Manipulation of β-carotene levels in tomato fruits results in increased ABA content and extended shelf life
Summary
Tomato fruit ripening is controlled by the hormone ethylene and by a group of transcription factors, acting upstream of ethylene. During ripening, the linear carotene lycopene accumulates at the expense of cyclic carotenoids. Fruit-specific overexpression of LYCOPENE β-CYCLASE (LCYb) resulted in increased β-carotene (provitamin A) content. Unexpectedly, LCYb-overexpressing fruits also exhibited a diverse array of ripening phenotypes, including delayed softening and extended shelf life. These phenotypes were accompanied, at the biochemical level, by an increase in abscisic acid (ABA) content, decreased ethylene production, increased density of cell wall material containing linear pectins with a low degree of methylation, and a thicker cuticle with a higher content of cutin monomers and triterpenoids. The levels of several primary metabolites and phenylpropanoid compounds were also altered in the transgenic fruits, which could be attributed to delayed fruit ripening and/or to ABA. Network correlation analysis and pharmacological experiments with the ABA biosynthesis inhibitor, abamine, indicated that altered ABA levels were a direct effect of the increased β-carotene content and were in turn responsible for the extended shelf life phenotype. Thus, manipulation of β-carotene levels results in an improvement not only of the nutritional value of tomato fruits, but also of their shelf life.
Introduction
Plants have evolved several mechanisms for seed dispersal, one of which is the development of fleshy fruits with attractive organoleptic characteristics, such as fleshiness, colours and flavours able to attract frugivore animals for seed dispersal. Tomato (Solanum lycopersicum L.) is a model system for the study of fruit ripening, mainly due to many genetic and postgenomic resources available for this species (reviewed in refs. Gascuel et al., 2017; Giovannoni et al., 2017; Klee and Giovannoni, 2011; Seymour et al., 2013). Tomato fruit development comprises an initial phase of postanthesis cell division, followed by one of cell expansion, during which concentrations of the hormones ethylene and abscisic acid (ABA) are both low (Zhang et al., 2009). Immediately, after the mature green (MG) stage of ripening, a transient peak in ABA content occurs, followed by a switch of ethylene production from System 1 (autoinhibitory) to System 2 (autocatalytic) and a peak in ethylene production (Klee and Giovannoni, 2011; Seymour et al., 2013). Several other events follow, such as fruit softening, the accumulation of sugars and organic acids, and a change of colour from green to red, due to the accumulation of the linear carotene, lycopene (Giuliano et al., 1993; Klee and Giovannoni, 2011). These changes are accompanied, at the molecular level, by extensive changes in gene expression (Alba et al., 2005; Carbone et al., 2005), with lycopene accumulation being highly associated with the up-regulation of genes encoding PHYTOENE SYNTHASE 1 (PSY1) and PHYTOENE DESATURASE (PDS) (Giuliano et al., 1993), and the down-regulation of genes encoding LYCOPENE β- and ε-CYCLASE (LCYb and LCYe) (Pecker et al., 1996; Ronen et al., 1999; Ronen et al., 2000). Many of these events have been demonstrated to depend on the presence of a functional ethylene receptor (Alba et al., 2005).
Besides ethylene, tomato fruit ripening is controlled by a cascade of transcription factors, some of which mediate input by other hormones, such as auxin and ABA (Giovannoni et al., 2017; Klee and Giovannoni, 2011; Seymour et al., 2013). Exogenous application of ABA at the mature green (MG) stage increases the amplitude of the ethylene peak and accelerates ripening, while the application of fluridone (a carotenoid biosynthesis inhibitor) or nordihydroguaiaretic acid (an inhibitor of ABA biosynthesis) has the opposite effect (Zhang et al., 2009). Knockout mutations of ZEAXANTHIN EPOXIDASE (ZEP) or silencing of 9-cis-EPOXYCAROTENOID DIOXYGENASE (NCED) results in decreased endogenous ABA and increased ethylene (Galpaz et al., 2008; Ji et al., 2014), respectively. In contrast, silencing of three CYP707A2 isoforms, encoding ABA 8′-hydroxylases acting in ABA catabolism, or of ABA uridine diphosphate glucosyltransferase (SlUGT75C1), which produces esterified ABA-glucose, generated over-ripe fruits with increased ABA levels (Ji et al., 2014; Sun et al., 2017). A similar phenotype was observed in RNAi lines for SlPP2C1, a group A type 2C protein phosphatase involved in ABA signalling (Zhang et al., 2018). These observations suggest that ABA influences fruit ripening in different ways, depending on the mode (external or endogenous) and timing of the application. Since ABA is synthesized from β-xanthophylls (Figure 1A), an interesting corollary of the above hypothesis is that carotenoid composition may itself play a role in controlling tomato fruit ripening. At the MG stage, levels of β-xanthophylls are high, and during ripening, they decline, due to the down-regulation of LCYb genes (Pecker et al., 1996; Ronen et al., 2000), possibly affecting ABA levels.
Several reports have described the metabolic engineering of plant carotenoid contents (Giuliano, 2014; Giuliano, 2017). Overexpression of a LCYb gene from Arabidopsis under the control of the ripening-associated PDS promoter leads to ripe tomato fruits that accumulate high levels of β-carotene (Rosati et al., 2000). Apart from the transgene, these engineered lines are perfectly isogenic with their lycopene-accumulating parental genotype, making them a good system to study the possible influence of carotenoid composition on fruit ripening. Using two independent transgenic lines, we conducted a system-wide study of the effect of increased β-carotene levels on tomato fruit ripening and shelf life. Our data suggest that the increase in the β-carotene content results in higher ABA content, which in turn has an effect on fruit ripening and shelf life.
Results
LCYb-overexpressing fruits exhibit higher β-carotene levels and increased shelf life, which does not correlate with antioxidant activity
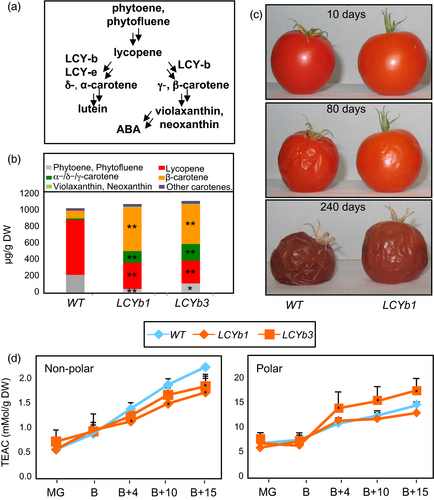
Transgenic tomato lines overexpressing Arabidopsis LCYb under the control of the chromoplast-associated PDS promoter accumulate β-carotene in ripe fruits (Rosati et al., 2000; Figure 1B,C). Homozygous T4 lines derived from two independent transformation events were grown in the greenhouse, and fruits were harvested at five stages of ripening: mature green (MG), breaker (B), breaker+4 or pink (B+4), breaker+10 or ripe (B+10) and breaker+10 or over-ripe (B+15 and B+40). Expression of AtLCYb peaked at B and B+4 (Figure S1). A fourfold to 10-fold increase in β-carotene and other cyclic carotenes and a twofold to threefold decrease in linear carotenes were observed in B+10 fruits compared to WT. Xanthophylls were undetectable in WT fruits, but became detectable in LCYb-overexpressing fruits (Figure 1C, Table S1).
LCYb-overexpressing fruits stored at room temperature for several months exhibited, relative to WT, a significant increment in shelf life, increased firmness and reduced water loss (Figure 1D). No significant alteration in the time elapsing between anthesis and fruit breaker stage was observed (Figure S2), indicating that the alteration was confined to the late stages of fruit development. Since the observed changes in carotenoid levels in LCYb-overexpressing fruits are likely to affect fruit antioxidant activity, which is known to impact fruit shelf life (Zhang et al., 2013), we measured the antioxidant activity in both polar and nonpolar extracts of WT and LCYb-overexpressing fruits at five ripening stages (MG, B, B+4, B+10 and B+15; Figure 1D). No significant differences compared to the WT were observed at early stages (MG and B). At later stages (B+4 through B+15), LCYb-overexpressing fruits displayed a reduction in the antioxidant activity of the nonpolar fraction, while that of the polar fraction varied in opposite directions in the two transgenic lines, with the LCYb3 line showing an increase and the LCYb1 line a decrease with respect to the WT.
Increased firmness and decreased water loss of LCYb-overexpressing fruits correlate with altered cell wall and cuticle composition
The firmness of WT and LCYb-overexpressing fruits was measured with a hand-held penetrometer at five different ripening stages. At MG and B, the firmness of the two types of fruits was similar, and then starting at B+4 and until the end of ripening, LCYb-overexpressing fruits exhibited significantly increased firmness (Figure 2A). One of the components of fruit firmness, evapotranspiration, was found to be significantly lower in LCYb-overexpressing fruits (Figure 2B). Total cell wall extracts from the pericarp of B+10 fruits were fractionated by sequential extraction with water, chelating agent, dilute alkali and concentrated alkali (Huisman et al., 1998). Significant increases were observed in the abundance of total cell wall material, water-soluble solids and dilute alkali-soluble solid fractions of LCYb-overexpressing fruits. Additionally, the WSS fraction of LCYb-overexpressing fruits showed a significant increase in the abundance of galacturonic acid, which is present in the backbone of HG and RG-I polysaccharides (Figure 2C). The occurrence of methyl-esterified pectins in the WSS fraction was analysed by immunodot analysis using the LM20 monoclonal antibody, which recognizes highly methyl-esterified HG epitopes. A lower abundance of methyl-esterified pectins was observed in the WSS fraction from LCYb-overexpressing fruits, compared to WT, while the ChASS fraction did not show significant differences (Figure 2D).
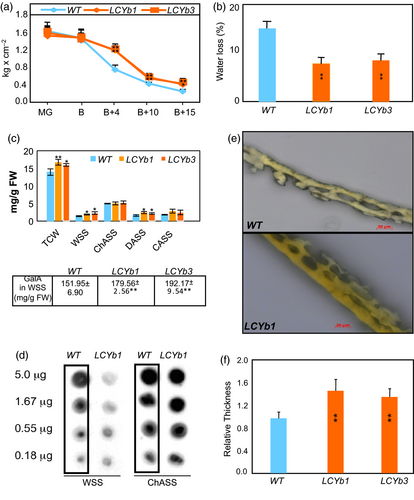
Cuticle thickness was also significantly increased in LCYb-overexpressing B+10 fruits (Figure 2E,F). We further investigated the chemical composition of the cutin polymer and the associated cuticular waxes using gas chromatography–mass spectrometry (GC-MS). The amounts of all cutin monomers were significantly higher in the LCYb-overexpressing fruits than in WT. Among the cuticular waxes, the major very-long-chain acyl derivatives were relatively unchanged in LCYb-overexpressing fruits, while the triterpenoid components α-amyrin, taraxasterol, ψ-taraxasterol and δ-amyrin were all >2-fold greater than WT levels (Table S2).
Metabolic remodelling in LCYb-overexpressing fruits
A total of 72 phenylpropanoids were measured in both the flesh and the cuticle of B+10 fruits, using liquid chromatography coupled with high-resolution mass spectrometry (LC-HRMS; Table S3). Most compounds showed an overaccumulation in LCYb vs WT fruit cuticles. A notable exception was found in the group of phenolic compounds, such as 1-caffeoyl-1-beta-D-glucose, 4-p-coumaroylquinic acid, chlorogenic, coumaric, dicaffeoylquinic acid and ferulic acids, which showed a slight decrease in the flesh of LCYb-overexpressing fruits relative to WT; on the contrary, stronger positive variations were observed for flavonoids and flavonoid glycosides: 26 out of 48 metabolites showed significantly higher levels in LCYb-overexpressing fruits, with catechin/epicatechin, eriodictyol, kaempferol and quercetin conjugates showing the largest increases (Table S3).
Additionally, the levels of 58 metabolites (20 amino acids, 19 sugars/polyols, eight organic acids and 11 others) were quantified by GC-MS in ripe fruits of two LCYb-overexpressing lines (Schauer et al., 2005; Table S4). Thirteen compounds (five amino acids, seven sugars/polyols and putrescine) showed significant changes in both transgenic lines. The amino acids alanine, aspartate, phenylalanine and proline, the sugars mannitol and sucrose, the organic acid 2-oxo-butyric acid and the polyamine putrescine were lower in LCYb-overexpressing fruits. In contrast, the sugars erythritol, galactinol, glucose, glucoheptose and melibiose, and the sugar phosphate fructose 6-P were higher. When averaged between the two LCYb lines, all changes were less than twofold in magnitude (Table S4), suggesting that changes in primary metabolism were minor.
LCYb-overexpressing fruits exhibit increased ABA and decreased ethylene production and altered expression of the RIN and NOR ripening regulators
ABA is synthesized from violaxanthin and neoxanthin, which are increased in LCYb-overexpressing fruits (Figure 1A,B). ABA accumulation during ripening was measured using LC-HRMS (Figure 3A, Table S5) and showed very distinct kinetics in WT and LCYb-overexpressing fruits: in WT fruits, ABA levels peaked at the B stage and then progressively declined until B+15, while in LCYb-overexpressing fruits they showed a much larger peak at the B stage, followed by a further increase to 17-fold WT levels at B+15. ABA catabolites (phaseic acid, dihydrophaseic acid, ABA-Glc and 7-hydroxy-ABA) also showed increased levels in LCYb-overexpressing fruits (Table S5). These data indicate that LCYb overexpression during ripening causes accumulation of β-carotene and β-xanthophylls, and that the increased flux through the β-branch of carotene biosynthesis results in increased levels of ABA and its downstream catabolites.
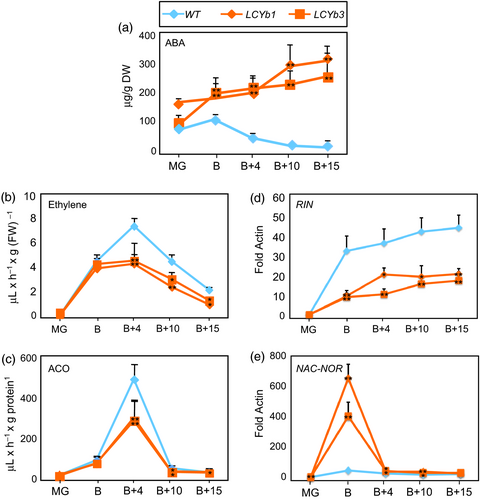
We also measured ethylene emission by intact fruits and the enzymatic activity of ACC oxidase, the last enzyme in the ethylene biosynthetic pathway. Ethylene production and ACC oxidase activity both peaked at B+4 in WT fruits, and the peak showed an approximately 50% reduction in LCYb-overexpressing fruits relative to WT (Figure 3B,C).
The kinetics of expression of two key regulators of fruit ripening, RIN (Vrebalov et al., 2002) and NAC-NOR (Giovannoni et al., 2004; Osorio et al., 2011), were analysed by quantitative real-time RT-PCR (qRT-PCR) in WT and LCYb-overexpressing fruits (Figure 3D,E). The RIN transcript was strongly repressed starting at the B stage and throughout the whole ripening process, while, on the contrary, NAC-NOR displayed a large increase in expression at the B stage.
Systems analysis of WT and LCYb-overexpressing fruits
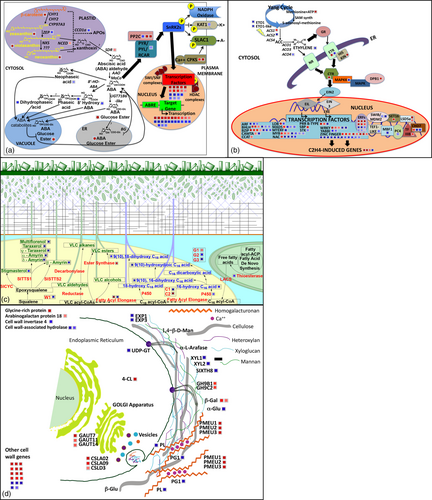
Transcript profiling was performed on fruits at three ripening stages (MG, B and B+10) in the WT and two LCYb-overexpressing lines using the EU-TOM3 Affymetrix microarray (Tables S6, S8 and S10A,B). Genes up- or down-regulated >1.5-fold in both transgenic lines with respect to WT, with a P-value ≤0.05, were considered differentially regulated and are shown as MapMan representations in Figure S3. A total of 123 transcripts were found to be differentially regulated in both the MG and B stages, 137 in both B and B+10 and 148 in both MG and B+10 (Figure S4, Table S12). GO enrichment analysis (Tables S7, S9 and S11A,B) showed a series of enriched GO terms in up-regulated genes (tetrapyrrole/chlorophyll and protein binding and amine metabolism at the MG and B+10 stages, respectively) and in down-regulated ones (carbohydrate and nucleotide metabolism). We also performed a manual annotation of differentially regulated transcripts involved in well-known aspects of fruit ripening, including ethylene metabolism and regulation, cell wall remodelling, cuticle biogenesis, primary metabolism, phenylpropanoid, carotenoid and apocarotenoid pathways (including ABA). All of the aforementioned classes were represented in differentially regulated genes, with ethylene- and cell wall-related genes showing the highest number of differentially expressed (particularly down-regulated) representatives (Tables S6, S8 and S10A,B). Interestingly, a series of key genes in the phenylpropanoid pathway (PHENYLALANINE AMMONIA-LYASE (PAL) and CHALCONE SYNTHASE (CHS) at the MG stage; PAL and 4-COUMARATE:COA LIGASE 2 (4CL2) at the B stage; CHS and DIHYDROFLAVONOL 4-REDUCTASE (DFR) at the B+10 stage) were also overexpressed in LCYb-overexpressing fruits compared to the WT.
The levels of 28 additional transcripts involved in fruit ripening control were measured in B+10 fruits using through qRT-PCR (Table S13). The majority of transcription factors, including RIN, TAGL1 (Itkin et al., 2009; Vrebalov et al., 2009), CNR (Manning et al., 2006), HB-1 (Lin et al., 2008), AP2a (Chung et al., 2010; Karlova et al., 2011) and TDR4/FUL1 (Bemer et al., 2012), were down-regulated in LCYb-overexpressing fruits. Down-regulated transcripts also included the NEVER-RIPE ethylene receptor, GR, CTR1 and EIN2, participating in ethylene signalling (Barry et al., 1996; Fu et al., 2005), and ACS2, ACS4 and ACO1 genes (Barry et al., 2000; Bidonde et al., 1998) involved in ethylene biosynthesis. Notable exceptions to this pattern were the NAC-NOR transcription factor (Giovannoni et al., 2004; Osorio et al., 2011), and the E4 and E8 ethylene-inducible genes (Cordes et al., 1989), which were up-regulated in LCYb-overexpressing fruits. Several genes involved in cell wall degradation/remodelling were also down-regulated, such as POLYGALACTURONASE 1 (PG1), PECTATE LYASE (PL), α-L-ARABINOFURANOSIDASE 1 (ARF1), EXPANSIN 1 (EXP1), β-D-XYLOSIDASE 1 and 2 (XYL1, XYL2) and XYLOGLUCAN ENDO-TRANSGLUCOSYLASE/HYDROLASE 4 and 8 (SIXTH4, SIXTH8) genes. Exceptions included the PECTIN METHYLESTERASE 1 and 2 (PMEU1, PMEU2) genes, which were up-regulated, consistent with the decreased levels of pectin methyl-esterification noted above.
The metabolic and transcriptional alterations observed in LCYb-overexpressing fruits at B+10, associated with ABA, ethylene, cuticle and cell wall metabolism, are summarized as ad hoc MapMan charts (Thimm et al., 2004) in Figure 4. LCYb overexpression in fruits resulted in extensive perturbations of ABA and ethylene metabolism and signal transduction, and also of cuticle and cell wall biogenesis.
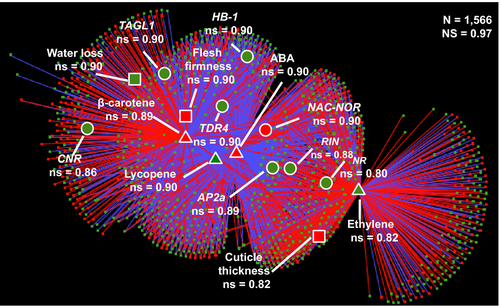
In order to identify co-regulated traits (e.g. metabolites, hormones, transcripts, phenotypic traits), we chose 1612 variables that are differentially regulated in LCYb-overexpressing with respect to WT fruits. The Pearson correlation coefficient values (ρ) for the resulting trait pairs (Table S14) were used to build a correlation network (Diretto et al., 2010a), including correlations |ρ| > 0.90 (Figure 5, Table S15). The overall ‘network strength’ (i.e. the average of all |ρ| values (Diretto et al., 2010a) was very high (0.97), indicating that the variables are tightly co-regulated. Several metabolites, hormones and phenotypic traits associated with fruit ripening grouped as a tight cluster, in a region populated by known ripening regulators. Notably, ABA had a central position in this network and was strongly co-regulated with NAC-NOR (ρ = 0.99), RIN (ρ = −0.99) and AP2a (ρ = −0.99), and less so with ethylene, NR, HB-1, CNR and TAGL1. NAC-NOR, RIN and AP2a were also strongly co-regulated with ethylene (ρ = 0.96, 0.98 and 0.97, respectively). Interestingly, the CNR ripening regulator (Manning et al., 2006) was more strongly co-regulated with ABA (−0.90) than with ethylene (0.73). We also constructed subnetworks centred around ABA and ethylene. The ABA network (Figure S5A, Table S16A) included the vast majority of transcription factor genes known to control fruit ripening: NAC-NOR, TDR4, AP2A, HB-1, RIN, TAGL1 and CNR. Of these, NAC-NOR showed positive co-regulation with ABA, while all other genes showed negative co-regulation (Tables S15 and S16A). Additional genes strongly co-regulated with ABA included genes involved in ABA signal transduction; genes for ethylene biosynthesis, sensing and signal transduction; and genes for carotenoid, chlorophyll and cell wall metabolism (Table S16A). In the second network, centred around ethylene (Figure S5B; Table S16B), strongly co-regulated genes were involved in ethylene biosynthesis, sensing and signal transduction, but also key ripening regulators and genes involved in ABA signal transduction (Tables S15 and S16B). This is consistent with recent suggestions of substantial crosstalk between the networks controlling these hormones during ripening (Galpaz et al., 2008; Ji et al., 2014; Sun et al., 2017).
Abamine treatment of LCYb-overexpressing fruits reduces ABA accumulation, increases ethylene production and reverses the long shelf life phenotype
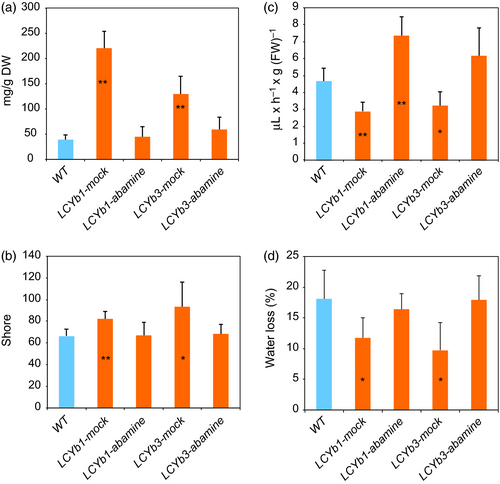
Abamine is a well-known inhibitor of NCED enzymatic action and of ABA biosynthesis (Han et al., 2004). To better investigate the ABA role in the extended shelf life phenotype, we treated LCYb-overexpressing fruits with abamine at the MG stage. The treatment resulted in a reduction of ABA in LCYb-overexpressing fruits to levels similar to WT ones (Figure 6A). As a result, ethylene production was increased to levels slightly higher than those of WT fruits (probably as a result of the injection), while flesh firmness and water loss reverted to WT levels (Figure 6B–D).
Discussion
LCYb overexpression in tomato fruits resulted in increased β-carotene content and fruit firmness and in extended shelf life. At the biochemical level, this phenotype was accompanied by a modification of cell wall composition and polymerization, of cuticle thickness and chemical composition, of primary metabolite and phenylpropanoid profiles in the fruit pericarp. This is, to our knowledge, the first time that metabolic engineering of carotenoid biosynthesis has been reported to have such profound and pleiotropic effects on fruit ripening. Until now, carotenoid composition has merely been considered as an output of the regulatory circuit controlling fruit ripening.
Metabolic alterations in LCYb-overexpressing fruits
Some of the metabolic perturbations observed in LCYb-overexpressing fruits can be attributed to their delayed ripening: compounds such as alanine, aspartate, proline, mannitol and putrescine increase during normal fruit ripening (Carrari et al., 2006), and show a decrease in ripe LCYb-overexpressing fruits compared to WT ones. Other metabolic perturbations can be attributed to the observed changes in gene expression: for instance, the decrease in sucrose and increase in glucose levels correspond to an induction, at the B stage, of ACID INVERTASE, which hydrolyses sucrose into glucose and fructose. The functional role of acid invertase is very well characterized in tomato fruits, with quantitative trait loci, genome-wide association studies and reverse genetic approaches, all indicating its importance for determining soluble solid content (Fridman et al., 2004; Tieman et al., 2017) and aspects of fruit development and seed yield (Zanor et al., 2009). Also, overexpression of ACID INVERTASE has been observed in tomato fruits overexpressing an ABA response element binding factor (SlAREB1) which leaves ethylene levels unaltered (Bastias et al., 2011), indicating that this trait may be under direct ABA control. Interestingly, LCYb-overexpressing fruits share considerable commonalities with those of the well-characterized ripening mutants rin, nor, NR and ap2 (Karlova et al., 2011; Osorio et al., 2011) with decreases in alanine, aspartic acid, glutamic acid, glutamine, phenylalanine and proline all being observed in rin, nor and NR and the changes in aspartic acid, glutamine, phenylalanine and proline also being observed in ap2. Glucose also showed consistent trends between ripening mutants and LCYb-overexpressing fruits, being overaccumulated in all the genotypes; while sucrose, present at higher contents in nor, rin and NR, displayed an opposite trend, that is reduction, in LCYb-overexpressing fruits.
Additional metabolic fluctuations that can be attributed to ABA accumulation are the increase in flavonoids. The extent of changes (>10-fold for some flavonoid glycosides) suggests they represent direct effects of the genetic manipulation and thus of the increase in ABA in LCYb-overexpressing fruits, compared to the less pronounced alterations observed in primary metabolites, which are likely to represent secondary effects. This hypothesis finds support in a series of previous studies linking ABA and flavonoid levels in apple (Lu et al., 2017), soybean (Gupta et al., 2018) and tomato (Mou et al., 2015). In agreement with biochemical data, a series of key structural phenylpropanoid genes was up-regulated in LCYb-overexpressing fruits: for instance, PHENYLALANINE AMMONIA-LYASE (PAL) at the MG and B stages, and CHALCONE SYNTHASE (CHS) at the MG and B+10 stages. ABA has been shown previously to promote PAL and CHS expression (Yu et al., 2015; Zhang et al., 2017) and PAL activity (Jiang and Joyce, 2003).
Purple tomatoes, overexpressing Del and Ros transcription factors from snapdragon and accumulating large amounts of anthocyanins, display extended shelf life, a phenotype attributed to the increased total antioxidant activity caused by anthocyanin accumulation (Zhang et al., 2013). While we cannot exclude that the increase of flavonoid levels in the peel of LCYb-overexpressing fruits influences its permeability, and hence, water loss and total antioxidant activity do not seem to have a causal relationship with the extended shelf life of these fruits: the fluctuations of total antioxidant activity in the polar fraction did not correlate with fruit shelf life, while those in the nonpolar fraction showed a negative correlation. This finding is not surprising, since β-carotene has been reported to have a lower antioxidant activity compared to lycopene (Bohm et al., 2002). Thus, the conversion of large part of lycopene into β-carotene in LCYb-overexpressing fruits is consistent with the observed decrease of antioxidant activity in the nonpolar fraction.
Alterations in cell wall and cuticle composition
The extraction and subsequent fractionation of the cell walls of LCYb-overexpressing fruits yielded higher amounts of total cell walls, water-soluble and diluted alkali-soluble solids per unit weight compared to WT, indicating alterations both in the content and in the solubility of cell wall polymers. The higher content of the pectic backbone sugar GalA in the WSS fraction suggested a higher content of pectins, which were less methyl-esterified than those from WT fruits. The regulated swelling and disassembly of primary cell walls and the modification of the middle lamellae between adherent primary cell walls are thought to be important factors contributing to tissue softening during tomato fruit ripening. The increased abundance in LCYb-overexpressing fruits of linear, low-esterified homogalacturonan is likely to influence both cell adhesion and fruit texture. Pectins secreted to the cell wall possess methyl-ester side chains, which are removed by pectin methylesterase (PME) as a prerequisite for polygalacturonase (PG) action (Wakabayashi et al., 2003). PME can play dual and contrasting roles within the plant cell wall: on the one hand, it generates blocks of de-esterified galacturonic acid residues within the pectin polymer that can be cross-linked by calcium, thus strengthening the cell-to-cell adhesion; on the other hand, the same de-methyl-esterified blocks may be more susceptible to degradation by PG. While PG-mediated polyuronide depolymerization during ripening does not appear to be the primary determinant of tomato fruit softening (Brummell and Harpster, 2001; Giovannoni et al., 1989; Uluisik et al., 2016), experimental data suggest a role for PMEs in determining fruit firmness: silencing of PMEU1 resulted in faster softening during fruit ripening (Phan et al., 2007), while silencing PMEU2 results in loss of tissue integrity during fruit senescence (Tieman and Handa, 1994). Additional genes contribute to tomato fruit softening during ripening: EXP1 and PL silencing resulted in a moderate increase in fruit firmness throughout ripening (Brummell et al., 1999; Uluisik et al., 2016; Wang et al., 2019). The expression of genes involved in cell wall remodelling in LCYb-overexpressing fruits is consistent with their phenotypes: PL, PG and EXP1 are down-regulated, while PMEU1/2 are up-regulated compared to WT. Taken together, these results indicate that LCYb overexpression affects the ripening-associated pathway leading to pectin solubilization and cell wall disassembly.
LCYb-overexpressing fruits also had significantly higher amounts of cutin monomers and alicyclic wax components relative to WT, but unchanged levels of linear, very-long-chain wax compounds. Our results suggest a regulatory effect of ABA on cuticle deposition in fruits, consistent with previously published data in tomato (Curvers et al., 2010; Martin et al., 2017) and Arabidopsis (Seo et al., 2011; Zhang et al., 2005) leaves.
Crosstalk between carotenoids, ABA and ethylene in the control of fruit ripening
Carotenoids, and more specifically 9-cis-epoxyxanthophylls, synthesized from β-carotene, are metabolic precursors of ABA (Figure 1A; Giuliano et al., 2003; Qin and Zeevaart, 1999). The increase in ABA levels observed in LCYb-overexpressing fruits is an indirect consequence of the increase in the β-carotene pool. This is, to some extent, unexpected: the rate-limiting step for ABA biosynthesis in leaves is believed to be the cleavage of 9-cis-epoxyxanthophylls by the NCED dioxygenase (Giuliano et al., 2003; Qin and Zeevaart, 1999; Thompson et al., 2000). However, the level of β-carotene in WT tomato fruits is only 1.37-fold higher than that of ABA and β-carotene and ABA levels are strictly co-regulated in LCYb-overexpressing fruits (Tables S16 and S17), suggesting that in tomato fruits, β-cyclization is rate-limiting for ABA biosynthesis. Since the β-cyclization step is itself regulated during ripening (Pecker et al., 1996), this has important implications for the regulatory circuits controlling tomato fruit ripening (see below).
Crosstalk between the ABA and ethylene signalling pathways has been described in Arabidopsis, where a screen for extragenic enhancers or suppressors of ABA-insensitive abi1 mutant resulted in alleles of the constitutive ethylene response mutant ctr1 and ethylene-insensitive mutant ein2 (Beaudoin et al., 2000). Blocking of ethylene biosynthesis before véraison in grape, a nonclimacteric fruit, results in inhibition of ABA biosynthesis and of fruit ripening (Sun et al., 2010). This indicates that the crosstalk between ABA and ethylene is probably present in different species and in both climacteric and nonclimacteric fruits. Both positive and negative crosstalk have been reported in tomato fruits between ABA and ethylene, depending on the time and mode of application of the former hormone: exogenous application of ABA during early fruit development induces fruit ethylene biosynthesis and accelerates ripening in a fashion dependent on the RIN ripening regulator (Mizrahi et al., 1975; Zhang et al., 2009); exogenous application of ABA or nordihydroguaiaretic acid, an inhibitor of ABA synthesis, respectively, accelerated or delayed fruit ripening, with a simultaneous higher and lower emission in ethylene (Zhang et al., 2009). Furthermore, reduced endogenous ABA levels in fruits of the tomato hp3 mutant resulted in increased ethylene production (Galpaz et al., 2008). Crosstalk of ABA and ethylene during in tomato fruit ripening has also been observed in fruits with altered expression of transcription factors such as NAC1 (Meng et al., 2016) and ZFP2 (Weng et al., 2015).
In LCYb-overexpressing fruits, the increase in ABA levels results in a corresponding decrease in the ethylene peak, a phenotype exactly symmetric to that of the hp3 mutant (Galpaz et al., 2008). These data support a negative crosstalk between the two hormones. How does this negative crosstalk occur in LCYb-overexpressing fruits? Our data indicate that ABA levels are strongly co-regulated with transcript levels of several ripening regulators, including NAC-NOR and RIN (Table S15 and S16A). RIN (Vrebalov et al., 2002) encodes a MADS-box transcription factor (Ito et al., 2017; Li et al., 2017), and NAC-NOR (Giovannoni et al., 2004) belongs to the NAC domain family of transcription factors, many of which are involved in responses to ABA (Nakashima et al., 2012). Both NAC-NOR and RIN are necessary for ethylene synthesis and fruit ripening. NAC-NOR acts upstream of RIN (Tigchelaar et al., 1973), and RIN binds promoter elements of ripening-associated genes in a CNR-dependent fashion (Fujisawa et al., 2013; Martel et al., 2011). Increased PG gene expression and increased susceptibility of ripe fruits to Botrytis cinerea require NOR, but not RIN (Cantu et al., 2009; Dellapenna et al., 1987), while induction during ripening of transcripts involved in ethylene biosynthesis is completely abolished in nor, but only partially in rin fruits (Osorio et al., 2011). ABA accumulation in rin fruits is similar to WT fruits, while in nor fruits it is decreased (McGlasson and Adato, 1976). Two recent studies suggested strong regulatory relationships between NOR and ABA: SlAREB1, a transcription factor involved in ABA signalling, directly regulates NOR expression (Mou et al., 2018) and tomato de penjar-type cultivars, characterized by extended shelf life, displayed a nor mutation, increased ABA levels and reduced water loss (Kumar et al., 2018). An interesting hypothesis is that ABA could regulate directly the expression of NAC-NOR and/or RIN. A series of ABA response elements such as ABRE, DRE, LTRE, MYB and MYC is localized in the promoter regions of NOR and RIN (Table S18).
Additional mutants are known to affect β-carotene biosynthesis in tomato fruits, such as old gold and Beta, which result respectively in an impairment or an enhancement, of the fruit-specific CYC-b cyclase (Ronen et al., 2000). An old gold allele shows increased fruit firmness, although its fruit ABA content has not been studied (Silletti et al., 2013). Beta alleles carry a poorly characterized chromosomal introgression from green-fruited tomato species, which results in complex vegetative and fruit phenotypes (unpublished data). Additional ABA biosynthesis mutants, such as flacca and sitiens, exhibit a wilty phenotype and reduced plant and fruit size (Nitsch et al., 2012). These characteristics of the mutants, the fact that the mutations are expressed throughout vegetative growth and fruit ripening, and the hypothesized dual role of ABA in regulating ripening (see below), complicate the interpretation of the mutant phenotypes.
A model to explain the phenotypic alterations of LCYb-overexpressing fruits
Based on the data gathered, we propose a model to explain the phenotypes observed in LCYb fruits. First, β-carotene levels appear to be the main driver of ABA levels during late ripening, as suggested by the parallel increase of the two metabolites in LCYb-overexpressing fruits. Second, while at the MG stage ABA acts as a trigger for ripening (Zhang et al., 2009), during late ripening it turns into a negative regulator of both ethylene production and fruit ripening (this paper). This dual role of ABA in regulating tomato fruit ripening is supported by the results obtained with RNAi lines where NCED silencing is driven by the fruit-specific E8 promoter: these lines show decreased ABA and increased ethylene levels during late ripening (Sun et al., 2012). A dual role of ABA in regulating fruit ripening has been also described in peach, where its application at the S3 stage represses ripening, while application at the S4 instead accelerates it (Soto et al., 2013).
In our model (Figure 7), primary effects include those known to be under direct ABA control, such as phenylpropanoid content, cuticle thickness, cutin and triterpenoid composition; secondary effects include instead those not directly associated with ABA, such as cell wall and primary metabolite composition. Since β-carotene in fruits is mainly synthesized as a result of CYC-B activity (Ronen et al., 2000), and CYC-B expression is negatively affected by ethylene (Alba et al., 2005), we hypothesize that a negative feedback loop, involving CYC-B, β-carotene, ABA and ethylene, is active during late fruit ripening, in which ethylene represses CYC-B, lowering the levels of ABA and thus enhancing its own levels and accelerating ripening. In LCYb-overexpressing fruits, lycopene β-cyclase activity and β-carotene levels are no more under negative ethylene control, and thus, ABA levels remain high during late ripening, repressing ethylene production and extending shelf life.
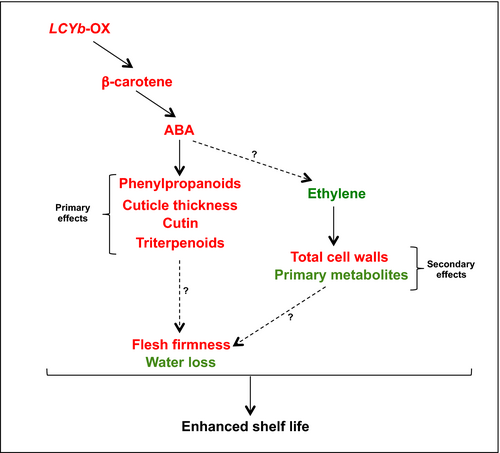
Our model is strongly supported by the pharmacological experiment shown in Figure 6: abamine, a known inhibitor of NCED activity, decreases ABA levels in ripe LCYb-overexpressing fruits and simultaneously decreases fruit firmness and water loss and increases ethylene production. This is a strong suggestion that β-carotene exerts its effects on fruit ripening through its cleavage product, ABA, and not through the alteration of the fruit antioxidant potential, as in the case of the Del/Ros tomatoes. Additional studies with inhibitors of ABA synthesis and sensing will shed more light on the role of this hormone in the control of tomato fruit ripening.
Methods
Plant material and fruit sampling
The LCYb transgenic tomato plants have been previously described (Rosati et al., 2000). Growth of plants was as previously described (Giliberto et al., 2005). Time to breaker was measured by tagging flowers at anthesis. Fruits were harvested at five ripening stages (mature green, MG; breaker, B; and 4, 10 and 15 days after the breaker stage, B+4, B+10 and B+15); at least six fruits from three different plants (three biological replicates) were harvested, cut into small pieces and frozen in liquid nitrogen. Pooled fruits for each biological replicate were stored at −80 °C for a maximum of 6 months before biochemical and transcriptomic analyses.
Analysis of ABA content
Frozen pericarp tissues at five ripening stages were lyophilized and ground to a fine powder. At least three different pericarp pools (biological replicates) were analysed for each genotype. 200 mg was extracted for each replicate as previously described (Welsch et al., 2008). LC-HRMS was carried out using a Finnigan Surveyor Plus HPLC System (Thermo Electron), equipped with a 3 μm Hypersil GOLD C18 reverse-phase column (150 × 4.6 mm; Thermo Electron) as reported before (Ross et al., 2004). Internal standard-based quantification was carried out using the MS data and the quantification software available in the Xcalibur 2.0 software package (Thermo Fisher Scientific, Bremen, Germany). Retention times and MS2 fragmentation patterns were used for identification by using authentic reference standards (trans-ABA from OlChemIm and (±)-ABA from Sigma, St. Louis, MO, USA).
Biochemical and phenotypic assays
Ethylene production was measured on freshly harvested fruits as described (Thompson et al., 1999). At least 10 fruits, with uniform size and pigmentation, were analysed for each line and developmental stage using a Carlo Erba Fractovap 4200 gas chromatograph (Carlo Erba Spa, Milan, Italy) equipped with a flame ionization detector (FID) and 1-m-long alumina column (80–100 mesh) at 80 °C. A calibration curve was performed using known concentrations of ethylene. ACO enzymatic activity was assayed according to the protocol from Barry et al. (1996). Water loss was measured, for each genotype, on at least 10 fruits from three different plants, kept at constant temperature and relative humidity (22 ± 2 °C, 60 ± 5%). Fruit firmness was evaluated using two different mechanical tests and at least 10 fruits for each line. Pulp firmness was checked on peeled fruits with a 1 kg hand-held fruit pressure tester (Turoni, Cesena, Italy) equipped with an 8-mm probe. Pericarp thickness was measured using a calliper. Cuticle isolation was performed on fruits at B+10 as previously described (Saladie et al., 2006). At least four strips/fruit were included in paraffin and fixed in formaldehyde:acetic acid 1:1 (v/v) in 18 volumes of 70% alcohol for 48 h, followed by dehydration in an alcohol series (50%–10%) and a water wash. At least four sections of 10 μm per fruit were cut with a microtome and stained with 0.05% of toluidine blue. Cuticle thickness was determined using light microscope images (40× magnification) and the ImageJ image analysis software (http://rsb.info.nih.gov/ij).
Cell wall fractionation and composition analysis
Preparation and extraction of total cell wall (TCW) were carried out essentially as described in Orfila et al. (2002). Four fractions were extracted sequentially from TCW as reported (Huisman et al., 1998). Monosaccharide composition was determined by high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD, Ion Chromatography System, ICS 3000, Dionex, CA) as described (Lionetti et al., 2010). The column was a CarboPac PA20 column 3 × 150 mm (Dionex, CA), equipped with a CarboPac PA20 guard column 3 × 30 mm. Peaks were identified and quantified by comparison to a standard mixture of fucose, rhamnose, arabinose, galactose, glucose, xylose, mannose, galacturonic acid and glucuronic acid (Sigma). All data are expressed as mean ± S.D. Specific pectic epitopes were detected by immunodot assay with LM20 antibody obtained from PlantProbes (UK). Polysaccharide solutions (5 mg/ml) in 0.5% (w/v) ammonium oxalate buffer were spotted as 1 μl drops onto nitrocellulose membrane (Bio-Rad, Munich, Germany) in a threefold dilution series. Membranes were allowed to air dry at room temperature for 1 h and then blocked with 3% blocking reagent (GE Healthcare, Bucks, UK) in phosphate-buffered saline (PBS; Bio-Rad) for 1 h prior to incubation for 1.5 h with LM20. After extensive washes in PBS, membranes were incubated with anti-rat secondary antibody conjugated to horseradish peroxidase (GE Healthcare) and washed in PBS prior to detection with ECL detection reagent (GE Healthcare). For each analysis, at least three biological replicates were performed.
Cuticle composition analysis
Cuticular waxes and cutin were analysed as previously described (Yeats et al., 2012). Samples were separated by gas chromatography (5890 II, Hewlett Packard, Avondale, PA; 30 m DB-1, 0.32 mm i.d., df = 1 μm, J&W Scientific, Folsom, CA) with He carrier gas (1.4 mL/min) and mass spectrometric detection (MS; 5971N, Agilent Technologies, Palo Alto, CA, USA, EI, 70 eV, m/z 50–800, 1 scan per s.). For analyte quantification, an identical GC system was used, except that a flame ionization detector (FID) was used, which burned H2 (30 mL/min) in air (200 mL/min), and used N2 to shape the flame (20 mL/min). Analytes were quantified against the internal standard by manual integration of peak areas. For each analysis, at least three biological replicates were performed.
qRT-PCR and microarray analyses
RNA isolation and real-time qRT-PCR conditions were as previously reported (Diretto et al., 2010a). List of primers for each gene is reported in Table S10. Normalization to a housekeeping gene (actin) and to WT values was applied to raw data to obtain relative expression levels. For each genotype, at least three biological replicates were performed.
Microarray experiments were carried out using GeneChip® EU-TOM3 platform (Affymetrix, Buckinghamshire, UK) and an external service provided by IFOM (Fondazione Istituto FIRC di Oncologia Molecolare—Cogentech, Milan, Italy) as previously described (Mori et al., 2012). CEL files were subsequently analysed with RobiNA software (Lohse et al., 2012). Briefly, subsequent steps of quality assessment, data normalization and identification of genes differentially regulated between WT and LCYb-overexpressing lines (LCYb1 and LCYb3) fruits for each ripening stage were carried out. The raw data were then normalized using the RMA method. Statistical analysis of pairwise differential gene expression was performed using a linear model-based approach, applying a 0.05 cut-off for P-values after a false discovery rate (FDR) correction. Microarray experiments have been deposited to the GEO public repository (https://www.ncbi.nlm.nih.gov/geo) under the accession number GSE108415. For each genotype, at least three biological replicates were performed.
Primary metabolite analyses
Metabolite analysis of ripe (B+10) tomato peeled pericarp samples (300 mg) by GC-MS was carried out essentially as described in Lisec et al. (2006), with specific modifications for tomato tissues as described in Schauer et al. (2006). The GC-MS system used comprised an AS 2000 autosampler, a GC 8000 gas chromatograph and a Voyager quadrupole mass spectrometer (Thermo Finnigan, Manchester, UK). The mass spectrometer was tuned according to the manufacturer's recommendations using tris-(perfluorobutyl)-amine (CF43). Both chromatograms and mass spectra were evaluated using the MASSLAB program (ThermoQuest, Manchester, UK) with reference to libraries of the Golm Metabolite Database (Kopka et al., 2005; Schauer et al., 2005). For each genotype, at least three biological replicates were performed.
Nonpolar and semi-polar metabolite analyses
Nonpolar (carotenoids) and semi-polar (phenylpropanoid) analyses were carried out by liquid chromatography coupled to diode-array detector and atmospheric pressure chemical ionization–high-resolution mass spectrometry (LC-DAD-APCI-HRMS) or electrospray ionization (LC-DAD-ESI-HRMS), respectively, operating in positive and negative ion modes, as previously described (D'Esposito et al., 2017; Fasano et al., 2016; Su et al., 2015). Identification of carotenoids was performed as reported previously (Liu et al., 2014). Phenylpropanoid analysis was performed by comparing chromatographic and spectral properties with standards and reference DAD-HRMS spectra as previously reported (Fernandez-Moreno et al., 2016; Iijima et al., 2008; Moco et al., 2006). For each analysis, at least three biological replicates were performed.
Total antioxidant capacity
Fruit total antioxidant capacity was determined as previously described (Enfissi et al., 2010), by estimating the capacity of nonpolar extract to quench the ABTS+ radical via measuring Abs at 734 nm compared to the one of Trolox. Results were expressed as a TEAC in mm of Trolox per gram of DW. For each line, at least three biological replicates were performed.
Abamine pharmacological treatment
Abamine treatment of LCYb-overexpressing fruits was carried out as previously described (Brandi et al., 2011; Mou et al., 2016; Su et al., 2015) using, for each fruit, 1 mL of 1 mm abamine, dissolved in dimethylsulphoxide (DMSO). At least 5 MG fruits for each genotype were collected and subjected to treatment with abamine or DMSO (mock). ABA, fruit firmness, ethylene emission and water loss were evaluated as previously described.
Statistical and bioinformatic analyses
The significance of differences between WT and LCYb-overexpressing fruits was evaluated using Student's t-test (*P < 0.05, **P < 0.01). For an easier homogenization and interpretation of the results, all data were normalized on the WT values. When the levels of a metabolite or gene expression were ‘not detectable’, an arbitrary value was set, corresponding to 1/10 of the lowest value in the data set. Normalized data were log2-transformed and visualized on public and ad hoc metabolic maps using the MapMan software (Urbanczyk-Wochniak et al., 2006). For enrichment analysis, Solyc of genes up- or down-regulated was subjected to Gene Ontology Enrichment Analysis (GOEA) using the Plant MetGenMAP tool (http://bioinfo.bti.cornell.edu/tool/GO/GO_enrich.html; Joung et al., 2009). Over-represented GO terms in each category (biological process, molecular function and cellular component) were determined using the false discovery rate (FDR) statistical method and a p value ≤0.05 (Benjamini and Yekutieli, 2001). Correlation networks were generated by Cytoscape version 2.6.3 (www.cytoscape.org; Cline et al., 2007), as previously described (Aversano et al., 2017; Rambla et al., 2016), and visualized as force-directed layouts weighted with log2 (1−|ρ|) values.
Acknowledgements
The work was supported by the European Commission (Projects DISCO, grant agreement: 613513; and Traditom, grant agreement: 634561) to GG, a Special Research Opportunity Grant from the Natural Sciences and Engineering Research Council of Canada, the Canada Chairs Program and the Canada Foundation for Innovation to RJ and the United States Department of Agriculture—Agricultural Research Service and the US National Science Foundation (grant IOS-1539831) to JG, and benefited from the networking activities within the European Cooperation in Science and Technology Action FA1106 (Qualityfruit) and CA15136 (EUROCAROTEN). We thank Florian Wurst and Peter Beyer, Freiburg University (Germany), for help in ABA measurements; Asaph Aharoni and Yoseph Hirschberg for providing annotated tomato cuticle and ABA-relative gene lists, respectively; and Christian Chervin, Julien Pirrello and Mondher Boutzayen for help in fruit abamine treatments. The authors declare to have no conflict of interest.
Author contributions
GG, JG and GD designed the research. GD and AF selected independent transgenic lines. GD and SF performed phenotypic, microarray/qRT-PCR analyses and carotenoid/phenylpropanoid metabolomic analyses. CF and BM carried out cell wall analyses. NS and ARF performed primary metabolite determinations. ZW, LB and RJ carried out cuticle metabolomics. AJM and JKCR performed cuticle microscopy. All authors analysed data, wrote and agreed the final version of the paper.




