How femoral morphology informs our understanding of the evolution of ornithopod locomotion and body size
Editor. Laura Porro
Abstract
Ornithopoda is one of the three main ornithischian dinosaur clades in which secondary quadrupedality is represented. However, when it evolved from obligate bipedality remains controversial. Indeed, the ability to alternate between the two habits was inferred in some ornithopods based on ichnological observations and a mosaic of bipedal and quadrupedal skeletal features. Yet a straightforward inference of such ability is complicated by the concomitant evolution of enlarged body mass, hence confusing the interpretation of skeletal features as related to quadrupedality or weight support. Previous 3D geometric morphometrics studies distinguished locomotor specializations from enlarged body mass in various archosaur femora (e.g. Triassic pseudosuchians and avemetatarsalians and giant Jurassic–Cretaceous theropods). Our study better characterizes ornithopod specializations by analysing 41 femora using 3D geometric morphometrics and comparing the results with those of the aforementioned studies. Whereas hadrosaur femora possessed quadrupedal archosaurian features (e.g. anteroposteriorly straight shaft, a symmetric fourth trochanter and a more obtuse angle between the lateral condyle and the crista tibiofibularis), femora from ornithopods with indeterminate locomotion were distinct from those of the obligate bipedal ones by having a laterally-bowed shaft (i.e. oblique femur). Oblique femora seem associated with a wider-gauge stance, previously inferred as indicating static bipedal abilities in a quadrupedal dinosaur clade (Titanosauria). Our study disambiguated femoral specializations to locomotor habit and weight support in heavy ornithopods with indeterminate locomotion, adding evidence that hadrosaurs were obligate quadrupeds and suggesting that non-hadrosaurian ankylopollexians and Rhabdodontidae may have had some facultative static bipedal abilities, which remain to be investigated biomechanically.
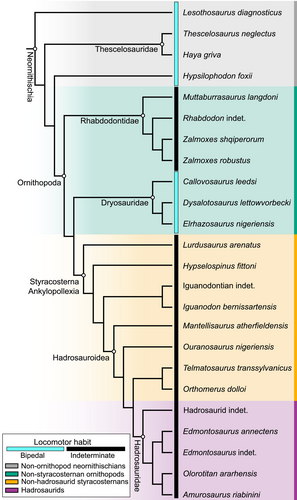
Ornithopoda is a clade of ornithischian dinosaurs which originated in the Middle Jurassic, with the oldest documented representative being the dryosaurid Callovosaurus leedsi from the Callovian (Ruiz-Omeñaca et al. 2006), although its origin might even be Early Jurassic (Dieudonné et al. 2021). Ornithopods were unarmoured herbivores with unique feeding specializations (e.g. narrow muzzle with multiple tooth rows; Norman & Weishampel 1985; Brett-Surman 1997; Norman et al. 2004). Their body mass ranged from a few kilogrammes in small dryosaurids such as Elrhazosaurus nigerensis to more than five tons in large hadrosaurids such as Parasaurolophus walkeri, and some could have evolved a body mass larger than 20 tons, such as Shantungosaurus giganteus (Seebacher 2001; Xing et al. 2014). The phylogenetic definition of Ornithopoda has changed repeatedly since it was first named, and robust ornithopod synapomorphies have been challenging to establish, mostly because of unstable relationships among early-diverging taxa (Brown et al. 2021). This instability is mostly related to the paraphyletic assemblage ‘hypsilophodontids’ being recovered as diverging earlier than Ornithopoda and marginocephalians (Boyd 2015; Madzia et al. 2018) or later as early-ornithopod relatives (referable or not to Ornithopoda; Farlow & Brett-Surman 1997; Sereno 1999; Butler et al. 2008; Rozadilla et al. 2016).
Avemetatarsalians are the only clade of tetrapods known to have evolved secondary quadrupedality (i.e. a reversion to quadrupedal habit from a plesiomorphically bipedal condition), and most occurrences are observed in ornithischian dinosaurs (e.g. Ceratopsia, Thyreophora). However, and in addition to phylogenetic uncertainties, estimations regarding ornithopod locomotion have been controversial since their first discovery. The earliest reconstructions of the first documented ornithopod Iguanodon (Mantell 1825) assumed it was quadrupedal based on its body proportions and, by extension, on the apparent resemblance between fossilized teeth of Iguanodon and those of modern iguanas (Brett-Surman 1997). Later, Leidy (1858) first suggested that Iguanodon and other ornithopods could have been bipedal, based on differences between fore and hindlimb proportions. This idea was later more generally accepted after the discovery of more complete specimens before the end of the nineteenth century in the Bernissart mines of Belgium. After studying these specimens, Dollo (1883) made several observations which supported a more bipedal locomotor habit in Iguanodon, including structural similarities between the pelvis and hindlimbs of birds and Iguanodon, and divergent lengths and autopod organization between the fore and hindlimbs. Moreover, Dollo (1883) suggested that the well-developed fourth trochanter of Iguanodon may have indicated strong lateral movements of the tail, inferring a possible aquatic habit consistent with a previous hypothesis from Owen (1875). Coupled with the discovery of bipedal dinosaur tracks from the Wealden group (Beckles 1862), Dollo (1883) argued that Iguanodon did not use its tail as a tripod for locomotion, but still suggested that its tail probably slid along the ground while walking. Therefore, this led to the reconstruction of a tripodal ‘kangaroo’ stance during the mounting of the Bernissart specimens. In a reconstruction of Camptosaurus, a close relative of Iguanodon, Gilmore (1909) commented that the metacarpals of Camptosaurus were short and stout, and probably more specialized for body weight support than to prehension. He therefore hypothesized that a quadrupedal rather than bipedal stance would have been more typical in Camptosaurus and closely related species. Later, Norman (1980, 1986) postulated that both Iguanodon bernissartensis and Mantellisaurus atherfieldensis (initially named I. mantelli and I. atherfieldensis respectively) were capable of switching from a bipedal to a quadrupedal stance but that Mantellisaurus was more likely to switch to a bipedal habit, as indicated by more quadruped-like forelimb proportions and the more robust manus of Iguanodon relative to Mantellisaurus.
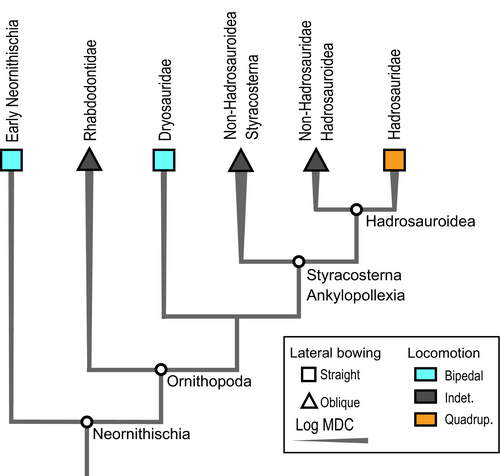
Furthermore, it was also suggested that hadrosaurids (i.e. the least inclusive clade containing Saurolophus osborni and Parasaurolophus walkeri; Fig. 1) were strictly bipedal animals based on observations such as hindlimb length relative to the forelimb and trunk lengths (Galton 1970) and the ‘non-graviportal’ morphology of the forelimbs, especially the manus with elongated metacarpals (Galton 1970; Norman 1980). However, hadrosaurids were later inferred to be facultative bipeds (i.e. intermediate between obligate bipedal and quadrupedal habit where both forms of locomotion are used; Grinham et al. 2019). This inference was based on additional osteological observations of the forelimb (Carrano 2001; Dilkes 2001) and ichnological evidence (Lockley & Wright 2001; Lockley et al. 2004; Díaz-Martínez et al. 2015), with the ability to switch from a dominant quadrupedal stance to a faster bipedal stance when required (Horner et al. 2004). More recently, (Maidment et al. 2012) argued in favour of an even more predominant quadrupedal habit in hadrosaurids based on osteological evidence from the forelimb (i.e. anterolateral process on the ulna, radius positioned medially to the ulna, reduction in carpal ossification, elongated and appressed metacarpals) indicating a rather pronated and weight-bearing manus. Considering that non-hadrosaurid iguanodontians presented a mosaic of bipedal and quadrupedal features, and that hadrosaurids possessed all major characteristic quadrupedal features, quadrupedality may have evolved in a stepwise manner, from early-diverging ankylopollexians (i.e. the least inclusive clade containing Camptosaurus dispar and Parasaurolophus walkeri) to later hadrosaurids (Fig. 1; Maidment et al. 2012; Maidment & Barrett 2012a, 2012b; Poole 2022).
However, the shift from bipedality to quadrupedality in ankylopollexians correlates with a gradual increase in body mass, which can render uncertain which proxy should be interpreted as indicative of locomotor habit or body size (Maidment & Barrett 2012a; Poole 2022). Moreover, while a consensus about obligate bipedal habit in early-diverging ornithopods has mostly been reached, the nature of facultative bipedality or quadrupedality remains unclear in later ornithopods, mostly among non-hadrosaurid ankylopollexians. As mentioned above, such shifts should correlate with the morphology of ornithopod skeleton, and especially limb bones such as the femur (Parrish 1986; Gatesy 1990; Carrano 1999). Indeed, the variation of femoral morphology relative to locomotor habit and body mass was previously investigated in closely-related archosaurs using 3D geometric morphometrics (3D GMM). This approach showed how femora of early archosauriforms (including ornithischians) (Pintore et al. 2022) and theropods (Pintore et al. 2024) may independently have specialized their shape in relation to locomotor habits (i.e. anterior femoral bowing, fourth trochanter symmetry) and body mass variations (i.e. distal shift of the fourth trochanter). Therefore, analysing various ornithopod femora using a similar 3D GMM approach could enable a better characterization of how their morphology specialized to increased body mass and shifts in locomotor habit, ultimately enabling a better understanding of how secondary quadrupedality may have evolved within this clade. As mentioned above, quadrupedality may have first evolved in non-hadrosaurid ankylopollexians. Therefore, taxa from this clade could offer a valuable opportunity to study how a facultative bipedal habit, if it existed, correlates with ornithopod femoral specializations, which is of a major interest for studies of the evolutionary history of ornithopods. Moreover, our findings could also inform about how secondary quadrupedality, and potential occurrences of facultative bipedal habit, may have evolved in other dinosaurs such as sauropodomorphs, ceratopsians and thyreophorans.
MATERIAL & METHOD
Sample
Our sample is composed of 41 femora from 23 species of Neornithischia (6 non-ornithopod neornithischians as outgroup and 35 ornithopods sensu Butler et al. 2008; Table 1). Specimens were chosen to best represent the locomotor transition from a bipedal to quadrupedal habit, and possible facultative bipedality, alongside the trend of increases in body mass from early neornithischians to later iguanodontians; within the constraints of availability and suitability for 3D digitization (photogrammetry, surface scans and CT scans; Fig. 1; Table 1). We considered most of the non-styracosternan neornithischians in our sample (except Rhabdodontidae which includes one species of Rhabdodon, the two species of Zalmoxes and Muttaburrasaurus; see below) as broadly obligate bipeds based on the literature, providing osteological correlates and timing of the locomotor transition from bipedality to quadrupedality across the ornithischian phylogeny (Maidment & Barrett 2012a; Poole 2022). Our outgroup comprises Lesothosaurus diagnosticus from the Early Jurassic; Haya griva and Hypsilophodon foxii from the Early Cretaceous; and Thescelosaurus neglectus from the Late Cretaceous (Fig. 1; Table 1). The three Haya griva specimens include two possible juveniles and one late juvenile to adult specimen (Barta & Norell 2021a). Rhabdodontidae are represented by the relatively small Rhabdodon (one indeterminate species of Rhabdodon from the Late Cretaceous of Fox-Amphoux, France) and two sympatric species of Zalmoxes (two Z. robustus and one Z. shqiperorum; Godefroit et al. 2009), both from the Late Cretaceous (Table 1). We attributed an indeterminate habit to Rhabdodon and the two Zalmoxes species in our sample because they have a mosaic of bipedal and quadrupedal features across their skeleton (e.g. for Zalmoxes: a wide ilium and an anterolateral process on the ulna which are considered quadrupedal features, but a pendant fourth trochanter which is considered a bipedal feature; Weishampel et al. 2003; Maidment & Barrett 2012a). The larger Muttaburrasaurus species from the Early Cretaceous, M. langdoni, is sometimes attributed to Rhabdodontidae (McDonald et al. 2010; McDonald 2012; Poole 2022), but it could be a member of Styracosterna (Madzia et al. 2020) or Hadrosauriformes (Agnolin et al. 2010; Bell et al. 2019) instead (Fig. 1; Table 1). Given the large body size and previous estimation of a quadrupedal habit for Muttaburrasaurus (Bishop et al. 2020), we did not attribute an obligate bipedal habit to it, contrary to most other non-styracosternan neornithischians in our sample, and therefore considered it as having an indeterminate habit. Dryosauridae contains the small bipeds from the Middle Jurassic Callovosaurus leedsi, the Late Jurassic Dysalotosaurus lettowvorbecki, and the Early Cretaceous Elrhazosaurus nigeriensis, for which relationships are somewhat unresolved (Boyd 2015; Madzia et al. 2018) but structured as presented in our composite phylogeny (McDonald 2012; Brown et al. 2021; Fig. 1; Table 1). Whereas all previously listed taxa are considered bipeds, quadrupedality (facultative to obligate) may have originated at the ankylopollexian node (Maidment & Barrett 2012a; Poole 2022; Fig. 1; Table 1). Because our sample did not include non-styracosternan ankylopollexians, we assume in our study that quadrupedality first evolved somewhere within Styracosterna. A large polytomy is often recovered in cladistic analyses for the non-hadrosauroid styracosternans (McDonald 2012; Madzia et al. 2020; Poole 2022). However, our sample includes only a few non-hadrosauroid styracosternans from the Early Cretaceous which have relatively clear phylogenetic relationships: several specimens of Iguanodontia including Iguanodon and Hypselospinus (see details below), and the more robust relative which diverged earlier, Lurdusaurus arenatus (Norman 2004; Galton 2009; McDonald et al. 2012; Fig. 1; Table 1). Our dataset of Iguanodontia is composed of I. bernissartensis from the Early Cretaceous, an indeterminate species from the Wealden group of the Isle of Wight, UK (NHMUK PV R120b–c, hereafter referred as ‘indeterminate iguanodontians’), and one specimen from the Valanginian of Wadhurst Clay formation of East Sussex, UK (NHMUK PV R1629d; Lydekker 1889; Table 1), historically referred as Iguanodon ‘hollingtonensis’ which may be attributed to Hypselospinus fittoni (Bonsor et al. 2023; Norman 2010). The Early Cretaceous Mantellisaurus atherfieldensis and Ouranosaurus nigeriensis are early-diverging relatives of the Late Cretaceous Telmatosaurus transsylvanicus and Orthomerus dolloi within the hadrosauroid clade (McDonald et al. 2012; Madzia et al. 2020; Bonsor et al. 2023); (Fig. 1; Table 1). Representatives of the Late Cretaceous Euhadrosauria (Madzia et al. 2020; i.e. Saurolophidae sensu Prieto-Márquez 2010) include several individuals of Edmontosaurus (2 E. annectens and 3 indeterminate specimens) and the two large Lambeosaurinae Amurosaurus riabinini and Olorotitan ararhensis (Fig. 1; Table 1).
| Higher clade | Species | Abb | Collection number | S | FL | MDC | Dig |
|---|---|---|---|---|---|---|---|
| Non-ornithopod Neornithischia | Lesothosaurus diagnosticus | Les | NHMUK PV R UB17 | R | 100.9 | 36.2 | SS |
| Neornithischia, Thescelosauridae | Haya griva | Hay | IGM 100/1324 | L | 166.9 | 74.3 | SS |
| Neornithischia, Thescelosauridae | Haya griva* | Hay | IGM 100/2013 | R | 129.4 | 50.8 | SS |
| Neornithischia, Thescelosauridae | Haya griva* | Hay | IGM 100/2015 | R | 124.3 | 53.9 | SS |
| Neornithischia, Thescelosauridae | Thescelosaurus neglectus | The | NCSM 15728 | R | 424.7 | 187.4 | SS |
| Non-ornithopod Clypeodontia | Hypsilophodon foxii | Hyp | NHMUK PV R 5830 | R | 102.1 | 33.5 | Ph |
| Ornithopoda, Rhabdodontomorpha | Rhabdodon indet. | Rha | MNHN 1939-11 | L | 533.7 | 248.3 | SS |
| Ornithopoda, Rhabdodontomorpha | Muttaburrasaurus langdoni | Mut | NHMUK PV R 9604 | R | 1038.4 | 463.4 | Ph |
| Ornithopoda, Rhabdodontomorpha | Zalmoxes robustus | Zal | NHMUK PV R 3819 | L | 304.6 | 119.5 | μCT |
| Ornithopoda, Rhabdodontomorpha | Zalmoxes shqiperorum | Zal | NHMUK PV R 4900 | R | 299.2 | 137.4 | Ph |
| Ornithopoda, Rhabdodontomorpha | Zalmoxes robustus | Zal | NHMUK PV R 3834 | R | 291.9 | 118.5 | Ph |
| Ornithopoda, Dryosauridae | Elrhazosaurus nigeriensis | Elr | MNHN F GDF332 | L | 228.2 | 88.4 | SS |
| Ornithopoda, Dryosauridae | Callovosaurus leedsi | Cal | NHMUK PV R 1608 | L | 279.4 | 105.9 | Ph |
| Ornithopoda, Dryosauridae | Dysalotosaurus lettowvorbecki | Dys | NHMUK PV R 12278 | R | 375.9 | 143.7 | Ph |
| Ornithopoda, Dryosauridae | Dysalotosaurus lettowvorbecki | Dys | NHMUK PV R 6861 | R | 189.5 | 68.2 | Ph |
| Ornithopoda, Dryosauridae | Dysalotosaurus lettowvorbecki | Dys | NHMUK PV R 12227 | R | 364.3 | 137.2 | Ph |
| Non-Hadrosauridea Styracosterna | Lurdusaurus arenatus | Lur | MNHN F GDF 1700 | L | 902.7 | 467.1 | Ph |
| Non-Hadrosauridea Styracosterna | Iguanodontia indet. | Igu | NHMUK PV R 120c | R | 795.5 | 400.3 | Ph |
| Non-Hadrosauridea Styracosterna | Iguanodontia indet. | Igu | NHMUK PV R 120b | R | 832.9 | 386.2 | Ph |
| Non-Hadrosauridea Styracosterna | Hypselopsinus fittoni | Hps | NHMUK PV R 1629d | L | 831.5 | 419.8 | Ph |
| Non-Hadrosauridea Styracosterna | Iguanodon bernissartensis | Igu | IRSNB R51 | L | 1037.3 | 524.3 | SS |
| Non-Hadrosauridea Styracosterna | Iguanodon bernissartensis | Igu | IRSNB R51 | R | 1029.3 | 515.5 | SS |
| Non-Hadrosauridea Styracosterna | Mantellisaurus atherfieldensis | Man | NHMUK PV R 5764 | L | 671.3 | 281.7 | SS |
| Non-Hadrosauridea Styracosterna | Mantellisaurus atherfieldensis | Man | NHMUK PV R 5764 | R | 670.2 | 280.2 | SS |
| Non-Hadrosauridea Styracosterna | Mantellisaurus atherfieldensis* | Man | NHMUK PV R 11521 | R | 597.2 | 240.8 | Ph |
| Non-Hadrosauridea Styracosterna | Mantellisaurus atherfieldensis* | Man | NHMUK PV R 11521 | L | 569.5 | 225.8 | Ph |
| Non-Hadrosauridea Styracosterna | Ouranosaurus nigeriensis | Our | GDF 300 | L | 850.1 | 331.9 | SS |
| Non-Hadrosauridea Styracosterna | Ouranosaurus nigeriensis | Our | MNHN F 1966.15 | L | 1048.6 | 336.5 | SS |
| Non-Hadrosauridea Styracosterna | Orthomerus dolloi | Ort | NHMUK PV OR 42955 | R | 498.1 | 195.7 | Ph |
| Non-Hadrosauridea Styracosterna | Orthomerus dolloi | Ort | NHMUK PV R 4914 | L | 461.1 | 183.6 | Ph |
| Non-Hadrosauridea Styracosterna | Telmatosaurus transsylvanicus | Tel | NHMUK PV R 3842 | L | 249.8 | 130.4 | μCT |
| Ornithopoda, Hadrosauridae | Hadrosaurid indet. | Had | NHMUK PV R 3646 | R | 1169.3 | 495.4 | Ph |
| Ornithopoda, Hadrosauridae | Edmontosaurus annectens | Edm | MNHN F AMN17 | R | 970.3 | 371.9 | SS |
| Ornithopoda, Hadrosauridae | Edmontosaurus annectens | Edm | UCMP 137278 | R | 705.1 | 256.3 | μCT |
| Ornithopoda, Hadrosauridae | Edmontosaurus indet. | Edm | UCMP 137406 | L | 642.9 | 247.3 | μCT |
| Ornithopoda, Hadrosauridae | Edmontosaurus indet. | Edm | UCMP 137415 | R | 995.3 | 351.1 | μCT |
| Ornithopoda, Hadrosauridae | Edmontosaurus indet. | Edm | NHMUK PV R 14369 | L | 1099.7 | 397.4 | Ph |
| Ornithopoda, Hadrosauridae | Amurosaurus riabinini | Amu | IRSNB Vert 067 | L | 611.8 | 218.2 | SS |
| Ornithopoda, Hadrosauridae | Amurosaurus riabinini | Amu | IRSNB Vert 067 | R | 625.2 | 231.9 | SS |
| Ornithopoda, Hadrosauridae | Olorotitan ararhensis | Olo | IRSNB Vert 068 | L | 1104.8 | 429.9 | SS |
| Ornithopoda, Hadrosauridae | Olorotitan ararhensis | Olo | IRSNB Vert 068 | R | 1105.5 | 425.2 | SS |
- Abb, genus abbreviation; Dig, digitization method (μCT/Ph/SS, microCT scan/photogrammetry/surface scan); FL, femoral length (mm); MDC, minimal diaphyseal circumference (mm); S, anatomical side (L/R, left/right).
- * Known juveniles.
Institutional abbreviations
GDF, Gadoufaoua collection, Musée National Boubou-Hama, Niamey, Niger; IGM, Mongolian Geological Institute, Ulaanbaatar, Mongolia; IRSNB, Institut Royal des Sciences Naturelles de Belgique, Bruxelles, Belgium; MNHN, Muséum national d'Histoire naturelle, Paris, France; NCSM, North Carolina Museum of Natural Sciences, Raleigh, USA; NHMUK, Natural History Museum, London, UK; UCMP, University of California Museum of Paleontology, Berkeley, USA.
3D digitization
We used different 3D digitization approaches to collect our sample (Table 1). Photogrammetry was performed using a Canon EOS R10 (Canon Inc.) camera with a 35 mm focal lens and a Nikon D5500 with a 50 mm focal lens for the smaller specimens (18 out 46 specimens). Surface reconstructions were conducted using the software Metashape Professional (Agisoft LLC) and Meshlab (Cignoni et al. 2008). CT scans were obtained from HiSpeed CT/i and micro-CT scans, from a Nikon Metrology XT H 225 ST. Resulting CT and micro-CT scans were segmented using the Avizo software (Hillsboro, Oregon, USA) to export 3D meshes (5 out of 46 specimens). The surface scanners Artec EVA and Space Spider (Artec 3D, Luxembourg), NextEngine (NextEngine Inc.), Range7 (Konica Minolta Sensing, Inc.) and their respective corresponding software Artec Studio Professional and Scan Studio Pro were used for 18 out of 46 specimens. Waltenberger et al. (2021) demonstrated that 3D models digitized from various approaches could reliably be analysed using a 3D GMM approach. In addition, several studies have highlighted that only minimal errors in the geometry of 3D models were introduced by combining photogrammetry, 3D surface scans and CT scans, especially at a broad comparative scale (Falkingham 2012; Fau et al. 2016; Soodmand et al. 2018; Díez Díaz et al. 2021).
3D geometric morphometrics
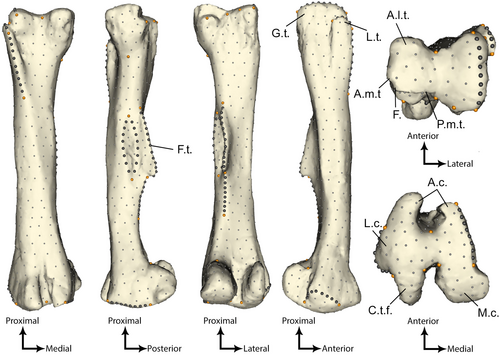
We used 3D geometric morphometrics (3D GMM) to study femoral shape variations across our neornithischian sample. This approach relies on the digitization of anatomical landmarks, making it well suited to study biological objects (Zelditch et al. 2012). The protocol detailed in Gunz et al. (2005), Gunz & Mitteroecker (2013) and Botton-Divet et al. (2016) was followed to digitize single anatomical landmarks and sliding semilandmarks along curves and surfaces. Indeed, such a high density approach is more suited to precisely visualize the entire shape variation of a biological object, and especially to circumvent the lack of single anatomical landmarks in certain areas such as the diaphyses of limb long bones (Gunz et al. 2009; Zelditch et al. 2012; Gunz & Mitteroecker 2013; Botton-Divet et al. 2015; Goswami et al. 2019).
We digitized 656 landmarks including 25 single anatomical landmarks, 96 sliding semilandmarks along curves and 535 along surfaces (Fig. 2; Table S1) using the Landmark software (v3.0.0.6; Wiley et al. 2005; IDAV 2007). The complete landmark configuration (i.e. including semilandmarks along surfaces) on one chosen specimen is here referred to as ‘the template’. We chose the Ouranosaurus femur GDF 300 as our template because it preserves prominent structures and seems to have had minimal taphonomic alterations of shape (Fig. 2). To further minimize the impact of taphonomic distortions, we digitized single anatomical landmarks on concavities rather than on convexities (e.g. on the borders instead of the tip of a tuberosity). In order to ensure that landmark coordinates from all specimens could be compared, we performed a series of semi-automatic projections of sliding semilandmarks and spline relaxations between the template, all specimens and their mean landmark configuration. The first step was to project all surface semilandmarks from the template onto all specimens from the sample using the placePatch function of the R package Morpho v2.8 (Schlager 2017). The next step was to perform five iterations of a spline-relaxation between the template configuration and every other specimen using the function relaxLM of Morpho. The final step was to perform three iterations of a spline-relaxation between the mean configuration and every specimen using the function slideLM of Morpho. A more detailed workflow is presented in Pintore et al. (2022). These three steps ensured that all landmark coordinates were geometrically homologous across all specimens and therefore ready for comparison (Gunz et al. 2005). A generalized Procrustes analysis (GPA) was performed using the gpagen function from the R package geomorph v3.3.1 (Adams & Otárola-Castillo 2013). GPA enables superimposing every specimen and homogenizing their position in a Cartesian coordinate system and isolating shape from size components (Gower 1975; Rohlf & Slice 1990; Zelditch et al. 2012). The remaining differences between superimposed configurations (i.e. Procrustes residuals) could then be interpreted as the shape variation between all specimens.
We computed a neighbour-joining tree (unrooted; Fig. S1) in order to visualize the global morphological variation using distance, nj and plot.phylo functions of the ape R package (Paradis et al. 2004). Next, we conducted a principal component analysis (PCA), which reduced the dimensionality of the variation and isolated several morphological components (Gunz & Mitteroecker 2013). The PCA enabled us to study morphological variations between ‘known’ bipeds and taxa with indeterminate locomotor habits. We performed repeatability testing by digitizing the same landmark configuration 10 times on 3 Mantellisaurus specimens (left and right NHMUK PV R5764, and left NHMUK PV R11521). Following the GPA on the resulting 30 configurations, a PCA demonstrated that the 10 configurations of each of the 3 specimens clustered together, therefore highlighting that biological variation prevailed over possible bias in operator ability to reproduce the landmark configuration (Fig. S2). The same person (author RP) did all landmark digitizing.
To study allometry related to body mass variations, PC axes of interest were further investigated using regressions with log-transformed minimal diaphyseal circumference (MDC). We used MDC because it is a reasonable proxy for body mass in analyses of scaling trends (Campione & Evans 2012). Indeed, it was not possible to estimate body mass because the current equations relying on MDC measurements differ between bipeds (i.e. only the MDC from the femur is needed) and quadrupeds (both humeral and femoral MDC are required; Campione & Evans 2012; Campione et al. 2014), and we did not have MDC data for humeri. Nonetheless, femoral MDC is still a reliable indicator of a more or less massive morphology, which indirectly and broadly correlates with body mass (McPhee et al. 2018; Pintore et al. 2022). We measured MDC using the software CloudCompare (https://www.cloudcompare.org). For informative purposes, we also measured the femoral length (FL; i.e. the maximal distance between proximal and distal ends) using the software MeshLab (Cignoni et al. 2008). To investigate evolutionary allometry across our sample, we computed Pearson's correlations, testing if the morphological variation correlated with log-transformed MDC and centroid size (i.e. the square root of the sum of the squared distances of all landmarks from their centroid). To account for the potential morphological resemblance between phylogenetically closely related taxa, we performed phylogenetic generalized least square regressions (PGLS) at the multidimensional (i.e. accounting for all PC axes) and unidimensional (i.e. along an isolated PC axis) levels and assuming Brownian motion (Adams 2014a).
Finally, we measured the angle between the crista tibiofibularis and the lateral condyle (Fig. S3) using OnScreenProtractor v0.5 (GNU GPLv3) because this parameter was previously demonstrated to correlate with shifts in locomotor habits, as well as indicating potential facultative bipedal habits (Pintore et al. 2022). We then compared the distribution of this angle within categories of locomotor habits using different statistics. We tested for normality using a Shapiro–Wilk test (p> 0.05 if normally distributed). We then tested for the equality of variances using a parametric Bartlett test for normal distribution and a non-parametric Mann–Whitney U test if not (p> 0.05 if variances are equals). Finally, we tested if the distribution significantly differed between groups with normal distributions and equal variances using a two-sample t-test. For testing differences between more than two normal distributions with unequal variances, we performed a Welch's anova. Finally, we conducted a Games–Howell post-hoc test to highlight which groups significantly differed from each other in the Welch's anova.
To further investigate the phylogenetic signal in our sample, we converted our composite phylogeny (Fig. 1) into hierarchical parenthesis format using the Mesquite software (Maddison & Maddison 2019), with all branch lengths set to 1. We computed a phylomorphospace by mapping the phylogeny onto the PCA using the plot.gm.prcomp of the geomorph package. The Kmult statistics, extended from the Blomsberg's K (Blomberg et al. 2003), enabled us to investigate how the morphological variation (i.e. global or isolated along each PC axis) compared to expectations under Brownian motion (Adams 2014b). A significant K value equal or greater than one indicates a strong phylogenetic signal whereas a value below one indicates likely evolutionary convergence.
We computed 3D visualizations in order to highlight the femoral features varying the most along each PC axis. To do so, we calculated a mean shape of all the femora of our dataset by performing a spline relaxation between the template landmark configuration and a mean landmark configuration extracted from the GPA. The associated thin-plate spine (TPS) deformation was used to warp the template mesh onto the mean landmark configuration to create the mean shape of all specimens. The first 3D visualizations were minimal and maximal theoretical shapes of each PC axis. This was done by interpolating the mean landmark configuration with the configurations at the most negative and positive extremes of the chosen PC axis. The second 3D visualizations were superimposed minimal theoretical shapes with vector displacements representing the distances between corresponding landmarks, highlighting which femoral features varied the most along each PC axis. Displacement vectors were computed using the segments3d function of the rgl R package (Adler & Murdoch 2020) and coloured with a heatmap gradient from blue to red using the ColorRamps R package (Keitt 2008; Botton-Divet 2017; Keitt et al. 2024).
RESULTS
Morphological variation
First, we investigated the global morphological variation in our ornithischian sample using the neighbour-joining tree, which reveals that the distribution of our taxa generally follows the phylogeny; except for dryosaurids being located closer to non-ornithopod neornithischians (Fig. S1). However, at a more focused level, the neighbour-joining tree highlights that some specimens of Zalmoxes, Iguanodon, Mantellisaurus and Edmontosaurus did not group consistently with other relatives from the same species when accounting for the global morphological variation of the femur (Fig. S1).
The first two PCA axes (PC1 and PC2) represent more than 50% of the complete morphological variation. PC1 (40.8%), which is broadly similar to the distribution showed in the neighbour-joining tree (Fig. S1), represents femoral anteroposterior bowing and a distal shift of the fourth trochanter whereas PC2 (11.2%) consists of mediolateral femoral obliquity (see below for more details and Fig. 3). Specimens are broadly distributed according to MDC along PC1, as shown by the increase in circle size toward the positive side of Figure 3A. Indeed, MDC ranges over 15-fold, from 33.5 mm to 524.3 mm (Table 1), and all specimens with an MDC smaller than 200 mm cluster together on the negative side of PC1, except Telmatosaurus and the two Orthomerus (Fig. 3, Tel, Ort; Table 1). Accordingly, no specimen with an MDC greater than 200 mm plots in the negative region of the PC1 axis except the Rhabodon, Muttaburasaurus and one individual each of iguanodontian indet. and Mantellisaurus. Whereas the aforementioned Rhabdodon, iguanodontian indet. and Mantellisaurus specimens still plot close to the midline of the morphospace, Muttaburasaurus is the only specimen with an MDC greater than 200 mm to plot very negatively along PC1 (Fig. 3, Mut; Table 1). The specimen on the most negative side of PC1 is the small early-diverging bipedal ornithischian Lesothosaurus diagnosticus (MDC: 36.2 mm) and the specimen on the most positive side is the Iguanodon bernissartensis holotype, a large styracosternan with an indeterminate locomotor habit (MDC: 515.5 mm; Fig. 3, Les, Igu; Table 1; Fig. S4). All non-styracosternan neornithischians are located in the negative part of PC1 (Fig. 3A). All hadrosaurids are clustered together on the positive side of PC1 (Fig. 3A). Generally, non-hadrosaurid styracosternans are located around the middle of PC1, except Telmatosaurus and one specimen (NHMUK PV OR 42955) of Orthomerus, which plot very positively along PC1 (Fig. 3A, Ort, Tel).
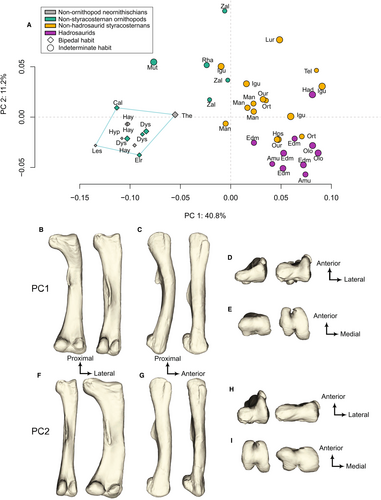
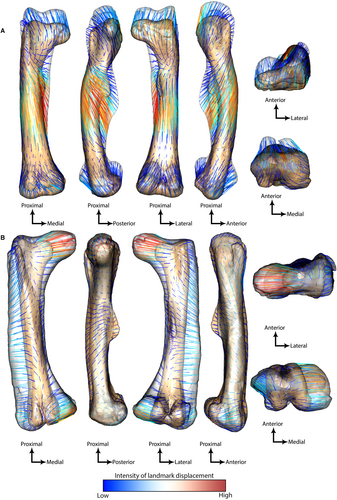
Theoretical shapes highlight that femora on the negative side of PC1 are anteriorly bowed with a fourth trochanter closer to the proximal epiphyses whereas femora on the positive side are straighter and more columnar, with a more distally located fourth trochanter (Fig. 3A–C). The longest displacement vectors are localized on the distal part of the fourth trochanter, indicating that this is the feature varying the most along PC1 (Fig. 4A). This demonstrates that the insertion site for caudofemoral muscles connecting the tail to the thigh is larger and more distally located in the straighter femora than in the most curved ones (Fig. 4A). Moreover, the fourth trochanter morphology is semi-pendant to pendant (i.e. asymmetric with a reversed distal slope) in the minimal theoretical shape and rounded and mostly symmetric in the maximal theoretical shape (Fig. 3C). The other longest displacement vectors are localized in the anterior part of the midshaft and the posterior-most part of the greater trochanter, demonstrating that anteroposterior bowing of the femur toward the anterior side is another feature varying greatly along PC1 (Fig. 4A). Other features with a large, but lesser, intensity of variation along PC1 are the prominence of the anterior condyles and the width of the epiphyses (Figs 3B, E, 4A). The maximal theoretical shape has prominent medial and lateral anterior condyles that form an intercondylar tunnel, whereas the minimal theoretical shape has a rather flat anterior surface of the distal epiphysis (Fig. 3D). The proximal and distal epiphyses appear relatively larger mediolaterally in the minimal theoretical shape than in the maximal one relative to the length of the diaphysis; hence highlighting the columnar morphology of hadrosaurids (Fig. 3B). That morphology is highlighted with long vectors oriented proximodistally on the proximal surface of the femoral head (i.e. the maximal theoretical shape appears longer than minimal theoretical shape in the proximal region when epiphyses are scaled equally; Fig. 4A).
The distribution along PC2 of our PCA is not linked to the variation of MDC (Fig. 3A), as demonstrated by specimens with the smallest and largest MDC being close together around the middle area of PC2 (see below for tests; Les, Igu, Hps; Fig. 3A). The specimen located on the most negative side is the right femur of the large hadrosaurid Amurosaurus riabinini whereas the specimen on the most positive side is the rhabdodontid Zalmoxes shqiperorum, which is more robust than the other species of Zalmoxes, Z. robustus (i.e. larger MDC for similar femoral length; Table 1; Figs 3A, S4, Amu, Zal). Among non-styracosternan neornithischians, the specimens of the earliest-branching taxa, and of most of the smallest (based on MDC) ‘known’ bipeds, are located in the negative region of PC2 and their latest relatives are located in the positive region of PC2, except for dryosaurids, which are located more negatively along PC2 than the rhabdodontids diverging earlier: Zalmoxes, Muttaburasaurus (the only non-styracosternan neornithischians with an indeterminate locomotor habit) and Rhabodon (Fig. 3A; rhabdodontids: Rha, Zal; dryosaurids: Cal, Dys, Elr). The distribution of styracosternans, which are assigned an indeterminate locomotor habit, does not follow an increase in MDC, with the large hadrosaurid Amurosaurus riabinini being located on the most negative side of PC2 and the very robust Lurdusaurus arenatus, possibly an early-diverging styracosternan, on the most positive side (Fig. 3A, Amu, Lur). All hadrosaurids are located on the negative side of PC2, except NHMUK PV R 3646 (Fig. 3A, Had).
Theoretical shapes demonstrate that the distribution along PC2 is essentially linked to femoral obliquity (Fig. 3F). This morphological variation is characterized by specimens on the most negative side of PC2 having a straight shaft, whereas specimens on the most positive side have a laterally deviated shaft, making the femur appear oblique (Fig. 3F), as indicated by the longest vectors localized on the medial side of the proximal and distal epiphyses and on the lateral side of the shaft (Fig. 4B). This obliquity is paralleled by a downward (distal) orientation of the crista tibiofibularis (Fig. 4B). This asymmetric orientation of the distal epiphysis forms an acute angle between the mediolateral axis of the distal epiphysis and the long axis of the shaft, whereas it is mostly perpendicular in the straight femora (Figs 3F, 4B). Similarly, the medial side of the femoral head is angled slightly upward (Figs 3F, 4B). Another feature varying less strongly is the width of the condyles, which are lateromedially wider in the maximal theoretical shape (i.e. oblique) than in the minimal one (i.e. straight; Figs 3F, H, 4B). In addition, the fourth trochanter is more prominent toward the posterior side in the minimal theoretical shape than in the maximal one, but both femora have an asymmetric distal slope of the fourth trochanter (Figs 3G, 4B), which is thus pendant. The angle between the crista tibiofibularis and the lateral condyle is also greater in the straight femora than in the oblique ones (Figs 3, 4B, 5; Table S2).
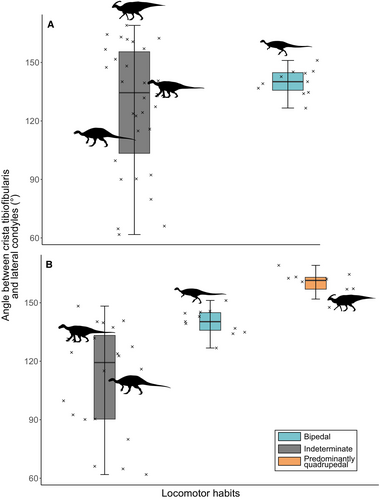
The mean angles between the crista tibiofibularis and lateral condyle do not significantly differ between obligate bipeds and taxa with indeterminate locomotor habit (Fig. 5A; Shapiro–Wilk test: p < 0.05 for taxa with indeterminate habit; Mann–Whitney U test: p > 0.05; two-sample t-test: p > 0.05). However, when considering all hadrosaurids as quadrupeds (as most recently hypothesized by Maidment & Barrett (2012a), Maidment et al. (2012) and Poole (2022)), the mean angles significantly differ between obligate bipeds, indeterminate habits and predominantly quadrupedal taxa (Fig. 5B; Shapiro–Wilk test: p > 0.05; Bartlett test: p < 0.05; Welch's anova: p < 0.05; Games–Howell post-hoc test: p < 0.05 for all combinations).
Phylogenetic signal
The multivariate K statistics show significant correlations between the distributions across the morphospace and the phylogenetic relationships at the multidimensional level (Kmult) and only for PC1 at the unidimensional level (K). The Kmult value is close to but below 1 (Kmult = 0.85; p < 0.01), indicating a smaller phylogenetic signal than intra-group variations. Even if the neighbour-joining tree indicated that the global morphological variation broadly follows phylogeny, dryosaurids and one Zalmoxes specimen plotted outside non-styracosteran ornithopods, which may have caused the phylogenetic signal to be less than expected under a Brownian motion model (Fig. S1). However, the phylogenetic signal is very high at the unidimensional level for PC1 (K = 4.16; p < 0.01), much greater than predicted by Brownian motion. This indicates that the morphological variation along PC1 is strongly structured according to the phylogeny.
The phylomorphospace highlights that non-styracosternan neornithischians are separated from hadrosaurids along PC1, and that non-hadrosaurid styracosternans are mostly located in the middle part of PC1 (Fig. 6). However, at a smaller scale, dryosaurids are located close to early-branching neornithischians whereas they are closer to rhabdodontids in the phylogeny (Figs 1, 6). In addition, some non-hadrosaurid styracosternans are located within the hadrosaurid cluster along PC1, such as Lurdusaurus, Telmatosaurus, Orthomerus, Ouranosaurus, Hypselospinus and Iguanodon bernissartensis (left femur only) (Figs 1, 6, S4, Hps, Igu, Lur, Man, Ort, Our, Tel).
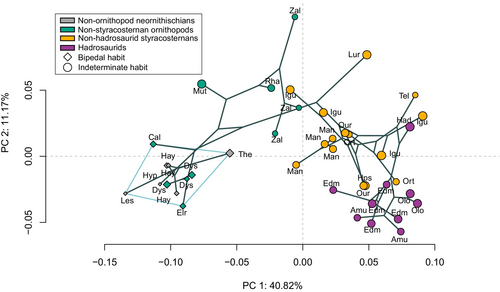
The K statistic is not significant for the distribution along PC2. Conversely, the phylomorphospace highlights that femoral obliquity varies homoplastically between non-styracosternan neornithischians (mostly bipedal excluding rhabdodontids) and styracosternans. Indeed, femoral obliquity globally increased from non-styracosternan neornithischians to non-hadrosaurid styracosternans and then decreased from non-hadrosaurid styracosternans to hadrosaurids (Figs 3, 5). However, while the variation also mostly follows the phylogeny within non-styracosternan neornithischians, femoral obliquity increased among rhabdodontids (which only include taxa with indeterminate locomotor habit) which diverged earlier than dryosaurids (which only include obligate bipeds) from styracosternans, which is similar to our results along PC1 (Fig. 1 vs Fig. 6). Thus, this observation highlights that small bipedal dryosaurids may have retained the more plesiomorphic condition (relative to the evolution of femoral obliquity) despite being more closely-related to Styracosterna than larger rhabdodontids with indeterminate locomotor habit. Unlike along PC1, the distribution of styracosternans followed the phylogeny along PC2, with a separation between non-hadrosaurid styracosternan and hadrosaurid clusters. However, the relative positions within each cluster do not seem to follow the phylogeny, which may partly explain also why the K value was not significant for the distribution along PC2 (Figs 1, 6).
Evolutionary allometry
First, we found a strong association between log-transformed centroid size and MDC (r2 = 0.97, p < 0.01), indicating that both femoral measurements can be used as indicator of body size. When performing Pearson's correlations in order to investigate the evolutionary allometry of femoral morphology along isolated axes, there is a significant and strong association between the distribution along PC1 and both log-transformed centroid sizes (r = 0.76, p < 0.01) and log-transformed MDC (r = 0.77, p < 0.01). However, there is no significant correlation with the distribution along PC2. The PGLS analysis factors out the phylogenetic resemblance between closely related specimens when studying allometry. The PGLS at the multidimensional level (i.e. all PC axes, the complete morphological variation) is significant (p < 0.01) but low for the log-transformed MDC (r2 = 0.05) and not significant for the log-transformed centroid sizes. When factoring out the effect of phylogenetic resemblance using the PGLS at a unidimensional level, the association between PC1 and log-transformed centroid size is significant but low (r2 = 0.12, p < 0.01) whereas it is stronger when using log-transformed MDC (r2 = 0.20, p < 0.01). Similarly to Pearson's correlations, there is no significant association with the distribution along PC2.
The Pearson's correlation for bipeds is only strong and significant for the association between PC1 and both log-transformed centroid size (r = 0.63, p < 0.01) and MDC (r = 0.67, p < 0.01), but not significant for taxa with indeterminate habit, further indicating that the allometric shift mostly occurred among bipedal taxa. No Pearson's correlation is significant along PC2 for bipedal taxa, regardless of body mass proxies. However, while no correlation is found between PC2 and taxa with indeterminate habit using log-transformed MDC, the correlation is significant and moderate when using log-transformed centroid sizes (r = −0.37, p < 0.05). This may suggest that log-transformed centroid size may provide more granularity when used as a body mass proxy than log-transformed MDC in 3D GMM morpho-functional analyses, but this should be investigated further. This negative correlation indicates a reversed allometric trend in femoral obliquity reverting to a straighter condition (plesiomorphic for the clade) in heavier hadrosaurids with indeterminate (presumably quadrupedal; see below) locomotor habit. When factoring out the effect of phylogenetic relationships using PGLS at the unidimensional level, associations between PC1 and both log-transformed centroid size (r2 = 0.39, p < 0.05) and log-transformed MDC (r2 = 0.39, p < 0.05) are moderate and significant for bipedal taxa only. However, and similar to most Pearson's correlations for isolated locomotor habits, there is no significant correlation along PC2, regardless of locomotor habit and body mass proxies when factoring out the effect of phylogenetic relationships.
These results together demonstrate a clear allometric trend along PC1, especially for bipedal taxa among which most of the allometric shifts of femoral shape seemed to occur. However, our results do not enable linking the evolution of femoral obliquity among taxa with indeterminate locomotor habit (non-hadrosaurid styracosternans and rhabdodontids) to an increase in body mass, but highlight that it reverted to a straighter condition among large later-diverging hadrosaurids with presumed obligate quadrupedal habits (Maidment et al. 2012).
DISCUSSION
Correlation between femoral specializations, & locomotor habit & body mass
While the morphological variation along PC1 in our ornithischian sample of femora is linked to an increase of body mass (broadly interpreted from the MDC measurements), it also relates to a shift in locomotor habit between ‘known’ bipeds and indeterminate habits, which presumably includes both more or less facultative bipeds and obligate/predominant quadrupeds (Norman 1980, 1986; Horner et al. 2004; Maidment et al. 2012; Poole 2022). Therefore, it does not seem possible to disentangle femoral features varying according to body mass from features linked to locomotor habits when relying only on the PC1 of ornithopod femora. However, observations about how most femoral shape variations along PC1 vary according to body mass or locomotor habits in other dinosaurs and their kin exist (Coombs 1978; Parrish 1986; Carrano 1999, 2001; Maidment & Barrett 2012a; Pintore et al. 2022, 2024). We now focus on four observations from ornithopods and how combining them with observations of early archosaur femora with different locomotor habits and body mass (Pintore et al. 2022) and giant theropods with obligate bipedal habit (Pintore et al. 2024) help to answer this study's main questions.
Distal shift of the fourth trochanter
First, the distal shift of the fourth trochanter (i.e. attachment site for caudofemoralis muscles) toward the midshaft is typically associated with a graviportal rather than cursorial morphology in archosaurs, as it may enable greater torques around the hip joint (Coombs 1978; Parrish 1986; Carrano 1999), which we also highlighted across early archosauriforms and theropods (Pintore et al. 2022, 2024). This shift is evident not only in bipedal sauropodomorphs and theropods, but also among robust armoured terrestrial aetosaurs and semi-aquatic crocodiles and phytosaurs (Pintore et al. 2022, 2024). Furthermore, the distal shift is associated with a more rounded and prominent (i.e. more anteriorly developed crest) shape of the fourth trochanter, another pattern evident among early archosauriforms, non-sauropod sauropodomorphs and theropods (Pintore et al. 2022, 2024). Therefore, we consider the distal shift of the fourth trochanter along the shaft of ornithopod femora as a specialization to body mass increase (Table 2).
| Locomotor habit | Body mass | |||||
|---|---|---|---|---|---|---|
| Anteriorly bowed femur | Fourth trochanter asymmetry | Femoral obliquity | Angle between distal condyles | Distal shift of the fourth trochanter | Prominence of anterior condyles | |
| Lesothosaurus | + | + | − | ~ | − | − |
| Thescelosaurus | + | + | − | ~ | − | ~ |
| Haya | + | + | − | ~ | − | − |
| Hypsilophodon | + | + | – | ~ | − | − |
| Muttaburrasaurus | + | ? | + | − | + | ~ |
| Rhabdodon | − | + | + | ~ | ~ | ? |
| Zalmoxes | + | ~ | + | ~ | ~ | ~ |
| Callovosaurus | + | + | − | ~ | − | − |
| Dysalotosaurus | + | + | − | ~ | − | − |
| Elhrazosaurus | + | + | − | ~ | − | − |
| Lurdusaurus | − | + | + | ~ | + | + |
| Iguanodon | − | ~ | + | − | + | + |
| Mantellisaurus | − | ~ | + | − | ~ | + |
| Ouranosaurus | − | + | − | ~ | + | + |
| Telmatosaurus | − | − | + | ~ | ~ | ? |
| Orthomerus | − | ~ | − | − | + | + |
| Edmontosaurus | − | − | − | + | + | + |
| Olorotitan | − | − | − | + | + | + |
| Amurosaurus | − | − | − | + | + | + |
- ? unknown (due to taphonomic distortion); − low; ~ medium; + high development. Meaning of medium condition in: fourth trochanter asymmetry, asymmetric but not pendant; angle between distal condyles, see significant discretization in Fig. 5B; distal shift of the fourth trochanter, intermediate condition between Lesothosaurus (most proximally located) and Olorotitan (most distally located); prominence of anterior condyles, prominent but without a closed intercondylar tunnel.
Fourth trochanter asymmetry
Second, the distal ridge of the fourth trochanter varies from asymmetric in obligate bipedal (pendant to non-pendant) to symmetric in taxa with indeterminate habit, which also correlates similarly with locomotor habits in early archosauriforms (Pintore et al. 2022) and other ornithischians (Maidment & Barrett 2012a). In most early-diverging bipedal neornithischians on the line to ornithopods, the most asymmetric condition represents the pendant fourth trochanter (i.e. reversed distal ridge; Dollo 1888; Sereno 1999; Norman 2004; Butler et al. 2007; Butler & Zhao 2009; Persons & Currie 2020). The pendant fourth trochanter is lost in styracosternans, which still have an asymmetric but not reversed distal ridge, whereas it is more symmetric in hadrosaurids (Table 2; Sereno 1999; Norman 2002; Horner et al. 2004; Brett-Surman & Wagner 2007; Persons & Currie 2020). Therefore, the loss of the pendant process in a still asymmetric fourth trochanter correlates with a rather indeterminate habit among non-hadrosaurid ornithopods and seems to indicate a shift from obligate bipedality to at least facultative bipedality, and to increasing quadrupedal abilities toward hadrosaurids (Maidment et al. 2012). Whereas the pendant process is clade-specific for ornithischians, it is lost early in thyreophorans (Coombs 1979; Galton 1982; Vickaryous et al. 2004) and marginocephalians (Dodson et al. 2004; Maryańska et al. 2004; Butler & Zhao 2009; Longrich 2011); hence presumably remaining as a ‘stem-ornithopod’ feature (Persons & Currie 2020). Therefore, we infer that a shift from a pendant to an asymmetric fourth trochanter is a specialization to changes of locomotor habit specific to ornithopods, and that the observation of such a shift should not be commonly applied to other dinosaur and archosaur clades without further investigations. However, a similar asymmetric semi-pendant process exists in early-diverging bipedal saurischians (Langer & Benton 2006), which could be of the same functional significance as the pendant fourth trochanter of early-diverging ornithischians (i.e. pendant and semi-pendant corresponding to different forms of an asymmetric fourth trochanter). Indeed, the fourth trochanter symmetry is correlated with variations in locomotor habits among early archosauriforms (Pintore et al. 2022), and the pendant process of early-diverging bipedal ornithischians could be part of a continuum from an asymmetric to a symmetric fourth trochanter, just like the ‘semi-pendant’ ridge in saurischians. Furthermore, the fourth trochanter asymmetry remains stable in most theropods, which are obligate bipeds, regardless of their body mass (Pintore et al. 2024). Whereas Maidment & Barrett (2012a, 2012b) suggested the pendant/asymmetric morphology as a locomotor specialization in ornithischians, Persons & Currie (2020) criticized that they did not address concomitant variations in fourth trochanter position along the shaft. We suggest this issue could be reconciled by our previous observations of a decoupling in the evolution of early archosauriform fourth trochanter morphology between body mass variations (i.e. more or less distally located) and locomotor habits (i.e. more or less symmetric) (Pintore et al. 2022, 2024). Our observation that these two features vary jointly along PC1, from small bipedal to larger indeterminate, possibly quadrupedal, ornithopod femora (Figs 3A, 4) hints at why this issue has been controversial from qualitative osteological observations alone.
Prominence of the anterior condyles
Third, we found that the anterior condyles are more prominent in femora of large-bodied taxa with indeterminate locomotor habits than in smaller obligate bipedal taxa (Table 2; Fig. 4A). This observation is consistent with findings from similar 3D GMM approaches applied to large bipedal theropods (Pintore et al. 2024) and obligate quadrupedal sauropods, which also have prominent anterior condyles (Lefebvre et al. 2022). Therefore, this femoral feature seems likely to be linked with an increase in body mass, regardless of locomotor habit, although prominence of the anterior condyles is more developed in ornithopods than in saurischians, with the presence of an intercondylar tunnel (Fig. 4A). We infer that the tendons and other tissues (e.g. aponeuroses/fascia) of the knee extensor (‘triceps femoris’; especially Mm. femorotibiales) muscles involved with this osteological feature (Godefroit et al. 2004; Horner et al. 2004; Averianov & Alifanov 2012) changed from broader and flatter morphology (with wider space between the anterior condyles in smaller taxa) to a more concentrated, subcircular morphology in taxa with the intercondylar tunnel. It is unclear why this feature evolved, but one clear consequence of the intercondylar tunnel is that it would prevent mediolateral ‘slipping’ of those soft tissues, constraining them to lie within the tunnel; probably more important if the femora were slightly more abducted (as suggested by oblique femoral morphology).
Anteriorly bowed femur
Fourth, on one hand an anteriorly bowed archosaur femur is associated with a bipedal locomotor habit, whereas a straight-shafted femur is more typical of a quadrupedal habit (Carrano 2001; Hutchinson 2001; Maidment & Barrett 2012a; Pintore et al. 2022). On the other hand, anterior femoral curvature is also negatively correlated with an increase in archosaur body mass (Carrano 2001) and was previously discussed as covarying between locomotor habit and body mass without clear distinction (Maidment & Barrett 2012a; Pintore et al. 2022). However, in saurischians, the femora of heavy bipedal sauropodomorphs and theropods are similarly anteriorly bowed and only quadrupedal archosaurs (at least the early archosauriforms studied) evolved a straightening of the shaft (Pintore et al. 2022). Therefore, our finding that the femoral morphology varies from anteriorly bowed in small early-diverging bipedal neornithischians to straight (columnar) in heavier ornithopods with indeterminate locomotor habit correlates with a locomotor shift toward quadrupedality in heavier ornithopods (Table 2; Figs 3A, 4B).
Therefore, while our results fit the inference (albeit one assumed here) that early-diverging neornithischians were mostly bipedal, they also inform about locomotor estimations in hadrosaurids. Indeed, the femoral morphology of the largest hadrosaurs in our sample seems to indicate that they were obligate quadrupeds because it follows similar trends in the fourth trochanter morphology and shaft straightness that were unambiguously observed in other robust quadrupedal archosauriforms (Figs 3A, 4; Pintore et al. 2022) consistent with other recent studies of hadrosaurids (Maidment & Barrett 2012a; Poole 2022). However, our results for femoral morphology alone do not suggest high disparity in hadrosaurid quadrupedal abilities as previously hypothesized (Maidment et al. 2012); it was also not supported by the results of Dempsey et al. (2023) for hadrosaur forelimbs. This could be investigated further by integrating other limb bones and/or other ornithopod taxa in morpho-functional studies. We were also able to clarify which femoral specializations relate to body mass and locomotor habits in non-hadrosaurid styracosternans, which have the most ambiguous locomotor habit of all ornithopods (Table 2). However, we did not clarify their locomotor habit, as the gradient of their femoral specializations is intermediate between those of bipedal neornithischians and presumably quadrupedal hadrosaurids (Fig. 3A); hence they could have been either quadrupeds or facultative bipeds. In addition, because increasing body mass follows ornithopod phylogeny, we were not able to discuss the potentially homoplastic nature of phylogenetic characters correlating with body mass. Indeed, it was previously demonstrated that such characters should be interpreted with caution because of the broadly convergent evolution of body mass variations among archosaurs (Pintore et al. 2022, 2024). Therefore, we caution that femoral specializations to increasing ornithopod body mass that are commonly coded in cladistic analyses could be homoplastic (i.e. position of the fourth trochanter (Weishampel et al. 2003; Butler et al. 2008), prominence of the anterior condyles (Norman 2002; Weishampel et al. 2003; Butler et al. 2008)). Moreover, such a pattern of variation between ornithopod phylogeny and characters related to body mass raises concerns regarding character correlation as the effect of body mass may be overweighted in phylogenetic analyses. Nevertheless, our findings illuminate some controversies about identifying femoral locomotor proxies for ornithopods by demonstrating how tightly the evolution of locomotor habit and body mass increase are related among ornithopods. Our results also inform about how the often rather simply termed/coded ‘fourth trochanter development’ could refer to both symmetry (which we show to be linked with locomotor habit) and position along the shaft and prominence, which is more likely to be linked to variation in body mass.
Femoral obliquity in heavy bipeds
Non-hadrosaurid styracosternans and rhabdodontids do have specific femoral features that set them apart from the rest of the sample along PC2 (Fig. 3A). Indeed, whereas most specializations to body mass and locomotor habits covary along PC1, non-hadrosaurid ornithopods and rhabdodontids are characterized by a femur that is more oblique in shape (i.e. mediolaterally bowed toward the lateral side) than other ornithopods from the sample (Table 2). Moreover, this femoral obliquity correlates with low angles between the crista tibiofibularis and the lateral condyle, which was demonstrated to be indicative of a more bipedal locomotor habit in early archosauriforms, whereas the highest angles (those that are more obtuse) are represented among presumably more quadrupedal hadrosaurids (Fig. 5B).
Allometric trend in femoral obliquity
The presence of an oblique femur was previously noted among rhabdodontids, dryosaurids, and early-diverging ankylopollexians, which, except for dryosaurids, were relatively large ornithopods (also termed ‘laterally bowed femur in anterior view’; Weishampel et al. 2003; Brusatte et al. 2017; Poole 2022). We find a high obliquity in the femora of the large rhabdodontid Muttaburrasaurus and the relatively large Rhabdodon and Zalmoxes shqiperorum (Table 2), but not in the less robust species Z. robustus and the dryosaurid Callovosaurus, which still have an oblique femur but to a lesser degree, and Dysalotosaurus and Elrhazosaurus, which have a straighter femur in anterior view (Table 2; Figs 3A, 7, S4; Cal, Dys, Elr, Mut, Rha, Zal). Furthermore, we note a similarly high femoral obliquity between large rhabdodontids, the very robust early-diverging styracosternans Lurdusaurus, indeterminate iguanodontians from the Wealden group, maybe the Iguanodon bernissartensis holotype and the later-diverging hadrosauroid Telmatosaurus (Table 2; Figs 3A, 7, S4; Mut, Rha, Zal, Igu, Lur, Tel). Later hadrosaurids did not maintain femoral obliquity and seem to have reverted to the plesiomorphic ‘straight in anterior view’ condition of small early-diverging bipedal neornithishians (Table 2; Figs 3A, 7, S4; hadrosaurids: Amu, Edm, Ol; early-diverging neornithischians: Hay, Hyp, Les, The). Therefore, we demonstrate an allometric trend in the evolution of femoral obliquity among non-hadrosaurid ornithopods that usually possess a mosaic of bipedal and quadrupedal characters (Figs 7, S4; Maidment & Barrett 2012a).
Covariation between femoral obliquity & locomotor habit
Moreover, we demonstrate the combination of high femoral obliquity with low angles between the crista tibiofibularis and the lateral condyle (i.e. indicative of a bipedal locomotor habit according to Pintore et al. 2022) around the styracosternan node, around which there is the most controversy about ornithopod locomotion (Maidment & Barrett 2012a; Poole 2022; Xu et al. 2018). Thus, we hypothesize that obliquity in the femur could have acted as part of the specializations that facilitated the locomotor shift toward quadrupedality with increasing body mass in non-hadrosaurid ornithopods (Fig. 7), perhaps aiding in support of a presumably more beveled knee joint (i.e. more inward-facing distal epiphyses in laterally-bowed femora) while ensuring mobility in larger-bodied bipedal taxa (i.e. as indicated by low angles in large taxa with indeterminate habit and laterally bowed femora). Femoral obliquity might then have reverted to a more ‘straight in anterior view’ condition in hadrosaurids, as in early-diverging neornithischians, whereas values of the angle between the crista tibiofibularis and the lateral condyle are greater (more obtuse angle) than in all other ornithopods and early-diverging neornithischians, indicating a more (obligate) quadrupedal habit (based on results from Pintore et al. 2022; Fig. 5B). Hence, our results add both femoral obliquity and the angle between the distal lateral condyles to the list of osteological proxies for locomotor estimation in ornithopods (Table 2), among which two (out of five) already involved the femur (a reduced fourth trochanter and femur longer than the tibia in quadrupeds; Maidment & Barrett 2012a).
Functional inference of femoral obliquity in dinosaurs
Because some early small ornithischian ichnofossils reveal shifts from a more narrow to a rather wide-gauge stance (Wilson et al. 2009), we hypothesize that early-diverging bipedal ornithopods may have evolved femoral obliquity with increases of body mass, perhaps to sustain some level of bipedal ability through a wider-gauge stance, which may have remained in early-diverging styracosternans and rhabdodontids (Fig. 7). Indeed, a more oblique femur in Zalmoxes has been suggested as indicative of an unusually wide-gauge stance among ornithopods (Weishampel et al. 2003). Similarly, titanosaurid sauropods have a more oblique femur, which is not laterally ‘bowed’ but more ‘outward-canted’ (i.e. the shaft of titanosaurids is not as circular in shape as in non-hadrosaurid ornithopods), but still resulting in a lower angle between the mediolateral axis of the distal epiphysis and the long axis of the shaft (Wilson & Carrano 1999). Wilson & Carrano (1999) inferred from osteological and ichnological observations that the laterally oblique femur of titanosaurs was linked to a wide-gauge stance, which may have improved static stability in large quadrupedal sauropodomorphs. They also suggested that this higher stability would have provided titanosaurs with some occasional bipedal abilities such as standing still on their hindlimbs for a short amount of time (termed ‘bipedal rearing’), as previously inferred in the clade based on the opisthocoelous morphology of caudal vertebrae and particular morphological traits of the pelvis (Borsuk-Bialynicka 1977; Carrano 2005; Jensen 1988). Occasional bipedal rearing ability has also been inferred in other sauropod clades based on the morphology of the camarasaurid axial skeleton (Jensen 1988) and modelling of shift in the centre of mass position in diplodocids and other sauropods that seem to have been unable to raise their neck very high (Alexander 1985; Upchurch et al. 2004; Mallison 2011). Such bipedal rearing positions appear comparable to the ‘tripodal’ position that was classically envisaged as the ornithopod bipedal stance (Dollo 1883; Jensen 1988; Wilson & Carrano 1999; Upchurch et al. 2004; Maidment & Barrett 2012a). While one femoral feature alone is not sufficient to infer such behaviour in non-hadrosaurid ornithopods, our findings demonstrate that only rhabdodontids (especially the larger specimens Muttaburasaurus, Rhabdodon and Z. shqiperorum; Fig. S4) and large non-hadrosaurid styracosternans shared femoral obliquity, consistent with a wide-gauge stance associated with improved static stability (Wilson & Carrano 1999; Day et al. 2002; Maidment et al. 2012). In addition, Mallison (2011) inferred that greater bipedal abilities in sauropods correlated with slower gait, often associated with wider-gauge stance because it may provide greater stability at the expense of reduced maximal velocity (Wilson et al. 2009; Maidment et al. 2012); hence consistent with our observation of an allometric trend in the evolution of femoral obliquity. Moreover, fitting Wilson & Carrano's (1999) hypothesis, styracosternan taxa with the most oblique femurs also had the most bipedal signal in the angle between the crista tibiofibularis and the lateral condyle (Fig. 5B). A more precise characterization of such bipedal abilities remains out of reach, but parallels with potential bipedality in titanosaurs and other sauropods suggest that ‘facultative bipedality’ could relate to a more static bipedal habit (i.e. standing still on the hindlimbs for reaching food or intimidation) than a sustainably cyclic bipedal locomotor mode. A more thorough osteological study integrating the whole hindlimb and axial morphology could yield further similarities of traits relating to the presumed bipedal-rearing of sauropods and facultative bipedality of ornithopods. Furthermore, biomechanical modelling (e.g. Mallison 2011) focusing on the biomechanical consequence of an oblique vs straight femur and acute vs obtuse distal lateral condyle angles in ornithopods could illuminate whether facultative bipedality, if it existed, can be inferred in ornithischian dinosaurs.
Variation in femoral obliquity through ontogeny in ornithopods
Weishampel et al. (2003) noted a shift from a more oblique to a straighter femur across the ontogeny of Zalmoxes and other ornithopods across the styracosternan clade. Accordingly, this femoral feature was listed as one of the many ontogenetically sensitive femoral features in a recent phylogenetic investigation of Ornithopoda, shifting from oblique to straight from juvenile to adult Zalmoxes, Tenontosaurus and Camptosaurus, and from straight in juvenile to oblique in adult Dryosaurus (Poole 2023). However, Weishampel et al. (2003) also noted that the femur of an adult Z. shqiperorum (NHMUK PV R4900) was more oblique in anterior view than Z. robustus femora of similar size, which is consistent with our observations (Fig. S4). Moreover, while our sample does not include any Zalmoxes juvenile, it does include juveniles of Mantellisaurus, which had some femoral obliquity but with little to no difference from the (most likely adult) holotype of Mantellisaurus (Figs 3A, S4, Man; Lockwood et al. 2021). The same observation applies between juvenile and adult Haya (Fig. S4). Finally, a similar approach applied to a sample composed of early archosauriform femora failed to detect a shift from quadrupedal to bipedal locomotor habit from juvenile to adult Mussaurus sauropodomorphs, even though the juvenile Mussaurus had a slightly lower bipedal signal than the adult (Pintore et al. 2022). Indeed the sample from Pintore et al. (2022), similar to that of this study, is phylogenetically large, rendering 3D GMM and PCA less likely to detect subtle ontogenetic changes relatively to other larger morphological variations. Therefore, we interpret the component of femoral obliquity we observed varying across the styracosternan node as not being ontogenetically related, but more relevant to the interspecific variations noted by Weishampel et al. (2003). Nevertheless, Kitchener et al. (2019) noted that ornithopods may have evolved a reduction of the fourth trochanter with the acquisition of a more quadrupedal habit by a heterochronic shift in which adults retained juvenile characters. Our result indicates that femoral obliquity paralleled a decrease in the fourth trochanter prominence along PC2. Thus, a similar heterochronic shift could be hypothesized at the evolutionary origin of femoral obliquity, which may have been more represented in juvenile bipedal early-diverging ornithopods than in adults, and which could have later been retained in heavier adult (perhaps facultatively) bipedal ornithopods.
CONCLUSION
Because an increase in body mass and a shift from bipedality to effectively obligate quadrupedality evolved at least partly in concert along the evolutionary history of ornithopods, it has historically been challenging to establish which hindlimb features correlated with which biological factor. We demonstrate that ornithopods evolved some femoral specializations to body mass and locomotor habits that are consistent with our and others' previous findings for other clades of dinosaurs and archosauriforms. By comparison with the previously demonstrated femoral specializations in closely related clades, it was therefore possible to better characterize the evolution of ornithopod femoral morphology with increasing body mass and changes in locomotor habit. The femoral shaft was anteriorly bowed in early-diverging bipedal ornithischians and straighter in later, presumably quadrupedal hadrosaurids. The fourth trochanter shifted distally from the most gracile to the most robust femora in our sample, whereas its morphology varied from more asymmetric (pendant and non-pendant) to more symmetric from bipedal early-diverging neornithischians to presumably quadrupedal hadrosaurids. Larger ornithopods also evolved more prominent anterior condyles. Whereas all of these femoral specializations covaried, only early-diverging ornithopods and non-hadrosaurid styracosternans evolved a strong femoral obliquity and a lower angle between the distal condyles (i.e. the crista tibiofibularis and lateral condyle; also correlated with a bipedal habit in early archosauriforms; Pintore et al. 2022). The evolution of femoral obliquity was distinct from the rest of the femoral specializations in ornithopods, and is observed only among the taxa with the most controversial locomotor habits (i.e. only in non-hadrosaurid styracosterans and rhabdodontids). Our results suggest that a higher femoral obliquity and lower angle between the distal condyles could have acted as a specialization to the parallel evolution of a larger body mass and a quadrupedal habit by providing a more stable, wide-gauge stance in non-hadrosaurid ornithopods. Therefore, as inferred in other large-bodied dinosaurs with a wide-gauge quadrupedal stance with similarly oblique femora (i.e. the outward-canted femur of titanosaurid sauropods), we hypothesize that ornithopods with the most oblique femora may also have been capable of some occasional bipedal abilities (i.e. facultative bipedality or static occasional bipedal rearing in a tripodal stance). Thus, our results agree with Maidment & Barrett (2012a, 2012b) and Maidment et al. (2012) that the locomotor habit of non-hadrosaurid iguanodontians may have included varying amounts of bipedal and quadrupedal abilities and that hadrosaurids had a predominantly quadrupedal habit. Thorough biomechanical investigations of femoral obliquity and the angle between the distal condyles could illuminate the timing and mechanisms behind the evolution of locomotor shifts in ornithopods. In addition, such research could potentially help resolve uncertainties about non-hadrosaurid styracosternan and rhabdodontid locomotion, and maybe better characterize what traits, if any, correspond to a facultative bipedal locomotor mode in dinosaurs and their cousins.
Acknowledgements
We are deeply grateful to the following people for providing high-quality data of ornithopod femora, which were essential to our study: C. Mallet (IRSNB), C. Kammerer (NCSM), B. Clark (NHM), M. Bellato, (MNHN). We also warmly thank curators for providing access to collections and exhibition, for helping with storing some specimens online, as well as extensive details about specimens: S.C.R. Maidment, M.E H. Jones, M.O. Day (NHM), P. Godefroit. A.-L. Folie (IRSNB), R. Allain, C. Colin, V. Pernegre, S. Daillie (MNHN). We thank the following people for facilitating the access to the specimens used in this study: A. Canoville (NCSM), D. Olivier, D. Brabant, J. Gônet (MNHN). We are also thankful to S. Tarquini, M. Proust, M. Camara for their help with 3D reconstruction of specimens, which helped saving time at the end of author RP's PhD. We also thank D. Hone, T. Raven and S.C.R. Maidment for helping carrying heavy ornithopod femora in the NHM collections during digitization. We thank RP's thesis examiners S.E. Pierce (Harvard University, USA), S.L. Brusatte (University of Edinburgh, UK) and S.C R. Maidment (NHM) for a thorough read and profound discussions about the early stages of this study, R. Lefebvre for insightful discussion about femoral obliquity in sauropods, and the rest of the GRAVIBONE team, C. Bader, C. Etienne, and P. Hanot, (MNHN) for discussions and their support. Finally, we thank two reviewers (S.C.R. Maidment and K.E. Poole) for their constructive feedback and stimulating discussions and S. Thomas for editorial work, which improved the quality of our manuscript.
Author contributions
Conceptualization Romain Pintore (RP), Alexandra Houssaye (AH), John R. Hutchinson (JRH); Data Curation RP, AH, JRH; Formal Analysis RP; Funding Acquisition AH, JRH; Investigation RP, AH, JRH; Methodology RP; Project Administration RP, AH, JRH; Resources AH, JRH; Software RP; Supervision AH, JRH; Validation AH, JRH; Visualization RP; Writing – Original Draft RP, AH, JRH; Writing – Review & Editing RP, AH, JRH.
Data archiving statement
Data for this study (including NHM specimens) are available in MorphoSource: https://www.morphosource.org/projects/000740467 (see Appendix S1 for individual DOIs).




