Noninvasive respiratory support preventing reintubation after pediatric cardiac surgery—A systematic review
Section Editor: David Faraoni
Abstract
Objective
To analyze the optimal postextubation respiratory support in pediatric cardiac surgery patients.
Design
Systematic review of randomized controlled trials.
Setting
Pediatric or neonatal intensive care units.
Participants
All aged children (<16 years) having cardiac surgery and postoperative invasive ventilation.
Intervention
Noninvasive respiratory support, including high flow nasal cannula (HFNC), conventional oxygen therapy (COT), noninvasive positive pressure ventilation (NIPPV), continuous positive pressure (CPAP), and noninvasive high-frequency oscillatory ventilation (NHFOV).
Measurement and Main Results
Studies were not pooled for statistical synthesis due to the limited number and quality of the included studies. Risk ratios with 95% confidence intervals were calculated for individual studies. A total of 167 studies were screened and six were included. The risk of bias was low in one, high in one, and had some concerns in four of the studies. Extubation failure (defined as reintubation) was the main outcome of interest. Risk ratio for reintubation was 0.10 (CI 0.02–0.40) and 1.07 (CI 0.16–7.26) in HFNC versus COT, 0.49 (CI 0.05–5.28) in HFNC versus NIPPV, 0.40 (CI 0.08–1.94) in HFNOV versus CPAP, 0.75 (CI 0.26–2.18) in HFNOV versus NIPPV, and 1.37 (CI 0.33–5.73) in CPAP versus NIPPV. Treatment durations did not differ between the groups.
Conclusion
We did not find clear evidence of a difference in reintubation rates and other clinical outcomes between different noninvasive ventilation strategies. Evidence certainty was assessed to be very low due to the risk of bias, the small number of included studies, and high imprecision. Future quality studies are needed to determine the optimal postextubation support in pediatric cardiac surgery patients.
1 INTRODUCTION
Cardiac surgery constitutes a great challenge for patients' respiratory physiology due to hemodynamic alteration and systemic inflammation after cardiopulmonary bypass (CPB) which may lead to fluid accumulation, pulmonary edema, pleural effusion, and increased resistance in pulmonary circulation.1-3 In addition, muscular weakness and diaphragmatic fatigue are common after cardiac surgery and can further compromise respiratory recovery after extubation.4, 5 These problems are emphasized in pediatric patients due to inherent respiratory organ anatomy and physiology.6, 7
A need for reintubation is common after pediatric cardiac surgery, as the reintubation rate has been reported to be up to 20%.8, 9 During past decades, the use of noninvasive respiratory support after extubation has gained popularity in preventing the risk of re-intubation or extubation failure.10 There are several noninvasive respiratory support modalities available. These modalities are based on high-flow oxygen and continuous, intermittent, bi-level, or oscillating positive airway pressure which helps keep the airways and alveoli open, helps to flush the airway dead space, and assists patients' work of breathing.11 Research on the most favorable respiratory support modality in pediatric intensive care unit (PICU) patients has been published previously12-17
Thus far, the knowledge on which noninvasive respiratory support modality would be the most beneficial concerning extubation failure in pediatric cardiac surgery patients is lacking. We aimed to review and summarize the existing research data to answer this question.
2 METHODS
2.1 Search and screening process
A search for this systematic review was performed on August 1, 2023. We searched PubMed, Scopus, and Web of Science databases from inception to August 2023. The complete search strategy is described in supplementary file 1. Two authors screened the abstracts and evaluated full reports in Covidence software. Cases of discrepancy were solved by mutual decision.
2.2 Inclusion and exclusion criteria
We included studies focusing on pediatric cardiac surgery patients. The children were aged less than 16 years at the operation. Cardiac surgery was defined as any heart procedure that was done invasively using CPB. Percutaneous cardiac interventions were excluded. We included only parallel-group randomized controlled trials, as these studies are designed to show interventions clinical efficacy. Crossover studies and cluster randomized studies were excluded. All studies that reported observational data or did not report original data were excluded. Furthermore, we excluded non-English reports.
2.3 Intervention
We included all noninvasive respiratory support modes (standard/conventional oxygen therapy, high-flow nasal cannula (HFNC), continuous positive airway pressure (CPAP), noninvasive intermittent positive pressure ventilation (NIPPV), bi-phasic positive airway pressure (BIPAP), noninvasive neurally adjusted ventilatory assist (NIV-NAVA), and noninvasive high-frequency oscillatory ventilation (NHFOV). We did not have any criteria regarding the control intervention.
2.4 Outcomes
Our main outcome was the reintubation rate after extubation. Our secondary outcomes were the noninvasive respiratory support duration, PICU treatment duration, adverse event rate, and mortality.
2.5 Data extraction
One author extracted the data and the other author validated the extracted data to reduce unforced extraction errors. The following information was extracted from each of the papers: authors, funding, competing interests, inclusion and exclusion criteria, intervention definition, control definition, outcome definitions, number of included patients, number of events, and main outcome measures.
2.6 Risk of bias and evidence certainty
The risk of bias was assessed by two authors independently according to Cochrane's Risk of Bias 2.0 tool. Robvis Shinyapp was used to generate the risk of bias plots. In the risk of bias assessment, the lack of blinding was not an issue as the outcome assessment was considered not to be influenced by the knowledge of the intervention.
Evidence certainty was assessed by the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) framework. Evidence certainty was ranked from very low to high.
2.7 Statistics
We aimed to conduct a meta-analysis where we would have used inverse variance (DerSimonian and Laird—random effects method) in the pooling of the studies. However, the number of included studies was small and the comparisons only consisted of one or two studies. Thus due to heterogeneity in the interventions and the limited reporting we did not pool the results. Instead, we calculated risk ratios (RR) with a 95% confidence interval and mean difference (MD) with CI for the individual studies that have presented these in forest plots. RevMan 5.4.1 was used in the analyses.
This study has been conducted according to the guidelines in the Cochrane Handbook and reported according to the PRISMA (Preferred reporting items in systematic reviews and meta-analysis) guideline. PRISMA Checklist is available in supplementary materials.
2.8 Protocol registration
Protocol was registered to Prospero.
3 RESULTS
3.1 Search results
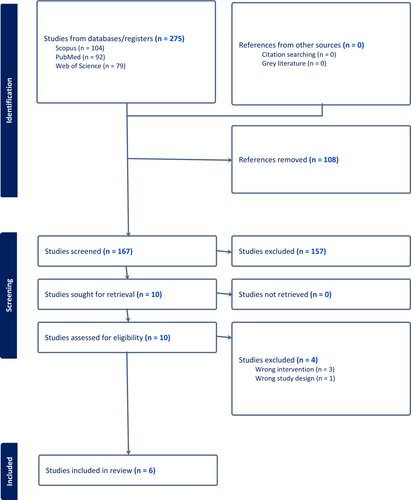
Initial search retrieved 167 abstracts, of which 10 full texts were further assessed and six studies included.12, 13, 18-21 (Figure 1).
3.2 Study and patient characteristics
All of the studies were parallel-group randomized controlled trials. Studies were conducted in China, India, Iran, and Italy. The number of participants varied between 80 and 121. The included patients were mostly infants, as the mean age was below 12 months in all but one trial. The patient characteristics were comparable between the intervention arms. (Table 1) The most common diagnoses were atrium septal defects and ventricular septal defects in the studies and the diagnoses were comparable between the intervention and control arms in the studies. (Table S1) One study did not present the diagnosis of the patients included in the study. (Table S1) All of the study authors reported not having any competing interests, and funding information was reported in three of the six studies. (Table 1) Three studies analyzed HFNC, three NIPPV, two COT, two NHFOV, and two CPAP. Inclusion criteria were relatively similar between the studies, as the most variation came from the age criterion (Table 1).
| Study | Country | Study design | Blinding | No. of participants | Inclusion criteria | Age | Weight | Intervention | Comparator | Main outcome | COI | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | ||||||||||
| Enayati et al 2021 | Iran | RCT | None | 92 | 1–24 months congenital heart surgery and cardiopulmonary bypass | 8.1 months | 10.2 months | 6.50 kg | 6.70 kg | HFNC | COT | PaCO2, PaO2/FiO2 ratios, respiratory failure, need for reintubation, development of atelectasis, pneumothorax, pleural effusion, and length of ICU stay. | None to report |
| Kumar et al 2022 | India | RCT | None | 121 | < 4 years age, elective cardiac surgery for congenital asyanotic cardiac defects using cardiopulmonary bypass | 47 months | 43 months | 14.1 kg | 12.4 kg | HFNC | NIPPV | PaCO2, PaO2/FiO2 ratios | None to report |
| Testa et al 2014 | Italy | RCT | None | 89 | <18 months of age, elective cardiac surgery with cardiopulmonary bypass | 3.8 months | 3.0 months | 4.6 kg | 5.0 kg | HFNC | COT | PaCO2 post exutabtion | None to report |
| Wu et al 2021 | China | RCT | None | 80 | Infants with congenital heart defect requiring cardiac surgey and needing 12 h of mechanical ventilation postoperatively | 2.3 months | 2.2 months | 4.4 kg | 4.2 kg | NHFOV | CPAP | postextubation respiratory failure and reintubation, the average PaCO2 level, the average oxygenation index (OI), and pulmonary recruitment in the early extubation phase. | None to report |
| Wu et al 2022 | China | RCT | None | 92 | < 3 months, congenital cardiac defect and needing cardiac surgery | 1.4 months | 1.5 months | 3.6 kg | 4.0 kg | NHFOV | NIPPV | reintubation, long-term noninvasive ventilation (NIV) support (more than 72 hours), and the time in NIV | None to report |
| Zheng et al 2022 | China | RCT | None | 83 | < 6 months, with stable hemodynamics after extubation and correction of ASD or VSD | 1.8 months | 1.7 months | 4.4 kg | 4.6 kg | NIPPV | CPAP | extubation failure rate and the level of PCO2 | None to report |
3.3 Risk of bias
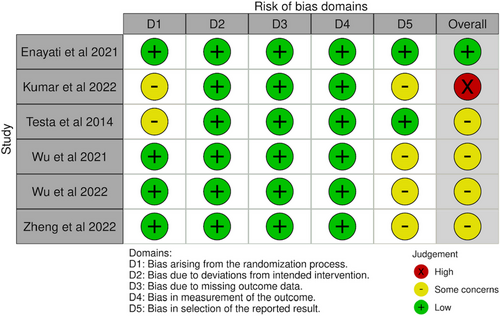
Overall risk of bias was low in one, had some concerns in four studies, and was high in one of the included studies. (Figure 2) Most issues were due to the selection of reported results, as trial protocols were not published.
3.4 Main outcome
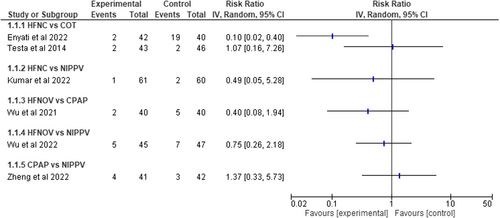
The main outcome of interest, the reintubation rate after extubation, was assessed in all of the included studies. HFNC was compared to COT in two studies (total of 171 children) and RR for reintubation was 0.10 (CI 0.02–0.40) and 1.07 (CI 0.16–7.26). One study included 121 children and compared HFNC to NIPPV and the RR was 0.49 (CI 0.05–5.28). HFNOV was compared to CPAP in one study (80 infants) and the RR was 0.40 (CI 0.08–1.94). One study with 92 children compared HFNOV to NIPPV and the RR was 0.75 (CI 0.26–2.18). Finally, CPAP was compared to NIPPV in one study with 83 infants and the RR was 1.37 (CI 0.33–5.73). (Figure 3) Evidence certainty was ranked as very low, due to heterogeneity, risk of bias, imprecision, and low number of included studies per ventilation strategy.
3.5 Secondary outcomes
Length of stay postoperatively at the PICU was assessed in three studies. Two compared HFNC to COT, and they found a difference of −0.79 days (CI −1.02 to −0.56 days) and − 1.20 days (CI −2.89 to 0.49) indicating a possible benefit of HFNC compared to COT. (Figure S1) One study with 121 children compared HFNC to NIPPV and the MD in the length of stay was −0.10 days (−0.41 to 0.21; Figure S1).
Noninvasive ventilation treatment duration was assessed in three studies. One study with 80 children compared HFNOV to CPAP and the difference was −0.20 days (−0.35 to −0.05) favoring NHFOV. (Figure S2) HFNOV was compared to NIPPV in one study and it did not find evidence of a difference (MD -0.0 days, CI −0.61 to 0.61; Figure S2). One study compared CPAP to NIPPV and the difference in treatment duration was −0.1 days (CI −0.25 to 0.05; Figure S2).
Pneumothorax rates were compared in three studies. Two studies compared HFNC to COT and did not find evidence of a difference (RR 0.62, CI 0.18–2.14; RR 1.07, CI 0.07–16.57; Figure S3). One study compared HFNOV to CPAP, but the effect estimate had high uncertainty due to a low event rate (RR 0.20, CI 0.01–4.04; Figure S3). Mortality during the hospitalization period was assessed in two studies. Both had such a low event rate, that the comparisons had high uncertainty. (Figure S4).
4 DISCUSSION
Several noninvasive respiratory support strategies have been compared in randomized controlled trials. However, as the main goal of postextubation respiratory support is to prevent extubation failure, this is clinically the most important outcome. The rate of extubation failure was analyzed in all of the included studies, but due to the low event rates (typically <10%) combined with relatively small sample sizes (80–121 patients), were the trials underpowered. Thus, conclusions on the best noninvasive support for the prevention of extubation failure in pediatric cardiac surgery patients cannot be drawn based on the currently available evidence.
As stated in this review, there is a need for studies on the most favorable noninvasive respiratory support strategy in pediatric cardiac surgery patients, although the topic has been examined in other pediatric patient groups. In general PICU patients, HFNC has been shown to be associated with higher rates of extubation failure than NIPPV or CPAP.22 In preterm neonates, NIV-NAVA and NHFOV have been promising postextubation respiratory support options.16, 23 In NIV-NAVA, the noninvasive ventilation is synchronized by recognizing the electronic activity of the diaphragm with a special esophageal catheter.24 Two previous studies have analyzed NIV-NAVA in cardiac surgery patients. A case–control study reported that NIV-NAVA did decrease reintubation rate or length of stay.14 A crossover randomized trial compared NIV-NAVA to CPAP and reported that NIV-NAVA reduced breathing work and improved synchrony.15 However, despite these promising findings in other patient groups, there are no parallel group randomized studies analyzing the efficacy of clinically relevant outcomes in cardiac surgery patients. NHFOV has gained popularity and favorable evidence in recent years. The high-frequency oscillation has been shown to improve gas exchange and functional residual capacity by providing a continuous distending pressure, and by that decreasing the infant's muscle work for breathing.25 Among preterm neonates, NHFOV has been shown to reduce extubation failures compared to NIPPV and CPAP.16, 17 NHFOV was analyzed in two included trials and compared to CPAP and NIPPV. The trials were rather small and the results had clear imprecision, but it seemed that NHFOV might have potential in the prevention of extubation failures.12, 13 Reporting on adverse events was limited in all of the included trials in this systematic review. None of the included studies aimed to assess costs related to treatment, as ventilators able to provide NHFOV are more expensive than those providing CPAP, NIPPV, or HFNC. In adult patients, HFNC was non-inferior to BiPAP in a large randomized trial.26 A recent meta-analysis rated that NIPPV was the best, followed by HFNC and CPAP in preventing postoperative pulmonary complications in adult patients.27 However, these findings cannot be transferred to pediatric cardiac surgery patients, due to differences in the respiratory physiology.
4.1 Strengths and limitations
This systematic review was conducted according to a preregistered protocol without any protocol deviations. Most limitations for this review come from the quality of the included studies, and the low number of included studies. Only one of the included studies was judged to be at low risk of bias, other studies had issues mostly with possible selective reporting. All the studies were too small to detect clinically relevant differences practically in all analyzed outcomes. Thus, the results have high imprecision. Furthermore, the clinical indications and patient characteristics were heterogeneous in the included studies, which leads to clear heterogeneity, and thus we decided to not pool any of the results together.
4.2 Recommendations for future studies
Future studies are needed with large enough sample sizes to precisely estimate the two most clinically relevant outcomes, reintubation rate and length of stay. Cost-effectiveness analyses based on the respiratory support expenses compared to a possible reduction in the length of intensive care unit stay would be beneficial in making guidelines. Future studies should also be conducted rigorously and follow good reporting and conducting guidelines to reduce possible sources of bias.
5 CONCLUSION
Several postextubation noninvasive respiratory support strategies after pediatric cardiac surgery have been compared in randomized trials. Based on the results of these studies, a conclusion of superiority or inferiority of any noninvasive respiratory support cannot be made. Evidence certainty was assessed to be very low. Future evidence in larger trials is needed to determine the optimal postextubation respiratory support.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST STATEMENT
None to report.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




