Sly-miR159 regulates fruit morphology by modulating GA biosynthesis in tomato
Summary
Fruit morphology is an important agronomical trait of many crops. Here, we identify Sly-miR159 as an important regulator of fruit morphology in tomato, a model species of fleshy-fruit development. We show that Sly-miR159 functions through its target SlGAMYB2 to control fruit growth. Suppression of Sly-miR159 and overexpression of SlGAMYB2 result in larger fruits with a reduced length/width ratio, while loss of function of SlGAMYB2 leads to the formation of smaller and more elongated fruits. Gibberellin (GA) is a major phytohormone that regulates fruit development in tomato. We show the Sly-miR159-SlGAMYB2 pathway controls fruit morphology by modulating GA biosynthesis. In particular, we demonstrate that Sly-miR159 promotes GA biosynthesis largely through the direct repression of the GA biosynthetic gene SlGA3ox2 by SlGAMYB2. Together, our findings reveal the action of Sly-miR159 on GA biosynthesis as a previously unidentified mechanism that controls fruit morphology in tomato. Modulating this pathway may have potential applications in tomato breeding for manipulating fruit growth and facilitating the process of fruit improvement.
Introduction
Fruit morphology is a major trait targeted for selection during domestication of fruit-producing crops, which has resulted in dramatic increase of shape variation in cultivated fruits compared to their wild ancestors (van der Knaap and Ostergaard, 2018; Monforte et al., 2014). Uncovering key genes and pathways controlling fruit morphology will not only help understand the process of domestication but also facilitate breeding new varieties for crop improvement. Furthermore, as fruit morphology is determined by the coordination of cell division and expansion that are fundamental cellular processes required for the development of all plant organs (van der Knaap et al., 2014; Snouffer et al., 2020; Tanksley, 2004), unravelling the molecular basis of fruit morphogenesis will also provide insights in general mechanisms of plant organogenesis.
Tomato (Solanum lycopersicum) is a model species for studying fleshy fruit development. Quantitative trait studies have revealed a number of loci that control fruit morphology in tomato, and several, including fw2.2, fw3.2, ovate, sun, locule-number (lc) and fasciated (fas), have been well characterized (van der Knaap et al., 2014). fw2.2 and fw3.2 are two important QTLs controlling fruit size in tomato. The FW2.2 gene encodes a Cell Number Regulator family protein that represses cell division (Frary et al., 2000; Guo and Simmons, 2011), and FW3.2 is a homolog of the Arabidopsis cytochrome P450 gene KLUH that positively regulates organ growth (Chakrabarti et al., 2013). The other four loci are major regulators of fruit shape in tomato. The gene underlying ovate is the founding member of the Ovate Family Protein class and acts as a negative regulator of fruit elongation (van der Knaap and Tanksley, 2001; Lazzaro et al., 2018; Snouffer et al., 2020; Wu et al., 2015, 2018). In contrast, the IQD family gene SUN promotes fruit growth in the proximal-distal axis and its overexpression results in longer berries (Wu et al., 2011, 2015; Xiao et al., 2008, 2009). lc and fas have similar functions in controlling the locule number in tomato. Plants carrying the mutant alleles of LC and/or FAS produce larger and flat-shaped fruits with more locules (Chu et al., 2019; Cong et al., 2008; Munos et al., 2011), thus these two genes affect both fruit size and shape in tomato development. LC was mapped near the tomato ortholog of WUSCHEL (WUS), while FAS likely encodes the tomato ortholog of CLAVATA3 (CLV3) (Chu et al., 2019; Munos et al., 2011; Xu et al., 2015). As the WUS-CLV pathway plays a conserved role in meristem development (Han' et al., 2020; Somssich et al., 2016), LC and FAS may act together to control fruit morphology by regulating meristem activities. These loci play prominent roles in shaping tomato fruits during domestication. In particular, ovate, sun, lc and fas can explain up to 71% of the fruit shape variation in cultivated tomatoes (Rodriguez et al., 2011). However, this observation also reflects a highly reduced genetic diversity in domesticated tomato varieties, in which the strong effect of these loci may overshadow the roles of other QTLs and limits the isolation of additional regulators of fruit morphology. Therefore, new strategies in addition to classic quantitative assays may need to be applied for further dissecting the gene network controlling fruit morphogenesis in tomato.
microRNAs (miRNAs) are a type of non-coding small RNA that act in posttranscriptional gene regulation in eukaryotes. They play important roles in major developmental and physiological processes in plants, such as meristem function, organ morphogenesis and stress response etc (Liu et al., 2018; Song et al., 2019; Tang and Chu, 2017). In tomato reproductive development, a number of miRNAs have been shown to be preferentially expressed in the ovary and/or at early fruit stages that are critical for determining fruit morphology (Mohorianu et al., 2011). Consistent with their expression patterns, some of these miRNAs have been implicated in regulating fruit shape and size. For example, overexpression of Sly-miR156 leads to the formation of abnormal carpel and fruit structures (Silva et al., 2014), and downregulation of Sly-miR160 increases fruit length and decreases fruit width, which results in elongated, pear-shaped fruits (Damodharan et al., 2016). As an miRNA often regulates a suite of target genes that in turn elicit a cascade of signalling events, further characterizing these fruit/ovary expressed miRNAs may help identify a set of related genes and pathways that control fruit morphogenesis.
In this study, we focused on the function of Sly-miR159 that is specifically expressed in the ovary and early fruit development (da Silva et al., 2017) (this study). Previous studies have shown that ectopic expression of Sly-miR159 impacts fruit set and ovule development in tomato, indicating its important role in fruit organogenesis (da Silva et al., 2017). However, whether and how endogenous Sly-miR159 affects fruit development, particularly fruit morphology, remain largely unknown. Furthermore, miR159 has a highly conserved sequence across plant species and targets the GAMYB-like transcription factors to mediate gibberellin (GA) signalling in a number of developmental processes, including fruit initiation in tomato (Achard et al., 2004; Allen et al., 2007; Millar et al., 2019; da Silva et al., 2017; Vallarino et al., 2015). But how the miR159-GAMYB pathway cooperates with GA to control tomato fruit shape and size is still not clear, though exogenous application of GA and enhancement of internal GA responses in pro mutant both have marked effect on tomato fruit morphology (Carrera et al., 2012; Hu et al., 2018; Marti et al., 2007).
Here we characterize the function of Sly-miR159 by downregulating its activity with Short Tandem Target Mimic (STTM) (Tang et al., 2012) and CRISPR/Cas9 (Du et al., 2020) technologies. The STTM159 and mir159CR plants show strong alteration in fruit morphology, producing larger and more flattened fruits with an increased number of locules. Furthermore, overexpression of SlGAMYB2, the major Sly-miR159 target, also results in a similar flat-fruit phenotype, while the loss of function of SlGAMYB2 has an opposite effect and leads to the formation of smaller and more elongated fruits. These results suggest that the Sly-miR159-GAMYB2 pathway has a vital role in regulating tomato fruit morphology. Moreover, we also show that Sly-miR159 affects fruit morphology by promoting GA biosynthesis, and this regulation is largely mediated by the direct repression of the GA biosynthetic gene GA3ox2 by SlGAMYB2. We propose that the regulation of GA biosynthesis by the Sly-miR159-GAMYB2 module represents a previously unidentified mechanism that control fruit morphology in tomato.
Results
Suppression of Sly-miR159 results in morphological changes of tomato fruits
MiR159 is a highly conserved miRNA that plays important roles in many developmental and physiological processes across plant species (Millar et al., 2019). The functions of miR159 have also been partly characterized in tomato (Lopez-Galiano et al., 2019; da Silva et al., 2017). In particular, overexpression of Sly-miR159 has been shown to affect fruit initiation and ovule/seed development (da Silva et al., 2017). Consistently, our quantitative PCR data indicate that Sly-miR159 is more highly expressed in the fruit compared to most other organs (Figure S1a). Furthermore, our and other’s tissue-specific analyses also reveal a strong expression of Sly-miR159 in the developing ovaries, fruits and seeds (da Silva et al., 2017) (Figure S1b–m). These results all suggest an important role of Sly-miR159 in ovary and fruit development. However, as the effect of Sly-miR159 loss-of-function has not been characterized in detail, the role of Sly-miR159 in regulating fruit organogenesis is still not clear.
In order to elucidate the native function of Sly-miR159, we utilized STTM technology to block its activity and analysed the phenotypes of STTM159 transgenic plants. We generated three independent STTM159 lines, and all of them had a significantly reduced level of Sly-miR159, indicating the success in knocking down the endogenous miR159 in tomato (Figure S2j). In the vegetative stages, the STTM159 plants are taller than wild type (Figure S2a); this phenotype resembles that of the target mimic 35S:MIM159 and supports the repressive role of miR159 on stem elongation (da Silva et al., 2017). STTM159 also has slightly wrinkled leaves (Figure S2b), suggesting Sly-miR159 may control leaf patterning similar to the role that miR159 members act in Arabidopsis (Allen et al., 2007), though its effect appears to be less prominent.
Similar to the mild phenotype in vegetative organs, the flower morphology is not strongly changed in STTM159 either. We only observed a slight increase in the size of floral petals and stamens in STTM159, but organ shape is largely unaltered as compared to wild type (Figure S2c,d). However, suppression of Sly-miR159 shows a greater impact on the development of ovaries and fruits. The most striking phenotype of STTM159 is the formation of flattened ovaries/fruits with a decreased shape index (length/width) compared to wild type (Figure 1a–c; Figure S2g,h; Table S1). In addition, STTM159 plants also have more carpels in the developing ovaries, which leads to an increase of locule number in the fruits (Figure S2e–g,i). Furthermore, we also found that the ovary/fruit perimeter and area of STTM159 are enlarged in both longitudinal and transverse dimensions, which results in heavier fruits in comparison with wild type (Figure 1f; Table S1). Sly-miR159 is generated from a single precursor (www.mirbase.org). We also used CRISPR/Cas9 to mutate the pre-Sly-miR159 and generate a loss-of-function mutant of Sly-miR159 (mir159CR) (Figure S3a–c). The mir159CR mutant shows a similar decrease in fruit shape index and increase in fruit perimeter/area as STTM159 when compared to wild type, further confirming the loss-of-function effect of Sly-miR159 on fruit shape formation (Figure S3d–k).

Fruit morphology is determined throughout a process from the development of ovary to the enlargement of fruit. To assess more in detail how fruit morphology changes over time in STTM159 as compared with wild type, we measured the ovary/fruit length and width and calculated the shape index from the stage-7 ovary to the ripening fruits (stages according to Brukhin et al. (2003)). Our results show the reduction in ovary/fruit shape index can be detected before anthesis from stage 11, becomes more dramatic as the ovary/fruit develops and plateaus at 10DPA (days post anthesis) (Figure 1a–c). Furthermore, the growth rate in the medio-lateral axis (width) of STTM159 fruits is higher than that of wild type (Figure 1d), while fruit growth in the proximal-distal direction (length) is similar in both plants (Figure 1e); this shows that the reduction of fruit shape index in STTM159 is primarily attributed to the increased growth of fruit width in comparison with wild type.
To determine how the growth of different internal tissues contributes to the overall morphological changes of fruits in STTM159, we measured the sizes of pericarp, septum, columella and locules in the fruits of STTM159 and wild type at anthesis. Our results show that the area and thickness of pericarp and septum are both reduced in STTM159, but the locule and columella areas are increased as compared with wild type (Figure S6a,b,f,g; Table S1); this indicates that suppression of Sly-miR159 results in a dramatic growth change of fruit tissues, which may contribute to the alteration of fruit shape and size in STTM159.
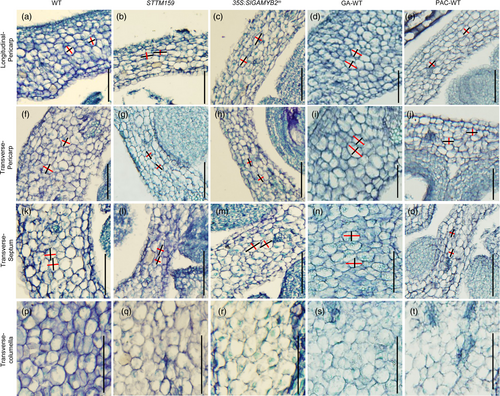
To further dissect the cellular basis of these morphological changes, we also analysed the cell number and size in the pericarp, septum and columella of STTM159 and wild-type fruits at anthesis. In both pericarp and septum, we found STTM159 has more but smaller cells than wild type (Figure 4a,b,f,g,k,l; Table S2), which suggests the thinner pericarp and septum in STTM159 are primarily caused by the reduction in cell size. In contrast, the size of columella cells is not significantly different in STTM159 compared to wild type, so the increased cell number is likely the major reason for the larger columella area of STTM159 fruits (Figure 4p,q; Table S2). Furthermore, we also measured the anticlinal (Y) and periclinal axis (X) of pericarp cells and observed a decreased anticlinal cell length in STTM159 compared to wild type in both longitudinal and transverse sections (Figure 4a,b,f,g; Table S2). This indicates the smaller pericarp cells in STTM159 are mainly resulted from the reduction of anticlinal cell growth. However, the periclinal axis of these cells is modestly increased on the transverse plane in comparison with wild type (Figure 4f,g; Table S2), which shows a trend of growth enhancement along the fruit perimeter of STTM159. This phenotype, together with the increased pericarp cell number, may be responsible for the longer fruit perimeter and larger fruit area in STTM159. Moreover, the reduced anticlinal cell length in the pericarp, and the relatively weaker elongation of columella tissues in the distal-proximal axis (Figure 4; Figure S6; Table S2), may act in cooperation to restrict the growth increase in the longitudinal direction in STTM159. The combined effect of these tissue and cellular changes makes the enlargement of STTM159 fruits more prominent in the transverse plane and results in a reduced fruit shape index as compared to wild type.
The miR159-targeted SlGAMYB2 controls fruit shape and size
SlGAMYB1 and SlGAMYB2 are two major target genes of Sly-miR159 (Karlova et al., 2013; da Silva et al., 2017). They are both expressed in the ovaries and fruits (da Silva et al., 2017), suggesting a potential role in regulating fruit organogenesis. We assessed the expression of these two genes using qPCR and found they are expressed at higher levels in the St11 and anthesis flowers than in the flowers at St7, which is opposite to the pattern of Sly-miR159 expression at these stages (Figure S4a–c). Furthermore, we detected increased transcription of both genes in STTM159 relative to wild type in the anthesis ovaries (Figure S4d,e). We also confirmed the cleavage of SlGAMYB1/2 by Sly-miR159 in the fruit using 5′-RACE (Figure S4f). These results all corroborate that SlGAMYB1 and SlGAMYB2 are downstream target genes of Sly-miR159 in ovaries/fruits. As the expression of SlGAMYB2 was more strongly increased than that of SlGAMYB1 when Sly-miR159 was suppressed at anthesis (Figure S4d,e), we speculated that SlGAMYB2 is a major target that mediates the function of Sly-miR159 in early fruit development. Therefore, we focused on investigating the role of SlGAMYB2 in the following studies. We used the constitutive 35S promoter to overexpress the native SlGAMYB2 gene and a Sly-miR159-resistant form of SlGAMYB2 (SlGAMYB2m) in tomato and obtained multiple 35S:SlGAMYB2 and 35S:SlGAMYB2m transgenic lines, all of which have higher expression of SlGAMYB2 than wild type. We selected one representative plant from each transgenic population and analysed their phenotypes (Figure 2; Figure S4). Compared to wild type, the most striking morphological change in these SlGAMYB2 overexpression plants is the formation of larger, heavier and more flattened fruits that phenocopies STTM159 (Figure 2c–h; Figure S4n–s; Table S1). Furthermore, this reduction of fruit shape index is mainly resulted from the increase in the medio-lateral axis (Figure 2e–g; Figure S4p–r), which is largely similar to the fruit shape change in STTM159 as well. In addition, 35S:SlGAMYB2m has a taller plant stature, slightly enlarged leaves and flowers and an increased number of carpels/locules (Figure S4g–m), which also resemble the morphological features of STTM159 (Figure S2).
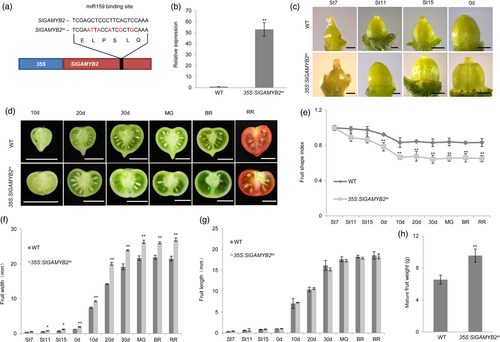
We also carried out a detailed examination of the ovary/fruit morphological changes in the development of 35S:SlGAMYB2m, as the reduction of fruit shape index is more dramatic in 35S:SlGAMYB2m compared to 35S:SlGAMYB2 (Figure 2c–g and Figure S4n–r), which is consistent with the much higher level of SlGAMYB2 expression in the former than the latter (Figure 2b; Figure S4o). Our results show that the decrease of fruit shape index in 35S:SlGAMYB2m is evident from ovaries at stage 11 (Figure 2c–e) and primarily attributed to the increase in ovary width at the same stage (Figure 2f); this suggests the fruit growth patterns of 35S:SlGAMYB2m resemble those of STTM159 (Figure 1). To support this hypothesis, we performed detailed tissue- and cellular-level analyses on the fruits of 35S:SlGAMYB2m and observed largely similar changes as seen in STTM159. For example, the 35S:SlGAMYB2m fruits have decreased area and thickness of pericarp and septum in comparison with wild type, which are likely attributed to the smaller size of cells in these tissues (Figure 4a,c,f,h,k,m; Figure S6a,c,f,h; Tables S1 and S2); These fruits also have increased pericarp cell number and periclinal cell length (Table S2), which may account for their larger perimeter and size as compared to wild type (Table S1). Moreover, the important features that determine the flattened fruit shape in STTM159, such as the reduced anticlinal growth of pericarp cells and specific enlargement of columella in the transverse direction, are detected in 35S:SlGAMYB2m as well (Figure 4; Figure S6; Table S1). These phenotypic similarities strongly support our model that SlGAMYB2 acts as a major effector of Sly-miR159 in fruit morphogenesis.
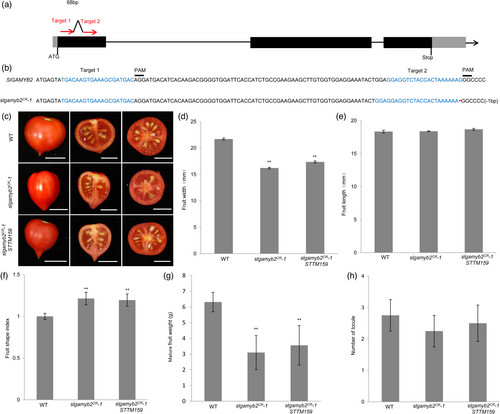
To further test this model, we generated three independent CRISPR/Cas9 engineered SlGAMYB2 mutants (slgamyb2CR), each of which carries deletions and/or insertions in the coding region of SlGAMYB2 (Figure 3a,b; Figure S5e). The slgamyb2CR plants produce smaller and lighter fruits with a shorter width, which results in increased fruit shape index (Figure 3c–g; Figure S5a–d). We also crossed one of these slgamyb2CR mutants, which contains a homozygous 1-nt deletion, with STTM159 to generate the slgamyb2CR STTM159 double mutant. We found the fruit phenotype of slgamyb2CR STTM159 resembles that of slgamyb2CR (Figure 3b–h) and is opposite to that of STTM159 and 35S:SlGAMYB2m (Figures 1 and 2). These results together confirm the important role of SlGAMYB2 as a major target of Sly-miR159 to control fruit development in tomato.
Sly-miR159 regulates fruit morphology by modulating GA biosynthesis
To characterize the molecular mechanisms that underlie the regulation of Sly-miR159 on fruit morphology, we carried out comparative transcriptomics of ovaries at anthesis in STTM159 and wild-type plants. We identified 5723 differentially expressed genes (DEGs) and performed a GO analysis of these DEGs to investigate the major molecular pathways that Sly-miR159 functions on (Appendix S1 and AppendixS2). In the top ranked GO categories, we found a number of terms associated with organ growth such as “cell proliferation”, “cellular process”, “developmental process”, “growth”, “reproduction” and “reproductive process”, etc (Appendix S2A), which suggests that Sly-miR159 may act through some growth-regulating genes in these terms to control fruit size and shape. In particular, “cell proliferation” contains a number of cyclin genes directly involved in cell cycle control (Appendix S2B) (Scofield et al., 2014), and “cellular process ” includes a class of genes encoding key components of cytoskeletal structures implicated in the regulation of cell division and differentiation (AppendixS2C) (Kost and Chua, 2002). These genes potentially function as important effectors to mediate the impact of Sly-miR159 on cell proliferation and growth during fruit development. Furthermore, we also found sets of genes related with the major fruit shape QTLs in the DEGs, including members of the OVATE, SUN and CLE families (Appendix S3), but many of them are not present in the most enriched GO categories. In addition, loss of Sly-miR159 results in up- or down-regulation of these genes and even the genes in the same family do not show expression changes in the same direction. These indicate a complex relation of Sly-miR159 with these key fruit shape regulators, which may involve layers of direct, feedback and/or feed-forward circuits.
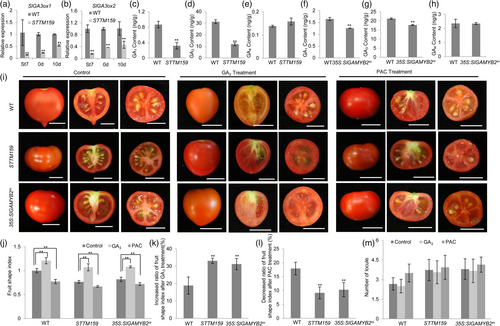
Other than these regulators of plant organ growth, a suite of genes in the DEGs that are involved in the signalling pathways of phytohormone GA also drew our great interest (Appendix S4). In particular, two genes that encode GA biosynthetic enzymes SlGA3ox1 and SlGA3ox2 have decreased expression in STTM159 as compared with wild type. qRT-PCR also confirmed this result and showed that the transcription of SlGA3ox1/SlGA3ox2 was not only reduced in the ovaries at anthesis but also in the stage-7 ovaries and young fruits at 10-DPA (Figure 5a,b). These results indicate Sly-miR159 may activate GA biosynthesis in these critical stages of ovary/fruit development by promoting the expression of SlGA3ox1 and SlGA3ox2.
Gibberellin has been shown to strongly impact fruit initiation and growth in tomato (Carrera et al., 2012; Garcia-Hurtado et al., 2012; Hu et al., 2018; de Jong et al., 2009; Marti et al., 2007; Olimpieri et al., 2007; Serrani et al., 2007, 2008). Specifically, application of the GA inhibitor paclobutrazol (PAC) reduces the length/width ratio of tomato fruits and results in similar tissue and cellular changes as observed in STTM159 and 35S:SlGAMYB2m; these include a thinner pericarp with decreased cell size and increased cell number and specific reduction of pericarp cell growth in the anticlinal direction that results in decreased cell shape index (Figure 4; Figure S6; Tables S1 and S2). In contrast, GA3 treatment exerts an opposite effect on tomato fruit growth at both tissue and cellular levels, which leads to the formation of elongated fruits with thicker pericarp and septum (Figure 4; Figure S6; Tables S1 and S2 (Carrera et al., 2012; Marti et al., 2007; Serrani et al., 2007)). These phenotypic analyses are consistent with our gene expression results and imply that Sly-miR159 may act through GA to control tomato fruit development.
To assess the possible regulation of Sly-miR159 on GA biosynthesis, we first quantitated the levels of active GAs (GA1, GA3 and GA4) in the ovaries of STTM159 and wild type at anthesis. We detected that GA1 and GA3 levels were markedly decreased in STTM159 in comparison with wild type, while the abundance of GA4 was not significantly changed (Figure 5c–e). Moreover, we applied GA3 and PAC on wild type and STTM159 plants and found the fruit shape change in STTM159 is more responsive to GA3 but less sensitive to PAC than that in wild type, as the STTM159 fruits showed stronger increase and weaker decrease of shape indices than wild-type fruits when treated with GA3 and PAC, respectively (Figure 5i–l). Furthermore, the reduced fruit length/width ratio in STTM159 was also restored to the wild-type value by GA3 treatment (Figure 5j). These results suggest the alteration of fruit morphology, particularly the decrease of fruit shape index, in STTM159 is largely attributable to the lower level of active GAs in ovaries/fruits; this is likely caused by the down-regulation of SlGA3ox1 and SlGA3ox2 when the function of Sly-miR159 is suppressed (Figure 5a,b). However, treatment of GA3 and PAC did not show a strong impact on the number of fruit locules in wild type and STTM159 (Figure 5m), indicating the increased locule number in STTM159 is likely due to misregulation of a different pathway.
SlGAMYB2 directly represses SlGA3ox2 in tomato fruit development
As SlGAMYB2 is a major target that mediates the function of Sly-miR159 in controlling fruit morphology (Figures 2 and Figure 3; Figures S4 and S5), we asked whether SlGAMYB2 carries out this role by suppressing GA biosynthesis. We first compared the active GA (GA1, GA3 and GA4) levels in the anthesis ovaries and detected a lower amount of GA1 and GA3 in 35S:SlGAMYB2m than in wild type (Figure 5f–h). We also treated 35S:SlGAMYB2m plants with GA3 and observed a change in fruit shape from flattened to an elongated shape (Figure 5i). Furthermore, the responses of fruit shape formation to GA3 and PAC are similar in 35S:SlGAMYB2m and STTM159; the shape indices of 35S:SlGAMYB2m fruits are more dramatically increased upon GA induction and decreased less in PAC treatment as compared to those in wild type (Figure 5j–l). These results together support our hypothesis that SlGAMYB2 acts downstream of Sly-miR159 to suppress GA biosynthesis in tomato fruit growth.
As we have shown earlier, two GA biosynthetic genes SlGA3ox1 and SlGA3ox2 are down-regulated in STTM159, which raises the possibility that SlGAMYB2 may modulate the transcription of these two key genes to impact GA production in tomato. To further validate this regulation, we assessed the expression of SlGA3ox1 and SlGA3ox2 in the fruits of 35S:SlGAMYB2m and wild-type control at anthesis. The qPCR result showed that only SlGA3ox2 was repressed in 35S:SlGAMYB2m, while the level of SlGA3ox1 is comparable to that in wild type (Figure 6a,b), suggesting SlGA3ox2, but not SlGA3ox1, is a putative target of SlGAMYB2. To test whether SlGAMYB2 directly binds to SlGA3ox2, we performed an electrophoresis mobility shift assay (EMSA) to assess the in vitro interaction of the SlGAMYB2-GST recombinant protein with four segments (P1–P4) in the 5′-regulatory region of SlGA3ox2, which all contain a putative recognition site of the R2R3-MYB transcription factors (Figure 6c; Figure S7a,b). The results showed that only two of these segments, P2 and P3, had strong affinity with SlGAMYB2-GST, whereas the other two (P1 and P4) failed to interact with it (Figure 6d,e; Figure S7c,d). Furthermore, we examined whether SlGAMYB2-GST also bound a region in the promoter of SlGA3ox1 that had a R2R3-MYB-binding sequence, but we could not detect any shifted bands in the experiment (Figure S7e). Together, these results corroborate the gene expression analysis and suggest that SlGAMYB2 only targets SlGA3ox2 in controlling GA biosynthesis.
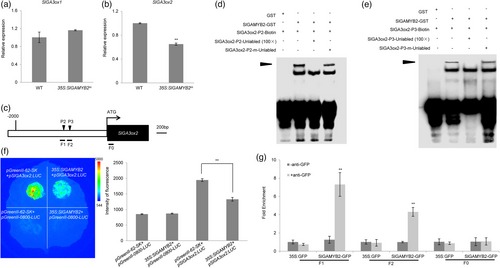
To further examine whether SlGAMYB2 interacts with SlGA3ox2 in plant cells, we carried out a transient expression assay in N. benthamiana using firefly luciferase (LUC) as the reporter. This analysis showed that the LUC activity driven by the SlGA3ox2 promoter was significantly reduced from the control when SlGAMYB2 was co-expressed in the same tissue (Figure 6f), which supports the direct and negative regulation of SlGAMYB2 on SlGA3ox2 in planta. Last, we utilized the 35S:SlGAMYB2m transgenic tomato line to conduct a chromatin immunoprecipitation (ChIP) experiment in the ovaries. As the SlGAMYB2m protein is also fused with a GFP tag, we immunoprecipitated it with a GFP antibody and detected a specific enrichment of two SlGA3ox2 promoter regions (F1 and F2) that encompass the P2 and P3 probe sequences, respectively (Figure 6g). These results provide strong evidence that SlGAMYB2 directly binds SlGA3ox2 in vivo to repress its transcription, which in turn reduces GA biosynthesis and impacts fruit morphogenesis in tomato.
Discussion
In this report, we identify Sly-miR159 as an important regulator of fruit morphology in tomato. miRNAs have been shown to play vital roles in many aspects of tomato development. In particular, several miRNAs have been found to be highly expressed in the fruit and impact fruit growth when their functions are modified (Damodharan et al., 2016; Silva et al., 2014; da Silva et al., 2017). However, the molecular and cellular mechanisms by which these miRNAs act to control fruit morphogenesis remain to be elucidated. Here we show that suppression of endogenous Sly-miR159 impacts fruit shape and size by affecting the internal fruit tissues, and these morphological changes are attributed to the alternation of cell proliferation and cell growth patterns in early fruit development. Furthermore, we find the Sly-miR159-targeted SlGAMYBs, especially SlGAMYB2, mediate the function of Sly-miR159 in controlling these phenotypes. More importantly, we reveal that the Sly-miR159-SlGAMYB2 pathway acts to modulate fruit morphology through the direct regulation of GA biosynthesis in young fruits. Together, our work exhibits a comprehensive analysis of the cellular and molecular roles of Sly-miR159 in fruit morphogenesis and demonstrates a key mechanism of the miRNA-associated control of fruit development in tomato. In addition, we use a reverse genetic approach to characterize the function of Sly-miR159 in fruit shape regulation. This strategy does not rely on the genetic diversity of available tomato germplasm and can specifically target small transcription units such as non-coding RNA genes, which may overcome the limitations of classic quantitative assays to discover new regulators of tomato fruit development. Moreover, as the regulation of miR159 on GAMYBs and the central GA signalling components are conserved in higher plants (Daviere and Achard, 2013; Millar et al., 2019), our results may also shed light on the genetic basis of fruit shape formation in other important crops.
Gibberellin is a major plant hormone that affects cell division, expansion and differentiation in many plant species. In tomato fruit development, GA plays vital roles in fruit set, growth and maturation (Garcia-Hurtado et al., 2012; Marti et al., 2007; Olimpieri et al., 2007), but how its activity is regulated during these developmental processes is largely unknown. Our study uncovers such a pathway that can help elucidate the molecular mechanisms governing the function of this key plant hormone in fruit morphogenesis. Furthermore, as miR159 and its targeted GAMYBs have been identified as critical downstream effectors of the GA-signalling pathways in a number of species (Millar et al., 2019), our results reveal another important action of the miR159-GAMYB module to operate upstream of GA and regulate its biosynthesis. It is worth noting that GA levels are not significantly altered in the tomato fruits that overexpress Sly-miR159 (da Silva et al., 2017). These Sly-miR159-overexpressing fruits are defective in ovule development and seed setting, suggesting that a significant portion of the Sly-miR159-induced GA production in fruit development is probably from ovules and seeds. This may also explain why overexpression of Sly-miR159 does not result in a dramatic change of fruit shape in their results (da Silva et al., 2017). In addition, these authors also show that SlGAMYB2 expression is not affected by GA, which indicates the uniqueness of SlGAMYB2 in the GAMYB family. It will be interesting to investigate whether other GAMYBs also act in a similar manner as SlGAMYB2 in GA-related processes, which may further our understanding of the functional relationship of this important transcription factor family with GA in plant development.
Although STTM159 and 35S:SlGAMYB2m plants have similar phenotypes, suppression of Sly-miR159 appears to show a stronger impact on fruit shape and GA biosynthesis than overexpression of SlGAMYB2 (Figures 1, 2 and 5). This implies that other genes may function in parallel with SlGAMYB2 to mediate the regulation of Sly-miR159 on GA in tomato fruit development. The most possible candidate of such genes is SlGAMYB1, as its expression is altered by gain and loss-of-function of Sly-miR159 (Figure S4; da Silva et al., 2017) and the homolog of SlGAMYB1 acts redundantly with that of SlGAMYB2 to regulate organ growth in Arabidopsis (Millar and Gubler, 2005). Furthermore, our transcriptomic data also revealed a number of important growth regulators in the DEGs between STTM159 and wild-type fruits, which may also operate as downstream effectors of Sly-miR159 in controlling fruit morphogenesis. In particular, a suite of core cell cycle genes and genes encoding key cytoskeletal components are present in the most enriched GO categories of these DEGs, suggesting Sly-miR159 may be involved in the regulation of cell proliferation and growth in tomato fruits. Consistent with these results, our phenotypic assays uncovered that cell number, size and dimension in various fruit tissues are affected by suppression of Sly-miR159 (Figure 4; Table S2), which sculpts the final shape of the STTM159 fruit. Specifically, the increase of pericarp cell number and periclinal cell axis may largely account for the longer fruit perimeter and width, and the reduction of pericarp cell length in the anticlinal direction combined with the modest distal-proximal elongation of columella tissues may be responsible for the unaltered fruit length in STTM159 as compared to wild type. Moreover, 35S:SlGAMYB2m fruits and PAC-treated wild-type fruits also exhibit similar morphological and cellular changes, which not only supports our proposed model that Sly-miR159 negatively regulates SlGAMYB2 that functions through GA to control fruit development but also implies that GA may act on the cell cycle and cytoskeleton genes downstream of Sly-miR159 to modulate critical cellular processes during fruit development. Further investigating the relation of GA with these key genes will help elucidate the molecular mechanisms of GA in controlling cell proliferation and growth, which is the most important cellular function of GA, but has not been fully understood to date. It is also worth noting that alteration of GA activity may not be responsible for all the phenotypic abnormalities caused by the misexpression of Sly-miR159 and SlGAMYB2, as GA3 application does not lower the increased locule number in STTM159 and 35S:SlGAMYB2m. This implies additional factors are required for the regulation of locule number by Sly-miR159. In our transcriptomic results, the expressions of two LC/FAS-related meristematic regulators, SlCLV1 (FAB; Solyc04g081590) and FIN (Solyc11g064850), are decreased in STTM159 compared to wild type (Appendix S3) and their mutants have increased number of locules (Xu et al., 2015). In addition, another CLE gene in the same family with FAS, SlCLE13 also has decreased expression in STTM159 (Zhang et al., 2014) (Appendix S3). Further analysing these genes will likely provide new insights in understanding the regulation of Sly-miR159 on locule number and fruit structure in the future.
Unlike the strong loss-of-function phenotype of the Arabidopsis miR159 at vegetative stages, suppression of Sly-miR159 appears to have only minor effects on the growth of vegetative organs. This is a bit surprising as the expression of Sly-miR159 is detected in most aerial parts of the tomato plant (Figure S1a). One possible explanation is that the induced SlGAMYBs upon suppression of Sly-miR159 require other factors to exert their roles in vegetative growth. In Arabidopsis, the miR159-targeted GAMYB protein MYB33 interacts with the TCP transcription factor TCP4 to control the development of various organs (Rubio-Somoza and Weigel, 2013). The close homolog of TCP4, LANCEOLATE (LA), is a central regulator of leaf morphogenesis in tomato (Ori et al., 2007). Whether LA functions with SlGAMYBs to control vegetative growth in tomato will be an interesting question to address in this respect. On the other hand, the specificity of Sly-miR159 action on fruit growth is a useful attribute for tomato breeding. Modification of fruit shape with minimal influence on vegetative organs can be achieved by engineering only Sly-miR159, thus greatly simplifying the selection process and facilitating the programme targeted for fruit improvement.
Methods
Generation of transgenic tomato lines and plant growth conditions
Construction of plasmids for generating STTM, CRIPSR/Cas9 and GUS reporter transgenic tomato lines was described in detail in Method S1. All the constructs were introduced in tomato (S. lycopersicum cv Micro-Tom) using the Agrobacterium tumefaciens-mediated transformation (Chetty et al., 2013). The presence of the transgene in each line was verified using PCR, and Sly-miR159 and SlGAMYB2 were also sequenced in mir159CR and SlGAMYB2CR to examine the resulted mutations. The reduced or increased expression of Sly-miR159 and SlGAMYB2 was confirmed in STTM159, 35S:SlGAMYB2 and 35S:SlGAMYB2m using qRT-PCR. STTM159 and slgamyb2CR were also crossed to generate the slgamyb2CR STTM159 double mutant.
Primers for PCR and qRT-PCR analyses are listed in Appendix S5. All the plants were grown in the greenhouse at 25 °C under natural daylight with a 16-h light/8 h dark regime.
RNA extraction and quantitative RT-PCR analysis
Total RNA was extracted from various tissues of wild-type and different transgenic tomato plants (details in the results section and figures) using TRIzol reagent (Thermo Scientific, Waltham, MA, USA). The first-strand cDNA was synthesized using a TAKARA first-strand cDNA synthesis kit (TaKaRa, Tokyo, Japan). Quantitative real-time PCR (qRT-PCR) was performed with SYBR Green detection, and relative gene expression was analysed using the 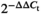 method (Livak and Schmittgen, 2001) from three biologically independent samples. Tomato β-Actin (Solyc11g005330) and U6 (Solyc01g103580) were used as the internal control for quantitation of mRNA and miRNA, respectively. Primers used for reverse transcription and qRT-PCR are listed in Appendix S5.
method (Livak and Schmittgen, 2001) from three biologically independent samples. Tomato β-Actin (Solyc11g005330) and U6 (Solyc01g103580) were used as the internal control for quantitation of mRNA and miRNA, respectively. Primers used for reverse transcription and qRT-PCR are listed in Appendix S5.
Rapid amplification of cDNA ends (5′-RACE)
Total RNA was extracted from wild-type ovaries at anthesis as described earlier, from which mRNA was enriched using the Oligotex mRNA Mini Kit (Qiagen, Valencia, CA). 5′-RACE was carried out following the published procedure (Zhai et al., 2014), and the amplified products were gel purified, cloned and sequenced. Primers used for 5′-RACE are listed in Appendix S5.
β-glucuronidase (GUS) staining
The GUS staining experiment was conducted following the protocol in Huang et al. (2012) with modifications. Briefly, different tomato tissues (details in Figure S1) were fully immersed in the staining buffer (0.5 mg/mL X-gluc (YEASEN, Shanghai, China), 100 mm Na2HPO4, 100 mm NaH2PO4, 10 mm EDTA, 0.5 mm K4Fe(CN)6, 0.5 mm K3Fe(CN)6, 0.01% Triton X-100), vacuum-infiltrated and stained at 37 °C for 12 h. After staining, the plant materials were treated with 75% ethanol to remove chlorophyll and photographed under a stereoscope (Leica, Wetzlar, Germany).
Phenotypic and histological analyses
To analyse the size and shape of ovaries/fruits in tomato development, ovaries were collected at the floral stages St7, St11, St15 and anthesis and photographed under a stereomicroscope (Leica), while fruits were harvested at the stages of 10d (days post anthesis), 20d, 30d, mature green (MG), breaker (BR) and red ripe (RR), hand-dissected and imaged using a digital camera. The length and width were measured from 10 ovaries/fruits for each line using the ImageJ software (https://imagej.nih.gov/ij/). To quantitate fruit weight, RR fruits on the first inflorescence were collected and weighed. In total, 10 fruits of each genotype were used to calculate the average fruit weight. For histological analysis, ovaries at anthesis were fixed with FAA and embedded in paraffin. These ovary samples were sectioned using the RM2265 microtome (Leica), stained with 1% Toluidine blue and observed under a compound microscope (BX51, OLYMPUS, Tokyo, Japan). Analyses of fruit length, width, perimeter, as well as the internal tissue measurements were performed in ImageJ. Cell size and shape analyses were conducted in the comparable cell layers of different plants, using previously published methods in Cheniclet et al. (2005) and Musseau et al. (2017). Ten fruits from each line were assessed for each morphological trait.
RNA-seq
Total RNA was extracted from three biologically independent pools of the wild type or STTM159 ovaries at anthesis as described in “RNA extraction and quantitative RT-PCR analysis”. RNA-seq libraries were constructed using the Stranded mRNA-seq (NR602, Vazyme, Nanjing, China) kit. Sequencing was performed in Genergy Bio (China) using Illumina Novaseq platform to produce 150 bp pair-end reads. FastQC (v0.11.5) was used to perform basic statistics on the quality of the raw reads. Trim_galore (v0.4.4) was used to remove adapter and low quality reads. Clean reads were mapped to tomato genome by STAR (v2.7) (Dobin et al., 2013). The expression values and transcript assembly of genes were analysed by StringTie (v1.3.6) (Pertea et al., 2016). Identification of DEGs was conducted using DESeq2 (Padj < 0.05) (Love et al., 2014).
Gibberellin quantification and GA3/paclobutrazol (PAC) treatment
About 500 mg of ovaries from wild type, STTM159 and 35S:SlGAMYB2m were harvested at anthesis and sent to the facilities in Institute of Chemistry, Chinese Academy of Sciences (Beijing 100190, China) and Wuhan Metware Biotechnology Co., Ltd (Wuhan, 430070, China) for GA quantification. GAs were extracted from three independent pools of ovaries and analysed based on the published protocols using mass spectrometer (Chen et al., 2013; Li et al., 2017).
For GA3 and PAC treatments, 10 mL of 0.1 mm GA3, 0.1 mm PAC (purity ≥ 95.0%, Sigma-Aldrich, Darmstadt, Germany) or control solution (0.095% ethanol) was applied to the roots of 30-day--old plants every day for 10 days. The effects of GA3 and PAC treatment on fruit morphology and tissue/cellular structures were analysed as described in section “Phenotypic and histological analyses”.
Protein–DNA interaction assays
Interactions of SlGAMYB1 or SlGAMYB2 protein with the promoter sequences of SlGA3ox1 or SlGA3ox2 were analysed in vitro using electrophoretic mobility shift assay, in Nicotiana benthamiana leaves with the dual-luciferase reporter system, and in tomato ovaries using ChIP. Detailed procedures of these experiments are provided in Method S2, S3 and S4.
Accession numbers
The RNA-Seq raw data in this study have been submitted to the GenBank databases under accession number PRJNA692259. Gene sequences can be found under the accession numbers: SlGAMYB1 (Solyc01g009070), SlGAMYB2 (Solyc06g073640), SlGA3ox1 (Solyc06g066820), SlGA3ox2 (Solyc03g119910), Tomato β-ACTIN (Solyc11g005330) and U6 (Solyc01g103580).
Acknowledgements
We thank Dr. Guiliang Tang (Michigan Technological University, USA) and Dr. Yuling Jiao (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, China) for gifts of vectors; Dr. Xuemei Chen (University of California, Riverside, USA), Dr. Beixin Mo (Shenzhen University, China), Dr. Vivian Irish (Yale University, USA) and Irish lab members for helpful discussions and suggestions on the manuscript. We are also grateful for the assistance from the Instrumental Analysis Center of Shenzhen University (Lihu Campus) and Central Research Facilities of College of Life Sciences and Oceanography on microscopy experiments. This project was supported by Guangdong Innovation Research Team Fund (2014ZT05S078), National Natural Science Foundation of China (NSFC) Grants (31772322) and Guangdong Special Support Program for Young Talents in Innovation Research of Science and Technology (2019TQ05N940).
Conflict of interest
The authors declare no conflict of interest.
Author contributions
P.Z. and T.H. designed the study, conducted experiments, analysis and interpretation of data and drafted the article. F.W., Y.D., F.Z. J.D. and Y.Z. conducted experiments, analysis and interpretation of data. P.Z., P.T., D.L. and T.H. conducted bioinformatics analysis. P.Z. and T.H. wrote the manuscript.




