salicylic acid (SA) or Pst infection (Liu et al., 2019). TaCIPK14 and TaCIPK15 exhibited the different expression patterns in response to SA treatment or Pst infection (Liu et al., 2019), which suggested that TaCIPK14 and TaCIPK15 had functional divergence during the wheat-Pst interaction. In this study, we functionally characterized TaCIPK14 and TaCIPK15 and assessed their roles in wheat response to Pst. We demonstrated that TaCIPK14 positively regulates wheat susceptibility to Pst, whereas TaCIPK15 positively regulates wheat resistance to Pst. Then, we targeted knockout of TaCIPK14 by CRISPR/Cas9 technology. The triple-recessive homozygous mutant plants of TaCIPK14 expressed BSR to Pst without any effect on agronomic traits. Our results indicated that TaCIPK14 can be used as a potential target for breeding broad-spectrum and durable disease-resistant wheat varieties. In addition, this study serves as a basis towards advancing our understanding of the CBL-CIPK complex in susceptibility during fungal pathogen infection.
Results
Sequence analysis and subcellular localization of TaCIPK14 and TaCIPK15
According to the sequences identified in the wheat genome, the full-length coding sequences (CDSs) of TaCIPK14 and TaCIPK15 were obtained from wheat cv. Suwon11 by reverse transcription PCR (RT-PCR). The full-length CDS of TaCIPK14 is 1335 bp, encoding 444 amino acids. The full-length CDS of TaCIPK15 is 1317 bp, encoding 438 amino acids. Blastn analysis of the wheat cultivar Chinese Spring genome identified three copies of TaCIPK14 and three copies of TaCIPK15 on the A, B, and D sub-genomes, respectively. TaCIPK14 obtained from Suwon11 shared 100% nucleotide sequence identity with the copy on chromosome 4D (TaCIPK14-4D), and contained 35 nucleotide variations relative to other two copies on chromosomes 4A and 4B (TaCIPK14-4A and TaCIPK14-4B; Figure S1a). TaCIPK15 obtained from Suwon11 shared 100% nucleotide sequence identity with the copy on chromosome 5D (TaCIPK15-5D), and contained 78 nucleotide variations relative to other two copies on chromosomes 5A and 5B (TaCIPK15-5A and TaCIPK15-5B; Figure S1b).
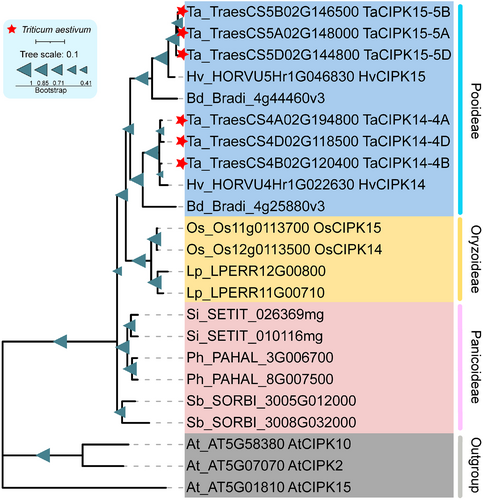
Amino acid sequences of OsCIPK14, OsCIPK15, TaCIPK14, and TaCIPK15 were aligned. The amino acid sequences of OsCIPK14 and OsCIPK15 were almost identical and differed in only a single amino acid substitution except for the five additional amino acids. TaCIPK14 and TaCIPK15 share only 81.31% nucleotide sequence identity and 80.04% amino acid sequence identity (Figure S2). A phylogenetic tree was constructed and revealed that the TaCIPK14 and TaCIPK15 are orthologues of OsCIPK14/15. However, TaCIPK14 and TaCIPK15 exhibited differentiation with a farther genetic relationship than OsCIPK14 and OsCIPK15 (Figure 1).
In silico sequence analysis revealed that TaCIPK14 and TaCIPK15 contain a catalytic kinase domain and a regulatory domain (Figure S2). The activation loop containing three conserved phosphorylatable residues (threonine, serine, and tyrosine) was found in the catalytic kinase domain. The NAF motif, which mediates CIPK binding to CBL, was found in the regulatory domain (Figure S2). In addition, the GFP fusion protein of TaCIPK14 and TaCIPK15 was mainly distributed in the cytoplasm and nucleus of wheat protoplasts (Figure S3a,c). The expression of TaCIPK14 and TaCIPK15 was confirmed by western blotting with a GFP-antibody (Figure S3b,d).
TaCIPK14 interacts with several TaCBLs
The CBL-CIPK pathway is conserved in different species. Therefore, we predicted that one or several CBLs in wheat may interact with TaCIPK14. To test this hypothesis, we analysed the interaction between TaCBLs and TaCIPK14 using the yeast two-hybrid assay. The results showed that TaCIPK14 interacts with all seven TaCBLs (TaCBL1.1, TaCBL1.2, TaCBL2, TaCBL3, TaCBL4, TaCBL6, and TaCBL9) in yeast (Figure S4a). To further investigate the interaction strength of different combinations, β-galactosidase activity analysis was performed. The results showed that the interactions of TaCIPK14 with TaCBL2, TaCBL4, or TaCBL6 exhibited the stronger interactions with higher β-galactosidase activity compared with other TaCBLs (Figure S4b).
Knockdown of TaCIPK14 reduces wheat susceptibility to Pst
To determine the role of TaCIPK14 and TaCIPK15 during the wheat response to Pst, the barley stripe mosaic virus (BSMV)-induced gene silencing (VIGS) system was used to knockdown the expression of TaCIPK14 and TaCIPK15. Photobleaching was observed on leaves infected with BSMV:TaPDS-as at 10 days post-inoculation (dpi) (Figure 2a, Figure S5a), indicating that the VIGS system is functioning. The mild chlorotic mosaic symptoms were observed on leaves expressing the VIGS constructs of TaCIPK14 and TaCIPK15, respectively (Figure 2a, Figure S5a). We inoculated the BSMV-treated wheat plants with Pst virulent race CYR31 and avirulent race CYR23, respectively. Fewer uredia were produced in TaCIPK14-knockdown plants (BSMV:TaCIPK14-1/2as) than that in control plants (BSMV:γ) at 15 dpi with CYR31, indicating increased resistance in TaCIPK14-knockdown plants (Figure 2b). However, sporulation of CYR31 showed no difference between TaCIPK15-knockdown (BSMV:TaCIPK15-1/2as) and control plants (Figure S5b). All leaves infected with CYR23 exhibited chlorotic and necrotic symptoms (Figure 2b, Figure S5b). A few fungal uredia surrounding the necrotic areas were observed in TaCIPK15-knockdown plants but not in TaCIPK14-knockdown and control plants (Figure 2b, Figure S5b). In comparison with the control plants, the fungal biomass of CYR31 was decreased by 27.8%–33.9% in TaCIPK14-knockdown plants (Figure 2c). No significant changes in fungal biomass of CYR31 were observed between TaCIPK15-knockdown and control plants (Figure S5c). After inoculation with CYR23, no significant changes in fungal biomass were observed between TaCIPK14-knockdown and control plants (Figure 2c). However, the fungal biomass of CYR23 was significantly increased by about 64%–66% in TaCIPK15-knockdown plants compared to that in control plants (Figure S5c).
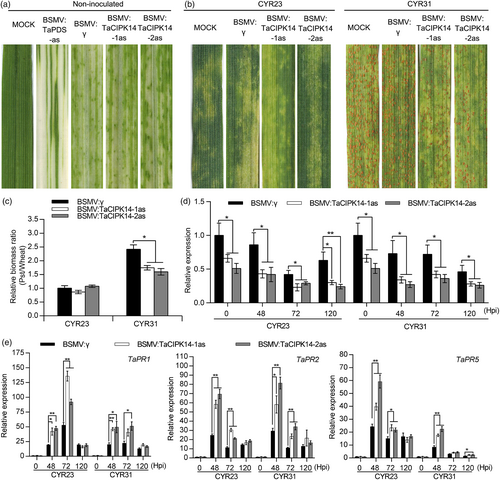
Then, silencing efficiency of TaCIPK14 and TaCIPK15 was analysed by quantitative RT-PCR (qRT-PCR). In comparison with the control plants, the transcripts of TaCIPK14 were reduced by about 31%–62% at 0, 48, 72, and 120 h post-inoculation (hpi) with CYR23 and about 34%–63% at 0, 48, 72, and 120 hpi with CYR31 in TaCIPK14-knockdown plants (Figure 2d). The transcripts of TaCIPK15 were reduced by about 34%–63% at 0, 48, 72, and 120 hpi with CYR23 and about 37%–63% at 0, 48, 72, and 120 hpi with CYR31 in TaCIPK15-knockdown plants compared to that in control plants (Figure S5d). Therefore, the expression levels of TaCIPK14 and TaCIPK15 were successfully silenced by VIGS.
To determine whether transcript accumulation of defence-related genes was affected by knockdown of TaCIPK14 and TaCIPK15, the transcript levels of three pathogenesis-related (PR) genes were measured. The results showed that the transcript levels of TaPR1, TaPR2, and TaPR5 were significantly increased in TaCIPK14-knockdown plants compared to that in control plants, albeit inconsistently (TaPR1 and TaPR2 at 48 and 72 hpi with CYR23 and CYR31; TaPR5 at 48, 72 hpi with CYR23 and 48 hpi with CYR31). Subsequently, the transcript levels of TaPR1, TaPR2, and TaPR5 in TaCIPK14-knockdown plants were rescued to control equivalent levels at 120 hpi compared to that in control plants (Figure 2 e). In contrast, compared with control plants, the transcript levels of TaPR1, TaPR2, and TaPR5 in TaCIPK15-knockdown plants were slightly induced by Pst at 48 and 72 hpi, and rescued to control equivalent levels at 120 hpi (Figure S5e).
To determine whether the phenotypic changes between control and TaCIPK14/TaCIPK15-knockdown plants are associated with fungal growth and development, the Pst-infected leaves were analysed microscopically (Figure S6a). In TaCIPK14-knockdown plants, the number of haustoria, hyphal length, and infection area were significantly decreased compared to that in control plants inoculated with CYR31 (Figure S6a–d). In contrast, the hyphal length and the infection area in TaCIPK15-knockdown plants were significantly higher than that in control plants at 72 and 120 hpi with CYR23 (Figure S7a–c). To clarify whether the silencing of TaCIPK14 and TaCIPK15 led to the changes in host resistance level to Pst, the accumulation of H2O2 around the Pst infection sites was detected by DAB staining. The area of H2O2 accumulation was significantly increased in TaCIPK14-knockdown plants compared to the control at 48 and 72 hpi with CYR31 (Figure S6e,f). In contrast, the area of H2O2 accumulation was significantly lower in TaCIPK15-knockdown plants than that in control plants at 72 hpi with CYR23 (Figure S7d,e). Taken together, the results indicate that silencing of TaCIPK14 reduces wheat susceptibility to Pst, whereas silencing of TaCIPK15 reduces wheat resistance to Pst.
Overexpression of TaCIPK14 enhances wheat susceptibility to Pst
To further investigate the role of TaCIPK14 during the wheat-Pst interaction, we developed transgenic wheat plants in which TaCIPK14 was overexpressed. Eight positive transgenic plants were identified from 24 T0 plants by PCR with specific primers (Figure S8b). The T1 generation plants, derived from relevant T0 generations, were further identified by PCR and qRT-PCR (Figure S8c,d). The expression level of TaCIPK14 was upregulated in six positive T1 generation plants compared to Fielder control plants (Figure S8d). Chlorotic and necrotic areas appeared on leaves inoculated with CYR23 at 15 dpi (Figure S8e). A few fungal uredia surrounding the necrotic areas were observed on leaves of these six positive T1 lines but not in other plants, indicating that TaCIPK14-overexpression (OE) lines exhibited enhanced susceptibility to Pst (Figure S8e). Compared with Fielder plants, fungal biomass was significantly increased in five of the six positive T1 lines inoculated with CYR23 (Figure S8f). Three T1 lines with the highest transcript levels among the five lines, TaCIPK14-OE15, TaCIPK14-OE17, and TaCIPK14-OE23, were selected for further experiments.
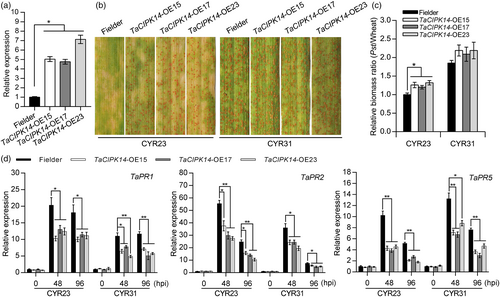
At the T2 generation, the plants from TaCIPK14-OE15, TaCIPK14-OE17, and TaCIPK14-OE23 were identified by PCR with specific primers (Figure S8g). qRT-PCR assay showed that the transcript level of TaCIPK14 was 4.76–7.13-fold higher in the three TaCIPK14-OE lines than that in Fielder (Figure 3a). Then, the positive T2 plants were challenged with CYR23 and CYR31, respectively. At 15 dpi with CYR23, the T2 generation plants expressed increased susceptibility with production of a few fungal uredia compared to Fielder plants (Figure 3b). In contrast, the T2 transgenic lines infected with CYR31 exhibited a similar susceptible phenotype with production of extensive uredia compared to Fielder plants (Figure 3b). Compared with Fielder plants, fungal biomass was significantly increased by 20%–32% in TaCIPK14-OE plants inoculated with CYR23 (Figure 3c). In addition, the PR genes (TaPR1, TaPR2, and TaPR5) showed lower transcript levels in the transgenic plants than that in Fielder plants after inoculation with CYR23 and CYR31, respectively (Figure 3d). Compared to Fielder plants, TaCIPK14-OE plants showed greater hyphal length and infection area of Pst and less H2O2 accumulation (Figure S9a–d). These results suggest that elevated expression of TaCIPK14 enhanced wheat susceptibility to Pst infection and decreased host defence response.
Targeted knockout of TaCIPK14 by CRISPR/Cas9 technology
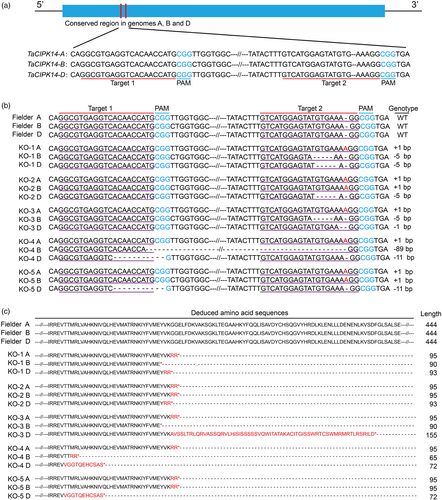
We designed two guide RNAs (gRNAs) for targeting the CDS at the 5′ end of TaCIPK14-4A, TaCIPK14-4B, and TaCIPK14-4D, respectively, based on the conserved sequences among these three copies of wheat (Figure 4a, Figure S1a). The gRNA1 and gRNA2 cassettes driven by the TaU6 promoter were simultaneously cloned into the vector VK005-6 (Figure S10a). We then transformed the CRISPR/Cas9 vectors into Fielder by Agrobacterium-mediated transformation. We identified 22 transformed plants, 18 (81.8%) of which showed the integration of Cas9, the gRNA cassette, and Bar fragments into the genome (Figure S10b).
To identify the mutation types, we designed specific primers to sequence the target region of the A, B, and D genomes (Figure S1a, Table S5). We identified three mutant plants (T0-4, T0-12, T0-21) from 18 transgenic plants by genome-specific PCR and Sanger sequencing (Figure S11a,b). To further verify the specific mutation type, the PCR products were subjected to TA-cloning and subsequent Sanger sequencing. Two mutant plants (T0-4 and T0-12) had mutations for gRNA2, and one mutant plant (T0-21) had mutations for gRNA1 and gRNA2 (Table S1). However, the mutations were present in only two of three diploid genomes in each plant of these three mutant plants (Figure S11a,b, Table S1). T0-4 had heterozygous mutations in TaCIPK14-4A and TaCIPK14-4B, but no mutation in TaCIPK14-4D. T0-12 had heterozygous mutations in TaCIPK14-4B, chimeric mutations in TaCIPK14-4D, but no mutation in TaCIPK14-4A. T0-21 had heterozygous mutations in TaCIPK14-4B and TaCIPK14-4D, but no mutation in TaCIPK14-4A (Table S1).
To investigate whether the mutations could be transmitted to the next generation, we self-pollinated these three mutant plants that carried mutations in the two diploid genomes, and genotyped individual T1 progeny using TaCIPK14 allele-specific primers. We selected 71 T1 progenies derived from the T0 plants for further genotyping. The mutations detected in T0 plants were transmitted to the T1 generation without new mutations. The mutations that were heterozygous in the T0 generation segregated in a Mendelian fashion (homozygous/heterozygous/WT = 1:2:1) in the T1 generation derived from lines T0-4, T0-12, and T0-21 (Table S1). The transmission of homozygous and heterozygous mutations from 12 T1 plants to their T2 offspring was further analysed by PCR and sequencing. Again, the homozygous mutations were 100% transmitted, whereas the heterozygous mutations segregated in a Mendelian fashion (Table S2). We obtained three T2 transgenic wild-type lines and seven double-recessive homozygous T2 mutant lines by genetic segregation (Figure S12a, Table S2). These results demonstrate that the mutations observed in primary transformed bread wheat plants (T0) can be stably transmitted to subsequent generations.
To obtain a triple-recessive homozygous mutant by genetic segregation, we crossed T2 lines aabbDD (T2-4-1) with AAbbdd (T2-12-12) and AAbbdd (T2-21-12), and crossed T2 lines aabbDD (T2-4-13) with AAbbdd (T2-12-1) and AAbbdd (T2-21-2), separately. We obtained 37 F1 plants (Figure S12a, Table S3). Then, individual F1 progenies were genotyped by PCR and sequencing using genomic-specific primers. As expected, all the F1 plants were triple heterozygotes. Then, we self-pollinated F1 plants, and further genotyped individual F2 progenies by RCR and sequencing using genomic-specific primers. We obtained two single-recessive homozygous mutant lines, four double-recessive homozygous mutant lines, and five triple-recessive homozygous mutant lines with specific genotypes from 281 F2 progenies (Figure 4b, Figure S12a, Table S3). All the mutations detected in F2 plants were from hybrid parent T2 lines, without occurrence of new mutations. All mutations caused frameshifts at the C-terminal regions in the predicted amino acid sequences and maintained the wild-type sequences in other regions of the loci (Figure 4c), indicating that all mutant lines had completely lost expression of TaCIPK14.
To investigate the off-target effects in the three T2 double-recessive homozygous mutants (T2-4-1, T2-21-2, and T2-12-1) and the five F2 triple-recessive homozygous mutants (KO-1, KO-2, KO-3, KO-4, and KO-5), seven potential genome-wide off-target sites for these two gRNAs were identified (Table S4). Then, genome-specific PCR and sequencing were performed to identify the off-target effect. We found no mutations at those selected potential genome-wide off-target sites (Table S4). We then used primer sets designed to specifically amplify Cas9, the gRNA cassette, and Bar sequences to determine whether these mutant lines were transgene-free plants without plasmid DNA (Figure S10a). We did not obtain transgene-free plants from these mutant lines (Figure S10c). We obtained transgene-free plants from the F4 triple-recessive homozygous mutant line KO-1 by genetic segregation (Figure S10d). The transgene-free triple-recessive homozygous mutant plants were defined as KO-1(Cas9-). These Tacipk14 mutant plants were selected for further experiments.
Triple-recessive homozygous mutant plants showed BSR to Pst
We further evaluated the resistance of the Tacipk14 mutant plants to Pst by inoculation with CYR31. At 15 dpi, the five F2 triple-recessive homozygous mutant lines expressed enhanced resistance with a significant reduction in sporulation compared to Fielder control plants (Figure S12b). However, the T2 double-recessive homozygous mutants exhibited a similar phenotype with production of extensive uredia compared to Fielder plants (Figure S12b). Moreover, compared with Fielder plants, the fungal biomass was significantly reduced in the triple-recessive homozygous mutant lines but not in the double-recessive homozygous mutant lines (Figure S12c). These results suggested that only the triple-recessive homozygous mutation of TaCIPK14 confers resistance to Pst in wheat.
To determine whether transgene insertion has an effect on stripe rust resistance, the F4 transgene-free plants KO-1(Cas9-), together with other F4 single-, double-, and triple-recessive homozygous mutant lines that had transgene insertion (Figure S10d), were inoculated with Pst race CYR32. Only the triple-recessive homozygous mutants (KO-1, KO-1(Cas9-), KO-4), but not the single- or double-recessive homozygous mutants, exhibited enhanced resistance to Pst with production of fewer uredia and fungal biomass compared to Fielder control plants (Figure S12d,e). These results suggested that the transgene insertion has no effect on wheat resistance to Pst.
To test whether the triple-recessive homozygous mutants confer BSR resistance to Pst, we challenged these plants with five virulent Pst races (CYR29, CYR31, CYR32, CYR33, and CYR34). The three T3 transgenic wild-type wheat lines (T3-4-11, T3-12-6, and T3-21-9) (Figure S10e, Figure S12a, Table S2) and Fielder plants challenged with the same Pst races were used as controls. It is worth noting that the current prevalent Pst races in China are CYR32, CYR33, and CYR34 (Liu et al., 2017). At 15 dpi, the triple-recessive homozygous mutants, but not the transgenic wild-type wheat lines, exhibited enhanced resistance with production of fewer uredia compared to Fielder (Figures 5a,c,e,g,i, S13a–e). The fungal biomass also showed a significant decrease in the triple-recessive homozygous mutants (Figure 5b,d,f,h,j). In addition, the triple-recessive homozygous mutants had higher expression levels of TaPR1, TaPR2, and TaPR5 and lower expression levels of reactive oxygen species (ROS) scavenger-related gene TaCAT2 than Fielder during Pst infection (Figure S14a–d). Compared to Fielder plants, the triple-recessive homozygous mutants showed less hyphal length and infection area of Pst, and greater area of H2O2 accumulation and necrotic cell death triggered by Pst (Figure S15a–g). Taken together, the results indicate that knockout of TaCIPK14 confers BSR to Pst.
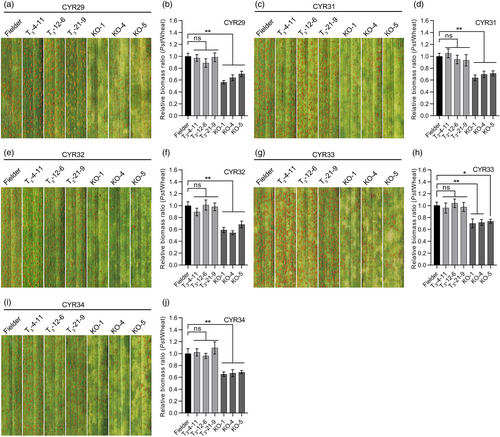
We further evaluated the adult-plant stripe rust resistance of the triple-recessive homozygous mutants by inoculation with a mixture of races CYR32, CYR33, and CYR34 in the field in 2021 and 2022. The results showed that the triple-recessive homozygous mutant plants exhibited enhanced resistance with fewer uredia and lower fungal biomass compared to Fielder control plants (Figure 6a–c). In 2021, the triple-recessive homozygous mutant lines (KO-1, KO-4, KO-5) showed average disease severities of 52.9%, 53.2%, and 53.2%, respectively, significantly lower than that of Fielder which exhibited an average disease severity of 60.4% (Figure S16g). In 2022, the triple-recessive homozygous mutant lines (KO-1, KO-4, KO-5) showed average disease severities of 54.4%, 49.5%, and 50.7%, respectively, significantly lower than that of Fielder plants which exhibited an average disease severity of 64.5% (Figure 6d). Collectively, the results suggested that knockout of TaCIPK14 contributes to the adult-plant stripe rust resistance in natural settings.

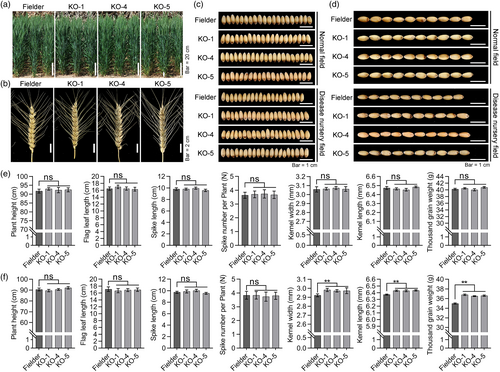
Agronomic yield traits of the triple-recessive homozygous mutant lines
To determine whether the Tacipk14 mutant plants had any negative effects on key agronomic traits, both greenhouse and field experiments were conducted. Without Pst inoculation, the seven yield-related traits of triple-recessive homozygous mutant lines were nearly identical to that of Fielder control plants under greenhouse and field conditions (Figure 7a–e, Figure S16a–f). With Pst inoculation, a dramatic reduction in kernel length, kernel width, and 1000 grain weight was detected in Fielder plants compared with the triple-recessive homozygous mutant plants under field conditions in 2022 (Figure 7c–f). The triple-recessive homozygous mutant plants inoculated with Pst displayed increased kernel length, kernel width, and 1000 grain weight compared with the Fielder plants under field conditions in 2021 and 2022 (Figure 7c,d,f, Figure S16h). These results demonstrated that knockout of TaCIPK14 reduces grain yield loss caused by stripe rust disease.
Discussion
The functions of TaCIPK14 and TaCIPK15 were differentiated in wheat defence response to Pst infection
Previous studies showed that gene duplication and separation of the latter stages are the two main objectives of evolution, resulting in the diversity of gene family members (Chothia et al., 2003). In Pooideae, Oryzoideae, and Panicoideae plants, CIPK14 is the paralogue of CIPK15. In rice, it was suggested that OsCIPK14 and OsCIPK15 are duplicated genes (Kurusu et al., 2010). TaCIPK14 and TaCIPK15 are orthologues of OsCIPK14/15 in wheat. They exhibited more differences in amino acid sequences than that in OsCIPK14/15 (Figure S2). Phylogenetic analysis showed that the intergeneric genetic relationship between CIPK14 and CIPK15 in Pooideae is stronger than the intrageneric relationship (Figure 1). However, in Oryzoideae and Panicoideae, the intergeneric genetic relationship between CIPK14 and CIPK15 is weaker than the intrageneric relationship (Figure 1). The results suggest that the common ancestor of Pooideae, Oryzoideae, and Panicoideae formed the CIPK14 and CIPK15 via gene duplication during the evolutionary process. Sequence differentiation of CIPK14 and CIPK15 occurred in Pooideae but not in Oryzoideae and Panicoideae due to a high-stress environment. Duplication and differentiation of genes lead to the diversity of gene functions, which play key roles in promoting the evolution of new features, organ differentiation, and better adaptation to changes in the environment (Flagel and Wendel, 2009). In rice, OsCIPK14/15 positively regulate MAMP-induced defence responses (Kurusu et al., 2010). In this study, TaCIPK15 was found to positively regulate defence responses to Pst, while TaCIPK14 negatively regulates defence responses to Pst, suggesting that the function of TaCIPK14 and TaCIPK15 is differentiated in wheat. In Solanum lycopersicum, SlCIPK6 was shown to positively regulate ROS production and Pto-mediated resistance against Pseudomonas syringae pv tomato DC3000 (de la Torre et al., 2013). However, AtCIPK6, an orthologue of SlCIPK6 in Arabidopsis, acts as a negative regulator in the PTI and SA-mediated immune response to P. syringae pv tomato DC3000 (Sardar et al., 2017). This indicates that AtCIPK6 and SlCIPK6 have contrasting functions in response to biotic stress. Thus, in line with previous studies, functional changes between CIPK homoeologues genes in different organisms may be a common phenomenon during the evolutionary process.
TaCIPK14-mediated wheat susceptibility to Pst may be via regulating the SA signalling pathway
SA is commonly known as phytohormone that regulates the defensive response against biotic stresses. Several studies have demonstrated that CIPKs regulate SA signalling in response to biotic stresses (Ma et al., 2020, 2021; Sardar et al., 2017). For instance, Arabidopsis CIPK14 and CIPK6 were reported to negatively regulate accumulation of SA and expression of defence marker gene PR1 in response to Pseudomonas syringae (Ma et al., 2021; Sardar et al., 2017). In addition, several studies showed that CBL-CIPK directly regulates the SA signalling pathway by targeting the components of the pathway (Liu et al., 2019; Xie et al., 2010). PR1, PR2, and PR5 are the marker genes for SA-mediated activation of SAR (Zhang et al., 2010). In this study, TaCIPK14-knockdown and TaCIPK14-knockout plants showed higher transcript levels of TaPR1, TaPR2, and TaPR5 with significantly lower fungal biomass compared to control plants when inoculated with virulent races. However, no significant change was found in fungal biomass compared to control plants in TaCIPK14-knockdown plants when inoculated with avirulent race CYR23. Suwon11, carrying the YrSu resistance gene, is resistant to CYR23 infection, but is highly susceptible to CYR31 (Cao et al., 2003). Therefore, we speculated that the significantly increased transcript levels of PRs caused by knockdown of TaCIPK14 may be not sufficient to change the resistance phenotype which is mediated by YrSu during the incompatible interaction. Taken together, our findings suggested that TaCIPK14-mediated wheat susceptibility to Pst may be via regulating the SA signalling pathway.
TaCIPK14 negatively regulated ROS production during the interaction between wheat and Pst
As a function of secondary signalling processes, ROS are indispensable in plant defence responses (Lehmann et al., 2015). In wheat, bursts of ROS as early as 12 hpi are associated with the activation of defence signalling in incompatible interactions with Pst (Wang et al., 2007). ROS are known to crosstalk with CBL-CIPK signalling in plants to regulate signal transduction processes (Ma et al., 2020; Narayanan et al., 2020). In this study, knockout of TaCIPK14 resulted in lower expression levels of ROS scavenger-related gene TaCAT2 during Pst infection. In the meantime, histological and cytological observations showed that H2O2 accumulation triggered by Pst was significantly increased in both TaCIPK14-knockdown and TaCIPK14-knockout plants. This result suggests that TaCIPK14 negatively regulates ROS accumulation in wheat defence against Pst. This observation is in agreement with previous studies in tobacco showing that TaCIPK14 overexpression resulted in the accumulation of lower contents of H2O2 under salt and cold stress (Deng et al., 2013), suggesting that TaCIPK14 responds via the same mechanism to deal with abiotic and biotic stresses. ROS are the main triggers of the hypersensitive response (HR). In this study, the triple-recessive Tacipk14 mutants showed more HR-associated cell death triggered by Pst than Fielder plants. Thus, we infer that TaCIPK14 mediates wheat susceptibility to phytopathogen attacks by suppressing ROS production.
As a member of plant calcium sensors, CBLs interact with CIPKs to form a CBL-CIPK complex to regulate plant development and stress responses (Tang et al., 2020). In this study, we found that TaCBL2, TaCBL4, and TaCBL6 may be involved in TaCIPK14-mediated immune responses due to the stronger interactions with TaCIPK14. Future studies will focus on identifying the exact upstream CBLs and downstream substrates of TaCIPK14 to reveal the details about how TaCIPK14 functions as a susceptibility factor in immune response to Pst.
Tacipk14 knockout mutant plants confer BSR to Pst
RNS-BSR refers to resistance against two or more races or strains of the same pathogen (Li et al., 2020). In the process of plant-pathogen interaction, complex and interconnected defence signalling pathways are activated following the perception of PAMPs or effectors. These defence signalling pathways involve many biological processes, including transcription regulation, epigenetic regulation, post-translational modifications, protein–protein interactions, and calcium signalling (Li et al., 2020). Disruption of the negative regulators in these signalling processes confers BSR (Li et al., 2020). For instance, rice OsMPK15 and soybean GmMEKK1, components of the MAPK cascade, negatively regulate PR gene expression and ROS accumulation. The osmpk15 mutant confers BSR against Xanthomonas oryzae pv. oryzae (Xoo) and multiple Magnaporthe oryzae strains, and loss function of GmMEKK1 confers BSR against multiple species of pathogens (Hong et al., 2019; Xu et al., 2018a). In this study, TaCIPK14, being an important component of the calcium signal transduction pathway, negatively regulates PR gene expression and the accumulation of ROS. Similar to previous studies, simultaneous editing of three homoeoalleles of TaCIPK14 confers BSR to multiple races of Pst. We are currently testing the resistance of Tacipk14 mutants to wheat leaf rust, stem rust, Fusarium head blight, powdery mildew, and sheath blight to examine the resistance spectrum of the Tacipk14 plants. Thus, this study provides a candidate gene for developing resistant varieties with improved BSR resistance to stripe rust.
Plant CRISPR/Cas9 genome editing may generate unintended off-target mutations. Moreover, such ‘off-target’ effects may cause unknown, nonquantifiable cellular signalling or physiological effects, which may impede functional analysis and gene activity studies (Kadam et al., 2018). In apple (Charrier et al., 2019), grape (Wang et al., 2018), and wheat (Li et al., 2021; Zhang et al., 2017, 2021a), editing activity was not detected by the analysis of off-target regions showing three or four mismatches with the guide RNAs. In our work, five potential genome-wide off-target sites were identified in the three T2 double-recessive homozygous mutants and five F2 triple-recessive homozygous mutants by PCR and Sanger sequencing (Table S4). In line with previous studies, no mutations were detected in any of the samples tested (Table S4). The results indicated that the enhanced resistance to stripe rust in the TaCIPK14-knockout wheat lines compared with the control is not related to off-target events.
Knockout of TaCIPK14 resulted in no yield penalty in wheat
Although disrupting susceptibility genes can generate germplasm with BSR against pathogens, some susceptibility genes have additional functions in plant growth and development (van Schie and Takken, 2014). Disruption of susceptibility genes may cause pleiotropic effects, including reduced growth, programmed cell death, low fertility, and loss of tolerance to other stresses (van Schie and Takken, 2014; Zaidi et al., 2018). However, some susceptibility genes function as suppressors of defence persistence, and knockout of these susceptibility genes is expected to have fewer pleiotropic effects and more suitable for application (van Schie and Takken, 2014). In this study, the transcript levels of defence-related marker genes in triple-recessive homozygous mutant lines were similar to those in Fielder control plants under normal growth conditions without Pst infection (Figure S14). Moreover, multiple key agronomic yield traits of the triple-recessive homozygous mutant lines were evaluated under normal growth conditions, and no significant differences were observed in these traits compared with Fielder plants (Figures 7, S16). Therefore, these results suggested that TaCIPK14 may function as a suppressor of defence persistence, and loss function of TaCIPK14 will be suitable for application. Previous studies showed that ectopic expression of wheat TaCIPK14 confers salinity and cold tolerance in tobacco (Deng et al., 2013). Thus, we are currently assessing the performance of TaCIPK14-knockout plants in the field under salinity, cold, and other abiotic stresses to better understand the breadth of their utility. In our future studies, we will cultivate the disease-resistant varieties by using this TaCIPK14-knockout mutant as a donor parent in backcrossing breeding programs, or directly knocking out TaCIPK14 in high-yield wheat varieties through CRISPR/Cas9 technology. Taken together, our work is an example of successful genome editing to generate an important BSR trait in wheat.
Experimental procedures
Plant materials and fungal strains
Wheat cultivar Suwon11 was used for VIGS assays in this study. Suwon11, carrying the YrSu resistance gene, is resistant to CYR23 and susceptible to CYR31 (Cao et al., 2003). Wheat cultivar Fielder was used as receptor material for generating TaCIPK14-OE transgenic wheat plants and TaCIPK14-knockout plants. Fielder, carrying the Yr6 and Yr20 genes (Chen, 2007), is resistant to CYR23 and susceptible to CYR29, CYR31, CYR32, CYR33, and CYR34. Pst races CYR23, CYR29, CYR31, CYR32, CYR33, and CYR34 were used for investigating stripe rust resistance.
Evaluation of stripe rust resistance and yield-associated agronomic traits
For assessments of stripe rust reactions at the seedling stage, wheat plants were cultivated under controlled greenhouse conditions and inoculated with Pst as described previously (Cao et al., 2003). Evaluation of disease resistance to stripe rust was performed according to a previous study (Liu et al., 2018). For adult-plant resistance evaluations, field experiments were conducted on Cao Xinzhuang experimental farm of Northwest A&F University in Yangling, China in 2021 and 2022. The triple-recessive homozygous mutant lines and Fielder control plants were grown under two distinct conditions: a normal field without Pst inoculation and a disease nursery field inoculated with Pst. For the stripe rust disease nursery field, all materials were inoculated at the jointing stage with a mixture of equal quantities of urediniospores of Pst races CYR32, CYR33, and CYR34. At the early grain-filling stage, disease severity was scored as the percentage of infected leaf area according to the modified Cobb scale (Peterson et al., 1948), and at least 100 plants in each replicate were scored. The proportion of the Pst sporulation area in each leaf was measured by Photoshop software according to a previous study (Peng et al., 2020). For all the materials grown in field and greenhouse conditions, multiple growth, development, and yield parameters were analysed with three replications as described previously (Zhang et al., 2021b).
Subcellular localization
To determine the localization of TaCIPK14 and TaCIPK15, the recombinant plasmids pCaMV35S:TaCIPK14-GFP, pCaMV35S:TaCIPK15-GFP, and pCaMV35S:GFP were constructed and independently transferred into wheat protoplasts by the polyethyleneglycol (PEG)-calcium method as described previously (Liu et al., 2018). The transformed wheat protoplasts were cultured at 22 °C for 18–48 h. Images were obtained with a confocal laser scanning microscope (Olympus microscope FV3000, Tokyo, Japan). Protein expression in wheat protoplasts was confirmed by Western blots.
Yeast two-hybrid assays
For interaction analysis of TaCIPK14 and TaCBLs, the MatchMaker Gold Yeast two-hybrid system (Clontech) was used. The coding sequences of TaCBLs were introduced into pGADT7 as prey, and the coding sequences of TaCIPK14 were introduced into pGBKT7 vector as bait. The bait and prey plasmids were co-transformed into yeast strain AH109 following instructions in the Yeast Protocols Handbook (Clontech). β-galactosidase activity analysis was conducted according to the Yeast Protocols Handbook (Clontech).
Virus-induced gene silencing (VIGS)
To confirm the specificity of the silenced fragments, the fragments of TaCIPK14 or TaCIPK15 showing no similarity with any other genes were selected after BLAST and siFi (version 3.2, Snowformatics) analysis of the wheat genome databases (http://wheat-urgi.versailles.inra.fr/) (Figure S1a,b). Two specific cDNA fragments of TaCIPK14 or TaCIPK15 were obtained by RT-PCR and subcloned into the BSMV vector. The details of VIGS assays were performed according to a previous study (Liu et al., 2018).
Analyses of gene expression and fungal biomass
Gene expression analysis was performed using qRT-PCR. Three or four leaves in each replicate were collected for RNA extraction. RNA extraction, cDNA synthesis, and qRT-PCR were performed following a previous study (Liu et al., 2018). TaEF-1α was used as the internal control gene for wheat to quantify the transcript levels of the specific gene according to the comparative 2–ΔΔCT method. Fungal biomass was analysed by quantitative PCR with DNA as template. For DNA extraction, three or four leaves in each replicate were collected at 12 dpi. To measure fungal biomass, the relative quantification of the Pst gene PstEF1α and the wheat gene TaEF-1α was analysed for calculating relative quantities of the Pst and wheat DNA as described previously (Yang et al., 2020).
Histological observations of fungal growth and host responses
For histological observations, the leaves inoculated with Pst were sampled. For histological observations of fungal growth, wheat germ agglutinin (WGA) conjugated to the fluorophore Alexa-488 (Invitrogen) was used to stain Pst infection structures. The stained Pst infection structures were observed with an Olympus BX-51 microscope (Olympus) under blue-light excitation (excitation wavelength 450–480 nm, emission wavelength 515 nm). For histological observations of host responses, H2O2 production in infected wheat leaves was detected by staining with 3,3′-diaminobenzidine (DAB; Amresco) as described previously (Wang et al., 2007), and observed with a BX-51 microscope (Olympus) under differential interference contrast optics. The number of haustorial mother cells and haustoria, hyphal length, infection area, and H2O2 area per infection site were measured using the cellSens Entry software (Olympus).
Generation of TaCIPK14-OE transgenic wheat plants
The coding sequence of TaCIPK14 was subcloned into pWMB110 to form the transformation vector pWMB110-TaCIPK14. The expression of TaCIPK14 was driven by the maize ubiquitin promoter (Figure S8a). The phosphinothricin acetyl transferase gene Bar, which is driven by the CaMV 35 S promoter, was used as a selectable marker gene (Figure S8a). The vector pWMB110-TaCIPK14 was transformed into wheat by Agrobacterium-mediated wheat transformation system. T0 to T2 generations were screened by PCR assay for detection of the inserted transgene fragments. Then, the corresponding wheat T1 and T2 transgenic lines were validated by qRT-PCR assay. The gene-specific primers were designed with the software of Primer 5.0 (Table S5).
Knockout TaCIPK14 by CRISPR/Cas9 system
Two gRNA target sites (named as Target 1 and Target 2) for TaCIPK14 on the conserved sequences of the wheat A, B, and D genomes were designed via the E-CRISP website (http://www.e-crisp.org) (Figure 4a, Figure S1a). The CRISPR/Cas9 vector used in this study was based on VK005-6 (Viewsolid Biotech) (Figure S10a). The two designed guide RNAs targeting TaCIPK14 were cloned into VK005-6 vector using the Plant Cas9/gRNA Plasmid Construction kit (Viewsolid Biotech), following the manufacturer's protocol. The knockout vector VK005-6-TaCIPK14 was transformed into wheat by the Agrobacterium-mediated wheat transformation system. The transgenic plants were validated by PCR assay for detection of the inserted Cas9, the gRNA cassette, and Bar fragments. To further analyse the positive transgenic plants, the genome-specific primers were designed to amplify the target region of the A, B, and D genomes (Figure S1a and Table S5). The PCR products were sequenced to identify the TaCIPK14 mutant plants. To determine the genotype of TaCIPK14 mutant plants, PCR products were cloned into the pMD-18T vector (Takara Biotech) followed by sequencing.
To investigate the off-target effects in the mutants, the potential genome-wide off-target sites for these two gRNAs were identified by using CasOT (http://eendb.zfgenetics.org/casot/) based on sequence similarity (Table S4). Site-specific genomic PCR and sequencing were used to identify the off-target effect. The primer sets are listed in Table S5.
Accession numbers
The sequences used in this study were assigned the following GenBank accession numbers: TaEF-1α (Q03033), PstEF1α (KNE93481.1), TaCBL1.1 (KU736847), TaCBL2 (KU736848.1), TaCBL3 (KU736849), TaCBL4 (KU736850.1), TaCBL6 (KU736851.1), TaCBL9 (KU736852.1), TaCIPK14 (KU736857.1), TaCIPK15 (KU736858.1), TaPR1 (AF384143), TaPR2 (DQ090946), TaPR5 (FG618781), and TaCAT2 (XM_044562031.1).
Acknowledgements
We thank Professor Larry Dunkle (Professor Emeritus at Purdue University, USA) for editing the manuscript. We thank Fengping Yuan, Hua Zhao, Xiaona Zhou, Liru Jian, and Qiong Zhang of State Key Laboratory of Crop Stress Biology for Arid Areas for their technical support. This work was supported by National Key R&D Program of China (2021YFD1401000), National Natural Science Foundation of China (32172381 and 31972224), Key Research and Development Program of Shaanxi (2021ZDLNY01-01), Natural Science Basic Research Program of Shaanxi (2020JZ-13), and the 111 Project from the Ministry of Education of China (B07049).
Conflicts of interest
The authors declare no conflicts of interest.
Author contributions
J. G. (Jun Guo), Z. K., and F. H. designed the experiments; F. H., C.W. (Ce Wang), H. S., S. T., G. Z. (Guosen Zhao), C. L., C. W. (Cuiping Wan), G. Z. (Gangming Zhan), and X. Y. performed the experiments; J. G. (Jia Guo) did the phylogenetic analysis; X. H. performed Agrobacterium-mediated wheat transformation; F. H., and J. G. (Jun Guo) wrote the paper. All authors commented on the article before submission.




