A randomized, double-blind, dose-controlled study of the use of dexmedetomidine alone for procedural sedation of children and adolescents undergoing MRI scans
Section Editor: Brian J Anderson.
Abstract
Background
Dexmedetomidine is a selective α2-adrenergic agonist originally approved for sedation of adults in the intensive care unit and subsequently approved for procedural sedation in adults undergoing medical procedures. Dexmedetomidine is widely used off-label for procedural sedation in children.
Aims
To evaluate efficacy and safety of monotherapy dexmedetomidine for magnetic resonance imaging procedural sedation of children ≥1month–<17years across three ascending doses.
Methods
Randomized, double-blind, dose-ranging study of procedural sedation recruited patients at USA and Japanese sites from February 2020 to November 2021. Patients were stratified into Cohort A (≥1month–<2years) or Cohort B (≥2–<17years). Cohort A loading doses/maintenance infusions: 0.5 mcg/kg/0.5 mcg/kg/h, 1.0 mcg/kg/1.0 mcg/kg/h, and 1.5 mcg/kg/1.5 mcg/kg/h. Cohort B loading doses/maintenance infusions: 0.5 mcg/kg/0.5 mcg/kg/h, 1.2 mcg/kg/1.0 mcg/kg/h, and 2.0 mcg/kg/1.5 mcg/kg/h. Primary endpoint was percentage of overall patients completing MRI without rescue propofol at the high versus low dose. Key secondary endpoint was percentage in each age cohort who did not require propofol at the high versus low dose.
Results
One hundred twenty-two patients received high- (n = 38), middle- (n = 42), or low-dose (n = 42) dexmedetomidine. A greater percentage completed MRI without propofol rescue, while receiving high- versus low-dose dexmedetomidine (24/38 [63.2%] vs. 6/42 [14.3%]) (odds ratio: 10.29, 95% confidence interval: 3.47–30.50, p < .001). Similar results were seen in both age cohorts. The most common adverse events were bradypnea, bradycardia, hypertension, and hypotension, and the majority were of mild-to-moderate severity.
Conclusions
Dexmedetomidine was well tolerated. The high dose was associated with meaningfully greater efficacy compared with lower doses. Based on these results, the recommended starting dose for procedural sedation in children ≥1month–<2years is loading dose 1.5 mcg/kg/maintenance infusion 1.5 mcg/kg/h; children ≥2–<17years is loading dose 2.0 mcg/kg/maintenance infusion 1.5 mcg/kg/h.
1 INTRODUCTION
Dexmedetomidine, a highly selective α2-adrenergic agonist, exhibits sedative, anxiolytic, and analgesic effects with minimal respiratory depression.1 Originally FDA approved for adult patients in the intensive care unit (ICU), dexmedetomidine subsequently was approved for procedural sedation in adults2 and has wide-spread off-label use for procedural sedation in children.
The present study was conducted to fulfill an outstanding post-marketing regulatory commitment following the approval of dexmedetomidine for procedural sedation in adults. The purpose was to provide controlled, dose-ranging data that could support the addition of an indication and recommended dose regimen for pediatric procedural sedation to the United States Prescribing Information.
Numerous observational, uncontrolled, unblinded studies of dexmedetomidine for pediatric procedural sedation have appeared, using various dosage levels and regimens, frequently in combination with other sedatives.3 The present study was a fully powered, randomized, controlled, double-blind, dose-ranging, multicenter study designed to provide data on the efficacy and safety of monotherapy dexmedetomidine across a range of ascending dose levels (low, middle, high) for procedural sedation in pediatric patients ≥1 month to <17 years of age undergoing magnetic resonance imaging (MRI) scans.
The primary objective was to assess the efficacy of dexmedetomidine monotherapy for pediatric procedural sedation as measured by the overall percent of patients at the high-dose versus low-dose level, who did not require concomitant propofol to achieve adequate sedation.
2 METHODS
This randomized clinical trial (ClinicalTrials.gov: NCT04237792) was conducted at 21 sites in the USA and Japan from February 2020 to November 2021. This study was conducted in compliance with all ethical principles of the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice guidelines and was approved by the Institutional Review Board or independent Ethics Committee at each participating institution. Informed consent was received from each patient's parent or guardian before any treatment was given. Patient assent was also obtained where appropriate for children and adolescents under the age of majority according to local institutional guidelines.
2.1 Inclusion and exclusion criteria
Key inclusion criteria were: male or female ≥1 month and < 17 years of age; American Society of Anesthesiologists Physical Status I, II, or III; required non-intubated, spontaneous breathing, moderate to deep sedation for an MRI study with an intensivist, anesthesiologist, or other qualified proceduralist. The MRI scan was expected to take ≥20 min up to 3 h. Key exclusion criteria were: pregnant/breastfeeding female patients; weight on Day 1 before randomization <10th percentile of weight for age and sex in the USA and Japan, >95th percentile of weight for age and sex in the USA, or >97th percentile of weight for age and sex in Japan; planned medical procedure during the MRI scan or post-MRI recovery period; and baseline SpO2 < 93% on room air.
2.2 Dose selection rationale
At the time of this study, no pediatric pharmacokinetic/pharmacodynamic (PK/PD) model relating plasma concentration of dexmedetomidine to target sedation for children undergoing procedural sedation was available. Therefore, selection of the dosing regimen was based on: (1) recommended doses for procedural sedation in adults2; (2) dosing experience and results of a previous open-label pediatric procedural sedation safety study4; (3) PK data from adult and pediatric ICU patients; and (4) simulated PK profiles at the projected doses for the current study based on a population PK model developed from pediatric ICU patients (Pfizer data on file).
Dose selection ranged from an expected minimally effective dose to maximum doses, intended to be representative of current clinical usage.5-7
Lower loading doses (LDs) were selected for patients <2 years old because of a potential increased risk of respiratory insufficiency in this group.5 In addition, due to the risk that a shorter duration and/or higher LD may be associated with increased adverse events (AEs),8 a conservative 10-min infusion interval was used for the dexmedetomidine LD, consistent with the current United States Prescribing Information for adults.2
Patients were stratified by two age groups: ≥1 month to <2 years (Cohort A) and ≥2 to <17 years (Cohort B); each group received dexmedetomidine at three ascending dose levels (Table 1).
| Dose level | Dexmedetomidine loading dose (mcg/kg) | Dexmedetomidine maintenance infusion dose (mcg/kg/h) |
|---|---|---|
| Cohort A (≥1 month to <2 years of age) | ||
| Low-dose level | 0.5 | 0.5 |
| Middle-dose level | 1.0 | 1.0 |
| High-dose level | 1.5 | 1.5 |
| Cohort B (≥2 years to < 17 years of age) | ||
| Low-dose level | 0.5 | 0.5 |
| Middle-dose level | 1.2 | 1.0 |
| High-dose level | 2.0 | 1.5 |
2.3 Treatment
This study included: screening, Day 1 (MRI scan and post-MRI recovery), Day 2 follow-up, and Day 29 long-term follow-up visits. Patients were randomized 1:1:1 to receive dexmedetomidine at the three dosing levels in each cohort.
Dexmedetomidine (Precedex™ [hydrochloride injection concentrate], Hospira, Inc. Lake Forest, IL) was provided by Pfizer, Inc. as 200 mcg/2 mL (100 mcg/mL) vials for IV infusion following dilution of the concentrate. Each vial was diluted by a third-party unblinded pharmacist. All patients of a specific weight received the same infusion rate regardless of dose to ensure the investigator remained blinded.
Dexmedetomidine dose adjustments were not permitted but could be discontinued for safety reasons at the discretion of the investigator. Treatment included an IV dexmedetomidine LD administered over 10 min immediately followed by an IV maintenance infusion (MI) throughout the procedure. Rescue propofol was available if adequate sedation was not achieved within 5 min after the start of the dexmedetomidine MI. The target sedation level was indicated by a score of two on the Pediatric Sedation State Scale (PSSS), defined as: quiet (asleep or awake), not moving during procedure, no frown (or brow furrow) indicating pain or anxiety, no verbalization of any complaint.9
Rescue propofol was administered as an initial 0.5 mg/kg bolus followed by 50 mcg/kg/min MI. Additional boluses of 0.5 mg/kg could be administered to reach the target sedation level, with a simultaneous increase in the MI rate of 25 or 50 mcg/kg/min after each bolus.
2.4 Outcome measures
2.4.1 Efficacy
The primary efficacy endpoint was the overall percentage of patients at the dexmedetomidine high-dose versus low-dose level, who did not require concomitant propofol. The key secondary efficacy endpoint was the percentage in the high- versus low-dose group in each age cohort that did not require concomitant propofol.
Additional secondary efficacy endpoints included: (1) percentage of time at the target PSSS score during the dexmedetomidine MI; (2) time from the start of the dexmedetomidine LD infusion to first propofol bolus administration; (3) total dose (mg/kg) of propofol required to complete the MRI scan at each dose level; and (4) emergence time (defined as the time to achieve a modified Aldrete score ≥9 post-MRI scan).10
In addition, the percentage of patients at the dexmedetomidine middle- compared with the high- and low-level who did not require concomitant propofol was examined.
2.4.2 Safety
Safety was assessed by the incidence, seriousness, and severity of treatment-emergent AEs.
Changes in blood pressure (BP), heart rate (HR), respiratory rate (RR), end tidal CO2 (EtCO2), and O2 saturation (SpO2) exceeding pre-specified limits or thresholds were recorded as AEs regardless of study discontinuation or medical intervention (Table 2).
| Adverse event | Reporting criteria |
|---|---|
| Bradycardia11 |
|
| Systolic hypotension (SBP) |
|
| Hypertension |
|
| Respiratory rate11 |
|
| SpO2 |
|
| Capnography |
|
- Note: Baseline values are the pre-study drug infusion values.
- Abbreviations: AEs, adverse events; DBP, diastolic blood pressure; ETCO2, end tidal CO2; RR, respiratory rate; SBP, systolic blood pressure; SpO2, O2 saturation.
Additional safety assessments included the percentage of patients who required mechanical ventilation or received an intervention to restore normal hemodynamic status, the incidence of protocol-specified AEs, including the incidence of protocol-specified AEs requiring intervention, and the number of withdrawal-related AEs.
All decisions to implement treatment or intervention for AEs were based solely on the clinical judgment of the investigator. If any protocol-specified AE did not respond to standard treatment, dexmedetomidine was to be discontinued and the patient treated as clinically indicated.
Mean change from baseline in vital signs (systolic BP [SBP], diastolic BP [DPB], mean arterial pressure [MAP], HR, and RR) and percentage of time outside of the stable range for hemodynamic parameters of SBP and HR (defined as maintenance of both SBP and HR within 30% of baseline) during treatment were monitored.
The Pediatric Anesthesia Emergence Delirium scale was used to assess patients for emergence delirium during recovery.14
2.5 Hypothesis and statistical analysis methods
The hypothesis was whether the dexmedetomidine high-dose level would be considered superior to the low-dose level in patients who do not require concomitant propofol to complete the MRI.
Power analysis indicated that a sample size of 40 patients at each dose level would provide 99% power in a two-sided test with α = 0.05 to detect a minimum significant difference of 35% in the primary efficacy endpoint, based on a Mantel–Haenszel test of proportions.
Data analysis was conducted on all treated patients. Primary and key secondary efficacy endpoints were analyzed using the Mantel–Haenszel test and odds ratios (95% CI). A post hoc Cochran–Armitage test was performed to test for a linear relationship among the three dose groups in the combined sample and each age cohort. The percentage of time at the target sedation scale was assessed using the Wilcoxon test with Hodges–Lehmann estimation of 95% CI. The amount of time from the start of the dexmedetomidine LD to the first propofol bolus administration was summarized using the Kaplan–Meier method and log-rank test. A Cox proportional model was used to estimate the treatment effect size with hazard ratios and 95% CI for treatment group pairs. Emergence time was summarized using the Kaplan–Meier method and log-rank test of homogeneity. The total amount and weight and time-adjusted amount of concomitant propofol was assessed using analysis of variance. Descriptive statistics (mean, SD, median, interquartile range [IQR], proportions) were used for safety analyses. Statistical analyses were performed using SAS, v9.2. A p value <.05 was considered statistically significant.
3 RESULTS
3.1 Patients
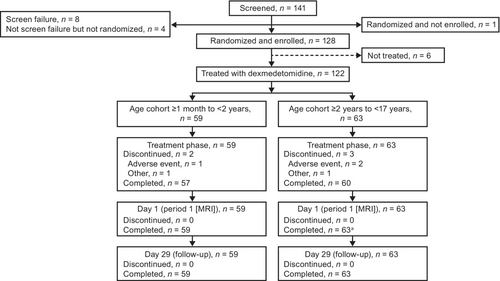
Of 141 patients screened, 128 were enrolled and randomized in the study. In total, 122 patients received treatment with dexmedetomidine at either the high-dose (n = 38; 18 and 20 in Cohort A and Cohort B, respectively), middle-dose (n = 42; 21 and 21, respectively), or low-dose (n = 42; 20 and 22, respectively) levels (Figure 1). Patient demographics were generally similar across both cohorts and all dosage groups (Table 3). Information on number of patients enrolled at each site and a description of Protocol Training, Source Data Verification/Review, and Site Monitoring procedures are provided in Table S1 and Appendix S1.
| Low dose (n = 42) | Middle dose (n = 42) | High dose (n = 38) | Total (N = 122) | |||||
|---|---|---|---|---|---|---|---|---|
| ≥1 month–<2 years (n = 20) | ≥2–<17 years (n = 22) | ≥1 month–<2 years (n = 21) | ≥2–<17 years (n = 21) | ≥1 month–<2 years (n = 18) | ≥2–<17 years (n = 20) | ≥1 month–<2 years (n = 59) | ≥2–<17 years (n = 63) | |
| Age, years | ||||||||
| Mean ± SD | 0.99 ± 0.612 | 6.68 ± 2.922 | 0.90 ± 0.488 | 7.40 ± 3.230 | 0.96 ± 0.384 | 6.63 ± 3.277 | 0.95 ± 0.499 | 6.90 ± 3.110 |
| Gender, n (%) | ||||||||
| Male | 8 (40.0) | 11 (50.0) | 12 (57.1) | 11 (52.4) | 10 (55.6) | 11 (55.0) | 30 (50.8) | 33 (52.4) |
| Female | 12 (60.0) | 11 (50.0) | 09 (42.9) | 10 (47.6) | 08 (44.4) | 09 (45.0) | 29 (49.2) | 30 (47.6) |
| Race, n (%) | ||||||||
| Black or African American | 02 (10.0) | 0 | 1 (4.8) | 2 (9.5) | 1 (5.6) | 02 (10.0) | 4 (6.8) | 4 (6.3) |
| Asian | 03 (15.0) | 11 (50.0) | 8 (38.1) | 08 (38.1) | 4 (22.2) | 07 (35.0) | 15 (25.4) | 26 (41.3) |
| White | 010 (50.0) | 10 (45.5) | 8 (38.1) | 09 (42.9) | 11 (61.1) | 09 (45.0) | 29 (49.2) | 28 (44.4) |
| Weight, kg | ||||||||
| Mean ± SD | 8.96 ± 2.395 | 25.30 ± 13.376 | 9.08 ± 2.140 | 26.04 ± 11.436 | 9.32 ± 1.382 | 24.36 ± 10.172 | 9.11 ± 2.010 | 25.25 ± 11.618 |
- Abbreviation: SD, standard deviation.
3.2 Primary and key secondary efficacy measures
For the high-dose group, 24/38 (63.2%) patients in the combined sample completed the MRI scan without concomitant propofol versus 6/42 (14.3%) patients in the low-dose group (odds ratio: 10.29, 95% CI: 3.47–30.50, p < .001) (Table 4). Similar results were seen in both Cohort A (50.0% vs. 15.0% [odds ratio: 5.67, 95% CI: 1.22–26.33, p = .022]) and Cohort B (75.0% vs. 13.6% [odds ratio: 19.00, 95% CI: 3.90–92.56, p < .001]) (Table 4).
| Age Cohort | Low dose | Middle dose | High dose | p values(a) | High dose versus low dose | High dose versus middle dose | Middle dose versus low dose | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Test statistic(b) (95% CI) | p values | Test statistic(b) (95% CI) | p values | Test statistic(b) (95% CI) | p values | |||||
| Percent of patients who did not require concomitant propofol to complete MRI†, n/N (%) | ||||||||||
| Overall | 6/42 (14.3) | 15/42 (35.7) | 24/38 (63.2) | <.001 | 10.29 (3.47, 30.50) | <.001 | 3.09 (1.24, 7.69) | .015 | 3.33 (1.14, 9.72) | .024 |
| Cohort A | 3/20 (15.0) | 2/21 (9.5) | 9/18 (50.0) | .013 | 5.67 (1.22, 26.33) | .022 | 9.50 (1.69, 53.33) | .006 | 0.60 (0.09, 4.01) | .597 |
| Cohort B | 3/22 (13.6) | 13/21 (61.9) | 15/20 (75.0) | <.001 | 19.00 (3.90, 92.56) | <.001 | 1.85 (0.48, 7.06) | .374 | 10.29 (2.29, 46.25) | .001 |
| Percentage of time at target sedation rating scale score (PSSS) of 2 during the dexmedetomidine maintenance infusion‡, mean ± SD | ||||||||||
| Overall | 77.2 ± 17.04 | 87.2 ± 11.88 | 91.1 ± 18.71 | 15.75 (8.93, 21.95) | <.001 | 6.41 (0.00, 10.34) | .011 | 9.37 (2.05, 16.07) | .004 | |
| Cohort A | 77.5 ± 15.94 | 82.5 ± 12.04 | 87.8 ± 23.93 | 12.07 (3.87, 22.32) | .007 | 9.37 (1.78, 16.67) | .021 | 3.88 (−3.44, 12.08) | .225 | |
| Cohort B | 76.8 ± 18.36 | 92.2 ± 9.72 | 94.1 ± 12.22 | 17.24 (7.74, 29.38) | <.001 | 0.00 (0.00, 6.49) | .213 | 15.10 (1.12, 25.00) | .006 | |
| Time from the start of dexmedetomidine loading dose infusion to the time of first propofol bolus infusion§, median (% censored) | ||||||||||
| Overall | 16.0 (14.3) | 31.5 (35.7) | N/A (63.2) | <.001 | 4.25 (2.19, 8.23) | <.001 | 2.09 (1.09, 3.99) | .020 | 1.93 (1.15, 3.22) | .005 |
| Cohort A | 16.0 (15.0) | 17.0 (9.5) | 62.0 (50.0) | .008 | 3.01 (1.27, 7.13) | .005 | 2.51 (1.13, 5.62) | .016 | 1.32 (0.67, 2.60) | .368 |
| Cohort B | 16.5 (13.6) | N/A (61.9) | N/A (75.0) | <.001 | 7.47 (2.45, 22.81) | <.001 | 1.68 (0.55, 5.14) | .358 | 3.43 (1.46, 8.08) | .001 |
| Amount of concomitant propofol required to successfully complete MRI (mg/kg)¶, mean ± SD | ||||||||||
| Overall | 4.83 ± 3.390 | 4.81 ± 3.539 | 3.43 ± 2.973 | −1.40 (−3.48, 0.68) | .181 | −1.38 (−3.62, 0.85) | .218 | −0.02 (−1.78, 1.74) | .984 | |
| Cohort A | 4.01 ± 2.335 | 4.73 ± 3.398 | 2.82 ± 1.594 | −1.19 (−2.99, 0.61) | .186 | −1.91 (−4.37, 0.56) | .124 | 0.72 (−1.28, 2.72) | .470 | |
| Cohort B | 5.56 ± 4.039 | 5.00 ± 4.039 | 4.51 ± 4.621 | −1.05 (−5.37, 3.28) | .621 | −0.49 (−5.88, 4.90) | .845 | −0.56 (−4.08, 2.96) | .747 | |
| Emergence time††, median (IQR) | ||||||||||
| Overall | 35.0 (49.0) | 42.5 (32.0) | 45.5 (28.0) | .117 | ||||||
| Cohort A | 30.0 (52.5) | 41.0 (21.0) | 38.0 (43.0) | .591 | ||||||
| Cohort B | 35.0 (32.0) | 46.0 (43.0) | 50.0 (27.5) | .207 | ||||||
- Abbreviations: HR, hazard ratio; IQR, interquartile range; MRI, magnetic resonance imaging; N/A, estimate not applicable due to the large proportion of censored patients; OR, odds ratio; SD, standard deviation.
- † Results were numbers (percentages) in each dose level; (a) p values were from Cochran-Armitage Trend Test; (b) statistical measurements were OR and Mantel–Haenszel test.
- ‡ Results were mean ± SD in % units at each dose level; (b) statistical measurements were location shift Hodges-Lehmann Estimation and Wilcoxon test.
- § Results were estimates of median survival time in minutes (percentages censored) at each dose level; (a) p values from the log-rank test of dose group homogeneity; (b) statistical measurements were HR based on the Cox Proportional Model Estimate and log-rank test for homogeneity.
- ¶ Results were mean ± SD in mg/kg unit at each dose level; (b) statistical measurements were mean difference and analysis of variance for pairwise comparison.
- †† Results were estimates of median survival time in minutes (IQR) at each dose level, with no censored data; (a) p values were from the log-rank test of dose group homogeneity.
A linear relationship was noted in the percentage of patients who did not require concomitant propofol among the three dexmedetomidine dose groups by a post hoc Cochran–Armitage Trend test in the combined sample (p < .001) and both Cohort B (p < .001) and Cohort A (p = .013).
3.3 Secondary efficacy measures
The percentage of time at the target PSSS score increased with increasing dexmedetomidine dose in the combined and separate age cohorts (Table 4). In the combined sample, patients were at the target sedation scale score for a mean time of 91.1% (SD 18.71) at the high-dose level. Moreover, the percentage of time at the target sedation level was higher in the high-dose versus low-dose level in the combined sample (location shift: 15.75%, 95% CI: 8.93–21.95, p < .001) and each age cohort (Cohort A: shift 12.07%, 95% CI: 3.87–22.32, p = .007; Cohort B: shift 17.24%, 95% CI: 7.74–29.38, p < .001). Comparisons of the high versus middle and middle versus low doses gave mixed results (Table 4).
The length of time to the first propofol bolus increased with increasing dexmedetomidine dose (Table 4, Figure S1). The time to the first propofol dose was greater in the high- versus low-dose group in the combined sample and in both age cohorts (combined sample: hazard ratio: 4.25, 95% CI: 2.19–8.23, p < .001; Cohort A: hazard ratio: 3.01, 95% CI: 1.27–7.13, p = .005; Cohort B: hazard ratio: 7.47, 95% CI: 2.45–22.81, p < .001) (Table 4).
In patients who required propofol, there was no evidence of differences in the amount of propofol given between the three dose levels in either cohort. There also was no evidence of differences in emergence time among the three dose levels in either age cohort (Table 4).
3.4 Exposure and safety
In Cohort A, the median (IQR) total dexmedetomidine dose on a weight-adjusted basis was 2.59 (1.04) mcg/kg, 2.01 (0.43) mcg/kg, and 0.88 (0.17) mcg/kg, in the high-, middle-, and low-dose groups, respectively. The median (IQR) duration of treatment in the three dose groups was 52.5 (40.0) min, 69.0 (24.0) min, and 56.0 (20.5) min, respectively.
In Cohort B, the median (IQR) total dexmedetomidine dose on a weight-adjusted basis was 3.19 (1.35) mcg/kg, 2.03 (0.45) mcg/kg, and 0.88 (0.18) mcg/kg, in the high-, middle-, and low-dose groups, respectively. The median (IQR) duration of treatment in the three dose groups was 57.5 (50.0) min, 66 (28.0) min, and 56.5 (20.0) min, respectively.
3.5 Adverse events
The overall proportion of patients with any AE was comparable across the three dose levels, ranging from 90.5% at the low dose to 94.7% at the high dose (Table 5). The majority (96.5%) of AEs were mild in severity, with the remainder moderate (Table 5). No severe AEs occurred. Only 5.7% (7/122) of patients experienced AEs requiring intervention. Interventions given for protocol-specified AEs included: lactated Ringer's solution bolus for hypotension (two patients); atropine for bradycardia (one patient); and glycopyrrolate for bradycardia and hydralazine for hypertension (in the one patient with a serious AE). Supplemental oxygen was allowed on a prophylactic basis (and was given to 41 patients) but only three patients received oxygen to treat AEs of bradypnea and/or hypoxia.
| AE, n (%) | Low dose (n = 42) | Middle dose (n = 42) | High dose (n = 38) | Total (N = 122) |
|---|---|---|---|---|
| Patients evaluable for AEs, n | 42 | 42 | 38 | 122 |
| Number of AEs, n | 102 | 96 | 91 | 289 |
| Patients with AEs | 38 (90.5) | 39 (92.9) | 36 (94.7) | 113 (92.6) |
| Patients with SAEsa | 0 | 0 | 1 (2.6) | 1 (0.8) |
| Patients with severe AEsb | 0 | 0 | 0 | 0 |
| Patients discontinued from study due to AEsc | 0 | 0 | 0 | 0 |
| Patients discontinued study drug due to AE and continue studyd | 1 (2.4) | 1 (2.4) | 1 (2.6) | 3 (2.5) |
| Cardiac disorders | 26 (61.9) | 24 (57.1) | 27 (71.1) | 77 (63.1) |
| Bradycardia | 24 (57.1) | 24 (57.1) | 27 (71.1) | 75 (61.5) |
| Rebound tachycardia | 0 | 0 | 1 (2.6) | 1 (0.8) |
| Sinus arrhythmia | 0 | 0 | 1 (2.6) | 1 (0.8) |
| Tachycardia | 3 (7.1) | 1 (2.4) | 1 (2.6) | 5 (4.1) |
| GI disorders | 1 (2.4) | 3 (7.1) | 1 (2.6) | 5 (4.1) |
| Abdominal pain | 0 | 1 (2.4) | 0 | 1 (0.8) |
| Nausea | 0 | 1 (2.4) | 1 (2.6) | 2 (1.6) |
| Vomiting | 1 (2.4) | 1 (2.4) | 0 | 2 (1.6) |
| Infections and infestations | 2 (4.8) | 0 | 0 | 2 (1.6) |
| Nasopharyngitis | 2 (4.8) | 0 | 0 | 2 (1.6) |
| Injury, poisoning, and procedural complications | 0 | 1 (2.4) | 2 (5.3) | 3 (2.5) |
| Anesthetic complication neurologicale | 0 | 1 (2.4) | 1 (2.6) | 2 (1.6) |
| Contusion | 0 | 0 | 1 (2.6) | 1 (0.8) |
| Investigations | 1 (2.4) | 0 | 0 | 1 (0.8) |
| Blood pressure increased | 1 (2.4) | 0 | 0 | 1 (0.8) |
| Nervous system disorders | 1 (2.4) | 0 | 0 | 1 (0.8) |
| Seizure | 1 (2.4) | 0 | 0 | 1 (0.8) |
| Psychiatric disorders | 0 | 0 | 1 (2.6) | 1 (0.8) |
| Agitation | 0 | 0 | 1 (2.6) | 1 (0.8) |
| Respiratory, thoracic, and mediastinal disorders | 34 (81.0) | 27 (64.3) | 22 (57.9) | 83 (68.0) |
| Bradypnea | 33 (78.6) | 27 (64.3) | 22 (57.9) | 82 (67.2) |
| Hypoxia | 6 (14.3) | 3 (7.1) | 1 (2.6) | 10 (8.2) |
| Tachypnea | 1 (2.4) | 0 | 0 | 1 (0.8) |
| Skin and subcutaneous tissue disorders | 1 (2.4) | 0 | 0 | 1 (0.8) |
| Rash | 1 (2.4) | 0 | 0 | 1 (0.8) |
| Vascular disorders | 23 (54.8) | 26 (61.9) | 27 (71.1) | 76 (62.3) |
| Diastolic hypertension | 3 (7.1) | 3 (7.1) | 4 (10.5) | 10 (8.2) |
| Diastolic hypotension | 1 (2.4) | 1 (2.4) | 0 | 2 (1.6) |
| Hypertension | 11 (26.2) | 17 (40.5) | 18 (47.4) | 46 (37.7) |
| Hypotension | 13 (31.0) | 11 (26.2) | 6 (15.8) | 30 (24.6) |
| Systolic hypertension | 1 (2.4) | 5 (11.9) | 3 (7.9) | 9 (7.4) |
| Withdrawal hypertension | 0 | 0 | 1 (2.6) | 1 (0.8) |
- Note: Patients are only counted once per treatment per event. Totals for the number of patients at a higher level are not necessarily the sum of those at the lower levels since a patient may report two or more different AEs within the higher-level category. Includes all data collected since the first dose of study drug. MedDRA v24.1 coding dictionary applied.
- Abbreviations: AE, treatment-emergent adverse event; GI, gastrointestinal; SAE, serious adverse event.
- a Serious AEs were defined as an event that resulted in death; was life-threatening (immediate risk of death); required inpatient hospitalization or prolongation of existing hospitalization; resulted in persistent or significant disability/incapacity (substantial disruption of the ability to conduct normal life functions); resulted in congenital anomaly/birth defect; or that was considered to be an important medical event.
- b Severe AEs were defined as an event that interfered significantly with patient's usual function.
- c Patients who have an AE record that indicates that the AE caused the patient to be discontinued from the study.
- d Patients who have an AE record that indicates that the action taken with study treatment was withdrawal of the study drug, but AE did not cause the patient to be discontinued from study.
- e Refers to emergence delirium.
Four patients experienced AEs of moderate severity: one in the low-dose Cohort A group (hypoxia), one in the low-dose Cohort B group (hypotension), and two in the high-dose Cohort B group (one with bradycardia, tachycardia, and hypertension and one with bradycardia).
One patient in the high-dose Cohort B group had a serious AE of hypertension. The patient developed tachycardia (HR: 178–184 beats/min) and hypertension (BP: 176/114) during dexmedetomidine MI following administration of glycopyrrolate, which was given for bradycardia. The hypertension was resolved with hydralazine, dexmedetomidine was stopped, and the patient completed the MRI scan while receiving propofol. Glycopyrrolate has been reported to elicit exaggerated hypertensive responses when used with high-dose dexmedetomidine.15
One patient in each dose group discontinued dexmedetomidine due to an AE: one in the low-dose Cohort A group (bradypnea), middle-dose Cohort B group (bradycardia), and high-dose Cohort B group (serious AE of hypertension). However, no patients discontinued the study and all patients completed the MRI scan.
3.6 Withdrawal-related AEs
One patient (a 3-year, 3-month-old male who received high-dose dexmedetomidine) had a brief (16 min) paradoxical agitation reaction. The reaction was considered mild, and the patient recovered without any intervention. Two additional patients had AEs of mild emergence delirium lasting 5–20 min: a 3-year-old (middle-dose group) and a 1-year, 9-month-old (high-dose group).
3.7 Vital signs
In both age cohorts, decreases in mean HR, SBP, DBP, and MAP were transient and consistent with the known pharmacological effects of dexmedetomidine. Minimal changes were seen at all doses for SpO2 and RR (Figures S2 and S3).
In the combined age cohorts, the ratio for the median (IQR) time outside of the hemodynamically stable range during the MRI scan and recovery period was 0.4 (0.4) and 0.3 (0.5), 0.4 (0.5) and 0.6 (0.4), and 0.3 (0.6) and 0.3 (0.7) at the low, middle, and high dose, respectively.
4 DISCUSSION
This study was undertaken to evaluate efficacy and safety of dexmedetomidine for MRI sedation of children between 1 month and 17 years of age. The results demonstrated that a greater percentage of children treated with the high dexmedetomidine dose completed the MRI scan without propofol rescue compared with the low dose (i.e., 24/38 [63.2%] vs. 6/42 [14.3%]) (odds ratio: 10.29, 95% CI: 3.47–30.50, p < .001). The primary endpoint in this study was met. Similar results were observed in the 1 month to <2 year and ≥2 to <17 year cohorts.
The greatest dexmedetomidine efficacy was demonstrated in the older age cohort, where 75% (15/20) of the patients treated with the high dose (LD 2 mcg/kg, MI 1.5 mcg/kg/h) completed the MRI scan without rescue propofol. Although the sample size in this dose subgroup is small, the results are consistent with the retrospective report from Mason et al (2008)6 on the use of high-dose dexmedetomidine as the sole sedative in children undergoing MRI. In their study, patients could receive up to three dexmedetomidine boluses if necessary and they reported a 97.6% success rate.6 The lower response rate observed in the present study likely reflects that this was a fixed-dose study in which patients received only a single bolus dose followed by a fixed MI.
The clinical response of the younger age cohort to the high-dose level of dexmedetomidine (LD 1.5 mcg/kg, MI 1.5 mcg/kg/h) was attenuated relative to the response observed at both the high-dose (LD 2.0 mcg/kg, MI 1.5 mcg/kg/h) and the middle-dose level (LD 1.2 mcg/kg, MI 1.0 mcg/kg/h) in the older age cohort (i.e., 50% vs. 75% and 61.9%, respectively). Whether these differences are attributable to PK and/or PD factors remains unclear. However, the simulated Cmax and steady-state dexmedetomidine concentrations at the high-dose level in Cohort A were lower by design than in Cohort B (Figure S4).
The key safety observations were that AEs occurred in most patients at all three dose levels but with few exceptions were mild in severity and did not require intervention. The safety profile was generally comparable across the three doses, did not worsen with increasing dose, and, from a risk–benefit perspective, supports recommendation of the high dose studied in each age cohort as the starting dose for pediatric procedural sedation. Of interest, emergence time was not materially prolonged with increasing dose levels (Figure S5).
In the absence of PK and modeling data, selection of dexmedetomidine dosing for this study was based primarily on clinical considerations. However, dexmedetomidine concentration versus time profiles at the projected doses for the study were simulated using a population PK model developed from ICU pediatric patients (Pfizer data on file). The simulated dexmedetomidine PK profiles following the high-dose (10 min LD and 1 h MI) treatment are presented in Figure S4. These simulated values overlap and exceed published target therapeutic concentrations for pediatric sedation derived from PK/PD models.16-18 However, the differences in patient populations, disease states, route of administration, and the use of dexmedetomidine in combination with other sedatives and analgesics make it difficult to ascertain the relevance of these results for the present study.
The strengths of this study include that it was a fully powered, prospective, double-blind, randomized, dose-controlled study with well-balanced treatment groups that support the generalizability of the study results to the pediatric population. Additionally, dexmedetomidine was studied as monotherapy at ascending fixed-dose levels, allowing for a clearer understanding of the efficacy and safety characteristics of dexmedetomidine itself, which is difficult or impossible to achieve when administering in a variable dosing manner and/or in combination with other drugs.
Study limitations include that the fixed-dosing regimens may have limited the investigator's ability to provide optimal sedation with dexmedetomidine. This may have resulted in greater propofol use that may have lowered the overall efficacy seen in the study. In addition, although adequately powered for the planned efficacy analyses, the small sample size, especially in the separate age cohorts, does not support the detection of uncommon or rare AEs.
5 CONCLUSION
The high dose of dexmedetomidine in this study was associated with meaningfully greater efficacy compared to lower doses in the combined sample and in each age cohort. Dexmedetomidine was well tolerated across all doses studied. The greatest efficacy was seen at the high-dose level in the ≥2 to <17 year age cohort. Efficacy was not as robust in the ≥1 month to <2 year cohort, even at the high-dose level. Based on these results, the recommended starting dose for procedural sedation in children ≥1 month to <2 years is LD 1.5 mcg/kg and MI 1.5 mcg/kg/h and in children ≥2 to <17 years, LD 2.0 mcg/kg and MI 1.5 mcg/kg/h.2
ACKNOWLEDGMENTS
We thank Dr. John W. Berkenbosch, MD, FAAP, FCCM, Professor of Pediatrics/Pediatric Critical Care, Norton Children's Hospital and the University of Louisville School of Medicine and Dr. Joseph Cravero, MD, Professor of Anesthesia, Harvard Medical School, Anesthesiologist-in-Chief, Department of Anesthesiology, Critical Care and Pain Medicine, Boston Children's Hospital, for their valuable input on the protocol design and study conduct. They were paid consultants to Pfizer in connection with the protocol design and study conduct. Medical writing support was provided by Alex Frings, PharmD, CMPP, of Engage Scientific Solutions (Fairfield, CT) and was funded by Pfizer. We would also like to thank Huifen Faye Wang, PhD (Pfizer, Inc.) for their support on the presentation and discussion of the dose PK simulation results.
FUNDING INFORMATION
This study was sponsored by Pfizer.
CONFLICT OF INTEREST STATEMENT
Umar Khan, Gregory B. Hammer, and Cassandra Duncan-Azadi were study investigators and have no conflicts of interest to declare. Yasuyuki Suzuki was a study investigator and reports commercial support from Pfizer, Inc. as Speaker and Consultant for Pfizer products. Sunring Chime and Phillip Chappell are full-time employees of Pfizer, Inc. and hold stock/stock options. Deborah Chiles was a full-time employee of Pfizer, Inc. at the time of this study and holds stock/stock options.
ETHICS APPROVAL STATEMENT
This study was conducted in compliance with all ethical principles of the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice guidelines and was approved by the institutional review board or independent ethics committee at each participating institution.
PATIENT CONSENT STATEMENT
Informed consent was received from each patient's parent or guardian before any treatment was given. Assent was also obtained from the patient where appropriate for children and adolescents under the age of majority according to local institutional guidelines.
CLINICAL TRIAL REGISTRATION NUMBER
ClinicalTrials.gov: NCT04237792.
Open Research
DATA AVAILABILITY STATEMENT
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Pursuant to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified patient data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.




