Emergency front-of-neck access in pediatric anesthesia: A narrative review
Section Editor: Britta S von Ungern-Sternberg
Abstract
Background and Objectives
Children undergoing airway management during general anesthesia may experience airway complications resulting in a rare but life-threatening situation known as “Can't Intubate, Can't Oxygenate”. This situation requires immediate recognition, advanced airway management, and ultimately emergency front-of-neck access. The absence of standardized procedures, lack of readily available equipment, inadequate knowledge, and training often lead to failed emergency front-of-neck access, resulting in catastrophic outcomes. In this narrative review, we examined the latest evidence on emergency front-of-neck access in children.
Methods
A comprehensive literature was performed the use of emergency front-of-neck access (eFONA) in infants and children.
Results
Eighty-six papers were deemed relevant by abstract. Finally, eight studies regarding the eFONA technique and simulations in animal models were included. For all articles, their primary and secondary outcomes, their specific animal model, the experimental design, the target participants, and the equipment were reported.
Conclusion
Based on the available evidence, we propose a general approach to the eFONA technique and a guide for implementing local protocols and training. Additionally, we introduce the application of innovative tools such as 3D models, ultrasound, and artificial intelligence, which can improve the precision, safety, and training of this rare but critical procedure.
1 INTRODUCTION
Securing the airway is a fundamental step to ensure ventilation and oxygenation in children undergoing general anesthesia, and it is a life-saving procedure in critically ill children. Failure to secure the airway and severe airway management complications are rare but can expose the patient to a potentially fatal outcome. A “Can't Intubate, Can't Oxygenate” (CICO) situation is characterized by inadequate oxygenation and rapid hypoxemia in which all the reversible causes of inability to oxygenate have been excluded and oxygenation is not achievable by all means of ventilation—bag-mask, supraglottic airway (SGA), and intubation. Poor ventilation in children, especially neonates and infants, is frequently due to laryngospasm and upper airway obstruction; these represent reversible causes that must always be excluded before proceeding to emergency front-of-neck access (eFONA), which should remain the absolute last resort. Unrecognized and untreated hypoxemia results in bradycardia and cardiac arrest, that can ultimately lead to brain damage and death. Patients undergoing Ear, Nose, and Throat (ENT) procedures and children under 1 year of age are at the highest risk for CICO situations.1-3
Most often, if the child is in a critical condition, with unstable cardiorespiratory parameters, awakening from anesthesia and re-establishing spontaneous breathing might not be viable options. In such cases, when bag-mask ventilation is unsuccessful, all measures to oxygenate fail and an ENT surgeon is not immediately available, an eFONA performed by the airway practitioner may be the only strategy to re-establish oxygenation and save the child's life.
No evidence-based consensus exists on the best practice and the minimum training requirements for pediatric eFONA. This leads to inconsistencies in various society's airway recommendations,4-7 and local standard operating procedures (SOP). These ambiguities transform an already challenging situation into an even greater one for the airway practitioner, as there is no clear advice on how to perform eFONA in children, especially in the youngest and most vulnerable.
An anonymized online survey of physicians at German perinatal centers revealed a need for SOPs, equipment, and training for CICO scenarios. Less than 1% of the 219 participants received specific training on infant cricothyroidotomy, and only 20% had access to a SOP for difficult airways in neonates with 69% reporting the lack of ready-to-use cricothyroidotomy kits at their facility.8
This review aims to summarize the current scientific literature and to provide a guide for best practices for eFONA in pediatrics, specifically in neonates and young children, with the limitation of evidence coming from animal and simulation studies and lack of clinical studies on the topic. This narrative review does not include elective tracheostomies.
2 METHODS
The databases Ovid Medline, PubMed, Web of Science, and Embase were searched independently by AKH and AT. We performed a review of the literature reporting the use of eFONA in infants and children. The following search terms were chosen: “pediatric emergency front-of-neck-access”, “pediatric or infant eFONA”, “pediatric or infant cricothyroidotomy or tracheotomy”, “pediatric or infant difficult airway”, “training pediatric emergency airway”, “scalpel tracheotomy in infants or children”, “needle cricothyroidotomy in infants or children”, and “animal models pediatric or infant emergency front-of-neck access”. The search was limited to English language and included animal and human studies. No date limits were set for the search. Eighty-six papers were deemed relevant by abstract, and their full text was obtained and examined further for compliance with the objectives of this educational review. For the eight remaining studies regarding the eFONA technique and simulations in animal models, we recorded their primary and secondary outcomes, their specific animal model, the experimental design, the target participants, and the equipment.
3 EPIDEMIOLOGY OF EMERGENCY FRONT-OF-NECK ACCESS: THE MAGNITUDE OF THE PROBLEM
A declared CICO event in children (i.e., oxygenation is not achievable by all means of ventilation—bag-mask, supraglottic airway (SGA), and intubation) can progress either to percutaneous FONA or surgical FONA. Technical aspects of percutaneous and surgical eFONA are described in detail in Table 1. The ideal technique should be one that is fastest, easiest to learn and remember, uses equipment that is readily available at the institution, leads to the highest success rate and least complications and can be performed by almost any team member in neonates and children.
| Technique | Method | Advantages (+) disadvantages (−) | Complications |
|---|---|---|---|
| Surgical |
Rapid sequence tracheotomy (RST) Midline skin incision with a 10-blade scalpel followed by careful exposure of the trachea using the scalpel and Backhaus clamps. Anterior luxation of it with a 3rd clamp. Vertical puncture of the trachea using scissors followed by a vertical incision of the first two tracheal rings to facilitate insertion of the age appropriate tracheal tube |
+ Fast execution after appropriate training. + simple equipment + very effective when trained + high success rate + Can be done in the smallest child − Cutting through the tracheal rings is often necessary − Good positioning with a shoulder roll is required − May require a an assistant − Securing the tracheal tube needs extreme care |
|
|
Scalpel bougie tracheotomy (SBT) Median 2–3 cm skin incision below the cricoid using an 11-blade scalpel. Careful exposure of the trachea followed by a longitudinal incision averaging the length of 2–3 tracheal rings. Insertion of an 8-Fr Frova intubating introducer into the distal trachea. Uncuffed 3.5 mm ID tracheal tube is placed over the catheter in a rotating motion. |
+ similar to eFONA in adults + Reduced risk of structural injury + Easier insertion of tracheal tube + high success rate + possible oxygenation through the catheter with Ventrain − Additional forces is required with a 3.0 mm ID tracheal tube − Larger incision required with a 3.5 mm ID tracheal tube − Ventrain is not readily available − Frova catheter is not readily available − Wrong channel can be formed |
|
|
| Percutaneous |
Catheter-over-needle cricothyroidotomy Cranio-caudal puncture of the cricothyroid membrane with needle. Insertion of cannula over needle. Needle removed and device connected to breathing bag. |
+ ready to use set + built in “stopper” to avoid posterior tracheal injuries + Small trauma to the skin − Cricothyroid Membrane often too small in children <8 years − High rate of failure and injuries |
|
|
Tracheal cannula Tracheal needle puncture (under aspiration, in 45°or lower) until loss of resistance. Insertion of cannula over needle. |
+ simple equipment − Risk of cannula kinking − High rate of posterior wall injury − Only temporary oxygenation solution with modified jet oxygenation |
|
Compared to adults, children and neonates are at significantly higher risk for difficult airway management and complications during general anesthesia.3, 9 The Anaesthesia PRactice in Children Observational Trial (APRICOT), a European prospective, multicenter cohort study, examined the incidence of serious critical events in over 30 000 children undergoing anesthesia. A secondary analysis found that the overall rate of difficult intubation—defined as Cormack-Lehane grading of 3 or 4, AND three or more attempts to insert the tracheal tube—was 0.28% in children aged 0 to 15 years but was significantly higher in neonates and children younger than 1 year, with rates of 1% and 1.1%, respectively.10 The PeDi Registry reported a rate of 2% for surgical airway in children with difficult airway (Cormack-Lehane 3 or 4, failed direct laryngoscopy or anatomical features suggesting for difficult airway) and 1% for cardiac arrest among complications of failed intubation and oxygenation.3 In the 4th UK National Audit Project11 five children, all below 10 years of age, required eFONA. Out of these three were successfully performed, one was attempted but was unsuccessful, and one was never performed as the child died on the way to the operating room. The only eFONA performed by an anesthesiologist with needle cricothyroidotomy was unsuccessful, but a subsequent intubation effort subsequently secured the airway. Furthermore, the 4th UK National Audit Project reported a high failure rate for needle cricothyroidotomy in adults; in children, the procedure might likely be even more demanding.11 Other in-hospital case reports similarly indicate that the skills and knowledge required for successful eFONA in children are insufficient.12-14 In most instances, an initial attempt at cricothyroidotomy was unsuccessful. If an adequate airway was eventually achieved, this was usually by an ENT surgeon through a tracheostomy.11-14
In the out-of-hospital setting, observational studies from national physician-staffed Helicopter Emergency Medical Services reported an approximate incidence of 0.01%–0.05% for eFONA; however, success rates were not elaborated further.15, 16 A systematic review by Morton et al., which included 69 studies with a total of 29 reported prehospital eFONA attempts in children, calculates a pooled success rate of 74% for prehospital eFONA in children, most of them being older than 10 years.17
4 EVIDENCE FROM ANIMAL MODELS
A systematic review conducted by Koers et al.,18 analyzing five studies with animal models that closely resemble the size of a child's trachea,19-23 concluded that the surgical scalpel method is superior to percutaneous techniques regarding success rate and complication rate. Reports of clinical cases support this finding and cast doubt on recommending needle cricothyroidotomy.13, 14
Results from animal studies are not easily transferable to children for several reasons. First, the methods and experimental settings employed in animal-based research lack standardization, thereby hindering valid comparisons and failing to accurately simulate real-life scenarios of CICO situations in children.
Second, a significant issue regarding representativeness arises from the small sample sizes typically employed and from the reduced number of study partecipants. Often, only two physician conduct eFONA procedures on a restricted number of animal subjects, using them for multiple interventions.21-24 This homogeneity may lead to modifications in the structure of the trachea due to repeated punctures, potentially compromising the reliability of subsequent attempts.21, 24
More recently, four studies involved rabbit cadaver models and standardized settings to simulate a more realistic scenario of eFONA in infants.25-28 Rabbit cadavers weighing 2.5–3.5 kg were used in these studies with the aim to better simulate the size of a small infant's trachea.
The synthesis from the recent four studies25-28 performed in animal models shows that the scalpel bougie tracheostomy is associated with higher success rate compared to the emergency tracheotomy and is associated with significantly fewer injured tracheal rings, and the participants expressed a preference for it due to the similarity with the adult surgical eFONA technique. However, they also share comparable limitations regarding their applicability to an actual CICO situation in the younger populations, like infants and neonates. The presence of a consistent individual assisting throughout all tracheotomies may have introduced bias to the results,25-28 as real-life situations with varying levels of operators' experience potentially lead to different success rates and performance times. Three of the four studies failed to consider the risk of intraoperative hemorrhage25-28 which is a prevalent complication during surgical tracheotomies. This complication can induce additional psychological stress on the practitioner and impede the visualization of the surgical site, significantly influencing the procedure's outcome.
Table 2 summarizes characteristics of recent emergency front-of-neck access in animal studies.
| Author | Prunty S.L. et al.21 | Stacey J. et al.22 | Metterlein T. et al.23 | Prunty S. L. et al.24 | Ulmer F. et al.25 | Both C.P. et al.26 | Thomas J. et al.27 | Riva T. et al.28 |
|---|---|---|---|---|---|---|---|---|
| Technique | Tracheal cannula | Tracheal cannula vs. Catheter-over-needle Tracheotomy | Catheter-over-needle-cricothyroidotomy | Catheter-over needle Tracheotomy vs. scalpel bougie technique (SB) | Rapid sequence tracheostomy (RST) | Scalpel bougie tracheostomy (SBT) | Scalpel bougie tracheostomy (SBT) | Rapid sequence tracheostomy (RST) vs. scalpel bougie tracheostomy (SBT) |
| Methods | Observational trial | Observational trial | Randomized trial | Observational trial | Observational trial | Observational trial | Randomized controlled trial | Randomized controlled trial |
| Procedure | Tracheal cannula:
|
Needle tracheotomy:
Quicktrach-Child: No detailed description of the procedure in the study |
Needle cricothyroidotomy:
|
COOK Melker set:
SB:
|
RST:
|
SBT:
|
SBT:
|
RST:
SBT:
|
| 1. Outcome |
Initial air aspiration: Total 93% (Direct aspiration 97%. Indirect aspiration 86%) |
Success rate: Cannula tracheotomy: 60%, no significant difference between 18G and 14G. Quicktrach-Child: 0% |
Successful placement and ventilation: 100% |
Success rate: Melker set: 100% SB: 75% |
Performance time: First to 10th attempt: 107 s ➔ 55 s (from skin palpation) |
|
|
Performance time: SBT 11 s faster than RST. (from first skin contact to connection to ventilation bag) |
| 2. Outcomes |
|
|
|
|
|
|
|
|
| Model | Six New Zealand White rabbits of ~4 kg | Five New Zealand White rabbits (3.2–5.3 kg) shaved, and pinned supine on a board at room temperature | 10 New Zealand White rabbits (3.5–5.4 kg) neck shaved, in supine position | Eight New Zealand White rabbits of ~4 kg |
2.8–3.2 kg Zika Zimmermann rabbits No mannequin head 10 new rabbits for each participants |
2.5–3.5 kg Zimmermann rabbits Infant mannequin head |
2.5–3.5 kg Zimmermann rabbits Infant mannequin head |
2.7–3.3 kg Zika-Zimmermann rabbits
|
| Process |
Six attempts with each method in each of the six rabbits. From the cricothyroid membrane downwards |
12 cannula eFONAs in each rabbit Alternating 18G and 14G cannulas From the tracheal cartilage proceeding caudal. Total of 13 attempts with Quicktrach-Child |
1 month before: practice with the set on manikins Performance of five procedures each |
Alternating between the two methods, four tracheotomies were performed on each rabbit. The first at the level of the first tracheal ring then advancing caudally |
|
|
|
|
| Participants | Two anesthesiologists, with significant experience in difficult airway | Two anesthesiologists, with significant expertise in emergency airway management | Two anesthesiologists: a first and a fourth-year residents | Two proceduralists, with expertise in emergency airway management | 50 Physicians trained in different areas in pediatrics and emergency medicine
|
29 Anesthesiologists: 23 senior physicians, six fellows, 16 previously took part in an eFONA workshop | 30 Physicians: 28 pediatric anesthesiologists, two pediatric intensivists | 30 Physicians: 15 pediatric intensivists, 15 pediatric anesthesiologists |
| Equipment |
18 gauge (18G) BD Insyte™ cannula (BD, Franklin Lakes)
|
|
|
|
|
|
|
RST:
SBT:
|
- Abbreviations: eFONA, emergency front-of-neck access, ID, inner diameter; RST, rapid sequence tracheostomy, SBT, scalpel bougie technique.
5 RECOMMENDED eFONA TECHNIQUES IN PEDIATRIC AIRWAY GUIDELINES
Little evidence exists regarding the performance of eFONA in a CICO situation in pediatric patients.13, 18-23, 29 Pediatric difficult airway management guidelines and algorithms focus on preventing a CICO situation and providing guidance for rescue maneuvers. For a considerable time, transcutaneous needle cricothyroidotomy has been the recommended preferred eFONA method for infants and children below 8 years of age.5, 30 The reason for choosing a transcutaneous technique assumes that anesthesiologists and nonsurgical specialists are more reluctant to perform a surgical procedure due to their limited surgical skills and have greater familiarity with Seldinger needle techniques, similar to central venous catheter placement.
Few pediatric unanticipated difficult airway guidelines have been published.4-7, 31 The UK-based Difficult Airway Society guidelines recommend seeking assistance from an ENT specialist who can perform a surgical tracheostomy or operate a rigid bronchoscopy as the initial step in such a situation.5 In these guidelines, in instances where an ENT specialist is not available, a percutaneous cannula cricothyroidotomy with trans-tracheal jet ventilation should be performed. If this approach fails, the last resource is a surgical cricothyroidotomy.5 Although the Association for Pediatric Anesthesia of Great Britain and Ireland (APAGBI) recommend percutaneous needle cricothyroidotomy5, 14 the 100% success rate in animals reported by Prunty et al.24 is in contrast to the 43% success rate reported in real life CICO-situations in adults11 and to two pediatric case reports that describe unsuccessful percutaneous cricothyroidotomy which was rescued by surgical tracheostomy.13, 14 With proper training the complication rate of the scalpel technique may be low.28
The All India Difficult Airway Association (AIDAA) guideline supports the surgical technique over the needle technique. However, they recommend tracheal needle puncture in children under 5 years of age if a surgeon is unavailable.6
The American Society of Anesthesiologists 2022 difficult airway guidelines for children recommend achieving an emergency invasive airway with any of the following techniques: surgical cricothyroidotomy, needle cricothyroidotomy if age-appropriate with a pressure-regulated device, large-bore cannula cricothyroidotomy, surgical tracheostomy, rigid bronchoscopy, or extracorporeal membrane oxygenation.4
In the recently published guidelines on neonatal and infant intubation, a clinical practice statement is made, that surgical tracheotomy should be performed when intubation fails, oxygenation and ventilation via a supraglottic device or face mask are severely impaired or impossible and spontaneous breathing cannot be restored.32, 33
The anatomical features of children should always be taken in consideration when an eFONA is going to be performed. Based on Navsa et al.,34 the mean dimensions of the cricoid membrane in neonates is 2.6 mm in height and 3.0 mm in length, indicating that a tracheal tube with an outer diameter exceeding 2.5 mm is too wide for the relatively narrow cricothyroid membrane, and may cause damage to the surrounding structures.34 This poses a challenge in implementing the above recommendations for cricothyrotomy, particularly in young children or for those children with craniofacial syndromes (i.e., Pierre Robin, Klippel-Feil, Down, etc). In addition, a recent study carried out on adolescents has shown that the cricothyroid membrane is smaller than previously thought even in that populations with the consequence that it might be difficult to introduce an ID 6.0 cannula.35
6 THE INFLUENCE OF HUMAN FACTORS ON PERFORMING eFONA
Dealing with airway emergencies and their outcomes means dealing with the “human factor”. This involves considering the impact of aspects of the individual team competencies, environment, processes, and culture on human performance.36 In addition, communication, teamwork, situation awareness, and leadership are key factors for success. CICO is one of the most stressful situations that a clinician can encounter. Stress can improve selective attention on a given task, but on the other hand it reduces situational awareness leading to underperforming, task fixation, and delay in decision-making to timely proceed to a front-of-neck access. These factors contribute significantly to morbidity and mortality.37 While in the emergency setting, activation of rehearsed responses with the trained equipment during simulation training and using cognitive aids can ease the deleterious effects of stress on the clinicians' performances.38, 39
CICO situations may often arise from lack of advanced planning, absence of second- and third- level strategies, deviation from algorithms, cognitive biases, poor communication, and not standardized or even unavailability of the proper equipment. Evidence should always guide the decision on when, what, and how perform a technique, especially in emergency situations. As preparation for the emergency reduces negative effects of human factors, addressing nontechnical skills might have much more impact on performance than the chosen access technique to the trachea.40, 41 For these reasons nontechnical skills in airway management should be an essential aspect of training.42, 43 Unfortunately, in children, eFONA is not performed at all or performed too late, because of the fear of harming a young patient. On the contrary, eFONA performed too early might cause severe and permanent anatomical and physiological damage; so may a tracheostomy performed late. Then, timing of eFONA performance must be chosen carefully but quickly and following the difficult airway algorithm and the emergency airway pathways.
Airway management including eFONA may be improved by the adoption and implementation of local practice guidelines and SOPs,44 use of preprocedural checklists45 and cognitive aids, collection of quality assurance data, and reporting of adverse events to registries.39 The American Society of Anesthesiologists 2022 difficult airway guidelines recommend considering a team debrief after all challenging airway encounter.4 They suggest identifying processes that worked well and opportunities for system improvement, and to provide emotional support to team members, particularly when there was patient harm.
7 NEW FRONTIERS AND ADVANCES IN eFONA: ULTRASOUND, ARTIFICIAL INTELLIGENCE, AND MACHINE LEARNING
Ultrasound has a role in training and anticipating difficulty in eFONA in an elective difficult airway. In fact, significant inaccuracy exists in locating the cricothyroid membrane in children of all ages by digital palpation.46 The ultrasound should not be used in an emergency setting but it has a potential use in premarking the incision site in the difficult airway management of an impalpable neck. This might be particularly true in detecting an infant's trachea, where soft tissues and small anatomical landmarks render surgical eFONA particularly prone to failure and complications, especially in the emergency setting.
Artificial intelligence (AI) is a broad term indicating a computer or machine that can achieve human-like goals using technology.47 Machine learning (ML) is one of the main field of AI; it uses pattern recognition and computational learning theory to recognize complex nonlinear relationships that exist among independent and dependent variables.48 This can be applied to images or video to analyze and derive predictions as in the so-called Machine Vision (MV).
A core problem in eFONA execution in children is the known inaccuracy of cricothyroid membrane identification by digital palpation.46 This can be addressed with the use of ultrasound: two techniques, transverse and longitudinal, have been described to identify the cricothyroid membrane49; in both, a skilled operator is needed, especially in time-dependent scenarios.50 Unfortunately, ultrasound skills are not evenly spread.51 Machine vision is an established domain in medicine52, 53; in anesthesia it is used in ultrasound techniques for regional anesthesia for helping nerve and vessels recognition.54, 55 Machine vision models are rising in the airway field too, for difficult intubation prediction56 and assistance in recognizing airway anatomy during intubation.57, 58 Machine vision in ultrasound for eFONA could help to develop models to recognize the cricothyroid membrane, hastening the procedure, and improving safety.
Another crucial point in eFONA performance is the decision-making process that is often delayed or absent.11 It is noted that under critical scenarios time's perception is altered.59 Several anesthesiologists59, 60 have advocated for a monitor or timer use in DA scenarios to not lose the time frame. The great potential of machine learning in monitoring has already led to the development of perioperative hypoxia prediction models61; in future, a possible direction could be the development of age-tailored alarms of critical hypoxia events that make declaration of the minutes left above a critical threshold of SpO2. In FONA, this could speed up and ease the transition between CICO and securing a neck access.
8 CURRENT TRAINING AND ROLE OF 3D PRINTING FOR PEDIATRIC AIRWAY MODELS
Airway practitioners caring for children need to be prepared to face CICO situations, remembering that they can happen not only at induction of general anesthesia but also—and possibly even more dangerously at extubation.62
Training for difficult airway management should regularly be planned in multidisciplinary teams through simulation-based training.63 In general, there is strong evidence on the efficacy of simulation-based training for invasive airway access.64
Training in performing eFONA in children poses a challenge. The use of rabbit cadaver training models might be limited by legislation regarding the manipulation of animal bodies, which broadly varies worldwide, rendering this specific training not always feasible or at least not with the same universal standards.
Simulation on manikins is an established way of training.65 However, the traditional models don't reflect accurately the anatomy of humans66, 67 and they lack of haptic qualities, lowering the simulation fidelity.68 This is particularly true for eFONA, that holds the issues of simulating a surgical procedure.69
3D printing, that is, the construction of a three-dimensional object from a Computer-Aided Design or a digital 3D model,70 is a growing field in medical models production.71 The greatest advantage of this technique is the possibility to produce high-resolution, accurate prints based on data acquired by actual CT scans. Specifically, for eFONA, a promising model has been developed by Weatherall et al.72 Based on a single dataset of a 21-month-old subject CT scan, the model stands out for the precise tactile qualities and the capability of replacing individual components easily and at relatively low cost, making it a practical and durable training option.
Moreover, as demonstrated by Chao et al.,73 setting up a 3D printing working group in a hospital is an affordable and feasible option. The same group has also produced high-fidelity and high-quality training phantoms for FONA. The printing data for models could be shared among centers. The spread of 3D printing could make training for eFONA and skill maintenance easily accessible, highly effective, comparable, and affordable worldwide (Figure 1). Consistent training should be made accessible to all staff members who may encounter a CICO situation, irrespective of their medical specialization or level of expertise.25 Given the notably steep learning curve observed during the initial four attempts, a minimum of four to five consecutive eFONA procedures should be performed during each training sessions.25
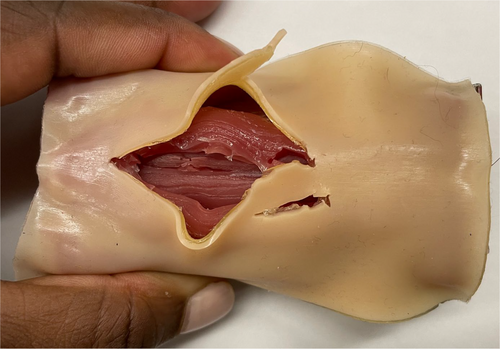
There is currently little evidence-based guidance on the optimal frequency of such training sessions to maintain practical skills, which seems to advocate a brush-up training after 3 months.74 The training regimen should not solely focus on the technical execution of the eFONA technique but also include comprehensive team-based training that covers team competencies like advanced planning, communication, decision-making, and procedural efficacy, addressing various aspects to mitigate the impact of the human factor.31, 36, 38, 39
9 PERSPECTIVES ON TECHNICAL ASPECTS OF eFONA
In the absence of definitive evidence based on human data, the selection of the preferred eFONA method for pediatric cases falls upon the individual institutions. From our perspective and based on the available evidence on animal studies, the scalpel bougie technique should be preferred in children under 8 years of age. Where available, the use of a Frova Intubating Introducer not only facilitates safe tracheal tube insertion, but also offers the advantage of initial possible oxygenation through the catheter using devices based on the Bernoulli principle and its variant, the Venturi effect, in cases where tube placement is challenging. A device applying the Venturi effect provides full ventilation in an obstructed airway situation, without the risk on air trapping and barotrauma. In this situation, bag mask ventilation and jet ventilation should be avoided.75
Moreover, the current cut-off of 8 years of age between surgical and percutaneous tracheostomy should be considered with caution, since it has been chosen upon previously published recommendations based on expert opinion, with age limit only indicative. The SBT procedure's resemblance to recommended surgical cricothyroidotomy in adults adds a sense of familiarity and may help reduce initial hesitation. Conversely, for children aged 8 and above, adherence to the DAS guideline is advised,31 meaning that a surgical cricothyroidotomy should be considered as first choice (Figure 2). To stabilize the trachea and identify the anatomical structures a laryngeal handshake can be used. The laryngeal handshake is performed with the nondominant hand, identifying the hyoid and thyroid laminae, stabilizing the larynx between thumb and middle finger, and moving down the neck to palpate the cricothyroid membrane with the index finger.76

It is important to note that each of the technique of eFONA are temporary airways that will require a formal tracheostomy by ENT surgeons performed in an operating room. During transfer, care should be taken to ensure the temporary eFONA access is not lost. We recommend a dedicated person for ensuring access is maintained during transfer or any interventions including cardiopulmonary resuscitation.
10 LIMITATIONS
The present review has several limitations, mainly due to the rarity of a CICO situation in children, and unidentified risk factors. Moreover, assumptions derive from studies performed in simulation and laboratory settings with animal models or mannikins. Translating findings from simulation and laboratory setting to a clinical setting is not a straightforward process. Finally, the pediatric age includes a wide age span, from neonates to infants and adolescents, all with different anatomical and physiological features; particularly for neonates and infants, effort to provide stronger evidence of the best clinical practices in CICO situation are still needed since current evidence is inconclusive. Even the recent published airway management guidelines in neonates and infants suggest a surgical approach leaving open which is the best technique.32, 33 Nevertheless, we have used the most recent evidence and literature to suggest possible clinical guidance, particularly on the surgical tracheostomy techniques. This is still a controversial area considering that several guidelines still recommend a needle tracheotomy as first choice.5, 30
11 CONCLUSION
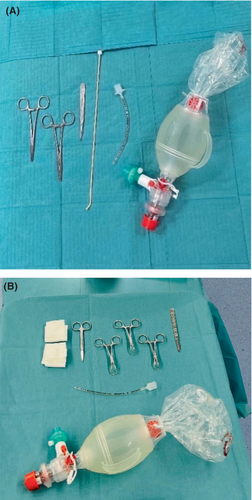
In situations where a Can't Intubate, Can't Oxygenate (CICO) scenario occurs, particularly in pediatric patients, practitioners must be ready, despite its rarity. Much of the current understanding comes from experimental animal studies, making its translation into clinical practice complex. Notably, children have varying physiological and anatomical features across different age groups, making a universal approach challenging. Despite a lack of human-derived evidence, each institution is tasked with selecting their preferred emergency front-of-neck access method. From our perspective, the use of a Frova Intubating Introducer should be recommended for children under 8 years. For those 8 years and older, a surgical cricothyroidotomy, in line with the DAS guidelines, is recommended. Regular and comprehensive training for all medical staff is crucial, with a focus not only on the technical aspects but also on team dynamics and decision-making. A CICO scenario should be clearly communicated, the attempt to oxygenate the patient by bag-mask ventilation, supraglottic airway device, or apneic oxygenation with high-flow oxygen should not be neglected at any time during the crisis and the necessary equipment should be accessible and standardized. The prompt availability of the necessary instruments and the setting in which the airway equipment is presented is crucial4, 44 (Figure 3). Dealing with a pediatric eFONA event is undeniably stressful, but proper preparation can bolster clinician confidence, ultimately improving patient outcomes.
KEY LEARNING POINTS
- A ‘can't intubate can't oxygenate’ situation is a rare but life-threatening situation and requires immediate intervention.
- Regular and repeated training is necessary to provide and maintain minimal skills on emergency front-of-neck access in children.
- This review concludes that surgical interventions, especially the scalpel bougie tracheostomy, have shown superiority in animal studies and could be considered as first choice in younger children under 8 years of age.
ACKNOWLEDGMENT
Open access funding provided by BIBLIOSAN.
CONFLICT OF INTEREST STATEMENT
Authors declare no COI. ND and CM are members of the Editorial Board of the Pediatric Anesthesia Journal.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




