Anesthetic-sparing effect of dexmedetomidine during total intravenous anesthesia for children undergoing dental surgery: A randomized controlled trial
Section Editor: Brian J Anderson
Abstract
Background
Dexmedetomidine, an α2-adrenergic agonist, reduces propofol and remifentanil requirements when used as an adjunct to total intravenous anesthesia in adults, but studies in a pediatric population are sparse. This study investigates the magnitude of dose-sparing effects of a postinduction dexmedetomidine bolus on propofol and remifentanil requirements during pediatric surgery.
Methods
In this randomized, double-blind, controlled trial, children aged 2–10 years undergoing elective dental surgery were assigned to one of four groups: placebo, 0.25 mcg/kg dexmedetomidine, 0.5 mcg/kg dexmedetomidine, and 1 mcg/kg dexmedetomidine. Maintenance with fixed-ratio propofol and remifentanil total intravenous anesthesia followed a bispectral index (BIS)-guided algorithm designed to maintain a stable depth of anesthesia. The primary outcomes were time-averaged maintenance infusion rates of propofol and remifentanil. Secondary outcomes in the postanesthetic care unit included sedation scores, pain scores, and time to discharge.
Results
Data from 67 patients were available for analysis. The median [interquartile range] propofol infusion rate was lower in the 1 mcg/kg dexmedetomidine group (180 [164–185] mcg/kg/min) versus placebo (200 [178–220] mcg/kg/min): percent change −10.0%; 95% CI −2.4 to −19.8; p = 0.013. The remifentanil infusion rate was also lower in the 1 mcg/kg dexmedetomidine group (0.089 [0.080, 0.095] mcg/kg/min) versus placebo (0.103 [0.095, 0.106] mcg/kg/min): percent change, −13.7%; 95% CI −5.47 to −21.0; p = .022. However, neither propofol nor remifentanil infusion rates were significantly different in the 0.25 or 0.5 mcg/kg dexmedetomidine groups. In the postanesthesia care unit, there were no differences in pain or sedation scores, and time to discharge was not significantly prolonged in any dexmedetomidine group.
Conclusion
Dexmedetomidine 1 mcg/kg reduced the propofol and remifentanil requirements during maintenance of anesthesia in children when administered as a postinduction bolus.
Trials Registration
ClinicalTrials.gov: NCT03422978, date of registration 2018-02-06.
1 INTRODUCTION
Dexmedetomidine is an α2-adrenergic agonist with anxiolytic, analgesic, and sedative effects, while typically causing minimal respiratory depression and only transient, well-tolerated hemodynamic changes.1, 2 It is a common adjunct to general and regional anesthesia within the pediatric population.1 Perioperative benefits of dexmedetomidine include reduced emergence delirium,3, 4 reduced postoperative pain and opioid requirements,5, 6 and reduced nausea and vomiting.5, 6
The intraoperative effect of dexmedetomidine in lowering anesthetic requirements in total intravenous anesthesia (TIVA) has been well demonstrated in adults7-10; specifically, reduced propofol requirements were reported during closed-loop anesthesia in patients given dexmedetomidine,7 and reduced remifentanil consumption during target-controlled infusion following intranasal dexmedetomidine.11 These findings indicate that dexmedetomidine may synergistically interact with both propofol and remifentanil to reduce requirements for both drugs. In the pediatric population, one retrospective study analyzed dexmedetomidine use alongside propofol-remifentanil TIVA in adolescent spinal surgery,12 finding that a continuous dexmedetomidine infusion reduced propofol infusion rates by 30%. In addition, studies using dexmedetomidine as an adjunct to propofol for pediatric sedation for investigational procedures have found that dexmedetomidine reduced propofol infusion rates.13-15 However, this effect has not been investigated prospectively in a pediatric surgical setting.
Although the safety of TIVA in children has been established,16 the increased dose requirements for propofol, compared to adults, can be associated with respiratory and cardiovascular complications during pediatric anesthesia.17 Additionally, minimizing anesthesia exposure may be an important consideration given concerns around associations with neurodevelopmental sequelae.18-20
This randomized trial aimed to explore dose–response relationships and quantify the anesthetic-sparing effects of varying doses of dexmedetomidine on propofol-remifentanil TIVA in children. We hypothesized that dexmedetomidine would cause a clinically significant reduction of 30% in propofol and remifentanil dosing required to maintain adequate depth of anesthesia as monitored through the bispectral index (BIS). The primary outcome measures were time-averaged changes in mean infusion rates of propofol and remifentanil compared to the placebo group. Secondary outcome measures were sedation scores after discontinuing the propofol-remifentanil infusion, pain scores and total fentanyl doses given in the postanesthetic care unit (PACU), and time to discharge from PACU.
2 METHODS
2.1 Study design
This was a randomized, double-blind, four-parallel arm study. Patients were randomized in a 1:1:1:1 ratio to receive a 10 mL bolus of 0.9% saline (placebo control) or 10 mL of normal saline containing 0.25 μg/kg dexmedetomidine (DEX-0.25), 0.5 μg/kg dexmedetomidine (DEX-0.5), or 1 μg/kg dexmedetomidine (DEX-1) after induction of anesthesia.
The trial was approved by the Institutional Ethics Board (H17-02157, University of British Columbia/Children's and Women's Health Centre of British Columbia Research Ethics Board, date of approval 2018-01-03), registered at ClinicalTrials.gov (NCT03422978, date of registration 2018-02-06), and authorized by Health Canada (212 359, date of no objection letter 2018-01-29). Written consent was obtained from parents or guardians of eligible children, and assent was obtained from children over 7 years old. The study was reported according to the CONSORT guidelines.21
2.2 Participants
The trial was conducted at BC Children's Hospital (Vancouver, Canada). Eligible patients were children 2–10 years of age scheduled for elective dental surgery, appropriate for TIVA, and assigned an American Society of Anesthesiologists Physical Status I or II. Patients were excluded if they required an inhalational induction of anesthesia, had neurological or cardiovascular conditions (including hypertension), had received preoperative sedatives or anxiolytics (e.g., benzodiazepines), had a weight <5th percentile or >95th percentile for age, or had hypersensitivity to dexmedetomidine or any other study medication.
2.3 Randomization
The equal allocation to four groups (control, DEX-0.25, DEX-0.5, and DEX-1) was completed via block randomization before study initiation using an Excel spreadsheet (Microsoft, Redmond, USA). Envelopes with a study ID and treatment group were prepared and assigned sequentially to each participant. All dexmedetomidine doses were diluted to a standard 10 mL volume with normal saline; an anesthesiologist not involved in the patient's care prepared a total syringe volume of 15 mL of study drug to account for the line dead space and some waste during the priming of the line, which would allow the patient to receive a 10 mL bolus of the study drug in all situations. After study drug preparation, the envelope was resealed. Both the participant and anesthesiologist were blinded to the allocation.
2.4 Anesthesia procedures
Patients underwent standard preoperative preparation with fasting, administration of acetaminophen (20 mg/kg) and ibuprofen (5 mg/kg), application of topical anesthetic to cannulation sites, and baseline measures of heart rate, noninvasive blood pressure, and oxygen saturation. Anesthesia was induced with a bolus of lidocaine (0.5 mg/kg), followed by a bolus of propofol (5 mg/kg) and remifentanil (3 μg/kg), and maintenance of TIVA commenced immediately following induction. Following the induction of anesthesia, bag-mask ventilation with 80% oxygen was administered. Following skin preparation to ensure a good signal was obtained, Bispectral Index (BIS) sensors were attached, and depth of hypnosis monitoring started. Then, endotracheal intubation facilitated mechanical ventilation to maintain an oxygen saturation (SpO2) ≥95% and endtidal carbon dioxide (ETCO2) between 35 and 45 mmHg. Following intubation, vital signs (BIS, HR, SpO2, and ETCO2) were monitored continuously and recorded every 5 s (Philips IntelliVue MX800, Philips Healthcare, Markham, Ontario) using custom data collection software.22 BIS was measured using the M1034B BIS Index Module with the MX800 patient monitor (Philips Healthcare, Markham, Ontario).
Approximately 3 min after induction of anesthesia, the prepared syringe of the study drug was delivered over 1 min using an infusion pump.
Propofol-remifentanil TIVA was maintained with separated pump infusions (Perfusor Space, B Braun, Mississauga, Ontario) initiated at 200 and 0.1 μg/kg/min, respectively. Following induction, additional bolus doses of propofol and remifentanil were given at the anesthesiologist's discretion until a satisfactory depth of hypnosis was reached. All bolus doses given at any point following the commencement of the infusions were recorded.
Depth of anesthesia was measured continuously by BIS. Every 5 min throughout the procedure, the anesthesiologist adjusted the propofol and remifentanil infusions proportionally to maintain a target BIS value between 50 and 60 following a protocolized algorithm: at or below a BIS of 50, the propofol infusion was reduced by 20 μg/kg/min and remifentanil by 0.01 μg/kg/min; above a BIS of 60 or following a patient response, the propofol infusion was increased by 20 μg/kg/min and remifentanil by 0.01 μg/kg/min, and a 500 μg/kg propofol bolus and 0.25 μg/kg remifentanil bolus given; if the BIS value was between 50 and 60, no change was made to the infusions. We adjusted the two infusions proportionally to allow our findings to be translated to those institutions and practitioners who mix propofol and remifentanil in the same syringe, which is an accepted practice.23 We anticipated that the selected range of 50–60, which is narrower than the conventional 40–60 recommendation for general anesthesia, would result in more frequent propofol and remifentanil dose adjustments, with the goal that tighter titration would enhance our ability to observe a dexmedetomidine effect.
In the last 15 min of the procedure, based on a warning from the dentist (a standard part of our operating approach during dental procedures), the anesthesiologist could titrate the anesthetic as they deemed appropriate to facilitate wake-up. The dentists administered local anesthetic at their discretion.
Rescue medications (glycopyrrolate, atropine, ephedrine, and epinephrine) were available as part of standard practice. Treatment of any hemodynamic alterations was at the attending anesthesiologist's discretion.
2.4.1 Postoperative follow-up
After the patient was transferred to the Post Anesthetic Care Unit (PACU), the patients were observed for 30 min. Sedation scores using the University of Michigan Sedation Scale (UMSS) and pain scores using the Face Legs Activity Cry Consolability scale (FLACC) or Faces Pain Scale – Revised (FPS-R) were recorded every 10 min.24 Time asleep was assessed through direct observation by a research assistant, every 10 min, including whether the child woke up spontaneously or when stimulated during routine nursing care. For pain scores at or above 5 out of 10, doses of fentanyl 0.5 μg/kg every 10 min were given intravenously, up to a total dose of 2 μg/kg.
2.5 Outcomes
The maintenance period was defined as the time after the standard weight-based induction boluses were delivered until the 15-min warning. This period included all boluses given after commencing the propofol and remifentanil infusions, with infusion rates from the infusion pump recorded every second until the 15-min warning. The primary trial outcome was the time-averaged propofol and remifentanil infusion rates during maintenance. Secondary outcomes were sedation scores, pain scores, total fentanyl doses given in the PACU, and time to discharge from the PACU. The quality of anesthesia depth titration was assessed as the percentage of time BIS fell between the target values of 50 and 60.
2.6 Statistical analysis
2.6.1 Sample size
A sample size calculated with a mean propofol infusion rate of 174 ± 42 μg/kg/min, based on historical data at our institution for children undergoing dental surgery, yielded 18 patients in each group to detect a 30% reduction of propofol consumption with a power of 90% and a critical alpha of .05, when Bonferroni corrected for three pairwise comparisons. Accounting for 20% losses or exclusions, recruitment of 88 patients was specified in the original protocol.
2.6.2 Data analysis
Data are presented as median (interquartile range [IQR]). The primary outcomes of mean infusion rates of propofol and remifentanil were assessed with Mann–Whitney U tests. Bootstrapped two-sided 95% confidence intervals (CI) were calculated for the between-group percentage differences. The quality of depth of anesthesia was defined as the percentage duration of the maintenance period in which the BIS was in the target range of 50–60. Secondary outcomes were compared with Kruskal–Wallis tests for continuous variables and Fisher's exact tests for categorical variables. Post hoc Bonferroni-corrected Mann–Whitney U tests were applied when appropriate.
Anesthesiologists sometimes entered rounded weights into the infusion pumps. Thus, comparisons of infusion rates were performed with original values and weight-corrected values, in cases where the infusion controller had a patient weight that differed from the actual patient weight; the weight-corrected values are presented here.
A critical alpha of .05, controlled for multiplicity for comparisons for the three dexmedetomidine groups against the placebo with the Bonferroni method, was considered statistically significant for all tests. All analyses were conducted in R 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria).
2.6.3 Exploratory analyses
Drug preparation error: After data collection from 88 patients, an initial review found that the number of patients suitable for analysis was fewer than 18 in the DEX-0.25 and DEX-0.5 groups. This was because some patients received an incorrect dexmedetomidine dose due to erroneous dead-space calculation (i.e., the independent anesthesiologist preparing the study drug did not account for the extra 5 mL of volume when preparing the dexmedetomidine syringe). The study's regulator (Health Canada) declined to permit a proposed extension of study randomization to achieve 18 cases per group. Accordingly, while we analyzed the data for all eligible cases who received the intended dexmedetomidine dose (primary analysis described above), we recognized that this analysis is underpowered. Hence, we have also analyzed all allocated cases that were not excluded for other reasons; that is, the eight cases excluded from the primary analysis due to the drug dosing error data were included in a linear regression model alongside the included data to examine the potential effect on the dose–response relationship between dexmedetomidine and reduction in anesthetic.
Shorter maintenance window: Considering the pharmacokinetics of dexmedetomidine, we also analyzed the data using a shorter 60-min window, from 10 to 70 min of maintenance of anesthesia, which aimed to optimize the likelihood of detecting an anesthetic-sparing effect, considering the pharmacokinetics of dexmedetomidine, that is, assuming an initial redistribution time of 6 min (rounded to 10 min from the start) followed by 60 min of observations based on an elimination half-life of 120 min.
Depth of anesthesia visualization: Intraoperative BIS values and infusion rates were visualized as a time series to explore the longitudinal effects of dexmedetomidine. The BIS monitors were often not applied during the first 3 min as the patients underwent endotracheal intubation and study drug administration. Hence, depth of anesthesia data were not available until after the BIS monitors were connected and the software had finished its initialization.
3 RESULTS
3.1 Study population
Data were collected between April 2018 and April 2020. A total of 88 patients were randomly allocated to the placebo control or dexmedetomidine treatment groups (DEX-0.25, DEX-0.5, and DEX-1), of whom 67 were included in the analysis of primary outcomes (Figure 1). Eight were excluded due to drug preparation errors in the DEX-0.25 and DEX-0.5 groups, of which seven were caused by underdosing from dead space volume correction error.
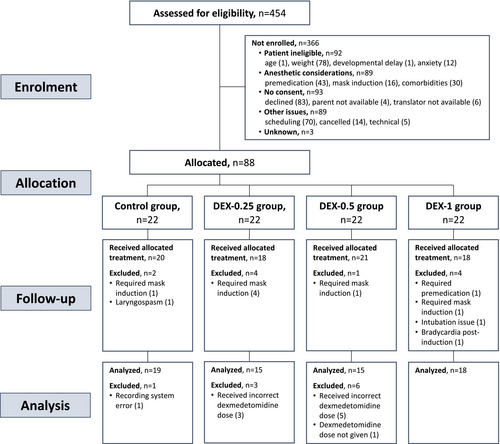
Patients' demographic and procedure characteristics were similar between groups, other than a shorter procedure duration in the DEX-0.25 and DEX-0.5 groups and a greater number of extractions in the DEX-1 group (Table 1). The overall median (IQR) age of participants was 3.4 years (2.9–4.2), and the maintenance period duration was 69.2 min (55.9–81.4).
| Control group (n = 19) | DEX-0.25 group (n = 15) | DEX-0.5 group (n = 15) | DEX-1 group (n = 18) | Total (n = 67) | |
|---|---|---|---|---|---|
| Age (years) | 3.5 (3.1–4.5) | 3.7 (2.8–4.3) | 3.0 (2.8–3.9) | 3.4 (2.9–4.1) | 3.4 (2.9–4.2) |
| Height (cm) | 96.2 (94.1–101.8) | 97.0 (93.2–104.6) | 96.1 (93.2–103.2) | 99.0 (89.2–102.6) | 96.9 (93.2–103.3) |
| Weight (kg) | 15.8 (14.7–16.9) | 14.7 (13.2–18.8) | 14.6 (13.8–17.4) | 15.0 (12.9–16.2) | 15.0 (13.7–17.5) |
| Procedure durationa (min) | 75.8 (50.8–88.4) | 63.1 (52.3–73.1) | 63.6 (48.4–73.0) | 76.4 (65.5–91.0) | 69.2 (55.9–81.4) |
| Number of extractions | 2.5 (1.2–4) | 3 (1–4) | 4 (1–4.5) | 4 (3.5–6) | 4 (1–5) |
| Number of restorations | 6 (4–8) | 6.5 (4–8) | 5 (2.5–7.5) | 6 (4–8.2) | 6 (4–8) |
- Note: Values are median (interquartile range).
- a Defined as the start of maintenance anesthesia to the 15-min warning by the dentist.
3.2 Propofol and remifentanil utilization
The median time-averaged maintenance propofol infusion rates were 180 (164–185) μg/kg/min for the DEX-1 group and 200 (178–220) μg/kg/min for the control group (percentage change, −10.0%; 95% CI, −2.4 to −19.8; p = .013) (Figure 2A). The median time-averaged remifentanil maintenance infusion rates were 0.089 (0.080–0.095) μg/kg/min for the DEX-1 group and 0.103 (0.095–0.106) μg/kg/min for the control group (percentage change, −13.7%; 95% CI, −5.5 to −21.0; p = .022) (Figure 2B).
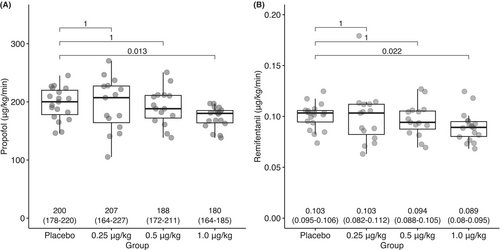
There were no significant differences in propofol or remifentanil utilization between the DEX-0.25 group versus control or the DEX-0.5 group versus control.
3.3 Depth of anesthesia
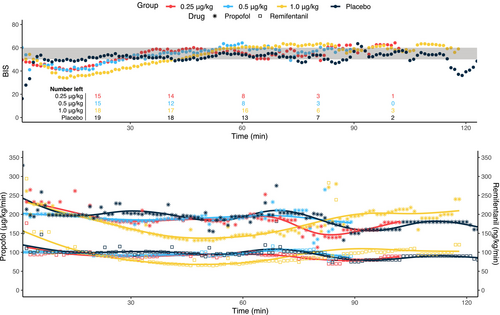
The recorded BIS data illustrate a depth of anesthesia overshoot from the dexmedetomidine bolus dose; the lowest BIS value occurred at 13 min for the DEX-0.25 group, at 14 min for the DEX-0.5 group, and at 13 min for the DEX-1 group (Figure 3). In response to the increased depth of anesthesia, there was a gradual reduction in propofol and remifentanil infusions, although the BIS values did not return to the target 50–60 range for 27 min in the DEX-0.25 group, for 29 min in the DEX-0.5 group, and most noticeably, for 43 min in the DEX-1 group. For the DEX-0.25 and DEX-0.5 groups, the reductions were small and transient, and thus not significant versus placebo. As the effect of dexmedetomidine wore off, beyond around 45 min, the propofol and remifentanil requirements rose again.
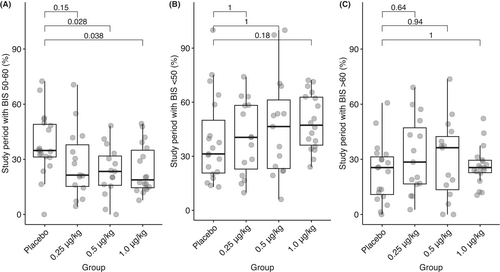
There were some differences in the consistency of maintaining the target depth of anesthesia (Figure 4). Overall, the control group had a median of 34.8% (31.2–49.0) of the maintenance period within the target range; the DEX-0.5 μg/kg group had a lower percentage within the target range (23.4% [15.9–31.8]; p = .028) as did the DEX-1 group (18.8% [14.6–35.0]; p = .038).
3.4 Postoperative follow-up
The duration asleep in the PACU was similar (Table 2). An overall difference between the four groups was found for the duration in the PACU, but no significant difference was found for any dexmedetomidine group compared with the control group. There were no significant differences in pain or sedation scores between the groups assessed at 10, 20, and 30 min. Overall, 14/67 (21%) children had significant pain (FLACC or FPS-R ≥ 5) and received one or more rescue fentanyl doses in PACU, with the proportion of children receiving fentanyl being similar between groups (Table 2). Overall, 15/67 (22%) patients had already received a dose of fentanyl in the operating room before transfer to PACU: 5/19 (26%) in the control group; 5/15 (33%) in the DEX-0.25 group; 4/15 (27%) in the DEX-0.5 group; and 1/18 (6%) in the DEX-1 group.
| Control group (n = 19) | DEX-0.25 group (n = 15) | DEX-0.5 group (n = 15) | DEX-1 group (n = 18) | p-Value | |
|---|---|---|---|---|---|
| Duration asleep in the PACU (min) | 23 (15.5–30) | 29 (21–34.5) | 27 (24–30) | 25.5 (22–29.8) | .348 |
| Duration in the PACU (min) | 60 (49.5–74) | 60 (51.5–74.5); p = 1.00 | 48 (46.5–54.5); p = .14 | 74 (67.8–89); p = .14 | .002 |
| Pain Scores (FLACC score or FSP-R) | |||||
| 10 min | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | .376 |
| 20 min | 0 (0–4.5) | 0 (0–0) | 0 (0–0) | 0 (0–0) | .125 |
| 30 min | 0 (0–6) | 0 (0–4.5) | 0 (0–2.5) | 2.5 (0–5) | .405 |
| Sedation Scores (UMSS) | |||||
| 10 min | 1 (0.5–2.5) [3] | N/A [0] | N/A [0] | N/A [0] | – |
| 20 min | 2 (0.5–3) [11] | 2 (2–2) [5] | 2 (1–3) [5] | 3 (2–3) [5] | .688 |
| 30 min | 1.5 (0–3) [12] | 1 (1–1) [9] | 1 (0–1.2) [12] | 1 (0–2) [12] | .790 |
| Number of patients receiving fentanyl | |||||
| None | 16 (84%) | 11 (73%) | 14 (93%) | 12 (67%) | .381 |
| 0.5 μg/kg | 1 (5%) | 3 (20%) | 1 (7%) | 2 (11%) | |
| 1–2 μg/kg | 2 (11%) | 1 (7%) | 0 (0%) | 4 (22%) | |
- Note: Values are median (interquartile range). When a metric was not assessed in the whole group, the number of patients with available data are shown in square brackets.
- Abbreviations: FLACC, Face Legs Activity Cry Consolability scale; FSP-R, Faces Pain Scale – Revised; PACU, postanesthesia care unit; UMSS, University of Michigan Sedation Scale.
3.5 Adverse event
One patient, 3 years of age and weighing 18.1 kg, experienced bradycardia after the administration of 1 μg/kg dexmedetomidine and received atropine rescue. Vital signs monitoring showed a minimum heart rate of 41 bpm and no significant reduction in expired carbon dioxide concentration during the bradycardic period.
3.6 Other exploratory analyses
3.6.1 Drug preparation error
In a linear regression model exploring the effect of dexmedetomidine dose on propofol and remifentanil infusion rates, including the eight additional cases excluded from the primary analysis had only a minor effect on the trend (Figure S1).
3.6.2 Shorter maintenance window
When using only the data for up to 70 min of maintenance period, the effect of dexmedetomidine on the anesthetic infusion rates was more pronounced for the DEX-1 group; the percentage difference was −25.8% (95% CI, −4.1 to −30.7; p = .003) for propofol and −23.1% (95% CI, −7.9 to 30.3; p < .001) for remifentanil (Figure S2). However, no change in significance was observed for the DEX-0.25 or DEX-0.5 groups.
4 DISCUSSION
In this randomized, double-blind trial, three different doses of dexmedetomidine, delivered as a bolus after the induction of anesthesia, were compared to a placebo in children undergoing elective dental procedures. The administration of 1 μg/kg of dexmedetomidine (DEX-1) resulted in lower propofol (−10.0%) and remifentanil (−13.7%) requirements, whereas the 0.25 μg/kg (DEX-0.25) and 0.50 μg/kg (DEX-0.5) doses did not decrease the propofol or remifentanil infusions rates significantly. There were some differences in the depth of anesthesia consistency, as assessed through the percentage of time BIS was within the target range of 50–60: The DEX-0.5 and DEX-1 groups had a lower percentage in range, with a trend toward greater anesthetic depth in a dose-dependent manner. After leaving the operating room, dexmedetomidine was not associated with differences in sedation, pain, rescue fentanyl, or prolonged time to discharge.
While our present trial demonstrates an anesthetic-sparing effect on propofol-remifentanil TIVA, the dose reduction was less than the hypothesized 30%. This contrasted with other previously reported results: With a 0.5 μg/kg/h continuous infusion of dexmedetomidine, Ngwenyama et al. reported a 30% reduction in propofol infusion rate, but not remifentanil infusion rate, in their retrospective study of adolescents undergoing spine surgery.12 Studies using dexmedetomidine as an adjunct to propofol for the sedation of children undergoing MRI examination15 and endoscopy procedures14 have demonstrated reduced propofol infusion rates by 35% (with 0.5 μg/kg dexmedetomidine) and total propofol dose by 43% (with 0.5 μg/kg then 0.15 μg/kg/h dexmedetomidine), respectively. Another study evaluating dexmedetomidine in among pediatric endoscopy procedures did not find a reduction in total propofol dose at a median dexmedetomidine dose of 0.25 μg/kg.13
One factor that may explain why we report a lower anesthetic-sparing effect was the administration of dexmedetomidine as a single bolus. Our median maintenance duration was 69 (IQR 56–81) min, and reductions in anesthetic infusion rates were not sustained over time. Previous studies in adults or children have used a dexmedetomidine bolus followed by continuous infusion7, 8, 14 or a continuous dexmedetomidine infusion without a loading dose.10, 12 Exploratory analysis with a 10–70-min window applied to the maintenance period found greater decreases in anesthetic infusion rates, including in the DEX-1 group.
The propofol dose reduction that can be achieved by including a single bolus of dexmedetomidine at the start of a TIVA general anesthetic adds to the list of the drug's clinical benefits. It may have particular utility in two clinical scenarios: Short but stimulating procedures such as dental surgery and adenotonsillectomy where a reduced propofol dose facilitates more rapid recovery of adequate spontaneous respiration and emergence from anesthesia, and for the many pediatric procedures amenable to a TIVA technique with spontaneous respiration via nasal cannulae. While these outcomes were not measured in this study, these scenarios indicate the possible significance of our findings and represent future testable hypotheses that are clinically relevant. Propofol has a narrow therapeutic index for the margin between loss of consciousness and apnea or hypopnea. Reducing the dose required to achieve a given depth of anesthesia by coadministering dexmedetomidine may increase the safety margin of this technique, which warrants further investigation.
There was one case of bradycardia observed in the DEX-1 group. Bradycardia is a known adverse effect following dexmedetomidine and has been previously reported: Dawes et al.25 observed transient bradycardia in an 8-year-old child of 35 bpm after a dexmedetomidine bolus dose of 0.7 μg/kg, recovering to normal levels without intervention. Four cases of transient bradycardia were also observed in a study investigating the effect of dexmedetomidine on myocardial repolarization at dexmedetomidine doses of 0.50–0.75 μg/kg, all recovering without treatment.26
The postoperative profile was similar between groups, except for an overall difference between the four groups for the time to PACU discharge. While the time to discharge was not significantly prolonged for any group in pairwise comparisons versus the control, it was numerically longer in the DEX-1 group and shorter in the DEX-0.5 group. Our study was not designed to detect differences in time to discharge, and the shorter duration in the DEX-0.5 group may simply be attributed to a shorter procedure duration. In a retrospective review, West et al.27 found a relationship between dexmedetomidine dose and increased duration of PACU stay (14.6 min per μg/kg), suggesting that this effect may only be significant when including doses >0.45 μg/kg.
4.1 Limitations
Firstly, our study was underpowered as a result of drug preparation errors during the conduct of the trial and the study regulator being unwilling to allow us to enroll replacement participants; this resulted in eight cases being lost overall from the DEX-0.25 and DEX-0.5 groups and may help explain why we did not detect a reduction in propofol–remifentanil infusions in these groups. We conducted an exploratory analysis to further investigate the effect of this data loss, but this secondary analysis did not significantly change the outcome for these groups. Secondly, dose–response relationships could not be fully evaluated because of variations in the consistency of BIS. Our anesthetic titration protocol may have been insufficiently aggressive. This resulted in BIS values falling below the target range after dexmedetomidine administration instead of lowered anesthetic infusion rates; we observed a dose-dependent percentage of time that the BIS was below the target range. Thirdly, as previously discussed, the trial design of using a single intraoperative bolus of dexmedetomidine may have reduced the observed anesthetic-sparing effects of dexmedetomidine.
5 CONCLUSION
In summary, a single postinduction 1 μg/kg dexmedetomidine bolus significantly reduced propofol and remifentanil requirements during maintenance of anesthesia in children undergoing dental surgery. This drug-sparing effect may have clinical utility in short, stimulating procedures, and facilitating the use of TIVA with spontaneous respiration. Our failure to detect a reduction in propofol–remifentanil infusion rates with lower bolus doses of dexmedetomidine may require further investigation.
ACKNOWLEDGMENTS
The authors thank the anesthesiologists, nurses, and dentists at BC Children's Hospital for accommodating our study procedures within a busy operating room schedule and the patients and their parents for their willingness to participate. MG holds a Michael Smith Health Research BC scholar award.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
Research data are not shared.




