Midlatency auditory evoked potentials during anesthesia in children: A narrative review
Abstract
The brain is considered as the major target organ of anesthetic agents. Despite that, a reliable means to monitor its function during anesthesia is lacking. Mid latency auditory evoked potentials are known to be sensitive to anesthetic agents and might therefore be a measure of hypnotic state in pediatric patients. This review investigates the available literature describing various aspects of mid latency auditory evoked potential monitoring in pediatric anesthesia.
1 INTRODUCTION
The relationship between anesthesia and the electrical activity of the brain has been described as early as in the 1940's.1 Development in this field has been extended to the investigation of the relationship between the brain activity and depth of anesthesia.2 Research of the brain activity as a surrogate to monitor the depth of hypnosis during anesthesia has mostly focused on the spontaneous electroencephalogram (EEG). However, the spontaneous EEG does not mature before adult age, which could influence its reliability in children.3 Although Mid-Latency auditory evoked potentials (MLAEP) are electrical brain activity, they are fundamentally different from the spontaneous EEG. These potentials are electric responses of the brain to an auditive stimulus. They occur at about 8–50 ms after the stimulus, which is after the auditory brainstem response (0–8 ms) and before the late cortical response (>50 ms). Needless to say, an impaired auditory pathway and/or inadequate delivery of the acoustic stimulus (poor fit of the earbuds in children) will make the recording of a MLAEP unreliable or even impossible.
A typical MLAEP waveform consists of 3 troughs (labelled with N for negative polarity) and 2 peaks (labelled with P for positive polarity) of a few microvolts (Figure 1). To put in perspective, the EEG is about ten times stronger and the ECG is about 100 times stronger. The weak signal of the MLAEP makes it susceptible to noise and diminishes its signal quality. The peaks and troughs are commonly labeled as N0, P0, Na, Pa and Nb and occur, in the awake adult, at about 9 ms, 12 ms, 16 ms, 25 ms and 36 ms, respectively, after the application of an auditive stimulus.4 The MLAEP is believed to be generated from the medial geniculate body and primary auditory cortex.5 In anesthetized adult patients, the peaks and troughs decrease in amplitude and the interval when they occur, which are called latencies, increases.5-9 Myelination of the brain in de developing child strongly influences evoked potentials, this makes it difficult to elicit these in infants having an age of less than 1 year and very difficult in infants less than 6 months of age. MLAEP mature after the first decade of life, around 10–12 years.10-13
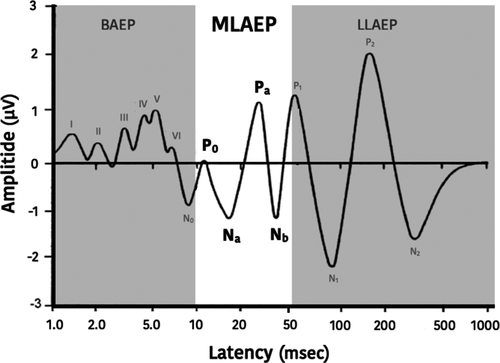
Mid-Latency auditory evoked potentials could be a parameter to measure the depth of hypnosis in anesthetized children. This narrative review summarizes the current literature concerning the use of MLAEP and its implications in anesthetized children.
2 METHODS
This study adheres to the Preferred Reporting Items for Systematic Reviews and MetaAnalysis (PRISMA) guidelines, since it was initiated as a systematic review. A literature search was performed with the assistance of an experienced librarian of the medical library (W.B.) at Erasmus University Medical Center in Rotterdam. The databases of Embase, Medline, Web-of-science, CINAHL, Cochrane, PubMed publisher and Google Scholar were consulted on April 30 2020. The query consisted, but not solely, of the following search terms: “evoked potential”, “anesthesia” and “children”. A detailed description of the search query per database can be found in Appendix S1.
Manuscript titles were screened for relevance by two authors (Y.C. and I.H.). Any type of clinical study investigating MLAEP during general anesthesia in children with any type of outcome measurements was considered relevant. Manuscripts published in languages other than English or Dutch, case reports and review manuscripts were excluded from analysis. When no consensus could be reached between the two authors about the relevance of a study, the abstract and/or full text was reviewed. When needed a third author (F.W.) was consulted to resolve the matter.
The quality of the studies was assessed with the “Quality Assessment of Diagnostic Accuracy Studies v 2” (QUADAS-2) tool.14 This tool systematically screens the risks of bias and applicability of the studies included in a systematic review in four key domains: patient selection, index test, reference standard, and flow and timing of the study. As recommended in the background document, the tool was tailored to suit our review question.15 The assessment for the “reference standard” was omitted, since all types of outcome measurements were marked as relevant (eg, MLAEP compared to clinical hypnotic depths, anesthetic use, hypnosis index of other types of monitors).
3 RESULTS
The search resulted in 1539 manuscripts of which 891 remained after removal of duplicates. Manual screening of titles, keywords and abstracts resulted in 45 manuscripts. A careful review of the remaining studies revealed that 15 included only adult patients, 6 were written in a language other than Dutch or English, four concerned patients from the pediatric intensive care unit, one was a case report, one investigated children without pharmacological sedation and in three cases the full text was unavailable. This resulted in the inclusion of 15 studies which were considered relevant for analysis in this review (Appendix S2). Due to the limited number of included studies and varying (primary) objectives, a meta-analysis was deemed futile.
3.1 Quality assessment of studies
Table 1 summarizes the quality of the studies according to the QUADAS-2 tool. Since none of the manuscripts described a randomized or blinded patient selection method, the risk for bias due to patient selection was rated “unclear” for all studies. The study by Alvarez et al. was only published as an abstract and was therefore missing details concerning “patient selection” and “flow and timing”.16 Accordingly, these domains were rated unclear for risk as well as for applicability concerns. Depth of hypnosis was assessed by three studies with clinical parameters, for example, movement and/or vocalization, without the use of validated tools.17-19 In one study the same researcher recorded the index values and assessed the depth of hypnosis.20 For these four studies, the risk of bias in the reference standard were rated as unclear. Two studies were rated unclear for their risk of bias in the domain of “Flow and Timing”, because one study was terminated prematurely due to change in the anesthesia practice 18 and the other failed to generate data from 4 of the 14 patients.21 Seven studies allowed premedication for their patients and were therefore rated as “unclear” for applicability concerns of patient selection.17, 18, 20-24 Studies were conducted with 3 different commercially available MLAEP hypnosis monitors or unprocessed MLAEP for analysis. Because each MLAEP derived hypnosis monitor has its own unpublished algorithm, it is unclear how different devices relate to each other. Therefore, the applicability concerns of the index test (MLAEP monitor) were rated “unclear” for all manuscripts.
| Study | Risk of bias | Applicability concerns | ||||
|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | |
| Liao et al. 2001 |

|

|

|

|

|

|
| Weber et al. 2004 |

|

|

|

|

|

|
| Alvarez et al. 2004 |

|

|

|

|

|

|
| Weber et al. 2005 |

|

|

|

|

|

|
| Ironfield et al. 2007 |

|

|

|

|

|

|
| Disma et al. 2007 |

|

|

|

|

|

|
| Daunderer et al. 2007 |

|

|

|

|

|

|
| Blussé van Oud-Alblas et al. 2008 |

|

|

|

|

|

|
| Blussé van Oud-Alblas et al. 2008 |

|

|

|

|

|

|
| Feuerecker et al. 2011 |

|

|

|

|

|

|
| Kuhnle et al. 2013 |

|

|

|

|

|

|
| Cheung et al. 2013 |

|

|

|

|

|

|
| Cheung et al. 2014 |

|

|

|

|

|

|
| Cheung et al. 2018 |

|

|

|

|

|

|
| Blussé van Oud-Alblas et al. 2019 |

|

|

|

|

|

|
Note
-
 Low Risk
Low Risk  Unclear Risk
Unclear Risk  High Risk.
High Risk.
3.2 MLAEP extraction
Unprocessed MLAEP (measurement of the actual latencies), were used as the index test by three studies.17, 21, 22 The remaining 12 studies used commercially available depth of hypnosis monitors. Three of the studies used the A-line monitor (Danmeter A/S, Odense, Denmark).19, 20, 23 To extract a reliable MLAEP from the EEG, measurements must be repeated several times (usually 250 to 1000 times). The A-line monitor uses the already collected data from the same patient to compute the MLAEP waveform faster (after 15 repetitions), that is, an autoregressive with exogenous input model.25 This results in the A-line Autoregressive Index (AAI) as a measurement for the depth of hypnosis.26
The AEP monitor/2 (Danmeter A/S, Odense, Denmark) is the successor of the A-line monitor and was used in 6 studies.16, 18, 24, 27-29 It circumvented situations in which the depth of hypnosis could not be measured due to absent or low-quality MLAEP (due to interference or (too) deep anesthesia) by analyzing the spontaneous EEG instead. The computed index value was called the composite A-line Autoregressive Index (cAAI).28
The studies by Cheung et al. were conducted with the aepEX PLUS monitor (Medical Device Management Ltd., Braintree, Essex, UK).30-32 Instead of an autoregressive model, it applied the more conventional “moving time averaging” technique to extract the MLAEP and to compute the aepEX index. This technique requires 256 repeated measurement to extract an entirely new MLAEP waveform, which takes about 37 s. Instead of computing an entirely new MLAEP waveform every 37 s, the MLAEP and aepEX index is updated every 0.3 s by discarding the first (oldest) measurement and adding the last (newest) measurement.30
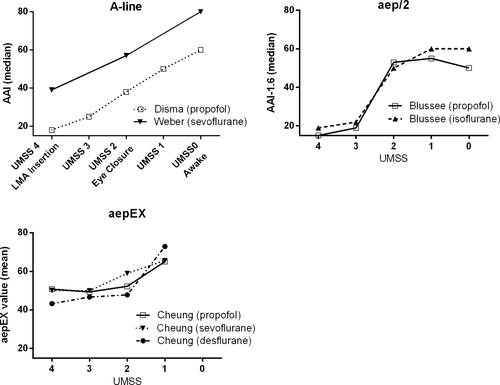
Seven studies using a commercially available MLAEP monitor reported the average index values observed during different depths of hypnosis.20, 23, 27, 28, 30-32 These values are illustrated in Figure 2. The primary objective of these seven studies was to evaluate the performance of a MLAEP monitor to detect different levels of depth of hypnosis in children (Table 2).20, 23, 27, 28, 30-32
| Study | Outcome | Results | Missing data due to monitor failure |
|---|---|---|---|
| Weber et al. 2004 | pka of AAIb during awake versus LMAc insertion (mean ± SE) | 0.99 ± 0.00 | Not reported |
| pk of AAI during awake versus eye closure (mean ± SE) | 0.77 ± 0.08 | ||
| pk of AAI during eye closure vs LMA insertion (mean ± SE) | 0.83 ± 0.07 | ||
| Disma et al. 2007 | Correlation between AAI and UMSSd | r: −.8237 (p: <.0001) | Not reported |
| Correlation between AAI and heart rate | r: .2382 (p: <.05) | ||
| Blussé van Oud-Alblas et al. 2008 | pk of cAAIe versus UMSS (mean ± SE) | 0.90 ± 0.08 | 26 data points (total 325 included) |
| pk during induction of cAAI versus UMSS (mean ± SE) | 0.84 ± 0.08 | ||
| pk during emergence of cAAI versus UMSS (mean ± SE) | 0.74 ± 0.02 | ||
| Blussé van Oud-Alblas et al. 2008 | pk during induction of cAAI versus UMSS (mean ± SE) | 0.61 ± 0.1 | 1 patient out of 21 patients |
| pk during Wake-up test of cAAI versus UMSS (mean ± SE) | 0.79 ± 0.05 | ||
| pk during Emergence of cAAI versus UMSS (mean ± SE) | 0.78 ± 0.03 | ||
| Cheung et al. 2013 | pk aepEX versus UMSS (mean [95% CI]) | 0.64 (0.55–0.72) | 43 patients out of 69 patients |
| AUCf (mean [95% CI]) | 0.77 (0.68–0.85) | ||
| Cheung et al. 2014 | pk of aepEX versus UMSS (mean (95% CI)) | 0.68 (0.44–0.92) | 59 patients out of 69 patients |
| AUC (mean [95% CI]) | 0.72 (0.62–0.82) | ||
| Cheung et al. 2018 | pk of aepEX versus UMSS (mean [95% CI]) | 0.68 (0.53–0.82) | 32 patients out of 45 patients |
| AUC (mean [95% CI]) | 0.89 (0.80–0.95) |
- a Prediction probability.
- b A-line autoregressive index.
- c Laryngeal mask airway.
- d University of Michigan sedation scale.
- e Composite A-line ARX index.
- f Area under the receiver operator characteristics curve.
Six studies investigated the MLAEP during different amounts of anesthetics or during certain anesthetic procedures, such as intubation and extubation17-19, 21, 22, 29 and 3 studies investigated the effect of MLAEP monitoring on efficiency of the anesthetic regime, such as recovery time from anesthesia and total anesthetics used (Table 3).16, 19, 24
| Study | Outcome | Results |
|---|---|---|
| Alvarez et al. 2004 | Recovery time: controls vs cAAIa vs BIS guided anesthesia respectively (seconds) | 419 vs 411 vs 396 (p = .993) |
| Mean Etsevob: controls vs cAAI vs BIS guided anesthesia (vol%c) | 1.71 vs 1.50 vs 1.87 (p = .442) | |
| Weber et al. 2005 | Propofol requirement of cAAI vs control group (mg kg−1 h−1: mean ± SD) | 4.2 ± 1.7 vs 6.4 ± 1.3 (p < .01) |
| Emergence time: cAAI vs control (minutes: mean ± SD) | 5.1 ± 3.7 vs 13.2 ± 8.2 (p < .01) | |
| Daunderer et al. 2007 | Latencies (Na, Pa, Nb, and P1) “Awake” vs “Intubation” or “Steady state” | Lower during “Awake” (p < .05) |
| Latencies (Na, Pa, Nb and P1) “Awake” vs “Extubation” | p > .05 | |
| Ironfield et al. 2007 | pkd of AAIe versus EtSevo of 0–1 year old (mean ± SE) | 0.53 ± 0.14 |
| pk of AAI versus EtSevo of 2 to11 years old (mean ± SE) | 0.53 ± 0.10 | |
| Feuerecker et al. 2011 | Latency of Na vs MACf sevoflurane of 2 months to 3 years old | r = 0.237 (p = .163) |
| Latencies vs MAC sevoflurane of 2 months to 3 years old (Spearman's correlation: Pa, Nb and P1 respectively) | 0.663, 0.783, 0.752 (p < .0001 for all) | |
| Latency of Na vs vol% sevoflurane of 2 months to 3 years old | r: .214 (p = .208) | |
| Latencies vs vol% sevoflurane of 2 months to 3 years old (Spearman's correlation: Pa, Nb and P1 respectively) | 0.688, 0.768, 0.735 (p < .0001 for all) | |
| Latency of Na vs MAC sevoflurane of 6 to 14 years old | r = 0.523 (p = .0003) | |
| Latencies vs MAC sevoflurane of 6–14 years old (Spearman's correlation: Pa, Nb and P1 respectively) | 0.734, 0.853, 0.868 (p < .0001 for all) | |
| Latency of Na vs vol% sevoflurane of 6–14 years old | r = .513 (p = .0003) | |
| Latencies vs vol% sevoflurane of 6–14 years old (Spearman's correlation: Pa, Nb and P1 respectively) | 0.728, 0.845, 0.860 (p < .0001 for all) | |
| Latencies (Na, Pa, Nb or P1) preopioid vs postopioid application | p > .05 | |
| Liao et al. 2011 | Correlation between AAI and EtSevo | R2 .01 (p = .01) |
| Time to spontaneous movement: AAI vs control (minutes: mean ± SD) | 3.9 ± 3.7 vs 6.1 ± 5 (p = .02) | |
| Time to fit for discharge: AAI vs control (minutes: mean ± SD) | 60.5 ± 10.0 vs 66.8 ± 9.0 (p = .03) | |
| Kuhnle et al. 2013 | Latencies (Na, Pa, Nb or P1) during serum propofol 0, 3, 6 and 9 µg ml−1 | p < .05 (except Na: 3 vs 6 µg ml−1) |
| Blussé van Oud-Alblas et al. 2019 | Model to predict cAAI with serum propofol concentrations (mean [CV%]) |
E0: 63.4 (14.9) Emax: 0.786 (6.1) γ: 6.85 (46.4) |
- a Composite A-line ARX index.
- b Endtidal sevoflurane.
- c Volume percentage.
- d Prediction probability.
- e A-line autoregressive index.
- f Minimum alveolar concentration.
Two studies investigated the effect of age on the MLAEP as the primary objective17, 18 and four manuscripts investigated age as their secondary objective.22, 30-32 A brief summary of these studies can be found in Table 4.
| Study | Outcome | Results |
|---|---|---|
| Ironfield et al. 2007 | pka of AAIb vs EtSevoc (age: 0–1 years) (mean ± SE) | 0.53 ± 0.14 |
| pk of AAI vs EtSevo (age: 2–11 years) (mean ± SE) | 0.53 ± 0.10 | |
| Daunderer et al. 2007 | Latencies (Na, Pa, Nb, P1) of <4 years and >4 years | NSd |
| Feuerecker et al. 2011 | Latencies (Na, Pa, Nb and P1) “infants” vs “schoolchildren” during “Awake” or 1.3vol%e or 2.6vol% sevoflurane |
Nb at 2.3vol%; p < .5 Na, Pa and P1; NS |
| Cheung et al. 2013 | EC50f and AUCg of aepEX vs UMSSh (age: 1–3 vs 3–6 vs 6–16 years) during propofol anesthesia | NS |
| Cheung et al. 2014 | EC50 and AUC of aepEX vs UMSS (age: 1–3 vs 3–6 vs 6–18 years) during sevoflurane anesthesia | NS |
| Cheung et al. 2018 | EC50 and AUC of aepEX vs UMSS (age: 1–3 vs 3–6 vs 6–18 years) during desflurane anesthesia | NS |
- a Prediction probability.
- b A-line autoregressive index.
- c Endtidal sevoflurane.
- d Not significant.
- e Volume percentage.
- f Half maximal effective concentration.
- g Area under the receiver operator characteristics curve.
- h University of Michigan sedation scale.
3.3 Propofol
Six studies described MLAEP during propofol anesthesia.20, 21, 24, 27, 29, 32 The effect of propofol on the components of the unprocessed MLAEP in children (with a mean age of 8.6 years) has been described by Kuhnle et al.21 They observed a dose related increase of latencies (Na, Pa, Nb and P1) and decreasing amplitudes.
Three studies investigated the performance of a MLAEP-based depth of hypnosis monitor during propofol anesthesia in children to detect the depth of hypnosis as defined by the University of Michigan Sedation Scale (UMSS).20, 27, 32 Disma et al. demonstrated that the A-line monitor had a spearman's correlation of r = −0.82 (p < .0001) in children 8 months to 7 years old receiving deep sedation for magnetic resonance imaging or esophagogastroduodenoscopy.20 In older children (10–20 years), Blussé van Oud-Alblas et al. used a wake-up test during scoliosis surgery to evaluate the AEP monitor/2 (the successor of the A-line monitor), which resulted in a prediction probability (pk) for the UMSS of 0.79 intra-operatively and 0.78 during emergence from anesthesia.27 A prediction probability describes the proportion in which the monitor predicts the correct value of any chosen parameter, for example, UMSS, dose of anesthetics. Its use has been a cornerstone in studies concerning monitoring the depth of hypnosis in children. In contrast to the study by Blussé van Oud-Alblas et al., a relatively low pk value to predict the UMSS of 0.64 for the aepEX monitor was observed by Cheung et al.32 The receiver operation characteristics (ROC), however, revealed an area under the curve (AUC) of 0.77.32 A recently published study by Blussé van Oud-Alblas et al. described a sigmoidal relationship between propofol serum concentration and AEP monitor/2 index values from a selection of the population from their previous published study.27, 29 They described the sigmoidal shape as steep for adolescents aged from 10 to 20 years, having a Hill coefficient of 6.85.29 This steep relationship was also noticeable between the aepEX PLUS monitor and propofol concentrations for children of 1 to 16 years, as predicted by the Propofol Paedfusor model, during the emergence period (propofol concentration of <2.0 mcg/ml).32 The Propofol Paedfusor model predicts the serum concentrations of propofol in a child after defining his/her age, weight and gender, and can be used for target-controlled infusion anesthesia in children.33-35
Implementation of an AEP monitor/2 guided propofol anesthesia in children was described by Weber et al. and resulted in a 34% decrease in propofol consumption and a reduction of 8 min in emergence time, compared to standard practice.24
3.4 Sevoflurane
Mid latency auditory evoked potentials during Sevoflurane anesthesia were described by 7 papers. Feuerecker et al. described the effect of increasing steady-state concentrations of sevoflurane on MLAEP in infants, schoolchildren and elderly.22 The peak latency of Pa, Nb and P1 increased significantly with increasing MAC levels of Sevoflurane in all age groups. The mean MLAEP peak latency Nb at 2.3vol% appeared later with increasing age, when comparing schoolchildren with infants (91.86 ms ± 14.10 vs 74.71 ms ± 10.15 p < .05). The administration of sufentanil had no influence on the peak latencies for all age groups. Daunderer et al. also observed increased peak latencies when anesthesia was maintained with sevoflurane (endtidal of 2.30vol% ± 0.43) or isoflurane (endtidal of 1.39vol% ± 0.19) compared to the awake child of 1–10 years old.17 However, they also found that the mean amplitude during steady-state anesthesia (2.30vol% sevoflurane and 1.39vol% isoflurane) was higher than during the awake state. No subgroup analysis for sevoflurane and isoflurane was performed.
The performance of the AAI during induction of anesthesia was evaluated by Weber et al.23 They used the pk to evaluate the ability and accuracy of the AAI to distinguish between the awake state, the moment of spontaneous eye closure and the moment of laryngeal mask (LMA) insertion. Weber et al. found a pk value of .99 (SE 0.00) for the AAI to distinguish between the awake state versus a clinical state of unconsciousness at LMA insertion. The awake state vs spontaneous eye closure resulted in a pk value of .77 (SE 0.08). Ironfield et al. investigated the AAI-1.6 as a predictor of sevoflurane concentration in infants (0–1 year) and older children (2–11 years).18 The performance of the AEP monitor/2 and BIS were compared using prediction probability. Index values during different endtidal concentrations of sevoflurane (2.5%, 2.0% and 1.5%) and 1 min preawakening were used. The mean pk values for the AAI to predict the endtidal sevoflurane in both age groups were low (0.53 ± 0.14 (0–1 year) vs 0.53 ± 0.10 (2–11 years)). However, this study was terminated early, because of a fundamental shift in patient population. Therefore, only nine children were enrolled. The effect of MLAEP guided anesthesia on recovery after sevoflurane anesthesia was investigated by Liao et al. and Alvarez.16, 19 After discontinuation of sevoflurane, Liao et al. found that the mean time to spontaneous movement was faster in de AAI group compared with the standard practice group (3.9 min ± 3.7 vs 6.1 ± 5.7, p = .02). In addition, in the AAI group the mean time until fit for discharge was significantly shorter than in standard practice (60.5 ± 10.0 vs 66.8 ± 9.0, p = .03).19 Alvarez et al., however, observed no such difference in recovery time when sevoflurane with N2O anesthesia was guided with the newer AEP monitor/2.16 They also did not find a difference between the mean endtidal sevoflurane concentration administered during anesthesia.
Cheung et al. investigated the performance of the aepEX in distinguishing unconsciousness from consciousness using the UMSS.30 In this study, the aepEX performed reasonably in terms of having a pk value of .68 and AUC of 0.72.
3.5 Isoflurane
Another study by Blussé van Oud-Alblas et al. investigated the AEP monitor/2 during isoflurane anesthesia in children with a mean age of 6.2 years.28 A pk value of .74 to predict the UMSS during emergence from anesthesia was observed. Daunderer et al. administered isoflurane or sevoflurane during the maintenance of anesthesia in children.17 They observed an increase in latencies after induction and a decrease after anesthetics were discontinued. Interestingly, they also found that the amplitude of the MLAEP was significantly higher during steady state compared during the awake state. However, no subgroup analysis was performed for children receiving isoflurane or sevoflurane during anesthesia maintenance.
3.6 Desflurane
The study of Cheung et al. was the only study investigating the MLAEP during desflurane anesthesia. They found that the aepEX monitor predicts the correct UMSS with a pk value of 0.68, while it could discriminate between unconscious and conscious state with a AUC of 0.89.31
3.7 Age
Comparing children aging >4 years to <4 years old, Daunderer et al. found a statistically nonsignificant increase of all latencies of the MLAEP in the older group.17 Feurecker et al., on the other hand, demonstrated a significantly increased Pa, Nb and P1 at 1.3vol% sevoflurane between infants (2 months to 3 years) and elderly patients (75–89 years). At 2.3vol% sevoflurane, an increased Na (schoolchildren (6–14 years) versus infants) and Nb (schoolchildren versus infants and elderly versus infants) were observed.22 The studies by Ironfield et al. and Cheung et al. investigated a commercially available MLAEP monitor and its performance (prediction probability and area under the ROC) between age groups of <1 year versus ≥1 year18 and <3 years versus 3–6 years versus ≥6 years.30, 31 No differences between the age groups were observed, however, in these studies. Table 4 summarizes studies concerning the effect of age on MLAEP.
4 DISCUSSION
Despite an extensive search and diligent screening of the current literature, we found no more than 15 relevant manuscripts reporting the use of MLAEP monitoring during anesthesia in children. Studies investigating the clinical performance of an MLAEP monitor as a depth of hypnosis monitor demonstrated a moderate to high correlation with the depth of hypnosis and an age-independent performance up to 14 years.17, 18, 20, 22, 23, 27, 28, 30-32 However, studies investigating the relationship between MLAEP and anesthetic drug dose showed conflicting results.18, 19, 21, 22 These differences might be explained by the algorithm used to compute an index value of the commercially available MLAEP devices.
Studies investigating the MLAEP waveform, observed increasing latencies and decreasing amplitudes when increasing the dose of anesthetics.21, 22 One study described an increased amplitude during anesthesia.17 When administering sufentanil to a child, the MLAEP waveform does not change.22 This might indicate that opioids don't have a clinically relevant effect on the depth of hypnosis and that the MLAEP waveform represents the depth of hypnosis component of anesthesia.
When investigating a commercially available MLAEP monitor, its relationship with the endtidal sevoflurane showed a weak correlation.18, 19 Liao et al. found a r2 of 0.03 for their linear regression model,19 while Ironfield et al. demonstrated a pk value of 0.53.18
It might be expected that this weak correlation and inconsistent change of the amplitude influences the performance of an MLAEP derived depth of hypnosis monitor. However, when analyzing the clinical performance of the A-line, AEP monitor/2 and aepEX monitor as a predictor for the UMSS during emergence, a pk value ranging from .64 to .78 was observed during all commonly used anesthetics (propofol, isoflurane, sevoflurane, and desflurane) in children.27, 28, 30-32
An even higher pk value (.99) was reported by Weber et al. when used to predict the awake state and movement of the patient during LMA insertion.23 We can assume that an awake person has an UMSS ≤1 and that LMA insertion is a significant physical stimulus; thus, having an UMSS ≥3 when a patient does not react during LMA insertion. Figure 2 illustrates the relationship between the index values and UMSS of each type of MLAEP monitor. Of note, the UMSS is as the name implies a tool to measure the depth of sedation. An UMSS of 4, in which the patient is unarousable, can be considered an adequate depth of hypnosis for anesthesia. However, when an even “deeper” hypnotic depth is needed or a measurement of the anesthetic depth is needed, the UMSS is not validated to measure this. The first commercially available MLAEP monitor designed for use as a depth of anesthesia monitor, the A-line, showed a linear relationship with the UMSS, while the newer models, that is, the AEP monitor/2 (which superseded the A-line) and the aepEX monitor, show a sigmoidal relationship. The sigmoidal relationship could suggest a binary behavior for the depth of hypnosis, that is, consciousness and unconsciousness, instead of a gradual relationship. By performing a Receiver Operating Characteristics (ROC) analysis, Cheung et al. investigated the performance of the aepEX to detect conscious (UMSS ≤1) and unconscious (UMSS ≥2) patients.30-32 They found an area under the curve (AUC) of 0.72–0.89, which implies that the aepEX is a reasonable device to distinguish between consciousness and unconsciousness.
While the EEG matures in early adulthood, MLAEP mature in the first decade of life.10-13 This might give the MLAEP a slight advantage over the EEG as a variable to assess the depth of hypnosis in anesthetized children. Six out of 15 studies investigated the effect of age on the MLAEP. Five studies comparing the MLAEP of children from different age groups showed no significant differences in latencies17 nor index values.18, 30-32 Only one study, by Feurecker et al., revealed increasing latencies with increasing age. This was, however, only apparent when comparing infants with elderly patients,22 indicating the consistency of the MLAEP as a parameter for assessing the depth of hypnosis in children up to the age of 14 years.
There are conflicting results concerning MLAEP guided anesthesia to improve the efficiency of the anesthetic regime. Weber et al. demonstrated that the time needed for emergence was reduced by 8 min and less propofol was needed when anesthesia was cAAI guided.24 During a day planned with many short procedures, an 8 min reduction in emergence time could allow more patients. However, in the study of Liao et al. AAI guided anesthesia only saved about 2 min until the patient moved after surgery and saved 6 min until the patient was fit for discharge, of which the clinical relevance can be questionable.19 Alvarez et al., on the other hand, could not demonstrate these benefits at all with cAAI guided anesthesia.16 Although Alvarez et al. had a larger number of patients included in their study compared to Weber et al., essential details are lacking from their study since only a published abstract could be found.
In daily practice, application of a MLAEP monitor in children can be challenging. Manufacturers of these monitors usually supply an in-ear headphone with the device, which cannot be adequately placed in the small ear canal of a child. Published manuscripts try to circumvent this problem by securing the earpieces with tape or replacing the headphones with their own over-ear headphones.18, 23, 30-32
The low voltage of the MLAEP waveform (a few hundred microvolts) makes it prone to noise and artifacts. This is especially evident when trying to measure MLAEP in awake young children or during the induction and emergence phase, making its clinical application challenging. This was also illustrated by the study of Daunderer et al. in which they described that only 1645 of the total 8544 AEP segments could be used for analysis.17 The studies by Cheung et al. reported that paired pk values during emergence from propofol, sevoflurane and desflurane could only be computed for respectively 26 out of 69, 10 out of 69 and 13 out of 45 patients.30-32 However, this could also be attributable to the failure of the BIS monitor.
Another consideration about the MLAEP waveform is that it is not fully developed in most children under 10 years.12 Despite that, MLAEP-based depth of hypnosis monitors can still compute a fairly reliable index value to indicate the depth of hypnosis.
Very few studies concerning the use of MLAEP monitoring in children during anesthesia are conducted. This review yielded only 15 studies concerning this subject. Due to the diversity in defined outcome parameters and methodological differences (eg, different anesthetics, premedication and type of monitor used) a comparative (meta)analysis was considered not meaningful. We therefore lack a statistically grounded recommendation in whether MLAEP monitoring is beneficial during anesthesia in children. This review, however, attempts to systematically highlight the current available literature, so that the reader can make an educated decision on whether MLAEP monitoring could benefit his or her anesthesia.
In conclusion, the current literature shows that MLAEP analysis can be used to reasonably assess the depth of hypnosis in children under anesthesia. Furthermore, its reliability does not seem to depend on the age of the child or the type of commonly used anesthetics according to the current published studies. However, only a few studies investigated the performance of an MLAEP monitor as a depth of hypnosis monitor and conclusions regarding its reliability should be made with caution.
ACKNOWLEDGMENT
We would like to thank the librarians, especially Wichor Bramer, at the medical library of the Erasmus medical center for their assistance in formulating and performing the search queries. We would also like to thank dr. Scoones for her English editing. This study was funded by departmental funding only.
CONFLICT OF INTEREST
All authors have no conflicts of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




