Targeting intracellular transport combined with efficient uptake and storage significantly increases grain iron and zinc levels in rice
Summary
Rice, a staple food for more than half of the world population, is an important target for iron and zinc biofortification. Current strategies mainly focus on the expression of genes for efficient uptake, long-distance transport and storage. Targeting intracellular iron mobilization to increase grain iron levels has not been reported. Vacuole is an important cell compartment for iron storage and the NATURAL RESISTANCE ASSOCIATED MACROPHAGE PROTEIN (NRAMP) family of transporters export iron from vacuoles to cytosol when needed. We developed transgenic Nipponbare rice lines expressing AtNRAMP3 under the control of the UBIQUITIN or rice embryo/aleurone-specific 18-kDa Oleosin (Ole18) promoter together with NICOTIANAMINE SYNTHASE (AtNAS1) and FERRITIN (PvFER), or expressing only AtNRAMP3 and PvFER together. Iron and zinc were increased close to recommended levels in polished grains of the transformed lines, with maximum levels when AtNRAMP3, AtNAS1 and PvFER were expressed together (12.67 μg/g DW iron and 45.60 μg/g DW zinc in polished grains of line NFON16). Similar high iron and zinc levels were obtained in transgenic Indica IR64 lines expressing the AtNRAMP3, AtNAS1 and PvFER cassette (13.65 μg/g DW iron and 48.18 μg/g DW zinc in polished grains of line IR64_1), equalling more than 90% of the recommended iron increase in rice endosperm. Our results demonstrate that targeting intracellular iron stores in combination with iron and zinc transport and endosperm storage is an effective strategy for iron biofortification. The increases achieved in polished IR64 grains are of dietary relevance for human health and a valuable nutrition trait for breeding programmes.
Introduction
Deficiencies of essential nutrients including iron, zinc, vitamin A, vitamin B-12, riboflavin, vitamin D and vitamin E are common and often coexist in affected populations. Iron and zinc deficiencies are widespread in developing countries but are also not uncommon in developed countries. Nearly 1.6 billion people in the world are affected by anaemia, to which iron deficiency anaemia contributes significantly (Stevens et al., 2013) and zinc deficiency is also reported to affect one-third of the world's population. Diet diversification, supplementation and food fortification are among the recommended approaches to relieve these deficiencies. However, these approaches are often difficult to implement due to various regional socio-economic factors. Furthermore, oral iron supplementation can lead to side effects including nausea and gastric pain and could increase the severity of infectious diseases, such as malaria (Oppenheimer et al., 1986; Sazawal et al., 2006). Food fortification usually causes colour and flavour changes because bioavailable iron molecules often react with other food molecules (Abbaspour et al., 2014; Hurrell, 2002). Biofortification for increasing iron in staple crops is therefore a promising strategy to overcome these constraints. Rice is the second most produced staple crop worldwide and consumed by more than half of the world population. Brown rice, which includes the aleurone layer of the seed coat (bran), is relatively rich in micronutrient content. However, to avoid rancidity during storage, rice is usually polished, consequently removing the nutrient-rich bran. The polished white rice (which is mostly the endosperm of the grain) contains mainly starch and is poor in iron, zinc and other micronutrients. Most of the commercially bred rice varieties contain only around 2 μg/g iron in the endosperm (Bouis et al., 2011). A high content of phytate, which binds iron and zinc, in cereal grains further reduces iron and zinc bioavailability. Furthermore, it has not been possible to increase the endosperm iron concentration in rice via conventional breeding because of the limited genetic variability of endosperm iron content in the rice germplasm (Bouis et al., 2011). Genetic engineering approaches, however, were successful in increasing the iron concentration in polished rice grains (Vasconcelos et al., 2017).
The continuously developing knowledge on iron uptake, storage and translocation in rice has driven the design of iron biofortification approaches during the last few years. FERRITIN (FER), a ubiquitous protein that can bind about 4500 iron atoms in a complex (Ford et al., 1984; Harrison et al., 1991), has been used to increase iron accumulation in the rice endosperm (Goto et al., 1999; Lucca et al., 2001; Oliva et al., 2014; Vasconcelos et al., 2003). Many laboratories also focused on strategies with potential to enhance iron uptake and translocation. For this, constitutive expression of NICOTIANAMINE SYNTHASE (NAS) involved in biosynthesis of deoxy-mugineic acid (DMA), that is phytosiderophores, has been a widely used approach, with 2- to 4.5-fold increased iron concentrations obtained in polished grains (Johnson et al., 2011; Lee et al., 2012; Masuda et al., 2009). As nicotianamine (NA) produced by NAS and mugineic acids (MAs) can also bind Zn, accompanied zinc increases in the lines overexpressing NAS are frequently reported. Among the other genes encoding iron transporters, YELLOW STRIPE LIKE 2 (OsYSL2) and IRON REGULATED TRANSPORTER 1 (OsIRT1) have also been transformed into rice either alone or in combination with genes related to MA synthesis and varied levels of iron increase were found in the rice grains (Ishimaru et al., 2010; Lee and An, 2009; Tan et al., 2015). YSL transporters are also known to transport zinc bound with MAs and NA. In comparison with the single-gene strategies, constructs expressing combinations of genes involved in iron transport and uptake could achieve higher endosperm iron concentrations in rice (Boonyaves et al., 2016; Trijatmiko et al., 2016; Wirth et al., 2009).
Cytosolic-free Fe concentrations are very low in plant cells because most of the iron is stored in chloroplasts, mitochondria and vacuoles in nontoxic forms and used for the synthesis of Fe-heme or Fe-S clusters (Finney and O'Halloran, 2003). Vacuoles have an important function in storing excess Fe and releasing it into the cytosol when the external Fe supply is suboptimal (Kim and Guerinot, 2007; Lanquar et al., 2010; Pich et al., 2001). In Arabidopsis, the vacuolar membrane located VACUOLAR IRON TRANSPORTER 1 (AtVIT1) is responsible for iron uptake into vacuoles (Kim et al., 2006). Oppositely, specific members of the NATURAL RESISTANCE ASSOCIATED MACROPHAGE PROTEIN (NRAMP) family mediate the efflux of iron from the vacuoles to the cytosol. NRAMP transporters localize to either intracellular vesicles, the vacuole or the plasma membrane depending on the plant species (Bereczky et al., 2003; Lanquar et al., 2005; Takahashi et al., 2011; Thomine et al., 2003). Arabidopsis AtNRAMP1, AtNRAMP3 and AtNRAMP4 mediate iron and cadmium transport, and AtNRAMP1 is preferentially expressed in roots (Curie et al., 2000; Thomine et al., 2000). AtNRAMP3 and AtNRAMP4 are localized to the vacuolar membrane, and their genes are induced by iron deficiency (Lanquar et al., 2005, 2010; Thomine et al., 2003). Among the seven NRAMP homologs identified in rice, OsNRAMP1 is involved in iron uptake (Curie et al., 2000) and is localized to the plasma membrane (Takahashi et al., 2011). Gross and colleagues (Gross et al., 2003) have discussed the homology between rice and Arabidopsis NRAMP genes in detail.
Utilizing vacuolar iron stores for increasing endosperm iron content has not been attempted to date. Here, we demonstrate that inter- and intracellular iron mobilization can be effectively combined for biofortification strategies in rice. To accomplish this, we expressed AtNRAMP3 under control of either the rice 18-kDa Oleosin (Ole18) or maize UBIQUITIN (Ubi) promoters together with the endosperm-specific expression of PvFER alone or in combination with the constitutive expression of AtNAS1. Most of the transgenic lines (background cv. Nipponbare) had significant increases of iron and zinc in both unpolished and polished grains, with maximum endosperm iron levels in lines expressing AtNRAMP3, AtNAS1 and PvFER together. Importantly, expression of all three genes in the popular rice mega-variety IR64 resulted in iron concentrations equalling more than 90% of the recommended iron increase in rice grains.
Results
Combined expression of AtNRAMP3, AtNAS1 and PvFER increases iron and zinc concentrations in polished rice grains
We transformed the rice cultivar Nipponbare (NB) with four constructs expressing different combination of genes, abbreviated NFUN (pCaMV35S::AtNAS1, pOsGLB-1::PvFER, pZmUbi::AtNRAMP3), NFON (pCaMV35S::AtNAS1, pOsGLB-1::PvFER, pOsOle18::AtNRAMP3), FUN (pOsGLB-1::PvFER, pZmUbi::AtNRAMP3) and FON (pOsGLB-1::PvFER, pOsOle18::AtNRAMP3) (Figure 1a). Together, 16 independent single insertion lines for each of the NFUN, NFON and FUN constructs and 13 single insertion lines for the FON construct were selected and grown in subsequent generations (Figure S1). Based on iron concentration analysis of T2 grains, we selected five or six lines for each of the four constructs with highest endosperm iron concentration for further analysis in subsequent generations (Figure S2). All of the NFUN and FUN lines had increased iron concentrations in polished T3 grains, ranging from 8.09 to 11.69 μg/g DW, respectively, as compared to 2.12 μg/g DW iron in the NB control (Figure 1b). Similarly, NFON and FON transgenic lines had increased iron concentrations in polished grains ranging from 7.64 to 12.77 μg/g DW, with NFON 16 having a more than six-fold higher endosperm iron concentration than the NB control (Figure 1b). In unpolished grains, NFUN and NFON lines had higher increases in iron concentrations compared to FUN and FON lines, with NFON 12 unpolished grains containing 29.75 μg/g DW iron (Figure 1b). Together, all transgenic rice lines had significantly higher iron concentrations in unpolished grains compared to NB grains. The transgene in NFON16, line with highest endosperm iron content, integrated into the first exon of a hypothetical protein encoding gene Os11 g0696100 (Figure S1b). In addition, all NFUN and NFON lines had significantly increased zinc concentrations in polished and unpolished T3 grains, ranging from 29.68 to 45.62 μg/g DW in polished grains as compared to 19.38 μg/g DW in the NB control and from 45.96 to 63.04 μg/g DW in unpolished grains compared to 29.54 μg/g DW in the NB control (Figure 2). Increases in iron and zinc concentrations were positively correlated in NFON lines. Copper concentrations were variable in some lines, while most lines had reduced manganese concentrations in the grains (Figure S3). Most transgenic plants were phenotypically similar to NB control plants and had comparable germination rates (Table S1 and Figure S4). Some of the NFON lines, however, had higher tiller numbers than NB (Table S1). With few exceptions, six grain-quality parameters measured in the transgenic lines (grain length, grain width, chalkiness, amylose content, starch content, protein content) were similar to NB control plants (Table S2).
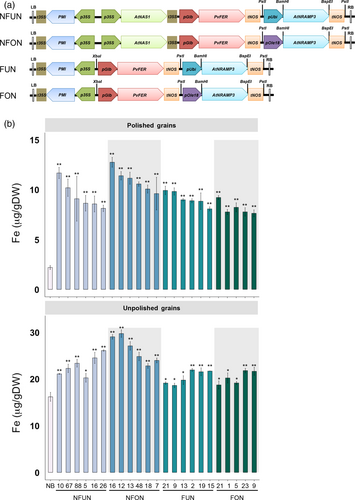
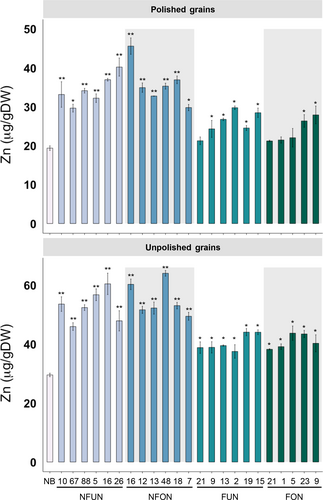
Together, the highest iron and zinc increases in the grains were observed in lines expressing the combination of NAS, FER and NRAMP (NFUN, NFON) as compared to the lines expressing FER and NRAMP only (FUN, FON) (Figures 1 and 2). The higher micronutrient concentrations in the NFON lines suggest that the Ole18 promoter is equally (and in some lines even more) effective for the expression of AtNRAMP3 compared to the Ubi promoter. Therefore, the Ole18 promoter would be preferable for targeted and localized expression in biofortification strategies.
Grain iron concentration and gene expression are positively correlated
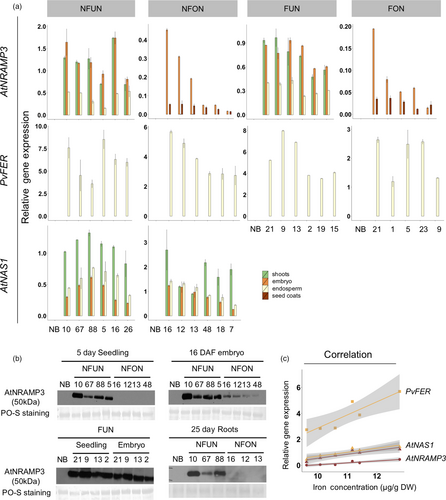
The expression of the transgenes was analysed in shoots and grains (embryo, endosperm and seed coat). As expected, AtNAS1 was expressed in all analysed tissues of NFUN and NFON lines, while expression of PvFER was detected only in the endosperm (Figure 3a). AtNRAMP3 was also expressed in all the tissues but more highly in shoots and embryos of NFUN and FUN transgenic lines (Figure 3a). As expected, AtNRAMP3 expression was restricted to the embryo and seed coat in NFON and FON lines (Figure 3a). This was further confirmed by the detection of the AtNRAMP3 protein in these specific tissues (Figure 3b). In NFON lines, transgene expression in the embryo and endosperm was positively correlated with increased iron concentration in the polished grains, indicating a synergistic action of NAS, FER and NRAMP (Figure 3c). As constitutive ectopic expression of AtNRAMP3 suppresses AtIRT1 expression in Arabidopsis plants (Thomine et al., 2003), we analysed the expression of the AtIRT1 rice homolog OsIRT1 in different tissues. Although the expression of OsIRT1 was variable in different transgenic lines, we found no indication that OsIRT1 expression was suppressed (Figure S5).
NFUN and NFON lines are more tolerant to iron-deficient growth conditions
We determined the iron and zinc distribution in the shoots and roots of selected transgenic lines for each construct when grown in iron-sufficient and iron-deficient conditions. In iron-sufficient conditions, most of the lines showed no significant increase in iron accumulation in the shoots and roots, except for NFON 16 that had a 1.5-fold higher iron concentration in shoots and 1.3-fold higher iron concentration in roots (Figure 4a). When grown in iron-deficient conditions, most of the NFUN, NFON and FUN lines had increased iron concentrations in the shoots whereas FON lines had similar iron concentrations as NB (Figure 4a). NFUN lines had the highest iron concentration in shoots, ranging from 1.4-fold to 1.6-fold higher than NB. The iron concentration in roots was lower in NFUN, NFON and FUN lines, with a nearly two-fold reduction in NFUN lines, while FON lines had similar root iron concentrations as NB roots (Figure 4a). In iron-sufficient conditions, NFON and NFUN lines also had increased shoot zinc levels compared to NB, but root zinc levels were similar in all transgenic lines and the control (Figure 4b). In contrast, in iron-deficient conditions shoot zinc concentrations were higher in NFON lines but 1.5-fold and two-fold lower in NFUN and FUN lines, respectively, compared to NB (Figure 4b). Shoot and root fresh weight was similar for transgenic plants and NB control when grown in iron-sufficient conditions (Figure 4c,d). In iron-deficient growth condition, however, NFUN, NFON and FUN lines had higher shoot and root fresh weight than NB, while FON transgenic plants showed no differences (Figure 4c,d). These results indicate that the expression of AtNRAMP3 and/ or AtNAS1 facilitates efficient iron translocation from roots to shoots, leading to increased tolerance to low-iron condition.

Furthermore, we analysed the expression of nine endogenous genes involved in iron homeostasis in shoots and roots of plants grown under iron-deficient and iron-sufficient conditions. In iron-sufficient conditions, most of the genes were expressed at similar levels in both transgenic and NB plants, but their expression was significantly different in iron-deficient conditions (Figure S6).
Transgenic plants do not accumulate more cadmium in grains
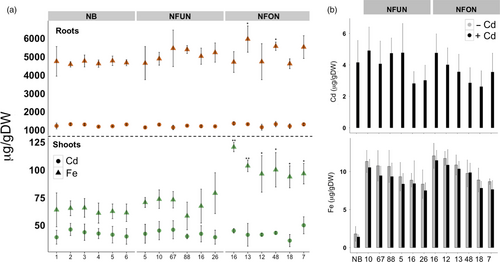
Arabidopsis lines that overexpress AtNRAMP3 accumulate more cadmium in leaves and roots (Thomine et al., 2000, 2003). We determined cadmium concentration in shoots, roots and grains of our transgenic rice plants grown in a hydroponic solution containing cadmium. The transgenic plants had similar cadmium concentrations compared to the NB control, ranging from 36.24 to 50.00 μg/g DW in shoots and from 1136.34 to 1356.58 μg/g DW in roots (Figure 5). The NFON plants had significantly higher iron concentration in the shoots as compared to NB and NFUN plants (Figure 5a). Importantly, cadmium concentrations in polished NFUN and NFON grains (2.62–4.9 μg/g DW) were comparable to NB grains (4.15 μg/g DW) (Figure 5b). Therefore, the ectopic expression of AtNRAMP3 in rice did not increase cadmium concentrations in the grains, shoots or roots of the transgenic plants.
IR64 lines expressing NRAMP, NAS and FER have dietary significant levels of iron and zinc in their grains
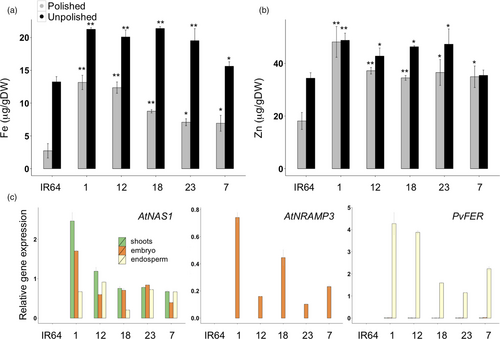
Based on the results with cv. Nipponbare, we transformed Indica rice cv. IR64 with the NFON construct (pCaMV35S::AtNAS1, pOsGLB-1::PvFER, and pOsOle 18::AtNRAMP3; Figure 1a). Five single insertion lines were selected for further analysis (Figure S7). The IR64 transgenic plants had increased endosperm iron concentration of up to 13.65 μg/g DW in the polished grains and 21.38 μg/g DW in the unpolished grains, as compared to 2.72 and 13.24 μg/g DW iron in the polished and unpolished grains of IR64 control plants, respectively (Figure 6a). Both polished and unpolished grains of the transgenic plants also had significantly increased zinc concentrations, with the highest zinc concentration of 48.18 μg/g DW in the polished grains of IR64_1 (Figure 6b). Expression of AtNAS1 was detected in shoots, embryo and endosperm, while AtNRAMP3 was expressed only in the embryo and PvFER only in the endosperm (Figure 6c). The transgenic plants were phenotypically similar to the IR64 control in greenhouse conditions, except for IR64_12 line which had a smaller grain size (Table S3).
Discussion
Iron biofortification in rice has been variably successful in increasing endosperm iron content. In general, strategies combining multiple genes achieve higher increases in rice endosperm iron content as compared to single-gene strategies. To provide 30% of the dietary estimated average requirement (EAR), rice lines containing 15 μg/g DW iron and 28 μg/g DW zinc in polished grains are required (Bouis et al., 2011). Transgenic rice lines reported to date have iron concentrations in the range of 6–19 μg/g DW (Johnson et al., 2011; Masuda et al., 2009; Trijatmiko et al., 2016; Wirth et al., 2009). The IR64 transformed lines expressing NAS and FERRITIN reported by Trijatmiko and colleagues (Trijatmiko et al., 2016) grown under field conditions contained around 15 μg/g DW iron in polished grains. These increases are promising; however, the best performing lines reported contain two transgene cassette copies inserted as an inverted repeat. Transgenic plants with an inverted transgene repeat could be problematic, both from a practical and from regulatory point of view. First, two or more insertions of transgenes often result in epigenetic transgene silencing in subsequent plant generations (Kumpatla and Hall, 1998; Rajeevkumar et al., 2015). Second, using an inverted transgene cassette repeat in breeding programs or crop production would require a regulatory approval of uncertain, but likely negative outcome. Consequently, iron-biofortified rice lines will be most successful when containing a single-copy transgene cassette insertion for high-iron concentration that is stably inherited and that can be bred efficiently into widely grown rice cultivars.
Here, we expand our biofortification strategies by combining increased iron uptake and storage with efficient intracellular iron mobilization. The expression of AtNAS1 increases NA and DMA synthesis, both of which facilitate iron transport within the plants (Alvarez-Fernandez et al., 2014; Wang et al., 2013 Aoyama et al., 2009; Rellan-Alvarez et al., 2008). Additionally, we reasoned that expression of AtNRAMP3 should remobilize iron from vacuole stores to the cytosol, which can then be transported to other tissues. By expressing AtNRAMP3 under control of the Ole18 promoter, we remobilized iron from the iron-rich aleurone layer for transport to the endosperm. The endosperm-specific expression of PvFER allows for effective storage of iron in the endosperm of the grains. We demonstrate that combining these genes can increase iron content more than six-fold higher in polished seeds in two major rice cultivars, Nipponbare and IR64. The transformed IR64 lines contain up to 13.65 μg/g DW iron in polished grains, which is more than 90% of the recommended target increase of 15 μg/g DW for rice grains. The highest zinc concentration of 48 μg/g DW in transformed IR64 lines is equivalent to 170% of the recommended increase of 28 μg/g DW.
Our transgenic rice lines expressing AtNARMP3, AtNAS1 and PvFER contain higher iron and zinc concentrations in the grains compared to lines expressing AtNRAMP3 and PvFER only. In NFON lines with highest iron grain concentrations, AtNAS1 is constitutively expressed while the AtNRAMP3 gene and PvFER are regulated by the rice Ole18 and Glb promoters, respectively. The Ole18 promoter directs strong expression in the embryo and aleurone layer of rice grains primarily from 7 to 17 days after flowering (DAF), whereas the Glb promoter is most active between 12 and 17 DAF mostly in the endosperm (Qu le and Takaiwa, 2004). The aleurone layer provides nutrients to the developing endosperm and together with the embryo and endosperm constitutes a symplast connected by plasmodesmata that allows efficient transport and distribution of nutrients during early grain filling (Ishimaru et al., 2011; Thomine and Lanquar, 2011; Zhao et al., 2014; Zhu et al., 2003). Therefore, it is likely that mobilizing iron from the vacuole to the cytosol in aleurone cells and embryo scutellum could facilitate iron transport to the endosperm. This is supported by the strong correlation between iron concentrations and transgene expression in the seed tissues of NFON lines, demonstrating that targeted expression of AtNAS1, PvFER and AtNRAMP3 is an effective strategy for increasing iron accumulation in the endosperm. Importantly, iron mobilization as a result of AtNRAMP3 expression did not affect growth and seed germination of the transformed plants. Endosperm zinc concentrations were also higher in most of NFUN, NFON, FUN and FON lines compared to the Nipponbare control. The highest zinc concentrations were found in NFUN and NFON lines that express all transgenes, consistent with previous reports that rice lines with high grain iron concentration, particularly those expressing NAS, usually have higher grain zinc concentration as well (Boonyaves et al., 2016; Douchkov et al., 2005; Lee et al., 2009; Wirth et al., 2009).
Our results also show that expression of AtNRAMP3 and/or AtNAS1 in NFUN, NFON and FUN lines increases their tolerance to low-iron conditions. The transgenic rice plants transport more iron from roots to shoots in iron-deficient growth conditions, similar to transgenic tobacco plants expressing AtNAS1 that accumulated more iron in leaves in low-iron growth conditions and therefore were more tolerant to iron deficiency (Douchkov et al., 2005). High-iron-biofortified rice expressing HvNAS, HvNAAT and HvIDS3 is also more tolerant to iron deficiency conditions in hydroponic culture and calcareous soil (Masuda et al., 2013). Expression of AtNRAMP3 alone can rescue the hypersensitive iron starvation phenotype of the Arabidopsis atnr3nr4 double mutant (Lanquar et al., 2005). Together, the tolerance of our transgenic lines to iron deficiency can be explained by the expression of AtNAS1 and increased cytosolic iron availability in vegetative tissues as the result of AtNRAMP3 expression. Similar to Arabidopsis plants expressing AtNRAMP3 that have reduced root and shoot zinc and manganese levels in iron deficiency conditions (Thomine et al., 2003), we also found reduced zinc concentrations in NFUN and FUN plants compared to the Nipponbare control in iron deficiency conditions. However, NFON and FON plants did not differ significantly from the Nipponbare control plants in their shoot and root zinc concentrations when grown in the same conditions. This is likely due to a localized expression of AtNRAMP3 in the NFON and FON plants.
Metal transporters often have a broader specificity; that is, transporters facilitating the uptake of essential metal ions including Fe, Zn, Cu and Mn may also transport heavy metal ions including cadmium (Cd). Particularly, Zn-specific transporters can cotransport Cd, and the NRAMP transporter family is known to transport Cd as well (DalCorso et al., 2010; Olsen and Palmgren, 2014; Papoyan et al., 2007). Thus, it is possible that expression of iron transporters to develop iron-biofortified rice may also cause the accumulation of nondesired toxic metals that could be detrimental to human health. The results of hydroponics experiments demonstrate that our transgenic rice lines did not have higher Cd concentrations in polished grains compared to the Nipponbare control. This supports other studies that also reported no increases in the Cd accumulation in grains of high-iron-biofortified rice (Masuda et al., 2012; Trijatmiko et al., 2016).
Together, the combinatorial and targeted expression of AtNAS1, AtNRAMP3 and PvFER is a novel and promising strategy for achieving significant iron and zinc increases in polished rice grains. The IR64 lines with highest iron concentration we report here are now important candidates for the development of agronomically robust rice mega-varieties with improved nutritional benefits for human health.
Experimental procedures
DNA construct, rice transformation and plant growth conditions
The full-length AtNRAMP3 genomic sequence was amplified using a forward primer containing a BamHI site and a reverse primer with a BspEI site (Table S4). The fragment was subsequently inserted into the BamHI- and BspEI-digested OsNAS2-PbskII(-) vector (Singh et al., 2017a,b) to generate pUbi::AtNRAMP3::tNOS fragment. In parallel, the PstI site in the Ubi promoter contained in the OsNAS2-PbskII(-) construct was changed by a point mutation to use the site for subsequent cloning steps. The pCAMBIA-1300PMI-NASFER vector containing the AtNAS1 and PvFER genes was cut at the PstI site and the pUbi::AtNRAMP3::tNOS fragment was inserted to generate the NFUN cassette. Similarly, pOle18 promoter sequence was amplified using a forward primer containing PstI and SphI sites, and a reverse primer containing a BamHI site, and then inserted into pUbi::AtNRAMP3-PbskII(-) digested with SphI and BamHI to generate pOle18::AtNRAMP3::tNOS. The pCAMBIA-1300PMI-NASFER vector was then digested at the PstI site and the pOle18::AtNRAMP3::tNOS fragment was inserted to generate the NFON cassette. For the FUN and FON constructs, pCAMBIA-1300PMI-NASFER was digested at the XbaI and PstI sites to obtain the pGlb::PvFERRITIN::tNOS fragment, which was subsequently inserted into pCAMBIA-1300PMI to generate the pCAMBIA-1300PMI-FER vector. The pCAMBIA-1300PMI-FER vector was cut at the PstI site, and the pUbi::AtNRAMP3::tNOS or pOle18::AtNRAMP3::tNOS fragments were inserted to generate the FUN or FON cassettes, respectively. These four vectors were transformed into Oryza sativa ssp. Japonica cv. Nipponbare, and NFON was additionally transformed into Indica IR64 using Agrobacterium tumefaciens strain EHA105 (Hood et al., 1993). Transformation, selection and regeneration followed an established protocol (Nishimura et al., 2006). Candidate transformants were first screened for the presence of AtNRAMP3, AtNAS1 and PvFER using PCR. Southern blot hybridization using digoxigenin (DIG) labelling was performed using PmlI-digested genomic DNA isolated from the transgenic lines to select lines with single-copy cassette insertions. The PCR-amplified PMI DNA fragment was used as a probe to detect the transgene cassette. The primer sequences used for cloning, sequence verification and PCR are provided in Table S4. Regions flanking the transgene in NFON16 were amplified using TAIL-PCR (Liu and Whittier, 1995). In brief, three consecutive PCRs were performed using three arbitrary primers and nested specific primers (Table S5). All TAIL-PCRs were performed in 20 μL reaction volumes. The first TAIL-PCR was performed using approximately 100 ng template DNA. The second PCR was performed using a 1:40 dilution of the preceding PCR and the third PCR using 1 μL of the PCR product of the second TAIL-PCR. Cycling conditions were as specified by Liu and Whittier (1995). PCR products were sequenced by Microsynth AG (Balgach, Switzerland).
The plants were grown in commercial soil ‘ETH Soil mixture 1' – Ricoter composition (25% field soil, sterile, 0-15 mm; 25% bark mulch; 20% sand, washed, 0–2 mm and 30% white peat, 7–20 mm) under greenhouse conditions in 80% humidity at 30 °C with 12-h light and 60% humidity at 22 °C with 12-h dark. Quantification of divalent metal ions and transgene expression was conducted using T2 and T3 grains.
Iron deficiency and cadmium excess treatment in hydroponic culture
Transgenic and Nipponbare (NB) seeds were germinated in vitro for 5 days in Petri dishes on a H2O-moistened filter paper and subsequently transferred into containers (with tip holes) containing 400 mL hydroponic solutions for 7 days. Solutions for hydroponic growth were prepared according to established protocols (Boonyaves et al., 2016; Kobayashi et al., 2005), that is, using 0.70 mm K2SO4, 0.10 mm KCl, 0.10 mm KH2PO4, 2.0 mm Ca(NO3)2, 0.50 mm MgSO4, 10 μm H3BO3, 0.50 μm MnSO4, 0.20 μm CuSO4, 0.01 μm (NH4)6Mo7O24, and 0.5 μm ZnSO4, with different iron concentrations added as Fe(III)-EDTA according to the treatment (normal condition: 100 μm; iron-deficient condition: 10 μm). For the iron-deficient condition, plants were grown in hydroponic solutions containing 10 μm Fe(III)-EDTA for 14 days. Plants grown in hydroponic solutions containing 100 μm Fe(III)-EDTA served as control. Solutions were changed every 2 days to avoid any precipitation and contamination. In case of Cd excess treatment, plants were grown in hydroponic solutions containing 50 μm CdCl2 and shoot and root samples were collected from 21-day-old seedlings. To analyse grain Cd concentrations, plants were grown until maturity in a hydroponic solution containing 10 μm CdCl2 in greenhouse conditions in 80% humidity at 30 °C with 12-h light and 60% humidity at 22 °C with 12-h dark.
Metal ion measurements
Grain samples were dehusked to obtain unpolished brown grains. The dehusked grains were processed using a grain polisher (Kett grain polisher ‘Pearlest’, Kett Electric Laboratory, Tokyo, Japan) for 1 min. Shoot and root samples from hydroponic culture were dried at 60 °C for 5 days. Two hundred milligrams of each ground grains sample and 50–100 mg of root or shoot samples were boiled in 15 mL of 65% v/v HNO3 solution at 120 °C for 90 min. Three millilitres of 30% v/v H2O2 was subsequently added and continuously boiled at 120 °C for 90 min. Metal concentrations were determined using inductively coupled plasma–optical emission spectroscopy (ICP-OES) (Varian Vista-MPX CCD Simultaneous ICP-OES). The wavelength used for iron, zinc, manganese and copper were 238.204, 213.857, 257.610 and 324.754 nm, respectively. The National Institute of Standards and Technology (NIST) rice flour standard 1658a was treated and analysed in the same manner and used as internal control for every measurement. Data were analysed using Student's t-test. The criteria of P < 0.05 and P < 0.01 were used to determine statistically significant differences among the tested lines and the control.
RNA extraction, cDNA synthesis and quantitative real-time PCR
Total RNA was extracted from roots and shoots of 5-day-old T3 generation seedlings using Trizol® reagent (Invitrogen of Thermo Fischer Scientific Inc., Massachusetts (MA), USA). To obtain total endosperm, embryo and seed coat RNA, whole seed coats, endosperm and embryo were separated manually from T3 rice grains at 16 DAF. Extraction buffer containing 0.15m NaCl and 1% sarcosyl sarcosyl (N-Lauroyl sarcosinate sodium) was added to the ground samples followed by purification with 8m guanidine hydrochloride buffer. The RNA was treated with DNase I (Thermo Fisher Scientific Inc., Massachusetts (MA), USA) to remove genomic DNA contamination. First-strand cDNA was synthesized using the RevertAid™ first-strand cDNA synthesis kit (Thermo Fisher Scientific Inc.). Quantitative RT–PCR was performed as previously described (Liu et al., 2011). In brief, qRT–PCR was performed in a 7500 Real-Time PCR System using the SYBR Green RT–PCR reagent kit following the manufacturer's protocol (Applied Biosystems, Carlsbad, CA). Each reaction was run in triplicates in a volume of 20 μL with an initial denaturation step at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 60 s. Data were analysed according to the manufacturer's instructions using the 7500 System SDS Software v1.4 (Applied Biosystems). The expression level of genes of interest was normalized to the expression of rice UBIQUITIN 5 (OsUBQ5).
Protein extraction and Western blotting analysis
Protein extraction and Western hybridization were carried out as described previously (Svozil et al., 2015). In brief, the frozen plant material was ground into a fine powder and proteins were extracted by incubation with SDS buffer containing 4% SDS, 40 mm Tris-base, 5 mm MgCl2 and 2x protease inhibitor mix (Roche Roche AG, Basel, Switzerland) for 20 min at room temperature (RT). Cell debris was removed by centrifugation at RT for 10 min at 16 000× g. The supernatant was stored at −80 °C for further use. For the Western blots, 30 μg of leaf extract and 50 μg of embryo extract was separated using SDS–PAGE on 10% SDS gels. After blotting, the nitrocellulose membranes were probed with an anti-AtNRAMP3 (1:1000) antibody for 1 h at RT and then followed by an anti-rabbit (1:5000, Roche) antibody for 1 h at RT. After detection of the immunofluorescence signal, the membranes were stained with Ponceau Red for 10 min.
Germination rate test
Thirty seeds of each tested line were imbibed in deionized water at RT for 2 days in the dark and then transferred to Petri dishes containing water-saturated filter paper for germination. Germination rates were scored every day. Three replications were performed using 30 seeds each time. The test was performed according to a published protocol (Cheng et al., 2013; Yang et al., 2012) with the above modifications.
Acknowledgements
We gratefully acknowledge the PhD fellowship support of Mrs. Jacqueline Imhof to TYW and support from an ETH research grant for this work to WG and NKB. We thank Prof. Rainer Schulin for providing access to ICP-OES and Björn Studer for kind assistance with metal measurements. We thank Irene Zurkirchen for the technical support in the greenhouse. We thank Wuyan Wang for assistance with analysing grain biochemical index and Komal Jhala and Simrat Pal Singh for assistance with IR64 transformation. We thank Dr. Sébastine Thomine (French Science Center for Scientific Research) for providing AtNRAMP3 antibody, Dr. Takaiwa (NIAS, Japan) for the Ole18 promoter and Prof. Keller (University of Zurich) for the ubiquitin promoter..
Conflict of interest
The authors declare that they have no conflicts of interest.
Author contributions
NKB conceived the study, NKB and TYW designed the experiments, TYW performed the experiments, TYW, WG and NKB interpreted the data, TYW and NKB wrote the manuscript, and WG and NKB edited the manuscript. All authors have read and approved the final manuscript.




