CRISPR/Cas9-mediated tetra-allelic mutation of the ‘Green Revolution’ SEMIDWARF-1 (SD-1) gene confers lodging resistance in tef (Eragrostis tef)
Summary
Tef is a staple food and a valuable cash crop for millions of people in Ethiopia. Lodging is a major limitation to tef production, and for decades, the development of lodging resistant varieties proved difficult with conventional breeding approaches. We used CRISPR/Cas9 to introduce knockout mutations in the tef orthologue of the rice SEMIDWARF-1 (SD-1) gene to confer semidwarfism and ultimately lodging resistance. High frequency recovery of transgenic and SD-1 edited tef lines was achieved in two tef cultivars by Agrobacterium-mediated delivery into young leaf explants of gene editing reagents along with transformation and regeneration enhancing morphogenic genes, BABY BOOM (BBM) and WUSCHEL2 (WUS2). All of the 23 lines analyzed by next-generation sequencing had at least two or more alleles of SD-1 mutated. Of these, 83% had tetra-allelic frameshift mutations in the SD-1 gene in primary tef regenerants, which were inherited in subsequent generations. Phenotypic data generated on T1 and T2 generations revealed that the sd-1 lines have reduced culm and internode lengths with no reduction in either panicle or peduncle lengths. These characteristics are comparable with rice sd-1 plants. Measurements of lodging, in greenhouse-grown plants, showed that sd-1 lines have significantly higher resistance to lodging at the heading stage compared with the controls. This is the first demonstration of the feasibility of high frequency genetic transformation and CRISPR/Cas9-mediated genome editing in this highly valuable but neglected crop. The findings reported here highlight the potential of genome editing for the improvement of lodging resistance and other important traits in tef.
Introduction
Tef [Eragrostis tef (Zucc.), Trotter] is an ancient grain cultivated in Ethiopia as a major staple food and cash crop for millions and a valued forage crop (Demeke and Marcantonio, 2019; Minten et al., 2018). Tef ranks first among cereals in the area of production in Ethiopia, covering about three million hectares and cultivated by more than six million farmers (Minten et al., 2018). Tef is tolerant to pests and diseases and suffers minimal post-harvest losses (Assefa and Chanyalew, 2018). Tef can grow relatively well in extremes of conditions not suitable for other cereals including a wider range of altitudes (up to 3000 m above sea level) and moisture extremes (Assefa et al., 2015), consequently rendering this grass more climate resilient than most crops. Tef grain is also valued as a high protein source, with a balanced and complete set of amino acids, and is a rich source of minerals including iron and calcium and is gluten-free, which contributes to food products suitable for people with celiac disease (Baye, 2014).
Over 50 years of research and development between 1960–2012 have brought incremental improvements in tef grain yield with genetic gains of 0.5 to 0.79 percent per year (Assefa and Chanyalew, 2018; Teklu and Tefera, 2005). However, tef yield remains very low compared to that of the other major cereals, averaging about 1.7 t/ha (Assefa et al., 2011; Cochrane and Bekele, 2018). Lodging, competition from weeds, grain shattering and low productivity of landraces (the types predominantly cultivated by farmers) are major limitations to tef production (Assefa et al., 2011). Lodging suppresses yield on average by 17% and substantially reduces the quality of harvested grain and straw (Assefa et al., 2011; Ketema, 1993).
Available tef germplasm pool manifests considerable genetic diversity in plant height, days to maturity, panicle type, caryopsis color and seed size, and plant characteristics contributing to lodging resistance/susceptibility (Assefa et al., 1999, 2011, 2015; Bayable et al., 2020). Decades of conventional breeding approaches to improve lodging resistance in tef have been of limited success (Assefa et al., 2011; Ketema, 1991; Tefera et al., 2003; Zhu et al., 2012). Recently, chemical mutagenesis has resulted in the successful development of a lodging resistant dwarf tef variety defective in the α-tubulin gene (Jöst et al., 2015). Introgression of this mutation into elite tef germplasm and agronomic evaluation is underway (Bekana and Assefa, 2021). Currently, all improved varieties of tef lodge to a varying degree (Cochrane and Bekele, 2018).
To combat lodging susceptibility, agronomically useful dwarfing genes have been extensively used in cereal crops (Liu et al., 2018; Quinby and Karper, 1954). The sources of the dwarfing genes are mainly spontaneous mutations, some of which have been under cultivation for about a century (Yamaguchi et al., 2016), while some were selected by humans thousands of years ago during early crop domestication (Asano et al., 2011). Semidwarf lines of different crop species have also been developed through induced mutagenesis (Shu et al., 2012). The most widely deployed and well-studied dwarfing genes are the reduced height-1 (Rht-1) gene in wheat (Peng et al., 1999) and sd-1 gene in rice (Ashikari et al., 2002; Sasaki et al., 2002; Spielmeyer et al., 2002) both of which were central to the Green Revolution of the 1960s and 1970s (Hedden, 2003). The wild-type alleles of these genes are involved in gibberellin (GA) signalling (RHT-1, a DELLA protein) and biosynthesis (SD-1, a GA 20-oxidase). Likewise, the semidwarfing gene uzu, a brassinosteroid (BR)-insensitive1 (BRI1) gene encoding a BR receptor (Chono et al., 2003), and sdw1/denso, a GA 20-oxidase orthologous to rice sd-1 (Jia et al., 2015), have both been used in barley breeding. On the contrary, mutations in GA biosynthetic genes causing GA deficiency were not useful in sorghum, due to associated undesirable stem growth phenotype (Ordonio et al., 2014). Rather, four independently inherited non-GA dwarfing genes (DW1–DW4), have been used extensively in commercial grain sorghum breeding mainly in the USA to significantly reduce sorghum plant height to improve lodging resistance and machine harvesting (Quinby and Karper, 1954). Cloning of these genes revealed that DW3 is an ortholog to maize BRACHYTIC2 (BR2), which encodes for ABCB1 auxin transporter (Multani et al., 2003), that DW1 encodes for a novel component of BR signalling (Hirano et al., 2017a) and DW2 encodes for AGC protein kinase involved in the regulation of stem elongation (Hilley et al., 2017). The gene encoding DW4 has not been cloned and its identity remains unknown. Studies of dwarfing genes have shown that sd-1, dw1, dw2 and dw3 are recessive, making them good candidates for targeted knockout mutagenesis using genome editing technologies. In tef, application of GA biosynthesis inhibitors, such as chlormequat chloride and paclobutrazol, have generated semidwarf and lodging resistant tef plants (Gebre et al., 2012; Plaza-Wüthrich et al., 2016), indicating that gibberellin deficiency induced through mutations in the tef SD-1 orthologue could be an avenue for generating semidwarf, lodging resistant tef lines.
Recent advances in genome engineering technologies, including CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats/CRISPR associated-9) and transcription activator-like effector nucleases (TALENS), have revolutionized the field of basic and applied plant biology. Plant traits of significance have been improved through this technology (Zhu et al., 2020). These, include resistance to bacterial blight in rice (Oliva et al., 2019), resistance to powdery mildew in wheat (Wang et al., 2014), virus resistance in cassava (Gomez et al., 2019), improvements in food quality traits in potato and soybean (Clasen et al., 2016; Haun et al., 2014), improvements in maize starch profile (Gao et al., 2020) and agronomic performance (Shi et al., 2017) including yield and yield components (Liu et al., 2021). Rapid domestication of underutilized crops has also been reported, demonstrating the potential of genome editing to improve the livelihoods of low-income farmers (Østerberg et al., 2017; Zsögön et al., 2018).
We report here the first successful production of semidwarf tef lines using CRISPR/Cas9 based genome editing of tef SD-1 gene. We present data on the nature and heritability of the mutations obtained and demonstrate the promise of genome editing for conferring lodging resistance in tef.
Results
Tef GA 20-oxidases
Using the rice GA 20-oxidases (GA20ox1-4, Sakamoto et al., 2004a,b) coding sequences (CDS) as baits, four tef GA 20-oxidases were identified from the tef genome (VanBuren et al., 2020) using CoGeBlast (Lyons et al., 2008). Based on nucleotide and deduced amino acid sequence identities, these GA 20-oxidases were named EtGA20ox1-4, with the corresponding rice GA 20-oxidases as shown in Figure 1a. Designation of the homeologs of each of the four tef GA 20-oxidases followed the corresponding A or B genomes. The four tef GA 20-oxidases CDS shared 61.7%–75.3% (Table S1) and 46.5%–69.3% amino acid identity with each other (Table S2). The level of similarity between each of the two tef homoeologous alleles (EtGA20ox1, EtGA20ox2 and EtGA20ox3) CDS ranged from 90.8%–95.4% nucleotide sequence identity and 88.2%–92.3% amino acid sequence identity (Tables S1 and S2). Based on the reference genome sequences, EtGA20ox4 has only one functional allele (Et_9A_061857), with the second allele represented by two shorter open reading frames (ORFs), Et_9B_065850 and Et_9B_064431 (Figure 1a) due to a mutation introducing a premature stop codon. The wild-type rice SD-1 (OsGA20ox2) was found to be closely related to EtGA20ox2 (EtSD-1, Figure 1a,b) with 83.5% nucleotide and 81.7%–84.4% amino acid identity, compared to that of the other tef GA 20-oxidases that shared 66%-76.3% nucleotide and 48.6%–66% amino acid identities (Tables S1 and S2).
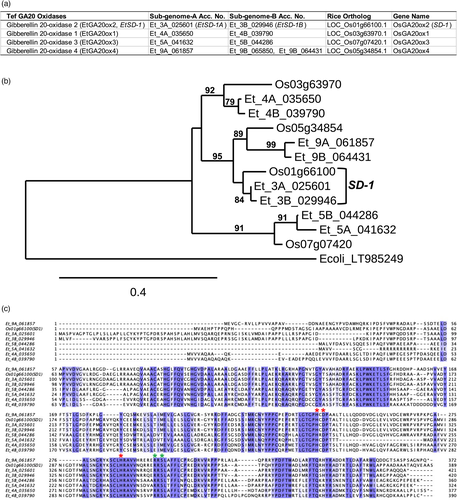
The tef SD-1 (EtSD-1) was chosen as a target for knockout mutation based on its similarity with the rice SD-1 gene. All identified tef GA 20-oxidases (EtGA20ox1-4) had the highly conserved HX(D/E)XnH triad motif, essential for binding Fe(II) and the two residues involved in 2-oxoglutarate binding (Figure 1c) common to 2-oxoglutarate/Fe(II)-dependent dioxygenases (2-ODDs; Farrow and Facchini, 2014).
Recovery of SD-1 edited tef lines
A simple Agrobacterium-mediated tef transformation and regeneration system were developed using young leaf tissue as explants (Figure 2) excised from 2- to 3-week-old seedlings (Figure 2a). The leaf base portion of the seedlings (Figure 2b) was sectioned transversely into 4–8 mm long segments, split into halves (Figure 2c) and inoculated with Agrobacterium harbouring either p8660 or p8702. Both binary vectors had the same Cas9 and gRNAs but differed in the promoters used to drive the morphogenic genes BBM, WUS2 and the Cre-recombinase (Figures S1 and S2). After transformation, green fluorescent protein (GFP) foci were visible within 3–5 days from both constructs. The GFP expressing cells grew into sectors of rapidly dividing embryogenic tissue (Figure 2d,e). Since negative selection was not used in this study (no antibiotic or herbicide selection), the rapidly growing GFP expressing sectors were excised manually under a fluorescence microscope from nontransformed tissues and subcultured onto fresh callus induction medium or regeneration medium. Transgenic GFP expressing tissues were regenerated (Figure 2f) after induction of Cre-recombinase expression to facilitate the excision of morphogenic genes. Rooted transgenic T0 plants were established in the soil, grown to flower stage and T1 seeds collected, which were shown to segregate for the GFP marker (Figure 2g,h).
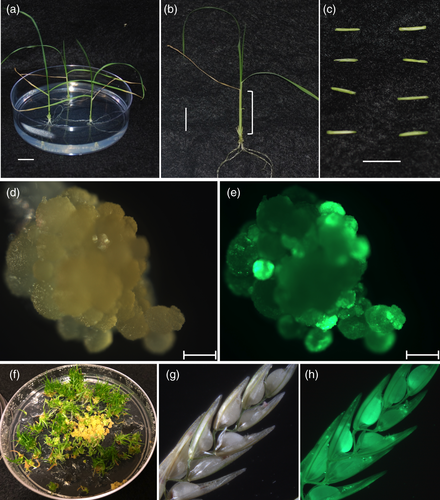
On average, it took 6–8 months to complete the entire process from wild-type seed germination through transformation and regeneration to the production of T1 transgenic seeds. Using this method, a total of 112 GFP expressing T0 tef plant lines were recovered in two tef cultivars (41 in Ada and 71 in Magna) with an average transformation efficiency using construct p8702 of 7.8% for Ada and 16.5% for Magna (Table S3). Of these, 20 T0 lines generated in Magna cultivar and seven lines in Ada cultivar were analyzed for mutations at the gRNA1 and gRNA2 target sites and characterized through subsequent generations. All the 27 lines were fertile and produced seeds, and four lines derived from p8660 in Magna background had delayed flowering (heading time), stunted growth, dark green leaves and produced less seeds than the wild-type control. These lines were excluded from further phenotyping studies.
High-efficiency tetra-allelic mutation in the Tef SD-1 gene
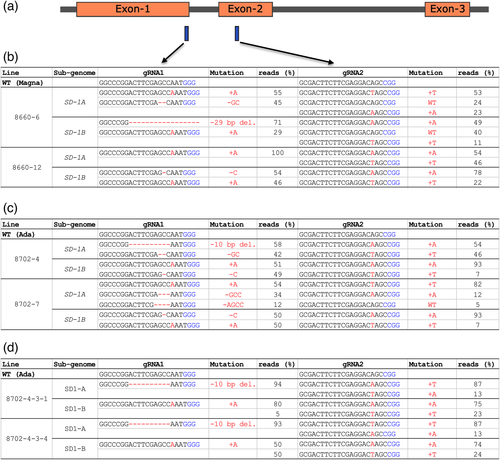
Two gRNAs were used to generate knockout mutations in the tef SD-1 gene. Positions of the SD-1 target site corresponding to exon-1 (gRNA1 target site) and exon-2 (gRNA2 target site) are shown in Figure 3a. Next-generation sequencing of the two target site amplicons from both subgenomes, A and B, revealed unprecedented editing efficiency of 100% as all lines had at least two or more alleles mutated at the target site. Characterization of the mutations across the two cultivars showed 19 of the 23 lines (83%) had tetra-allelic frameshift mutations (Figure 3b,c and Table S4). We also found four of the 27 lines (two in Ada and two in Magna) could potentially be clones based on next-generation sequence data. The frequency of tetra-allelic mutation was generally higher at the gRNA1 target site than at gRNA2 with 83% versus 56%, respectively. In almost all of SD-1 edited tef lines, the mutation types were simple insertion or deletions (InDels, Figure 3b,c, and Table S4). Insertions were mainly of single nucleotides while deletions ranged from 1 to 32 bp (Figure 3b,c, and Table S4). Chimeric mutations were also observed in some of the lines, mainly at the gRNA2 site. The observed mutations were heritable as confirmed through sequencing of subsequent generations (Figure 3d).
SD-1 mutation reduced culm length and plant height
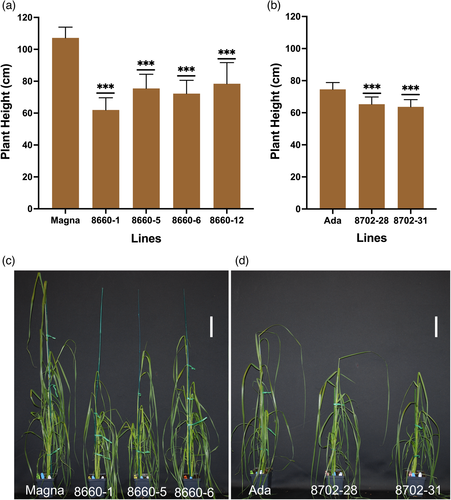
Null mutation in the SD-1 gene is known to affect culm and internode lengths in rice (Tomita and Ishii, 2018). Phenotypic effects of the SD-1 mutations in tef were evaluated by measuring four T1 Magna and two Ada lines for stem height during vegetative growth, and for culm, internode and panicle lengths at maturity (harvest). These lines had tetra-allelic knockouts at T0 and were subsequently confirmed through sequencing to carry the SD-1 knockout in the T1 progenies (Figure 3b,c and Table S4). There were no differences in germination rates between T1 seed from edited sd-1 lines and wild-type seed (data not shown). Visible differences in plant height started to emerge at about 2–3 weeks after planting. Measurements of stem height (as measured from soil level to the topmost collar) at 37 days after planting revealed that sd-1 lines in Magna background had a significant (P < 0.001) reduction in plant height (28%–42%; Figure 4a,c). In addition, two sd-1 Ada lines, 8702-28 and 8702-31, had 12% and 15% reductions (P < 0.001) in plant height compared to that in the unmodified Ada control (Figure 4b,d). Further characterization on T2 lines was conducted on Magna edited lines only as those derived from Ada lines (both edited and wild-type) suffered poor panicle exertion under the conditions of our study.
Quantitative PCR analysis identified null segregants in Magna sd-1 T1 generation lines. Of these, two null segregant T2 lines, 8660-6 and 8660-12 were subjected to further phenotypic characterization. Like the T1 generation, discernable plant height differences were observed in the T2 generation at the vegetative growth stage 2–3 weeks after planting. Measurements of plant height at 5 weeks showed significant (P < 0.001) differences between the wild-type and sd-1 lines, with 37% and 33% reductions in height relative to the Magna control for lines 8660-6 and 8660-12, respectively (Figure 5a). At harvest, the sd-1 lines had 10%–12% reduction in plant height (Figure 5b), 18% reduction in culm length (Figure 5d), and no significant difference in panicle (Figure 5c) and in peduncle lengths (data not shown) compared to that in the Magna control. Since culm length is the sum of all internodes (internodes 2–7) without the peduncle, we examined whether all, or only some, of the internodes were reduced in length. Individual internode lengths were not significantly different between the two sd-1 edited lines but were significantly reduced compared to that of the Magna control (Figure 5e). Average percent reductions in the lengths of internodes 2 through 6 ranged between 14%–19%, with no correlation between internode number and percent reduction in internode length. The average percent length reduction of basal internode-7 was seen to be significantly greater at 38%. Under growth chamber conditions, tiller number (both total and effective tillers) was not significantly different between sd-1 lines and the Magna control (data not shown).
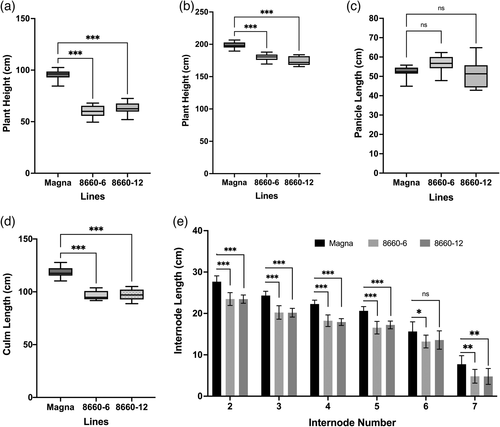
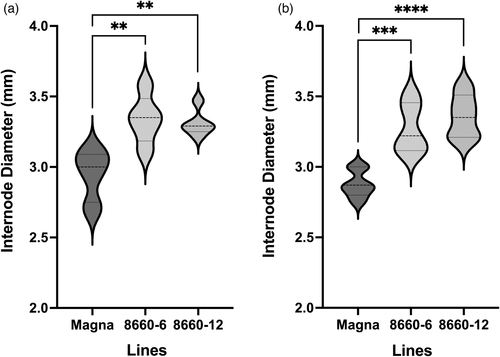
Internode diameter at harvest was measured on the sd-1 edited lines and controls from the two consecutive internodes at the base of greenhouse-grown T2 plants (internode-8 or -9). The sd-1 lines had significantly (P < 0.01) thicker internodes compared to that of the corresponding internodes of the wild-type Magna control (Figure 6a,b). No differences were observed in internode thicknesses at the mid-section of the plant (internode-3; data not shown). It was noted that both sd-1 lines and the wild-type control had a greater number of nodes (8 or 9) under greenhouse conditions than observed from plants grown in the growth chamber (up to seven nodes).
Application of GA3 rescued semidwarf phenotype of sd-1 lines
To study whether the semidwarfism seen in sd-1 lines could be rescued by application of GA3, two experiments were run. In the first experiment, GA3 was applied as a spray treatment for 11 consecutive days to greenhouse-grown 6-week-old wild-type Magna and the semidwarf line 8660-6 and increases in stem length were recorded. In GA3-untreated control (−GA3) stem length (measured as a difference in plant height before and after treatment) was increased by 34.2 cm for 8660-6 and by 55.5 cm in wild-type Magna. In GA3-treated plants (+GA3) of 8660-6 and wild-type Magna, stem length was increased by 87.5 and 95.9 cm, respectively. Relative to untreated controls, GA3 treatment increased stem elongation more in line 8660-6 (155% increase) than in wild-type control (82% increase), offsetting the effect of SD-1 knockout on stem elongation (Figure S3a,b). In the second experiment, in vitro germinated seedlings (from T4 generation) of sd-1 lines 8660-6 and 8660-12 along with a wild-type control were transferred to MS media with or without 50 µm GA3, and plant height was recorded after 11 days. Data collected showed increases in stem elongation in these two lines over the untreated controls (Figure S3c,d). Both greenhouse and in vitro experiments clearly demonstrated that the dwarfing phenotype in sd-1 lines could be rescued by GA3 application.
Estimation of lodging using image data
Numerical estimation of lodging in tef was done by calculating the height : width ratios from tef images collected over time using PlantCV (Gehan et al., 2017). Using these images, the shape of the plant was identified, and a reference line was placed at the root/soil base for every image to generate a height above the reference line measurement. To compensate for the distance from the camera, the height data were normalized with the area of the size marker in the images. The height above the reference line for each image was divided by the area of the size marker, to obtain a normalized height. Across all tef entries, plant height increased over time (Figure 7a). The control Magna and edited sd-1 lines reached maximum height by 8 weeks after planting. Tef produces many stems (tillers). Due to differential curvature, they do not all lodge together. Height alone, therefore, cannot be used as a determinant of lodging, and hence, it must be combined with plant width. As for height measurements, the width measurements were normalized with the size marker area, and the averages were plotted (Figure 7b). Up to 6 weeks after planting, the Magna control and sd-1 edited lines exhibited similar increases in width, with the width of the Magna control significantly (P < 0.05) increasing after 7 weeks compared to that of the sd-1 lines (Figure 7b).
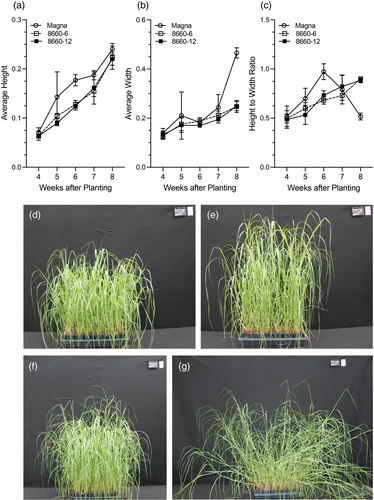
As indicated above, lodging includes changes in both the height and width of the plant. Therefore, the height : width ratio was calculated as an estimation of lodging (Figure 7c). In the sd-1 lines, the height : width ratio steadily increased over time. The control Magna had a greater increase in height : width ratio in comparison with sd-1 lines until 6 weeks after planting (Figure 7c) and thereafter decreased rapidly indicating a significant degree of lodging that was also confirmed by visually inspecting the image data (Figure 7e,g). In contrast, although the height : width ratio of the SD-1 edited lines increased over time (Figure 7c), there were no obvious visual indications of lodging (Figure 7d,f) during the study period.
Measurement of lodging using USDA-GRINS descriptor
A similar measurement of lodging at the heading stage, based on the USDA-GRINS descriptor that measures lodging on the scale of 1 to 9 showed a significant (P < 0.01) difference between sd-1 lines, with an average score of 1.2–1.3, and the Magna control, with an average lodging score of 5 (Figure S4).
Discussion
Successful production of gene-edited plants requires a robust transformation and plant regeneration system that, to date, has been a limiting factor for tef. Recent advances in the use of the plant transcription factors BBM and WUS2 (Gordon-Kamm et al., 2019; Lowe et al., 2016, 2018) and the co-expression of GROWTH-REGULATING FACTOR 4 (GRF4), and its cofactor GRF-INTERACTING FACTOR 1 (GIF1; Debernardi et al., 2020), have shown significant improvements in genetic transformation and frequency of recovery of transgenic and gene-edited events in otherwise recalcitrant monocot species.
Two prior reports showed successful transformation and regeneration of transgenic tef plants. A single transgenic tef plant expressing a reporter gene was first reported by Gugsa (2005) using an immature zygotic embryo as an explant. Gebre et al. (2013) also reported the generation of eight T0 transgenic tef lines expressing a GA 2-oxidase, an enzyme that deactivates bioactive GAs or their precursors (Yamaguchi, 2008) using an immature zygotic embryo as an explant. The transformation system we developed for tef is advantageous over earlier methods due to (i) the use of readily available young leaf explants, which circumvents the need for extra space and time required to produce a continuous supply of flowering tef plants as a source of explants, (ii) avoiding the tedious dissection and collection of immature zygotic embryos from one of the smallest cereal seed, and (iii) the co-expression of BBM and WUS2 to induce de novo somatic embryogenesis, which was critical for the successful recovery of transformed and gene-edited tef lines at a very high frequency. In our study, the transformation of tef using the young leaf as an explant was efficacious (Table S3) with constitutive expression of BBM and WUS2. These morphogenic genes were, however, known to have undesirable pleiotropic effects when expressed in regenerated plants, and finetuned expression or programmed excision of these genes have been reported to overcome such effects (Hoerster et al., 2020; Lowe et al., 2016, 2018; Wang et al., 2020). The construct p8702 had Cre expression driven by RAB17 promoter (Figure S2) for ABA or desiccation induced excision of these genes. Inclusion of ABA at 50 µm before regeneration was sufficient to induce the excision of these genes in our experiments.
Bioactive gibberellins (GA1 and GA4) are involved in diverse plant processes including seed germination, stem elongation, leaf expansion, floral transition and seed/fruit development (Achard and Genschik, 2009; Yamaguchi, 2008). Like rice and most other monocot species, tef has four GA 20-oxidase genes (Figure 1). Though transgenic RNAi lines of rice GA20ox1, that predominantly expressed in reproductive organs, are known to cause semidwarfism (Oikawa et al., 2004) and RNAi lines of GA20ox3 to cause semidwarf and disease resistance phenotype (Qin et al., 2013), only mutations in the GA20ox2 gene (sd-1) have been widely introgressed and utilized in rice production due to the beneficial effect on plant stature, lodging resistance, increased harvest index and responsiveness to nitrogen fertilizer application (Peng et al., 2021). Up to 10 alleles of SD-1, obtained through spontaneous mutation or conventional mutagenesis, are known so far (Peng et al., 2021) and more alleles are being introduced through genome editing (Biswas et al., 2020; Hu et al., 2019).
Inspired by the wide application of the SD-1 mutation in rice to combat lodging through reduced height, we generated multiple tef lines with knockout mutations in tef SD-1 gene. Tef being a self-pollinating allotetraploid (2n = 4× = 40; VanBuren et al., 2020), mutation of all four alleles of SD-1 gene was required to see the altered phenotype due to the recessive nature of sd-1 mutation (Hedden, 2003). Most of the tetra-allelic mutations obtained in both tef cultivars in this study (Figure 3 and Table S4) were frameshift mutations resulting in the introduction of a premature termination codon.
Disruption of SD-1 function results in an increase in the level of GA53, (a GA intermediate and a substrate for GA 20-oxidase) and reduced levels of bioactive GA1 precursor, GA20 and bioactive GA1 (Sasaki et al., 2002; Spielmeyer et al., 2002) leading to shorter internode and culm lengths as observed in sd-1 lines of indica (Chunhai and Zongtan, 1996) and japonica rice (Tomita and Ishii, 2018). Although we did not measure levels of bioactive GA in tef lines, the sd-1 tef lines have shown a reduction in culm length, due to the reduction in lengths of all internodes (internode-2 to internode-7; Figure 5e). This reduction in culm length (plant height) could be rescued by the application GA3 (Figure S3) indicating that the GA deficiency due to disruption of SD-1 gene was responsible for the observed semidwarfism in sd-1 tef lines. Like earlier findings in rice, we did not see significant differences in panicle lengths between sd-1 tef lines and the wild-type control. The absence of negative impacts of the sd-1 mutation on panicle length could be due to redundancies of the GA 20-oxidases (Figure 1), which were shown to have specificity in expression and function in different plant tissues and developmental stages (Sakamoto et al., 2004a,b).
Three types of lodging are known in cereal crop plants: stem bending (leaning), stem buckling and root lodging (Hirano et al., 2017b). In tef, the predominant form of lodging is considered as stem bending (Bayable et al., 2020; Ketema, 1983), although Van Delden et al. (2010) reported that root lodging was the predominant type of lodging in combination with weaker stem strengths. Resistance to stem bending lodging is the type conferred by SD-1 mutation in rice (Hirano et al., 2017b). Under field conditions, lodging in tef is typically measured following the method of Caldicott and Nuttall (1979), but various methods have been described both for field and greenhouse-grown plants (Bayable et al., 2020; Ben-Zeev et al., 2020; Blösch et al., 2020; Van Delden et al., 2010). Since our study was based on potted plants in the greenhouse, we used lodging indices that measured the degree of leaning on a 1–9 rating based on USDA-GRINS descriptors used for tef and also through time course image analysis using PlantCV functions (Gehan et al., 2017) that considered changes in plant shape based on height to width ratio over time. Based on observations from multiple greenhouse experiments lodging due to stem bending was the predominant type of lodging observed for the control Magna plants, which began during vegetative growth (5–6 weeks after planting) with most plants fully lodged by 8 weeks. Using both the USDA-GRINS rating scale and image analysis, the sd-1 edited tef lines were found to be significantly more resistant to lodging than the unmodified control (Figures 7 and S4). These observations were made under greenhouse conditions with no wind and rain, and the outcome could be different under field conditions where the wetness of the soil and the plant either from rain or dew have been reported to affect lodging (Van Delden et al., 2010).
Tef’s morphological characteristics including plant height, lengths of the panicle, the peduncle, the internodes (culm length), thickness of the basal internodes, angle of the panicle, tiller number, root characteristics and the relationship of these plant parts with each other are known to influence the degree of lodging in tef (Bayable et al., 2020; Blösch et al., 2020; Girma, 2019; Ketema, 1983; Van Delden et al., 2010), besides environmental conditions and agronomic practices (Ben-Zeev et al., 2020; Merchuk-Ovnat et al., 2020). The reduction in culm length and increased thickness of the first and second basal internodes are likely factors for the increased lodging resistance observed for the sd-1 tef lines. Further studies under field conditions are necessary to confirm and validate the lodging resistance reported in this study.
Conclusions
We developed a robust and reproducible tef transformation and CRISPR/Cas9 targeted gene editing system for this important food security crop and showed that an important trait such as lodging resistance can be achieved in a fraction of time and resources required by conventional plant breeding. We believe this will serve as an additional toolkit for breeders and product developers in their quest to improve tef to the benefit of Ethiopian farmers and tef producers elsewhere. We trust that improvement in lodging resistance in tef can facilitate a tremendous increase in tef productivity both directly through reducing losses due to lodging and indirectly through allowing a modernized tef production system including the use of yield-enhancing inputs such as fertilizers and machine harvesting of tef.
Materials and methods
Tef GA 20-oxidases and identification of Tef SD-1
Sequences of rice (Oryza sativa L.) GA 20-oxidases (OsGA20ox1-OsGA20ox4; Sakamoto et al., 2004a,b) were used as baits to retrieve orthologous GA 20-oxidases from the published tef genome (VanBuren et al., 2020) using CoGeBlast (Lyons et al., 2008). To construct a phylogenetic tree, the coding sequences (CDS) of GA 20-oxidases of both tef and rice were analyzed with the phylogeny.fr (http://www.phylogeny.fr/, accessed June 15, 2021) pipeline using the default ‘One Click mode’ (Dereeper et al., 2008). The One Click mode uses ‘optimized programs including MUSCLE (Edgar, 2004) for multiple alignments, Gblocks (Castresana, 2000) for alignment curation, PhyML (Guindon and Gascuel, 2003) for phylogeny and finally TreeDyn (Chevenet et al., 2006) for tree drawing to reconstruct a phylogenetic tree from tef and rice GA 20-oxidases’. Detailed descriptions of the programs are found at http://www.phylogeny.fr/.
Guide RNA (gRNA) design and constructs
Two guide RNAs (gRNA1 and gRNA2) each targeting both homoeoalleles of the tef SD-1 gene were designed using the CRISPOR program (Haeussler et al., 2016). The designed gRNA oligonucleotides were synthesized at Integrated DNA Technologies (Coralville, IA) after introducing 5'-compatible overhangs on forward and reverse gRNA oligos (Table S5). Assembly of the gRNA oligos into an expression system and subsequently into binary vectors followed the method described by Čermák et al. (2017). Briefly, the gRNA oligonucleotides were cloned into modular vectors pMOD_B2518 for gRNA1 and pMOD_C2517 for gRNA2. The two gRNA expression cassettes plus monocot codon-optimized Cas9 (pMOD_A1110) and a binary vector harbouring hygromycin phosphotransferase II (pTRANS_250d) were assembled using Golden Gate (Engler et al., 2008) and cloned in MAX Efficiency™ DH5α competent cells (Thermo Fisher Scientific, Waltham, MA). The cloned gRNA oligonucleotides and Cas9 were confirmed through sequencing using primers listed in Table S5. The resulting binary vector was named p8603. From this binary vector, the Zm-Ubipro::Cas9// Ta-U6pro::EtSD1sgRNA1// Ta-U3pro::EtSD1sgRNA2 cassette was excised as an AscI-PmeI fragment (AscI site blunted) that was cloned into the SwaI site of PHP81814 to generate p8660, and into the FseI site of the binary vector PHP78891 (after blunting) to generate p8702. The two recipient binary vectors, PHP81814 and PHP78891 harbour the morphogenic regulator genes from maize, BBM and WUS2, and a green fluorescent protein (GFP) marker gene ZsGreen (Lowe et al., 2016). The binary vectors p8660 and p8702 (Figures S1 and S2) were electroporated into thymidine auxotrophic (THY-) LBA4404 Agrobacterium tumefaciens (Lowe et al., 2016) and used for tef transformation. Binary vectors PHP81814 and PHP78891 and A. tumefaciens strain LBA4404 THY- harbouring the helper plasmid PHP71539 were kindly provided by Corteva Agriscience, Johnston, IA.
Transformation and regeneration of tef lines
Seedling and leaf explant preparation
Seeds of tef cultivars Magna, and Ada, accession numbers, PI 243908 and PI 524433, respectively, were obtained from the National Genetic Resources Program of the United State Department of Agriculture (USDA). Seeds were rinsed with distilled water, sterilized by agitating in 50% (v/v) commercial bleach containing 1–2 drops of Tween-20 for 10 min and rinsed five times with sterile distilled water. Between 12 and 15 seeds were germinated per petri dish, containing Murashige and Skoog (MS) basal medium (Murashige and Skoog, 1962) supplemented with 20 g/L sucrose (MS2) and 2.2 g/L gelzan. A sterile inverted sundae cup (https://www.webstaurantstore.com, item # 760SD12) was placed over the petri dish bottom, sealed and cultured at 28 °C for 10–14 days in a walk-in growth room maintained at 16 h day and 8 h dark with light intensity of 90 µmol/m2/s. After 10–14 days shoots were cut approximately 0.25 cm above the base using a sterile scalpel blade and the upper portion of leaves (above the topmost collar) was removed. The stem base consisting of folded young leaves was sectioned transversely to generate shoot material approximately 0.4–0.8 cm in length. The stem material was sliced longitudinally to generate two similar-sized explants per section and used for Agrobacterium inoculation.
Agrobacterium preparation and explant inoculation
The Agrobacterium LBA4404 THY- harbouring the binary vectors p8660 and p8702 was streaked on Luria broth (LB) containing 50 mg/L thymidine, 50 mg/L gentamicin and 100 mg/L spectinomycin to produce a master plate and cultured for 3–4 days at 28 °C and used as a master plate for up to 3 weeks by storing at 4 °C. One day prior to transformation, a fresh plate was re-streaked from the master plate and cultured at 28 °C for 24 h. On the day of transformation, the Agrobacterium culture was scraped from the medium and transferred to a liquid inoculation medium consisting of MS basal medium supplemented with 68.5 g/L sucrose, 36 g/L glucose, 6.79 µm 2,4-D, 100 µm acetosyringone and 0.02% (v/v) Alligare Surface nonionic surfactant (https://alligare.com). The Agrobacterium suspension was vortexed and adjusted to an OD600 = 0.5. Three millilitres of inoculation medium were pipetted into each well of a 6-well plate, and 25–30 explants were added to each well and vacuum infiltrated for 30 min. Explants were then removed using fine forceps, briefly touched onto a sterile filter to draw off excess inoculation medium and placed into Petri dishes containing MS2 medium supplemented with 10 g/L glucose, 10 µm 2,4-D, 100 µm acetosyringone and 50 mg/L thymidine and solidified with 4 g/L gelzan. Co-culture was performed for 3 days at 22 °C in the dark, after which explants were transferred to a callus induction medium consisting of MS basal medium supplemented with 30 g/L sucrose (MS3), 10 µm 2,4-D, 100 mg/L cefotaxime and 4 g/L gelzan and cultured for 4 weeks in the dark at 28 °C.
Selection of transgenic tissues and plant regeneration
Green fluorescent protein fluorescing callus units were observed 3 days post-inoculation and monitored on a weekly basis. Starting after 4 weeks, GFP fluorescing callus units were transferred onto a excision medium consisting of either MS3 supplemented with 10 µm 2,4-D and 50 µm abscisic acid (ABA), for tissues transformed with p8702 and cultured for 48 h in the dark at 28 °C followed by a transfer onto the regeneration medium. Tissues transformed with p8660 were subcultured directly to regeneration medium, consisting of MS3 supplemented with 6 µm 6-benzylaminopurine (BAP). All tissues were cultured on a regeneration medium for 3–4 weeks in a 16 h light 8 h dark cycle. Greening shootlets developing from the callus were transferred onto MS3 supplemented with 2 µm BAP and cultured for 3–4 weeks in 16 h light until individual shoots were regenerated. These were separated and transferred onto MS2 gelzan for root initiation and growth with an inverted sundae cup dome placed over the petri dish bottom.
Plant growth conditions
Rooted in vitro plantlets or tef seeds were planted in 3.5-inch pots containing Jolly Gardener Pro-Line C/V potting mix and grown in a walk-in growth chamber maintained at 29/22 °C day/night temperature and relative humidity (RH) of 50% with 12 h light at 500 µmol/m2/s. Greenhouse conditions were 29/22 °C day/night temperature and RH of about 50%. Day length was not controlled in the greenhouse. Tef seeds were planted in potting media by distributing (about 10–20 seeds per pot) on top of potting mix and then lightly covering with a 0.1–0.2 cm layer of potting mix. The potting mix was wetted with water before and after planting and placed in 18 pot-holding trays covered with transparent plastic domes. The domes were removed 5 days later, and the seedlings were thinned/transplanted to one plant per pot 8–10 days after sowing (three-leaf stage) and the plants were grown to maturity in a growth chamber or greenhouse. The plants were staked as necessary. Growth chamber and greenhouse-grown plants were fertilized three times a week with water-soluble fertilizers at a rate of 175–200 ppm using either Jack’s 15-5-15 Ca-Mg LX or 15-16-17 Peat-Lite, respectively.
Molecular characterization
To identify SD-1 gene mutations, genomic DNA was extracted from leaf tissues of T0, T1 and T2 plants as described previously (Gao et al., 2010) or using Qiagen DNeasy plant mini kit (Qiagen, Germantown, MD). The tef genome is an allotetraploid (2C = 2n = 4× = 40) consisting of two diploid subgenomes, designated as the A and B genomes. Amplicons of 117 and 97 bp, and 140 and 107 bp, are expected for the SD-1 gRNA1 target site (TS1) and SD-1 gRNA2 TS2 in the A and B genomes, respectively. Next-generation sequencing was used to evaluate the target site sequence changes. Target sequences were amplified first with Phusion Flash High Fidelity PCR Master Mix (F-548; Thermo Fisher Scientific), then secondary amplification used Phusion Master Mix F-531 (Thermo Fisher Scientific) following a standard PCR protocol. The Molecular Inversion Probe (MIP) primers used are listed in Table S6. The resulting PCR products were purified with a Qiagen PCR purification spin column, DNA concentrations measured with a Hoechst dye-based fluorometric assay and then sequenced directly with next-generation sequencing. To detect genome editing reagents and helper genes in the T1 and T2 generations, qPCR was performed using Qiagen QuantiTect Multiplex PCR Master Mix. The primers and probes used are listed in Table S7. The tef phytoene desaturase (PDS) gene was used as an internal control for PCR.
Measurements of morphological traits on T1 and T2 sd-1 plants
Morphological traits, including plant height, panicle, peduncle, internode lengths and tiller number were recorded at harvest as described earlier (Ketema, 1983). Briefly, panicle length was measured from the base of the inflorescence (beginning from the base of the lowest panicle branch to the tip of the panicle), and peduncle length (also called internode 1 in this study) was measured from the topmost node on the culm to the base of the inflorescence. Internode length was measured as the length between two consecutive nodes, with internode number 2 being the topmost after the peduncle, with increasing internode number towards the base of the plant. Culm length was determined by adding the lengths of all internodes other than peduncle and panicle. Plant height data were obtained by adding culm, peduncle and panicle lengths. For data collected at harvest, measurements were collected from ten plants per line and three stems per plant (pot). Plant height data were also collected 5 weeks after seed planting from 18–36 main stems by measuring stem height from the base at the soil surface to the topmost collar.
Measurements of lodging resistance
A total of three entries consisting of, two T2 generation of SD-1 knockout lines in Magna cultivar background and a wild-type control, were used for assessing lodging resistance. The SD-1 edited lines and the control plants were planted in three trays each with 18 plants per tray and grown in the greenhouse. Assessments of lodging were made at the heading stage (8 weeks after planting) based on individual pots from three replicated trays (total of 54 potted plants per line). Two methods were used for measuring resistance to lodging. The first used the USDA-GRINS morphological descriptors for cool-season grasses, including tef, where lodging was scored on a 1–9 scale, with 1 being no lodging and 9 being extreme lodging (https://npgsweb.ars-grin.gov/gringlobal/descriptordetail?id=110126). The second method employed image analysis technology. Images of tef plants in trays were collected at weekly intervals during the vegetative growth phase (weeks 4–8 after planting) using a Nikon D80 camera. Collected images were then analyzed using PlantCV (Gehan et al., 2017). Using PlantCV, tef plants were segmented from background objects and the ‘analyze_shape’ function was used to measure the width of the isolated tef plant images, while the ‘analyze_bound_horizontal’ function was used to set a reference line at the top of the pot-holding tray in order to estimate plant height. A size marker within the image was measured to normalize values since there could be a slight variation in camera position. The height, width and height_above_horizontal_bound/width ratio were used to estimate lodging.
GA3 treatment
GA3 (Catalog No. 7645, Sigma Aldrich, St. Louis, MO, USA) was used at final concertation of 50 µm prepared in Milli-Q water containing 0.1% (v/v) Surface (https://alligare.com) for greenhouse spray treatment or included in MS2 media containing 2.2 g/L gelzan. The untreated controls were Milli-Q water containing 0.1% (v/v) Surface without GA3 for greenhouse spray and MS2 media with 2.2 g/L gelzan without GA3. For greenhouse GA spray treatment, 6-week-old plants were used while for in vitro GA treatment, in vitro germinated 6-day-old seedlings were used. GA treatment lasted for 11 days after which plants were scored for stem elongation (plant height).
Data analysis
Plant morphological data including plant height, lengths of culm, internodes, panicle and peduncle were analyzed by one-way analysis of variance and Dunnett’s multiple comparisons test (comparison of means of edited events with wild-type control) using GraphPad Prism version 9.1.2 for macOS, GraphPad Software, San Diego, CA. Lodging data (height/width ratio) were analyzed using R (R Core Team, 2013).
Acknowledgements
This work was supported by the Donald Danforth Plant Science Center and through the Open Innovation Fund from Corteva Agriscience awarded to Getu Beyene Duguma. We thank Prof. Daniel Voytas, University of Minnesota, for providing vectors pMOD_B2518, pMOD_C2517, pMOD_A1110 and pTRANS_250d. The authors thank Corteva Agriscience (Johnston, IA) for providing binary vectors PHP81814, PHP78891 and A. tumefaciens strain LBA4404 THY- harbouring the helper plasmid PHP71539. Requests for Corteva Agriscience materials, including Agrobacterium strain LBA4404 (THY−), genetic elements and/or plasmids should be directed to William Gordon-Kamm, with completion of requisite Corteva Material Transfer Agreement and stewardship standards before the release of materials.
Conflict of interest
The authors declare no conflicts of interest.
Authors contributions
GB, DJM, NJT, EG, DG, SC, KA and GT conceived the experiment. GB, DJM, NJT, RDC, JV, NH, NW, MG, MM, MY, BL, CS, HG and WG-K designed the experiments and generated the data. GB, DJM, HG and MG analyzed the data. GB and DJM wrote the manuscript. All authors reviewed and commented on the manuscript.
Open Research
Availability of materials
Novel biological materials described in this publication may be available to academic requesters and other not-for-profit institutions solely for noncommercial research purposes upon acceptance and signing of a material transfer agreement between the requester institution and the applicable author institution for the particular materials. In some cases, such materials may originally contain genetic elements described in the manuscript that was obtained from a third party(s), and the authors may not be able to provide materials including third party genetic elements to the requestor because of certain third party contractual restrictions placed on the author's institution. In such cases, the requester will be required to obtain such materials directly from the third party. The authors and authors' institutions do not make any express or implied permission(s) to the requester to make, use, sell, offer for sale, or import third party proprietary materials. Obtaining any such permission(s) will be the sole responsibility of the requestor. Corteva Agriscience Agrobacterium strain, genetic elements and plasmids for making transgenic or edited plants would be available to academic requesters and other not-for-profit institutions solely for noncommercial research purposes upon acceptance and signing of a material transfer agreement with stewardship requirements.




