Intraoperative fluid administration volumes during pediatric liver transplantation and postoperative outcomes: A multicenter analysis
Section Editor: Francis Veyckemans
Abstract
Introduction
Fluid administration is an important aspect of the management of children undergoing liver transplantation and may impact postoperative outcomes. Our aim was to evaluate the association between volume of intraoperative fluid administration and our primary outcome, the duration of postoperative mechanical ventilation following pediatric liver transplantation. Secondary outcomes included intensive care unit length of stay and hospital length of stay.
Methods
We conducted a multicenter, retrospective cohort study using electronic data from three major pediatric liver transplant centers. Intraoperative fluid administration was indexed to weight and duration of anesthesia. Univariate and stepwise linear regression analyses were conducted.
Results
Among 286 successful pediatric liver transplants, the median duration of postoperative mechanical ventilation was 10.8 h (IQR 0.0, 35.4), the median intensive care unit length of stay was 4.3 days (IQR 2.7, 6.8), and the median hospital length of stay was 13.6 days (9.8, 21.1). Univariate linear regression showed a weak correlation between intraoperative fluids and duration of ventilation (r2 = .037, p = .001). Following stepwise linear regression, intraoperative fluid administration remained weakly correlated (r2 = .161, p = .04) with duration of postoperative ventilation. The following variables were also independently correlated with duration of ventilation: center (Riley Children's Health versus Children's Health Dallas, p = .001), and open abdominal incision after transplant (p = .001).
Discussion
The amount of intraoperative fluid administration is correlated with duration of postoperative mechanical ventilation in children undergoing liver transplantation, however, it does not seem to be a strong factor.
Conclusions
Other modifiable factors should be sought which may lead to improved postoperative outcomes in this highly vulnerable patient population.
1 INTRODUCTION
Pediatric liver transplantation requires tailored and highly specialized intraoperative management and presents many challenges for the anesthesiologist. Children with acute and chronic liver failure frequently have physiologic abnormalities that place them at increased risk of anesthetic complications. The various phases of liver transplantation are associated with hemodynamic alterations due to several factors including hypovolemia, acute blood loss, underlying abnormalities in systemic vascular resistance due to liver failure, cross clamping of the inferior vena cava and portal vein, and reperfusion of the grafted liver.1 Fluid management is an important part of the anesthetic care of these children, and unlike many other facets, is under the direct control of the clinical team. Increasing attention is being paid to liberal and conservative fluid management strategies in abdominal and nonabdominal surgeries and their impact on postoperative outcomes in both adults and children.2-6 Adult liver transplant studies have indicated an association between liberal fluid administration and postoperative respiratory complications as well as duration of mechanical ventilation.7 To date, no pediatric liver transplant studies have examined this association.
The aim of our study was to evaluate the association between the volume of intraoperative fluid administration and outcomes following pediatric liver transplantation. Our primary outcome was the duration of postoperative mechanical ventilation. Our secondary outcomes included intensive care unit (ICU) and hospital lengths of stay, and exploratory outcomes such as the volume of postoperative fluid administration and maximum postoperative vasoactive-inotropic score (VIS). We hypothesized that increasing intraoperative fluid volumes would result in longer durations of ventilation after pediatric liver transplantation.
2 METHODS
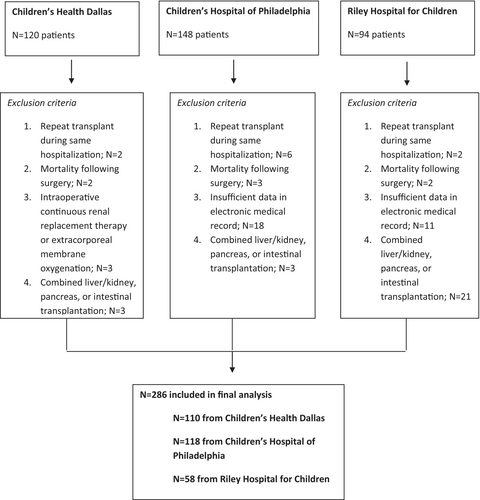
We performed a multicenter, retrospective cohort study at three pediatric liver transplant centers: University of Texas Southwestern Medical Center/Children's Health Dallas (CHD), University of Pennsylvania/Children's Hospital of Philadelphia (CHOP), and Indiana University Health/Riley Children's Health (RCH). For each site, Institutional Review Board approval was obtained, and requirement for written informed consent was waived. We used the electronic medical record at each site for data extraction at CHD and CHOP [Verona, WI], and Cerner, at RCH [North Kansas City, MO]. We included patients undergoing liver transplantation from 2010 to 2021 at CHD (patients were enrolled up until data analysis), from 2010 to 2020 at RCH, and from 2012 to 2020 at CHOP (electronic intraoperative documentation did not commence until 2012). We excluded (1) patients who died following liver transplantation during hospitalization whether still requiring mechanical ventilation at the time of death or not, (2) patients who required repeat liver transplantation during the same hospitalization, (3) patients who required intraoperative continuous renal replacement therapy or extracorporeal membrane oxygenation, (4) patients who underwent combined liver/kidney, pancreas, or intestinal transplantation, and (5) patients with insufficient information in the electronic medical record for the primary predictor or primary outcome variables.
2.1 Primary predictor definition
We calculated total intraoperative fluid administration using the following formula: total fluids = crystalloid (mL) + (5% albumin [mL] × 1.5) and indexed it to weight (kg) and duration of anesthesia (anesthesia start time to anesthesia stop time, hours). Albumin is multiplied by a factor of 1.5 because colloids are more potent intravascular volume expanders compared to crystalloids. A meta-analysis of studies comparing fluid resuscitation with colloid and crystalloid to crystalloid alone showed that greater fluid volumes are required to meet the same targets with crystalloids than with colloids, with an estimated ratio of 1.5.8 Crystalloids included dextrose and nondextrose fluids. None of the participating centers had any formal guidelines or policies in place by which to guide fluid administration in the perioperative period.
2.2 Outcomes definitions
Unless otherwise noted, outcomes were established a priori. Our primary outcome was duration of mechanical ventilation after surgery, which we calculated as the time from anesthesia stop to extubation. If the patient was extubated in the operating room upon completion of surgery, we defaulted the duration of mechanical ventilation to zero hours. Secondary outcomes included ICU length of stay (time from ICU admission following liver transplant to ICU discharge) and hospital length of stay (time from ICU admission following liver transplant to hospital discharge). Exploratory outcomes included fluid administration volume in the first 24 postoperative hours (calculated like intraoperative fluid volume except indexed to 24 h) and maximum postoperative VIS in the first 24 postoperative hours. The VIS is a score reflective of hemodynamic instability and has been shown to predict poor postoperative clinical outcomes.9 It is calculated by the following formula: VIS = dopamine dose (μg kg−1 min−1) + dobutamine dose (μg kg−1 min−1) + (100 × epinephrine dose [μg kg−1 min−1]) + (10 × milrinone dose [μg kg−1 min−1]) + (10 000 × vasopressin dose [U kg−1 min−1]) + (100 × norepinephrine dose [μg kg−1 min−1]). Post hoc outcomes included vascular (hepatic artery or portal vein) thrombosis in the postoperative period (identified through manual chart review) and postoperative acute kidney injury (AKI). We defined postoperative AKI as an increase in postoperative creatinine level by more than 1.5 times baseline or an increase of more than 0.3 mg dl−1 in any 48-h period within 7 days of surgery. Preoperative creatinine used as the baseline value was the most proximate creatinine level before surgery.
2.3 Confounders definitions
Maximum intraoperative VIS score was included in the model as a potential confounder, whereas maximum postoperative VIS was an exploratory outcome. None of the participating centers had any formal guidelines or policies in place by which to guide vasoactive administration in the perioperative period. Generally, at RCH, transplant surgeons request systolic blood pressure goals greater than 100 mm Hg after reperfusion of the grafted liver, and at CHD and CHOP, the requested systolic blood pressure goal is 90 mm Hg after reperfusion.
We identified major chronic conditions through manual chart review of past medical history and diagnosis of portal hypertension through review of progress notes. We identified the pediatric end-stage liver disease (PELD) score, and model for end-stage liver disease (MELD) score for patients over 12 years of age, through manual chart review or cross-checking institutional datasets maintained by the solid organ transplant teams. The MELD score has been validated as a predictor of survival in adults with liver failure.10 The score incorporates the patient's serum creatinine, bilirubin, sodium, and international normalized ratio values. The PELD score is specific to children but has been only weakly associated with outcomes in pediatric liver failure.11 The PELD score incorporates the patient's age, serum bilirubin, albumin, and international normalized ratio, as well as history of growth failure. These scores were adopted by the United Network for Organ Sharing for prioritization of patients awaiting liver transplantation in the United States. If certain criteria are fulfilled, children with acute liver failure who are in most need of liver transplantation are listed as status 1a and are given priority over children listed with a MELD/PELD score. Very sick, chronically ill children can be listed as status 1b, which also gives priority over children listed with a MELD/PELD score. The PELD scores included in our analysis are natural PELD scores without exception points.
We identified the remaining variables through manual review of progress notes, anesthesia records, and operative notes. We defined the preoperative hemoglobin as the most proximal hemoglobin value prior to surgery. We calculated the percent intraoperative time hypotensive by summing the durations of all episodes of intraoperative hypotension and dividing by anesthesia duration. Blood pressures were measured invasively and were available at 1-min intervals. We defined hypotension as mean arterial pressure greater than 30% below baseline, with baseline mean arterial pressure being the most proximate preoperative recording, usually from a noninvasive measurement on the floor prior to transfer to the operating room. To avoid the undue influence of artifact, we required each episode of hypotension to be at least 2 min long. We calculated duration of surgery as the time interval between “procedure start” and “procedure end.” We included intraoperative blood product administration as a “yes/no” binary predictor variable, as well as total blood product administration which included the sum volume of all blood products administered during surgery, normalized to body weight. We did not include urine output or total fluid balance in the analysis because urine output can be an unreliable indicator of fluid balance. It is often inaccurate during surgery due to technical problems (foley catheter kinks and obstruction) and there is an inherent propensity for mismeasurement by Foley bag. In addition, it can be influenced by diuretic administration. Total blood loss was identified in the anesthetic record. If missing, the documented blood loss from the surgeon's intraoperative note was used.
2.4 Statistical analysis
We calculated descriptive statistics as mean (SD) and median (IQR) for continuous variables and frequency and percent for categorical variables. We performed univariate linear regression analyses to investigate the association between patient demographics, clinical characteristics, and outcomes. An analysis of residuals confirmed the assumptions of linearity. We entered variables with p values <.25 from the univariate linear regression analyses as candidate variables for stepwise multiple linear regression analyses. We used stepwise multiple linear regression analyses to identify significant independent variables associated with clinical outcomes. Variables were assessed for collinearity. We considered p values <.05 statistically significant. Where variables were missing, those cases were excluded from that analysis. Any outlying values were included in analysis. This analysis plan was formulated before the data were examined and can be found in the study protocol.
We conducted two post hoc linear regression analyses. In the first, we excluded patients with immediate extubation in the operating room after surgery and in the second, we stratified patients into cohorts by age less than 2 years and age greater than or equal to 2 years. We also conducted two additional analyses based on reviewer feedback. We performed a univariate linear regression examining association between time-of-day surgery ended and duration of mechanical ventilation after surgery. The predictor variable “time-of-day” was categorized as “day” if surgery ended between 07:00 a.m. and 07:00 p.m., and “night” if it ended between 07:00 p.m. and 07:00 a.m. We also performed a univariate linear regression to examine the association between postoperative fluid volume administration, calculated similarly to intraoperative fluid volume, in the first 24 h after surgery and duration of mechanical ventilation. Additionally, we performed univariate and stepwise multiple logistic regression analyses to examine the association between intraoperative fluid volume and postoperative vascular thrombosis (hepatic artery and portal vein thrombosis) and postoperative AKI.
All analyses were conducted in SAS/STAT software Version 9.4 (SAS Institute Inc.).
2.5 Sample size considerations
To detect a nonzero r2 attributed to one independent variable using an F-test (or two-sided t-test) at a significance level (alpha) of .05, we would need at least 152 patients. The power is calculated assuming that the actual value of r2 is .05.
3 RESULTS
We identified a total of 362 pediatric liver transplants during the study period: 120 from CHD, 148 from, and 94 from RCH. We excluded a total of 76 patients, leaving 286 for study (Figure 1). The median age of the entire cohort was 2.6 years (IQR: 1.2, 9.3). Median age was 2.8 years (IQR: 1.6, 9.1) at CHD, 3.2 years (IQR: 1.0, 14.3) at RCH, and 2.3 years (IQR 0.9, 7.8) at CHOP. One hundred and twelve (39%) children were less than 2 years of age (36 of 110 [33%] at CHD, 22 of 58 [38%] at RCH, and 54 of 118 [46%] at CHOP). The median weight was 14.1 kg (IQR 9.0, 28.1) in the entire cohort (14.1 kg [IQR 11.0, 28.7] at CHD, 15.0 kg [IQR 8.51, 34.4] at RCH, and 13.7 kg [IQR 8.5, 23.5] at CHOP), and 144 (50%) patients were female. The three main indications for transplant were (1) fulminant or acute liver failure (n = 37, 13%), (2) chronic liver failure (n = 194, 68%), and (3) hepatoblastoma or metabolic disease (n = 55, 19%). Only 43 (15%) children received a split liver transplant and 14 (5%) received living donor transplants, all of which were performed at CHOP. The median maximum intraoperative VIS score was 13.0 (IQR: 7.5, 22.0). In 69% and 43% of patients, dopamine and epinephrine infusions were administered during surgery, respectively. In contrast to CHD and CHOP, where dopamine and epinephrine were the primary vasoactive infusions administered, norepinephrine and vasopressin were used most frequently at RCH. At Riley, 90% and 86% received norepinephrine and vasopressin, respectively, compared to 4% and 1% receiving these agents at the other two centers combined. Surgery ended during the day in 153 (53%) of patients, and at night in 133 (47%). The median intraoperative fluid administration volume was 12.5 mL kg−1 h−1 (IQR: 8.7, 16.9) for the entire cohort, and 14.8 mL kg−1 h−1 (IQR 11.9, 19.8) at CHD, 15.1 mL kg−1 h−1 (IQR: 9.4, 21.0) at RCH, and 9.6 mL kg−1 h−1 (IQR: 6.3, 12.1) at CHOP. Median intraoperative fluid volume was 12.2 mL kg−1 h−1 (IQR: 8.4, 16.5) in acute or fulminant hepatic failure patients, 12.5 mL kg−1 h−1 (IQR: 8.7, 16.8) in chronic liver failure patients, and 12.4 mL kg−1 h−1 (IQR: 8.7, 16.7) in hepatoblastoma and metabolic patients. The median proportion of intraoperative fluid comprised of albumin, when not weighted, was 0.37 (IQR: 0.24–0.51) in the cohort. Median intraoperative blood loss was 10.9 mL kg−1 (IQR: 4.3, 20.8) in the entire cohort, 10.3 mL kg−1 (IQR: 3.9, 20.7) in acute or fulminant hepatic failure patients, 10.9 mL kg−1 (IQR: 4.3, 20.8) in chronic liver failure patients, and 10.5 mL kg−1 (IQR: 4.0, 20.7) in hepatoblastoma and metabolic patients. None of the participating centers administered tranexamic acid during liver transplantation during the study period. Median volume of intraoperative blood products administered was 20.1 mL kg−1 (IQR: 8.9, 45.2) in the entire cohort, 23.1 mL kg−1 (IQR: 9.4, 45.7) at CHD, 18.3 mL kg−1 (IQR 7.3, 46.9) at RCH, and 18.9 mL kg−1 (IQR: 8.6, 41.1) at CHOP. Median red blood cell administration in the cohort was 15.3 mL kg−1 (IQR: 6.0, 32.0). Only 53 patients (19%) did not receive any blood products. The median duration of postoperative mechanical ventilation was 10.8 h (IQR: 0.0, 35.4), the median ICU length of stay was 4.3 days (IQR: 2.7, 6.8), and the median hospital length of stay was 13.6 days (9.8, 21.1) (Table 1). Ninety-one (32%) patients were extubated in the operating room at the end of surgery (20 at CHD, 71 at CHOP, and 0 at RCH). Median duration of mechanical ventilation in children not extubated in the operating room was 24.6 h (IQR: 10.0, 58.2) for the entire cohort, 36.3 h (IQR: 15.6, 70.3) at CHD, 13.5 h (IQR: 7.7, 24.1) at RCH, and 30.6 h (IQR: 10.3, 71.0) at CHOP.
| Characteristics | Value | Missing data (%) |
|---|---|---|
| Baseline | ||
| Age, years | 2.6 (1.2, 9.3) | 0 (0) |
| Sex, female | 144 (50) | 0 (0) |
| Weight, kg | 14.1 (9.0, 28.1) | 0 (0) |
| Race | ||
| White | 150 (52) | 1 (0.4) |
| Black | 49 (17) | |
| Other | 86 (30) | |
| Major chronic conditions | ||
| Prematurity | 31 (11) | 0 (0) |
| Chronic lung disease | 12 (4) | |
| Congenital heart disease | 19 (7) | |
| Chronic kidney disease | 14 (5) | |
| Biologic PELD score, mean (SD) | 22 (17) | 58 (25) |
| 1a designation | 20 (9) | |
| 1b designation | 38 (15) | |
| Biologic MELD score, mean (SD) | 23 (12) | 13 (25) |
| 1a designation | 8 (16) | |
| 1b designation | 2 (4) | |
| Indication for liver transplant | ||
| Fulminant liver failure | 20 (7) | 0 (0) |
| Acute liver failure | 17 (6) | |
| Chronic liver failure | 194 (68) | |
| Hepatoblastoma | 24 (8) | |
| Inborn error of metabolism | 31 (11) | |
| Portal hypertension | 163 (57) | 0 (0) |
| Pretransplantation ICU admission | 67 (23) | 0 (0) |
| Pretransplantation mechanical ventilation | 22 (8) | 0 (0) |
| Previous solid organ transplantation | 14 (5) | 0 (0) |
| Preoperative hemoglobin, g dL−1, mean (SD) | 10.3 (2.3) | 1 (0.3) |
| Intraoperative | ||
| Split liver transplant | 43 (15) | 0 (0) |
| Living donor transplant | 14 (5) | 0 (0) |
| Duration of surgery, h | 5.8 (4.8, 6.7) | 0 (0) |
| Blood loss, mL | 200.0 (100.0, 400.0) | 35 (12) |
| Blood loss, ml kg−1 | 10.9 (4.3, 20.8) | 35 (12) |
| Blood loss in living donor transplantation, ml kg−1a | 15.9 (11.7, 48.5) | 1 (7) |
| Red blood cell administration, mL kg−1 | 15.3 (6.0, 32.0) | 0 (0) |
| Plasma administration, mL kg−1 | 0.0 (0.0, 13.7) | 0 (0) |
| Platelet administration, mL kg−1 | 0.0 (0.0, 1.9) | 0 (0) |
| Cryoprecipitate administration, mL kg−1 | 0.0 (0.0, 0.0) | 0 (0) |
| Total intraoperative blood product administration, mL kg−1 | 20.1 (8.9, 45.2) | 0 (0) |
| Prothrombin or fibrinogen concentrate administration | 1 (0.4) | 0 (0) |
| Cold ischemia time, min, mean (SD) | 313 (133) | 81 (28) |
| Warm ischemia time, min, mean (SD) | 46 (19) | 26 (9) |
| Fluid administration, mL kg−1 h−1 | 12.54 (8.7, 16.9) | 0 (0) |
| Maximum vasoactive-inotropic score | 13.0 (7.5, 22.0) | 0 (0) |
| Time hypotensive, % of anesthesia time | 5 (1, 15) | 2 (1) |
| Postoperative | ||
| Duration of mechanical ventilation, h | 10.8 (0.0, 35.4) | 0 (0) |
| ICU length of stay, days | 4.3 (2.7, 6.8) | 0 (0) |
| Hospital length of stay, days | 13.6 (9.8, 21.1) | 0 (0) |
| Fluid administration in first postoperative 24 h, mL kg−1 h−1 | 2.9 (2.0, 4.0) | 0 (0) |
| Maximum vasoactive-inotropic score | 4.0 (0.0, 11.8) | 0 (0.) |
| Maintenance of open abdominal incision | 73 (26) | 0 (0) |
| Hepatic artery thrombosis | 29 (10) | 0 (0) |
| Portal vein thrombosis | 11 (4) | 0 (0) |
| Postoperative acute kidney injury | 132 (46) | 1 (0.3) |
- Note: Unless otherwise indicated, data are presented as median (IQR) or n (%).
- Abbreviations: MELD, model for end-stage liver disease, patients 12 years and older; PELD, pediatric end-stage liver disease, patients younger than 12 years.
- a Fourteen living donor transplantations.
Univariate linear regression showed a weak correlation between intraoperative fluid administration and duration of mechanical ventilation after liver transplant in our study population (r2 = .037, p = .001). Following stepwise multiple linear regression, intraoperative fluid administration remained correlated (r2 = .161, p = .04) with duration of mechanical ventilation after liver transplant. The following variables were also identified as independently correlated with duration of mechanical ventilation: center (RCH vs CHD, p = .001), and maintenance of open abdominal incision after transplant (p = .001). See Table 2 for full results.
| Univariate regression | ||||
|---|---|---|---|---|
| Estimate ± SE (95% CI) | p | r 2 | Units/Reference for estimate | |
| Primary predictor—Intraoperative fluid volume | 1.3 ± 0.4 (0.5, 2.1) | .001 | .037 | 1 mL kg−1 h−1 |
| Age | −0.6 ± 0.6 (−1.7, 0.5) | .29 | .004 | 1 year |
| Sex | ||||
| Female (reference) | .002 | |||
| Male | −5.0 ± 6.0 (−16.8, 6.8) | .40 | ||
| Weight | −0.1 ± 0.2 (−0.4, 0.2) | .53 | .001 | 1 kg |
| Race | ||||
| White (reference) | .006 | |||
| Black | 10.1 ± 8.3 (−6.3, 26.5) | .23 | ||
| Other | 4.4 ± 6.9 (−9.1, 18.0) | .52 | ||
| Prematurity, <37 weeks gestation | −9.5 ± 9.8 (−28.7, 9.7) | .33 | .003 | |
| Chronic lung disease | −6.2 ± 14.9 (−35.5, 23.2) | .68 | .001 | |
| Congenital heart disease | −10.6 ± 12.0 (−34.2, 13.1) | .38 | .003 | |
| Chronic kidney disease | 13.9 ± 13.8 (−13.4, 41.1) | .32 | .004 | |
| Biologic PELD score | −0.5 ± 0.3 (−1.0, 0.0) | .05 | .022 | 1 score |
| Biologic MELD score | −0.4 ± 0.7 (−1.7, 1.0) | .58 | .008 | 1 score |
| 1a/1b | ||||
| Neither (reference) | .007 | |||
| 1a designation | 21.0 ± 10.9 (−26.0, 25.7) | .70 | ||
| 1b designation | 11.9 ± 9.8 (−7.4, 31.2) | .23 | ||
| Transplant indication | ||||
| Fulminant liver failure (reference) | .014 | |||
| Acute liver failure | −12.1 ± 16.7 (−44.9, 20.7) | .47 | ||
| Chronic liver failure | −19.5 ± 11.9 (−42.8, 3.9) | .10 | ||
| Hepatoblastoma | −7.5 ± 15.3 (−37.5, 22.7) | .63 | ||
| Inborn error of metabolism | −20.2 ± 14.5 (−48.7, 8.3) | .16 | ||
| Portal hypertension | 1.0 ± 6.1 (−10.9, 12.9) | .87 | .000 | |
| Pretransplantation ICU admission | 11.7 ± 7.1 (−2.2, 25.6) | .10 | .010 | |
| Pretransplantation mechanical ventilation | 22.8 ± 11.1 (0.9, 44.7) | .04 | .015 | |
| Previous organ transplantation | 8.1 ± 13.9 (−19.2, 35.4) | .56 | .001 | |
| Preoperative hemoglobin | −0.5 ± 1.3 (−3.1, 2.1) | .71 | .001 | 1 g dL−1 |
| Center | ||||
| Children's Health Dallas (reference) | .076 | |||
| Children's Hospital of Philadelphia | −29.8 ± 6.5 (−42.5, −17.0) | .001 | ||
| Riley Children's Health | −25.7 ± 7.9 (−41.2, −10.1) | .001 | ||
| Split liver transplant | −18.4 ± 8.3 (−34.8, −2.1) | .03 | .017 | |
| Duration of surgery | 4.7 ± 1.8 (1.1, 8.3) | .01 | .023 | 1 h |
| Intraoperative blood loss | 0.0 ± 0.0 (0.0, 0.0) | .83 | .000 | 1 mL |
| Intraoperative blood product administration | 7.2 ± 7.7 (−8.0, 22.3) | .35 | .003 | |
| Total intraoperative blood product administration | 0.0 ± 0.1 (−0.1, 0.1) | .37 | .003 | 1 mL kg−1 |
| Cold ischemia time | 0.02 ± 0.01 (0.004, 0.03) | .003 | .042 | 5 min |
| Warm ischemia time | 0.07 ± 0.03 (−0.00, 0.13) | .04 | .016 | 5 min |
| Maximum vasoactive-inotropic score | 0.2 ± 0.1 (−0.1, 0.4) | .30 | .004 | 1 score |
| Intraoperative time hypotensive | −0.1 ± 0.2 (−0.5, 0.2) | .51 | .002 | 1% of anesthesia time |
| Maintenance of open abdomen after surgery | 33.8 ± 6.6 (20.9, 46.7) | .001 | .086 | |
| Stepwise regressiona | ||||
|---|---|---|---|---|
| Estimate ± SE (95% CI) | p | r 2 | Units/Reference for estimate | |
| Primary predictor—Intraoperative fluid volume | 0.9 ± 0.4 (0.0, 1.7) | .04 | .161 | 1 mL kg−1 h−1 |
| Center | ||||
| Children's Health Dallas (reference) | ||||
| Children's Hospital of Philadelphia | −12.1 ± 7.2 (−26.3, 2.1) | .09 | ||
| Riley Children's Health | −33.4 ± 7.8 (−48.8, −18.1) | .001 | ||
| Maintenance of open abdomen after surgery | 36.1 ± 7.3 (21.7, 50.5) | .001 | ||
- Abbreviations: ICU, intensive care unit; MELD, model for end-stage liver disease, patients 12 years and older; PELD, pediatric end-stage liver disease, patients younger than 12 years.
- a Only variables with significant p values are shown.
Univariate linear regressions for secondary and exploratory outcomes can be found in Tables S1 and S2.
Table 3 shows results of the stepwise multiple linear regression for our secondary and exploratory outcomes. Hospital length of stay was independently correlated (r2 = .229) with the following variables: age, chronic lung disease, pretransplant mechanical ventilation, total intraoperative blood product administration, and percent intraoperative time with hypotension. Postoperative fluid administration was independently correlated (r2 = .258) with weight and percent intraoperative time with hypotension. The maximum postoperative VIS was independently correlated (r2 = .419) with maximum intraoperative VIS, intraoperative fluid administration, and total intraoperative blood product administration. ICU length of stay was independently correlated with intraoperative time with hypotension, but with a very low r2 value of .0318.
| Outcome | Variable | Estimate ± SE (95% CI) | p | r 2 | Units/Reference for estimate |
|---|---|---|---|---|---|
| ICU length of stay, days | Intraoperative time hypotensive | 0.20 ± 0.09 (0.03, 0.36) | .02 | .018 | 1% of anesthesia time |
| Hospital length of stay, days | Age | −0.6 ± 0.2 (−1.0, −0.2) | .002 | .229 | 1 year |
| Chronic lung disease | 19.4 ± 5.4 (8.8, 30.0) | .001 | |||
| Pretransplantation mechanical ventilation | 14.5 ± 4.1 (6.4, 22.6) | .001 | |||
| Total intraoperative blood product administration | 0.10 ± 0.02 (0.07, 0.14) | .001 | 1 mL kg−1 | ||
| Intraoperative time hypotensive | 0.02 ± 0.07 (0.05, 0.31) | .008 | 1% of anesthesia time | ||
| Postoperative fluid administration, ml kg−1 h−1 | Weight | −0.04 ± 0.00 (−0.05, −0.03) | .001 | .258 | 1 kg |
| Intraoperative time hypotensive | 0.03 ± 0.01 (0.02, 0.04) | .001 | 1% of anesthesia time | ||
| Maximum postoperative vasoactive inotropic score | Maximum intraoperative vasoactive-inotropic score | 0.3 ± 0.1 (0.1, 0.4) | .001 | .419 | 1 score |
| Intraoperative fluid administration | 0.3 ± 0.1 (0.1, 0.4) | .003 | 1 mL kg−1 h−1 | ||
| Total intraoperative blood product administration | 0.04 ± 0.01 (0.01, 0.06) | .001 | 1 mL kg−1 |
- Abbreviations: ICU, intensive care unit; MELD, model for end-stage liver disease, patients 12 years and older; PELD, pediatric end-stage liver disease, patients younger than 12 years.
- a Only variables with significant p values are shown.
Nine children died after transplant during the hospitalization and were excluded. The median length of time from liver transplantation to death was 4.4 days (IQR: 1.8, 62.1). See Table S3 for characteristics of these patients.
3.1 Post hoc analyses
Median duration of mechanical ventilation in children not extubated in the operating room was 24.6 h (IQR: 10.0, 58.2) for the entire cohort. In a post hoc analysis excluding patients with immediate extubation in the operating room after liver transplant, stepwise multiple linear regression continued to show an association (r2 = .143) between duration of postoperative mechanical ventilation and intraoperative fluid administration (estimated duration of postoperative ventilation increased by 1.2 h [95% CI 0.1, 2.3] for every 1 mL kg−1 h−1 of fluid administration, p = .03), center (patients at RCH had a decrease in postoperative ventilation of 39.8 h [95% CI −57.5, −22.1] compared to patients at CHD, p = .001), and maintenance of open abdominal incision after transplant (duration of ventilation increased by 26.7 h [95% CI 9.9, 43.6], p = .002).
There were 112 patients in our cohort less than 2 years of age. Median duration of ventilation was 13.4 h (IQR: 0.0, 41.7) in this group, compared to 8.9 h (IQR: 0.0, 30.9) in children 2 years old or greater. In children less than 2 years, stepwise multiple linear regression showed associations between duration of postoperative mechanical ventilation (r2 = .228) and the following variables: maximum intraoperative VIS (duration of ventilation increased by 0.6 h [95% CI 0.1, 1.0] for every 1 point increase in score, p = .03), warm ischemia time (duration of ventilation increased by 0.6 h [95% CI 0.0, 1.1] for every 1 min increase, p = .04), and maintenance of open abdominal incision after surgery (duration of ventilation increased by 55.6 h [95% CI 31.9, 79.4], p = .001). In patients greater than or equal to 2 years of age, the following were correlated with duration of mechanical ventilation (r2 = .184): center (patients at RCH had a decrease in postoperative ventilation by 41.5 h [95% CI −59.8, −23.1], p = .001, compared to patients at CHD, and patients at CHOP had a decrease in postoperative ventilation by −28.5 h [95% CI 44.9, −12.0], p = .001 compared to patients at CHD) and maintenance of open abdomen after surgery (duration of increased by 25.5 h [95% CI 8.2, 42.7], p = .004).
Median duration of ventilation was 10.0 h (IQR: 0.0, 33.0) after surgery that ended during the day, and 11.3 h (IQR: 0.0, 35.8) after surgery that ended at night. Univariate linear regression did not show an association between time of day in which surgery ended and duration of mechanical ventilation (estimate ± SE −9.8 ± 7.7; 95% CI −25.0, 5.4, p = .2). Univariate linear regression also did not show an association between postoperative fluid volume administration and duration of mechanical ventilation (estimate ± SE −1.4 ± 2.5; 95% CI −6.3, 3.6, p = .6).
Forty children (14% of the cohort) developed either hepatic artery thrombosis or portal vein thrombosis in the postoperative period. Univariate logistic regression showed a significant but small association with intraoperative fluid administration and the resultant stepwise multivariable model showed an OR of 1.053 (95% CI 1.001, 1.107) of developing this outcome for every 1 mL kg−1 h−1 of intraoperative fluid administered. Postoperative AKI occurred in 132 children (46%). Univariate logistic regression showed for every 1 mL kg−1 h−1 of intraoperative fluid administered, the OR for developing AKI was 0.964 (95% CI: 0.932, 0.997). However, multivariable logistic regression did not show an association (Tables S4 and S5).
4 DISCUSSION
In this multicenter study of pediatric liver transplantation, we found that intraoperative fluid administration volumes were associated with duration of postoperative mechanical ventilation, even after controlling for confounders. However, the r2 value of the linear regression is low, indicating fluid administration is likely not a strong factor impacting this outcome. The effect size was an increase in postoperative ventilation by over 8 h for every 10 mL kg−1 h−1 of intraoperative fluid administration. It is difficult to compare our findings to similar studies, as our choice of analysis differs. In a metanalysis of studies in adult liver transplantation, where fluid management strategies were dichotomized as “restrictive” versus “liberal,” Carrier et al found that restrictive intraoperative fluid management is associated with a shorter duration of mechanical ventilation (mean difference − 13.04 h; 95% CI −22.20, −3.88).7 We included fluid administration as a continuous variable rather than dichotomizing as “liberal” versus “restrictive” to maintain granularity of the data. However, given the weak correlation, it is reasonable to assume we would not have found a difference in duration of ventilation had we dichotomized this predictor.
We are unaware of any pediatric liver transplant studies that have examined the association of volume administration and duration of postoperative ventilation. However, single center studies have compared immediate extubation versus without immediate extubation groups and have found no difference in the amount of crystalloid received.12-14 A recent study also failed to show that red blood cell or albumin administration volumes predict successful extubation in less than 24 h compared to greater than 24 h in pediatric living donor recipients.15 In these studies, the decision to extubate in the operating room was left to the discretion of the anesthesiologist, indicating that the volume of resuscitation did not impact the decision to extubate. In a post hoc analysis with immediate extubation patients (91 children, or 32% of our cohort) excluded, we still found the amount of intraoperative fluid administration correlates with duration of postoperative mechanical ventilation, however, again with a low r2 In this analysis, every 10 mL kg−1 h−1 of intraoperative fluid administration increased the duration of postoperative ventilation by over 12 h.
To calculate fluid volumes, we chose our crystalloid to colloid ratio based on a 2015 meta-analysis examining 24 randomized controlled trials performed in adults with a wide variety of disease states where crystalloid was compared to colloid for resuscitation to achieve prespecified hemodynamic end points. The authors found a pooled crystalloid to colloid ratio of 1.5 in the randomized studies, signifying greater fluid volumes are required to meet the same targets with crystalloids than with colloids.8 We feel it is important to account for the increased relative potency of albumin compared to crystalloid by weighting it more heavily in the calculation of administered fluid volumes. We also factored blood product transfusion volumes in our analysis. While the median volume of blood products administered was 20.1 mL kg−1 in our cohort, it was not predictive of a longer duration of postoperative ventilation. It was, however, associated with a longer hospital length of stay and maximum postoperative VIS (see below).
In addition to intraoperative fluid administration, we found a weak correlation between maintenance of an open abdomen at the end of surgery and duration of postoperative ventilation. Children with an open abdomen had ventilation increased by more than 36 h. This finding is obvious in clinical practice considering children are often left intubated because of the anticipated need for return to the operating room for closure. However, 34% of patients with open abdomens were extubated within 12 h of the end of surgery, which is an unlikely interval for return to the operating room for abdominal closure. Lastly, we found a weak correlation between center and duration of postoperative ventilation. Patients at RCH had a median duration of ventilation decreased by 33 h compared to the other sites. The reasons for this difference are unclear, as a comparative analysis of variables between the three sites in this study was not undertaken. It is possible this finding is due to a culture of earlier extubation at certain transplant centers, but this remains conjecture at this point.
We conducted a post hoc analysis separately examining children under 2 years of age and over 2 years of age given physiological differences between these age groups. Likely because of the smaller sample sizes when assessed separately, we did not find any correlation between amount of intraoperative fluid administered and duration of mechanical ventilation after transplant.
Multiple variables were independently correlated with hospital length of stay, although again with a low r2 limiting the strength of these relationships. Recipient age was associated such that for every 1-year increase in age, hospital length of stay decreased by 0.6 days. This agrees with study findings by Zhang et al, where recipient age less than 2 was associated with a higher odds of hospital length of stay over 30 days.16 We also found pretransplant mechanical ventilation was associated with an increased hospital stay by more than 14 days. In contrast, while Zhang et al found pretransplant ICU status and life support was associated with increased hospital length of stay, they did not find that pretransplant mechanical ventilation was associated.16 In another study of post liver transplant hospital length of stay in children, Covarrubias et al found pretransplant ICU admission was associated with longer length of stay, but they did not measure the impact of pretransplant mechanical ventilation.17 We were unable to find a relationship between pretransplant ICU admission and hospital length of stay, but we may simply have been underpowered to detect it.
In examination of exploratory outcomes, we found weight and percent intraoperative hypotensive time to be weakly associated with postoperative fluid administration. Both relationships showed a small effect. We found maximum postoperative VIS to be weakly associated with maximum intraoperative VIS, intraoperative fluid administration, and total blood product administration. While these relationships are not strong, the latter findings are curious. It is possible that intraoperative circulatory overload led to worsening hemodynamics postoperatively or fluid/blood therapy was used liberally intraoperatively to avoid hypotension, which was unmasked postoperatively.
We chose to exclude children who died in the postoperative period because they died while receiving mechanical ventilation. They tended to be younger, had higher PELD/MELD scores, and required three times as much blood product during the intraoperative course as the rest of the cohort.
While we did not find that intraoperative fluid administration is a strong factor impacting duration of postoperative ventilation after pediatric liver transplantation, in our data, 16% (r2 = .16) of the variance in the outcome was explained by fluid administration, as well as center and maintenance of an open abdomen after surgery. This suggests that fluid management should not be the primary concern when trying to improve postoperative outcomes. However, it is one of the few variables that are under the anesthesiologist's control during these complicated operations. Whether more restrictive or more liberal fluid management strategies should be used, with vasoactive agents utilized more frequently in lieu of fluid resuscitation, has been hotly debated in several clinical scenarios including liver transplantation.2-6, 18, 19 Furthermore, fast tracking pediatric liver transplant recipients with early extubation is a management strategy that is increasingly sought after, with intraoperative fluid management potentially playing a role.12, 14, 20, 21 We may require more tailored fluid management strategies during pediatric liver transplantation, but the best methods for prediction of fluid responsiveness remain unknown. A small study of pediatric patients with cirrhosis undergoing liver transplantation tested the ability of the dynamic preload variables pulse pressure variation, stroke volume variation, and plethysmographic variability index to predict fluid responsiveness. They found significant reductions in values of all three dynamic variables after fluid loading in fluid responders, but not in fluid nonresponders. However, the area under the receiver operating characteristic curves demonstrated only mild to moderate predictive ability for pulse pressure variation and stroke volume variation (0.67 and 0.68, respectively), and poor predictive ability for plethysmographic variability index (0.56). They also tested central venous pressure which similarly had a poor predictive ability with an area under the receiver operating characteristic curve test statistic of 0.57.22 Larger, preferably multicenter studies are needed to further test the predictive ability of these dynamic preload variables to guide fluid resuscitation. In general, collaborative pediatric transplant anesthesia research is desperately needed to develop best practices and standards of care for this highly vulnerable population. A potential strategy to promote collaborative research relationships among pediatric transplant anesthesiologists is through the auspices of society special interest groups.
The retrospective nature of our study has inherent limitations due to potential inaccuracies in charting. Additionally, despite our attempts to control for confounders, residual unmeasured confounding likely occurred.
5 CONCLUSION
The duration of postoperative mechanical ventilation is weakly correlated with the amount of intraoperative fluid administration in children undergoing liver transplantation. Other modifiable factors should be sought which may lead to improved postoperative outcomes in this highly vulnerable patient population.
ACKNOWLEDGMENTS
The authors acknowledge: Emily Melikman, Research Coordinator in the Department of Anesthesiology and Pain Management at the University of Texas Southwestern Medical Center, Dallas, TX, for her assistance with regulatory procedures, and Paula Hu, Research Coordinator in the Department of Anesthesiology and Pain Management at the Children's Hospital of Philadelphia, Philadelphia, PA for her assistance with regulatory procedures and data collection.
FUNDING INFORMATION
Departmental funding was received for the conduct of this study.
CONFLICT OF INTEREST STATEMENT
None of the study authors have any relevant conflicts of interest to disclose.
ETHICAL APPROVAL STATEMENT
For each site, Institutional Review Board approval was obtained, and requirement for written informed consent was waived. All study procedures were conducted in accordance with the Institutional Review Committees at each respective site.
INSTITUTIONAL REVIEW BOARD APPROVALS
Approval was obtained on 02/21/2020 at CHD (Gregory Meyers, board member), 06/03/2020 at CHOP (Dr. Barbara Engel, Chair), and 07/01/2020 at RCH (Michael Turik, Chair).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




