The effect of augmented reality on preoperative anxiety in children and adolescents: A randomized controlled trial
Section Editor: Nada Sabourdin
Abstract
Background and Aims
Virtual reality has been shown to be an effective non-pharmacological intervention for reducing anxiety of pediatric patients. A newer immersive technology, that of augmented reality, offers some practical advantages over virtual reality, and also seems to show beneficial effects on anxiety. The main objective of this study was to determine whether augmented reality could reduce preoperative anxiety in pediatric patients undergoing elective day surgeries. A secondary outcome was to document the level of satisfaction from pediatric patients toward augmented reality intervention.
Methods
Children and adolescents aged between 5 and 17 years old scheduled for elective day surgery under general anesthesia were randomly divided into two groups. Patients in the control group received standard care, whereas patients in the augmented reality group were accompanied by two virtual characters who taught them relaxation techniques and provided emotional and informational support. Anxiety was measured at the time of admission and at the time of induction using the short version of the modified Yale Preoperative Anxiety Scale.
Results
The analysis included 37 pediatric patients in the augmented reality group and 64 in the control group. Anxiety scores were statistically significantly lower in the augmented reality group than those in the control group at the time of admission (median difference [95% CI]: 6.3 [0–10.4], p = .01), while no difference was observed between groups at the time of induction (median difference [95% CI]: −4.2 [−5.2–4.2], p = .58). Most patients in the augmented reality group wished to wear the glasses again and reported to be very satisfied with the intervention.
Conclusion
To our knowledge, this study is the first large randomized controlled trial to provide empirical evidence of reduction in anxiety for children and adolescents using augmented reality prior to induction of general anesthesia.
1 INTRODUCTION
The hospital environment is unfamiliar and stressful for both children and parents. Children are highly susceptible to anxiety.1 Several studies report prevalence rates that range between 40% and 80% of pediatric patients showing signs of anxiety at some point in the perioperative period.2, 3 Preoperative anxiety can adversely affect patients in the perioperative period and beyond.4, 5 There is ample evidence that alleviating children's anxiety can improve recovery, mitigate postoperative pain, and improve the overall perioperative experience.6 Despite potential adverse side effects, the use of premedication remains a standard to control preoperative anxiety.7 Currently available non-pharmacological strategies for managing preoperative anxiety include parental presence during induction of anesthesia,1 various behavioral techniques for distraction—from clowns and music to immersive technologies8—and preprocedural educational tours of the perioperative setting.9
Over the last 5 years or so, researchers have increasingly studied immersive technology as a non-pharmacological solution for perioperative anxiety.10 Both virtual reality (VR) and augmented reality (AR) are immersive technologies that aim at extending an individual's sensory environment. Wearing a VR headset over their eyes, users are transposed to an artificial world via fully digital three-dimensional visualization, which provides a completely virtual closed environment. On the other hand, AR allows users to simultaneously view the real-world environment with a digital image overlay.11
There is substantial evidence showing that VR intervention is effective for perioperative anxiety.10 A systematic review revealed that seven out of eight VR studies reported a score of anxiety significantly lower in the VR groups compared to the control groups.12 The newer immersive technology of AR is increasingly used in many industries, including healthcare.13 More specifically, in pediatric care, it has been used in recent years as a mean to manage preoperative anxiety. To our knowledge, three case reports showed preliminary positive results of using AR14: (i) one child (age = 11) and his parents who opted for AR glasses over the VR headset as a playful distraction technique during preinduction intravenous access15; (ii) the satisfactory use of AR during the induction of general anesthesia in a case study of three children16 (mean age = 8.7); and (iii) in a case study with three children, AR was shown to reduce fear during pediatric otolaryngology procedures17 (mean age = 14.7).
The use of AR with pediatric patients has some potential advantages over that of VR. Both VR and AR can divert children's attention away from stressful procedures toward enjoyable and playful stimuli. Yet AR still allows children to be aware of their surroundings while interacting with anesthesia providers and to communicate with significant people around them.18 AR is unique in its ability to blend the unfamiliar surroundings of the perioperative setting of the hospital with playful holograms. Also, due to the visual contact with their surroundings, children are less likely to experience nausea, also known as “cybersickness.”19
Recent reports suggest that AR is a promising intervention to alleviate preoperative anxiety in children.16 However, further empirical evidence—based on standardized randomized-controlled trials with larger samples—is needed to establish whether AR could serve as an efficient and safe non-pharmacological solution to managing preoperative anxiety in patients.14 The primary aim is to determine whether anxiety would be significantly reduced in pediatric patients using AR prior to undergoing elective surgeries compared to standard care. The generated AR content included animation-based distraction and relaxation technique guidance.
2 METHOD
2.1 Study
This study was a prospective randomized clinical trial conducted at Centre mère-enfant Soleil of the Centre Hospitalier Universitaire (CHU) de Québec-Université Laval between July 2022 and December 2022. The trial was approved by the Research Ethics Committee of the CHU de Québec-Université Laval (#2022-4563). Recruitment was conducted by the research assistant. All patients and their parents received an appropriate explanation of the study upon arrival on the day of the surgery. Written informed consent was obtained from parents and assent was also given by all patients.
2.2 Patients
Inclusion criteria were patients from 5 to 17 years of age with an American Society of Anesthesiologist (ASA) physical status of 1 or 2, who presented at Centre mère-enfant Soleil of the CHU de Québec-Université Laval, for an elective day surgery in the following specialties: ophthalmology, dentistry, urology, otorhinolaryngology, orthopedics, and general surgery. Due to the size of the Hololens headband and the language level used in the AR software, it was determined before study enrollment that it would not be feasible to include patients <5 years of age. Exclusion criteria were patients who were accompanied by an adult who was not the parent; patients with an ASA physical status higher than 2; patients with intellectual or developmental disabilities; patients with a history of epilepsy; patients with visual impairment (best-corrected visual acuity <20/200; determined via parental report); patients who were actively followed at the Complex Care Clinic of the Centre mère-enfant Soleil; and patients on whom the AR headset did not fit appropriately.
2.3 Randomization
Randomization was conducted by an independent statistician prior to the study using an online tool (www.sealedenvelope.com) to randomize patients within groups such that an equal number was assigned to each condition using serially numbered sealed opaque envelopes according to the underlying random sequence list by the statistician. Group assignment was carried out by the research assistant by checking the designated group in the serially numbered envelopes.
2.4 Intervention and storyline
Patients in the control group received standard care without AR intervention. Standard care includes explaining what is being done and why, and saying comforting and supportive words during procedures. After admission, patients in the control group were invited to wait in the day surgery waiting room with their parents until surgery was ready to be performed. When the operating department called, the patients and their parents were accompanied by a beneficiary attendant to the operating room (OR) waiting area. When the surgical team was ready, the anesthesiologist brought the patient to the OR. Parents were asked to stay in the waiting area. Music, video games, or tablets were not allowed in the OR.
In addition to standard procedure, patients in the intervention group received AR intervention through Microsoft HoloLens 2 glasses. The animation follows the story of a character called "Constellation", who is a celestial body that lives among the stars and travels through the universe. Constellation has discovered a special civilization that comes from the North Star called the "Equanimous". The character tells patients that these magnificent beings have the mission of instilling strength and willpower in those who are lucky enough to meet them. One of them—Equoo—specifically chooses the patient to accompany him or her on this journey and teaches the patient relaxation techniques that can be used even outside of the hospital. After completing the baseline anxiety assessment in the day surgery waiting room, patients in the AR group were invited to put on AR glasses with the help of the research assistant. The simulation began with a 2-min presentation of the two main characters, Constellation and Equoo, and their mission (Phase I). Constellation then invited patients to imitate Equoo's movements and to perform progressive muscle relaxation20 for a 16-min duration (see Figure 1A). When relaxation exercises were completed, the entertainment mode was activated (Phase II), and patients were free to start animations as many times as desired (see Figure 1B, for an example). The third phase of the intervention took place in the waiting room of the OR. Two posters were placed on the wall to trigger breathing exercises and animations to promote relaxation. When looking at those posters with the AR glasses, patients were presented with a typical cardiac coherence exercise involving inhaling for 4 s as the ball goes up, then exhaling for the same amount of time as the ball goes down (see Figure 1C). The breathing exercise lasted 2 min and could be done as many times as desired. A minimum of 20 min is therefore required to complete the intervention before going to the OR. The fourth and final phase of the intervention took place in the OR and lasted approximately 2 min. At various times, Constellation reminded the patient to stay calm and to be courageous in order to reach his full potential. She also described certain sensations that the patient might feel as well as the actions that will be carried out by the anesthesiologist in the OR. The total duration of the AR intervention varied across patients according to the time they had to wait before surgery (as optimal timing is difficult in busy theaters) and the number of animations they decided to trigger. No patients received preoperative oral or intravenous medication in either the control or the AR group.

During the intervention, patients were free to remove them at any time. Patients who did not complete the progressive muscle relaxation or removed the AR glasses before entering the OR were considered as dropouts and were excluded from the study. The type of induction of anesthesia, either inhaled or intravenous, was left at the discretion of the anesthesiologist after discussion with the patient, independently of AR use. In our center, most pediatric inductions of anesthesia are inhaled, except for teenagers, who are often induced with an intravenous line or left the choice of inhaled or intravenous induction depending on clinical circumstances.
2.5 Outcomes
The primary outcome was preoperative pediatric anxiety as measured by the short version of the modified Yale Preoperative Anxiety Scale (mYPAS-SF). The mYPAS-SF is a validated perioperative pediatric anxiety instrument with observational measurements of anxiety in four categories (activity, emotional expressivity, state of arousal, and vocalization) with a range of 22.92–100, with higher scores indicating greater anxiety.21 The French version of the mYPAS-SF was administered at two time points: at the time of admission (baseline or T0) and immediately before induction of general anesthesia (T1). A blinded research assistant recorded the mYPAS-SF at T0 before assignment to intervention while the anesthesiologist (not blinded) administered the mYPAS-SF at T1. The secondary outcome was the level of satisfaction from patients toward AR intervention. After surgery, patients were invited by the research assistant to answer the question “I would like to wear the glasses again and meet with Equoo” using a Smiley Face 5-point Likert scale ranging from "not at all" to "very much". A higher score represents a higher level of satisfaction.
2.6 Sample size calculation
The power analysis was conducted using G*Power 3.1 (Heinrich Heine University, Düsseldorf, Germany). The sample size was based on a recent meta-analysis reporting an effect size of 0.71 for studies comparing preoperative anxiety of pediatric patients between VR and control groups.22 It was estimated that a sample size of 35 patients per group would enable to detect a between-group statistical difference with a 5% significance level () and a 80% power (). To account for potential dropouts, incomplete data and hardware malfunction a recruitment goal of patients was determined before the start of the study.
2.7 Statistical analyses
All data in this study are presented as the median (interquartile range) or numbers (%). The test of normal distribution was assessed using Shapiro–Wilk test. Chi-squared test was used to analyze categorical variables (gender, ASA physical class, past surgical procedures, and type of surgery). Age and differences in the mYPAS-SF scores between groups at each time point were analyzed using the nonparametric Mann–Whitney test. Within-group mYPAS-SF changes were analyzed using Wilcoxon signed-rank test. The effect size was the difference in medians, with 95% confidence intervals calculated by bootstrapping with replacement with 500 000 repetitions. Two-side p-value <.05 was considered as statistically significant and median difference or risk ratio with 95% confidence interval (CI) was reported.
3 RESULTS
One hundred thirty patients were assessed for eligibility on the day of surgery, and two patients were excluded (Figure 2). One was a non-French speaker, while the other one had a developmental disability. The trial ended just before Christmas holidays as the target number of participants was reached and the transition would be optimal for the medical staff. There was an unanticipated hardware malfunction due to battery depletion and application crashes during the intervention for four patients in the AR group; thus, the patients did not receive the allocated intervention and were not included in the analysis. During the follow-up period, the anesthesiologist failed to administer the mYPAS-SF at T1 for four patients in the AR group. In addition, 19 patients in the AR group discontinued the intervention because their surgery was earlier than anticipated (10 patients) or because they wished to stop the intervention before completion (9 patients). No patient was lost to follow-up in the control group; hence, a total of 101 patients underwent analysis (64 in the control group and 37 in the AR group).
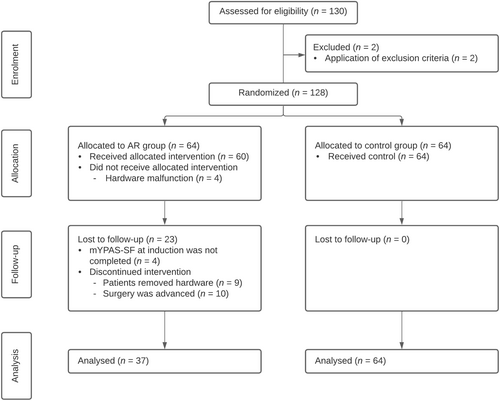
The median (IQR) age of the children was 9 (6–12). Sixty-one children (60.4%) were male, and 56 (55.4%) had undergone a previous surgical procedure. The most common surgical procedure was urology surgery (30.7%) followed by orthopedic (29.7%) and otorhinolaryngology (27.7%) procedures. Table 1 shows the differences between demographic and surgery characteristics in the two groups. Because the differences were not statistically significant, the two groups can be considered to be homogenous with regard to all the considered variables.
| Control group (n = 64) | AR group (n = 37) | |
|---|---|---|
| Age (year) | 9 (6–13) | 8 (6–11) |
| Gender (M/F) | 35 (55)/29 (45) | 26 (70)/11 (30) |
| Already underwent a surgical procedure | 35 (55) | 21 (57) |
| ASA class (1/2) | 54 (84)/10 (16) | 31 (84)/6 (16) |
| Type of surgery | ||
| Otolaryngeal | 17 (27) | 11 (30) |
| Ophthalmic | 2 (3) | 1 (3) |
| Orthopedic | 22 (34) | 8 (22) |
| Urology | 18 (28) | 13 (35) |
| Dental | 1 (2) | 1 (3) |
| Other | 4 (6) | 3 (8) |
- Note: Data are expressed as median (IQR) or numbers (%).
- Abbreviation: ASA, American society of anesthesiologist.
With regard to preoperative anxiety, the Mann–Whitney test revealed no significant statistical difference between groups for mYPAS-SF scores at the time of admission (T0) (p = .58, Table 2). However, mYPAS-SF scores were statistically significantly lower in the AR group compared to the control group at the time of induction (T1) (p = .01, Table 2). Wilcoxon signed-rank tests were further conducted to assess changes in mYPAS-SF scores within groups from T0 to T1. The analyses showed a significant statistical increase in mYPAS-SF scores from to T0 to T1 in the control group (Table 2, median difference [95% CI]: −6.3 [−10.4–0], p < .001). However, no significant statistical difference between T0 and T1 was found in the AR group (Table 2, median difference [95% CI]: 4.2 [0–6.25], p = .31). A Mann–Whitney test revealed no significant statistical difference regarding mYPAS-SF scores at T1 between all patients who received inhalation induction (76.2%) and those who received intravenous induction (23.8%, median difference [95% CI]: 0 [−.2–6.3], p = .22).
| Control group (n = 64) | AR group (n = 37) | Median difference (95% CI) | Risk ratio (95% CI) | p-value | |
|---|---|---|---|---|---|
| mYPAS-SF score | |||||
| Baseline (T0) | 22.9 (22.9–33.3) | 27.1 (22.9–33.3) | −4.2 (−5.2–4.2) | .58 | |
| Induction (T1) | 29.2 (22.9–44.8) | 22.9 (22.9–29.2) | 6.3 (0–10.4) | .01 | |
| Proportion of patients | |||||
| mYPAS-SF score > 30 at T0 | 19 (29.7) | 10 (27.0) | 1.1 (0.6–2.1) | .78 | |
| mYPAS-SF score > 30 at T1 | 28 (43.8) | 6 (16.2) | 2.7 (1.2–5.9) | .01 | |
| Increase in mYPAS score at T1 | 29 (45.3) | 8 (21.6) | 2.1 (1.1–4.1) | .02 | |
- Note: Data are expressed as median (IQR) or n (%). mYPAS-SF, short version of the modified Yale Preoperative Anxiety. T0: at the time of admission, T1: before anesthesia induction.
Twenty-nine patients (19 in the control group and 10 in the AR group) showed anxiety at T0 (mYPAS-SF scores >30) while 34 patients (28 in the control group and 6 in the AR group) showed anxiety at T1. Chi-squared tests revealed no statistically significant difference between the two groups according to the number of patients with anxiety at T0 (p = .78, Table 2). However, more patients showed anxiety in the control group than in the AR group at T1 (p = .01, Table 2). Moreover, the number of patients with an increase in mYPAS-SF from T0 to T1 was statistically significantly higher in the control group than in the AR group (p = .02, Table 2).
Most patients in the AR group wished to wear the AR glasses again and reported to be very satisfied with the intervention. The median (IQR) satisfaction score of patients with the AR intervention was 5 (4, 5) out of 5. Overall, patients wore the AR glasses for 20–135 min (M = 50.75, SD = 27.31). No patients reported having experienced eye pain or cybersickness.
4 DISCUSSION
The present RCT examined whether the use of AR during the preoperative phases—from admission up to induction of anesthesia—was effective at reducing anxiety in pediatric patients. To our knowledge, our study provides a first validation of the use of AR technology as a means to reduce anxiety in pediatric patients in a perioperative setting. The AR intervention—which combined the accompanying presence of virtual characters who provided guidance on relaxation and enjoyable distraction to children—proved to be effective to the extent that 24% fewer pediatric patients showed an increase in anxiety from admission to induction. At the time of induction, anxiety levels of 16.2% compared to 43.8% of the patients were above the well-documented cutoff score of 30-point on the mYPAS scale for the AR group and control group, respectively. The AR intervention was also associated with a very high level of satisfaction from the pediatric patients.
Some observations from the current study point to some potential practical benefits of adopting AR rather than VR as an immersive technology-based intervention. In their study, Caruso and his collaborators found that 22% of children discontinued the intervention by frequently removing the VR headset.23 Most of these children were younger (4 and 5 years old in samples of children aged up to 17 years old) so the headset may have been large and heavy on them.18 In the present research, 9 out of 60 pediatric patients removed the AR glasses (15%), and age did not seem to be a factor in the removal (the median age of those patients who removed the glasses was 9-year-old while that of patients who completed the study was 8-year-old). AR glasses also provide a better anesthesia facemask fit for inhaled induction than VR headsets, and anesthesiologists reported that the use of AR glasses made it possible for them to assess the child's eye movements during induction.15
Throughout the perioperative period—from admission to induction—patients had their AR glasses on from 20 to 135 min (M = 50.75, SD = 27.31). Following the relaxation exercises, patients were invited to use the AR glasses and interact with the virtual characters while staying in the OR waiting area until after the induction of anesthesia. In most previous studies on VR, patients are asked to use VR just before the surgery24 and, when reported, the time during which children keep their VR headset on is about 5 min. Perhaps due to the AR glasses being less bothersome (and less heavy), patients in our study wore their AR glasses for 51 min on average.16, 17 That patients willingly use AR for a longer period allows developers to include educational content and more interactive virtual objects.
The main goal of many interventions designed to reduce perioperative anxiety is to distract the patient's attention away from stressful stimuli. If an intervention is restricted to distraction immediately prior to induction, its use helps in the preoperative phase but does not educate children and adolescents on how to manage their fears and anxiety. In the present study, interactive educational guidance on relaxation was part of the AR intervention.23 One limitation of our study is that there was no group with either AR-only or relaxation techniques only. Further research would be required to dissociate the actual contribution of AR from that of relaxation and breathing to the reduction of anxiety. Also there was no measure of learnability and respiration related to the educational content on breathing and relaxation techniques. Another study limitation is the inability to ensure that the anxiety assessment at T1 was blind, which may have resulted in an overestimation of the effect of the AR intervention.
Across case reports and RCT studies, there is significant variability in both hardware and software of VR and AR technologies as well as great differences in content—virtual universe and storyline. A related issue is the need to adapt content to the age group; children of 5–8 years old and adolescents have very different interests (though, in the present study, satisfaction was as high for adolescents as it was for children). One recommendation is that researchers and users of immersive technologies as a means to reduce preoperative anxiety and provide educational information to children and adolescents, would benefit from a close collaboration with designers to develop content specifically tailored for pediatric patients and adapted according to age group and cultural background.25
The present RCT on AR in the pediatric theater may offer insights for applying this novel technology in daily practice. Given the constraints of pediatric care units, low-cost solutions that are simple to use and integrate by both patients and healthcare professionals are required. For health professionals involved in the treatment pathway, minimal initial training is necessary to learn how to use the AR device. It is also advised to create content with open-source tools that are cross-platform and compatible with multiple devices. Content should be culturally neutral and AI voice synthesis would simplify language translations. Giving all children the chance to benefit from AR is a logistical problem. Our estimations from 64 pediatric patients in the RCT suggest one AR device could cope with three surgeries every day. The AR intervention should begin about 30 minutes before the scheduled procedure to facilitate the rotation of AR devices to provide children with the best experience (a balance between avoiding prolonged time with the AR glasses and allowing for rich, educational, and enjoyable content).
Future research work should concentrate on providing more systematic (RCT-based) evidence of the effectiveness of AR interventions as a means to reduce anxiety in pediatric patients across a range of different medical procedures. As for development, we wish to develop further interactivity—so-called mixed reality whereby pediatric patients could interact with virtual information overlaid onto reality.
ACKNOWLEDGMENTS
The authors declare that the research was conducted in the absence of any personal or financial relationships that could be construed as a potential conflict of interest. We are thankful to Émilie Malouin and Sandrine Dugas for their assistance with data collection. We would also like to thank the nursing staff, respiratory therapists, and anesthesiologists working in the day surgery unit, operating room, and postanesthesia care unit for their contributions and support for the optimal flow of the study, patient care, and anxiety score collection. Thanks are also due to Steven Thomas for assistance with data processing. We would also like to acknowledge the exceptional contribution of Martin Thibodeau—creator of the EQUOO universe and of the AR intervention—to the care of pediatric patients at the Centre Hospitalier Universitaire (CHU) de Québec-Université Laval. Special thanks to Mélanie Grenier for the visual design and to Frima Productions for the animation. This work was supported by the ministère de l'Économie, de l'Innovation et de l'Énergie (Quebec Government), and the Fondation du CHU de Québec in the form of a Technological Maturation Grant awarded to Myriam Bransi and Sébastien Tremblay.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




