Pulmonary vein isolation by means of a novel cryoballoon technology in paroxysmal atrial fibrillation patients: 1-year outcome from a large Italian multicenter clinical registry
Abstract
Introduction
Recently, a new cryoballoon (CB) technology (POLARx; Boston Scientific) has come onto the market. Preliminary data have shown that its acute safety and efficacy are similar to those of the first-generation CB. The aim of this study was to assess the medium-term outcome of pulmonary vein isolation (PVI) with the POLARxTM CB in a large multicenter registry.
Methods
We prospectively collected data on 125 consecutive patients with paroxysmal atrial fibrillation (AF) who underwent PVI by means of a novel CB system. Two cases of transient phrenic nerve palsy occurred, with full recovery in the 48h post procedure; no major procedure-related adverse events were reported. During the 90-day blanking period, 4 (3.2%) patients experienced an early recurrence. After the blanking period, over a mean follow-up of 411 ± 62 days, 19 patients (15.2%) suffered an AF/atrial tachycardia (AT) recurrence. The 1-year freedom from AF/AT recurrence was 86.4% (n = 17): 10 (8%) patients had an AF recurrence, 6 (4.8%) had an AT occurrence and 1 (0.8%) suffered both events. Patients with AF/AT recurrences had both a shorter deflation time and total deflation time. Moreover, CB ablations with measured TTI < 90 s and TTI < 60 s were more frequent in patients without AF/AT recurrence (88.5% and 77.4%, respectively) than in those who experienced at least one AF/AT recurrence (67.5% and 55.0%, p = .001 and p = .005, respectively).
Conclusion
The novel POLARx cryo-balloon system is safe and effective for PV isolation, displaying a 1-year freedom from atrial arrhythmia recurrence of 86.4%, which is in line to that reported with AFA-Pro CB or RF ablation.
Clinical trial registration
Catheter Ablation of Arrhythmias with a High-Density Mapping System in Real-World Practice (CHARISMA). URL: http://clinicaltrials.gov/ Identifier: NCT03793998. Registration date: January 4, 2019.
Abbreviations
-
- AF
-
- atrial fibrillation
-
- AT
-
- atrial tachycardia
-
- CB
-
- cryoballoon
-
- CI
-
- confidence interval
-
- DMS
-
- diaphragm movement sensor
-
- ERAF
-
- early recurrences of AF
-
- HR
-
- hazard ratio
-
- LIPV
-
- left inferior PV
-
- LSPV
-
- left superior PV
-
- PN
-
- phrenic nerve
-
- PV
-
- pulmonary vein
-
- PVI
-
- PV isolation
-
- RF
-
- radiofrequency
-
- RIPV
-
- right inferior PV
-
- RSPV
-
- right superior PV
-
- TTI
-
- time-to-isolation
1 INTRODUCTION
Pulmonary vein (PV) isolation is the cornerstone of catheter ablation in patients with symptomatic atrial fibrillation (AF).1 Radiofrequency (RF)-based PV isolation, in combination with a 3D mapping system, and cryoballoon (CB) are the most widely used ablation techniques for PV isolation, and have shown similar safety and efficacy.2 Recently, a new CB technology (POLARx; Boston Scientific) has come onto the market. The novel POLARx CB has several similarities to the Arctic Front Advance-Pro (AFA) CB (i.e., double-layer balloon, 28 mm balloon size, and nitrous oxide cooling technology), but the inner balloon pressure is kept constant during the inflation and freezing phases. Thus, the inner balloon pressure of the POLARx is lower than that of the AFA-Pro during the freezing phase, resulting in a more compliant balloon. Preliminary data3-6 have shown that the acute safety and efficacy of POLARx are similar to those of the AFA-Pro CB, although the nadir temperatures of POLARx are lower than those of AFA-Pro. However, limited data on the medium-term outcome of PV isolation by means of this new CB technology are currently available.7-9 The aim of this study was to assess the medium-term outcome of PV isolation by means of the POLARx CB in a large multicenter registry.
2 METHODS
2.1 Patient population and study design
The Catheter Ablation of Arrhythmias with a High-Density Mapping System in Real-World Practice (CHARISMA) (ClinicalTrials.gov Identifier: NCT03793998) is a prospective, single-arm, multicenter cohort study designed to describe clinical practice regarding the approach to the ablation of various arrhythmias. The study complied with the Declaration of Helsinki, the locally appointed ethics committee approved the research protocol, and informed consent was obtained from all patients. In this paper, we present the analysis of the first 125 (out of 127, 98.5%) consecutive patients (July 2020 to October 2021) indicated for paroxysmal AF ablation who had undergone PV isolation by means of the POLARx system in seven Italian centers and had completed a minimum follow-up of 1 year. Two patients (out of 127, 1.5%) were excluded as they were lost to follow up. All patients were followed up at the same hospital, from the time of ablation to the last follow-up visit.
2.2 Ablation procedure
Oral anticoagulation was left uninterrupted on the day of the procedure in all patients, and continued in patients with a high thromboembolic risk, as indicated by their CHA2DS2-VASc score. Antiarrhythmic drugs, except for amiodarone, were usually discontinued ≥ 5 half-lives prior to ablation. The ablation procedure has already been described.10 In brief, procedures were performed under conscious sedation or general anesthesia. After transseptal catheterization, the CB catheter was inserted into the left atrium via a 15.9-F steerable sheath (POLARSheath, Boston Scientific). Mapping of the PVs was performed by means of a 20-mm inner lumen circular mapping catheter with 8 electrodes (POLARMap, Boston Scientific). The mapping catheter was advanced into each PV ostium and positioned as proximally as possible, in order to record PV potentials. A 28-mm, short-tip (5-mm tip) or long-tip (12-mm tip) CB (POLARx, Boston Scientific) was advanced, inflated, and positioned at each PV antrum. Optimal vessel occlusion was considered to have been achieved when selective contrast injection showed the absence of contrast backflow to the atrium. The standard set of lesions includes the left superior PV (LSPV), which is treated first, followed by the left inferior PV (LIPV), right inferior PV (RIPV) and right superior PV (RSPV). As per the nature of this project, we did not implement a standardized protocol regarding the number and duration of freezes nor the use of a bonus freeze, but a general strategy was shared among the centers. In the presence of a common ostium, the veins were treated as separate branches. The application time for each vein was calculated as the time-to-isolation (TTI), and the circular mapping catheter was positioned at the proximal part of the ostium before each freeze. Protocol-directed cryoablation was delivered for 180 or 240 s, according to the operator's preference, if solation was achieved in ≤ 60 s, or for 240 s if isolation occurred in > 60 s or when TTI was not available. The ablation endpoint was PV isolation, as assessed by entrance and exit block, verified at the end of the procedure by means of the POLARMap catheter, which was placed at the ostium of every PV. TTI was defined as the time from freeze initiation to the last recorded PV potentials. Total deflation time was defined as the sum of thaw time (to 0°C) and deflation time (0°C to 20°C). In order to avoid phrenic nerve (PN) palsy, continuous high-output pacing to capture the PN was performed during right-PV applications was performed. Cryoablation was immediately stopped if there was loss or reduction of PN capture or the minimum temperature of < 65°C was reached. Specifically, a movement sensor (Diaphragm Movement Sensor, DMS, Boston Scientific), in addition to conventional beside methods, was used to check nerve capture during CB ablation. Baseline DMS is automatically assessed when general PN pacing is started. The DMS cut-off was set at 60% of diaphragmatic excursion. The freeze cycle was terminated by double stop if the cut-off was reached and no PN capture was detected immediately.
2.3 Post-ablation management and follow-up
Complications were reported on the case report form and collected during follow-up. After ablation, anticoagulation therapy was continued for at least 3 months, and then according to the patient's stroke risk. Clinical evaluation and ECG were performed at 1, 3, 6, and 12 months. Holter ECG was performed at 3, 6 and 12 months post-ablation or in the case of symptoms. Arrhythmia recurrences within the first 3 months (blanking period) were classified as early recurrences and were not considered procedural failures. The primary endpoint of this analysis is the freedom from AF recurrence as defined as 1-year absence of AF or atrial tachycardia (AF/AT) lasting continuously for > 30 s, as recorded by any post-ablation ECG modality.
2.4 Statistical analysis
Descriptive statistics are reported as means ± SD for normally distributed continuous variables, or medians with 25th to 75th percentiles in the case of skewed distribution. Normality of distribution was tested by means of the non-parametric Kolmogorov–Smirnov test. Differences between mean data were compared by means of a t-test for Gaussian variables, and the F-test was used to check the hypothesis of equality of variance. The Mann-Whitney non-parametric test was used to compare non-Gaussian variables. Differences in proportions were compared by applying χ2 analysis or Fisher's exact test, as appropriate. Hazard ratios (HRs) and their 95% confidence intervals (CIs) were computed by means of Cox regression models, in which baseline parameters were taken as fixed covariates and endpoint events were taken as time-dependent covariates. The proportional hazard assumption was assessed by means of Schoenfeld residuals. A p value < .05 was considered significant for all tests. All statistical analyses were performed by means of STATISTICA software, version 7.1 (StatSoft, Inc., Tulsa, OK).
3 RESULTS
3.1 Study population
One-hundred twenty-five consecutive patients with a history of paroxysmal AF were enrolled. Besides AF, 20 (20.0%) patients had a history of atrial flutter/AT. The mean age was 60.3 ± 11 years, and 89 (71.2%) were males. Baseline clinical characteristics are shown in Table 1.
| Parameter | All patients (n = 125) |
|---|---|
| Age, years | 60.3 ± 11 |
| Male Gender, n (%) | 89 (71.2) |
| History of atrial flutter/atrial tachycardia | 20 (16.0) |
| LVEF, % | 58.2 ± 7 |
| BMI, kg/m2 | 26.7 ± 5 |
| Cardiomyopathy, n (%) | 12 (9.6) |
| Hypertension, n (%) | 53 (42.4) |
| Coronary artery disease, n (%) | 9 (7.2) |
| History of heart failure, n (%) | 3 (2.4) |
| COPD, n (%) | 2 (1.6) |
| Alcohol consumption (low to moderate), n (%) | 21 (16.8) |
| Current smokers, n (%) | 23 (18.4) |
| CKD, n (%) | 2 (1.6) |
| History of major bleeding, n (%) | 1 (0.8) |
| Antiarrhythmics, n (%) | 80 (64.0) |
| ACE-ARB, n (%) | 36 (28.8) |
| Betablockers, n (%) | 49 (39.2) |
| Statin, n (%) | 27 (21.6) |
| Diuretics, n (%) | 12 (9.6) |
| Antiplatelet agents | |
| •No therapy, n (%) | •116 (92.8) |
| •Single, n (%) | •8 (6.4) |
| •Double, n (%) | •1 (0.8) |
| TAO | |
| •No therapy, n (%) | •25 (20) |
| •VKA, n (%) | •8 (6.4) |
| •DOAc, n (%) | •92 (73.6) |
- Abbreviations: ACE, Angiotensin Converting Enzyme; ARB, Angiotensin Receptor Blockers; BMI, Body mass index; CKD, Chronic kidney disease; COPD, Chronic obstructive pulmonary disease; DOAc, Direct oral anticoagulant; LVEF, Left ventricular ejection fraction; VKA, Vitamin K antagonist.
3.2 Procedural characteristics
In the majority of the cases (100, 80%), procedures were performed under conscious sedation, whereas general anesthesia was applied less frequently (25, 20%). A total of 495 PVs were targeted in the 125 patients. PV isolation was achieved in all patients by means of cryoablation alone. A short tip was used in 77 (61.6%) of the cases, whereas a long tip in 48 (38.4%) of the cases. The mean number of freeze applications per patient was 5.0 ± 1.4 (1.2 ± 0.6 for LIPV, 1.3 ± 0.7 for LSPV, 1.3 ± 0.6 for RIPV and 1.2 ± 0.7 for RSPV). Fifty-three (42.4%) patients were treated with a single application to each of the PVs (393 PVs [80.2% of the total] were treated in a single-shot fashion). TTI was available in 301 (61.4%) PVs, the median TTI being 39 [30 to 61] s (median temperature at TTI = −48°C [−52 to −40]). The median nadir temperature was −57°C [−61 to −52], the median time to target at −40°C was 30 [27 to 33] s, the median thaw time to 0°C was 19 [15-22] s and the median deflation time (from 0°C to 20°C) was 25 [18 to 33] s. Complete occlusion of the PV was achieved in 372 (81.6%) out of 451 PVs with available information on the grade of PV occlusion. Detailed procedural data are reported in Table 2. Cryoablation was stopped early in 23 (3.7%) cases: in 11 cases (8 at the RSPV and 3 at the RIPV), owing to transient weakening of right hemi-diaphragm contractions measured by the DMS; in 9 cases (1 at the LSPV, 4 at the LIPV, 2 at the RSPV and 2 at the RIPV) because the minimum temperature cut-off (−70°C) had been reached or the temperature had decreased very rapidly, and in the remaining 3 cases owing to the operator's choice. At the end of the procedure, complete isolation of each PV was documented by the POLARMap catheter in all patients. Two cases of transient PN palsy occurred, with full recovery in the 48h post procedure; no major procedure-related adverse events were reported.
| Parameter | All PVs | LIPV | LSPV | RIPV | RSPV |
|---|---|---|---|---|---|
| Total number of PVs, n | 490a | 123 | 123 | 122 | 122 |
| Mean number of CBAs, n | 5.0 ± 1.3 | 1.2 ± 0.6 | 1.3 ± 0.7 | 1.3 ± 0.6 | 1.2 ± 0.6 |
| Single CBA, n (%) | 393 (79.9) | 102 (81.6) | 95 (76.0) | 95 (76.0) | 101 (80.8) |
| 2 CBAs, n (%) | 78 (15.9) | 19 (15.2) | 21 (16.8) | 22 (17.6) | 16 (12.8) |
| >2 CBAs, n (%) | 19 (3.9) | 2 (1.6) | 7 (5.6) | 5 (4.0) | 5 (4.0) |
| Patients treated with single CBA of each of the PVs | 52 (42.4) | ||||
| TTI measured per vein, n (%) | 301 (61.4) | 76 (61.7) | 94 (76.4) | 51 (41.8) | 80 (65.6) |
| TTI (sec) | 39 [30 to 61] | 37 [28 to 53] | 45 [33 to 71] | 45 [32 to 71] | 38 [26 to 53] |
| Temperature at TTI (°C) | −48 [−52 to −40] | −45 [−50 to −37] | −51 [−54 to −43] | −48 [−50 to −38] | −47 [−51 to −37] |
| Time to target (−40°C) (sec) | 30 [27 to 33] | 30 [28 to 34] | 29 [27 to 31] | 31 [27 to 35] | 29 [27 to 31] |
| Nadir temperature (°C) | −57 [−61 to −52] | −55 [−59 to −51] | −58 [−61 to −55] | −55 [−60 to −50] | −59 [−63 to −53] |
| Thaw time (to 0°C) (sec) | 19 [15 to 22] | 18 [15 to 20] | 20 [17 to 24] | 17 [14 to 21] | 20 [16 to 26] |
| Deflation time (0°C to 20°C) (sec) | 25 [18 to 33] | 24 [19 to 30] | 28 [20 to 40] | 20 [16 to 29] | 25 [18 to 38] |
- Abbreviations: CBA, Cryoballoon application; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; PV, pulmonary vein; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; TTI, time to isolation.
- a A left common ostium PV was present in 2 patients and right accessory PVs in 3 patients; data not included here.
3.3 Outcome
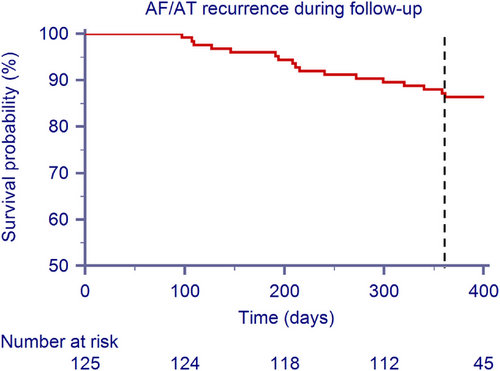
During the 90-day blanking period, 4 (3.2%) patients experienced an early recurrence. After the blanking period, over a mean follow-up of 411 ± 62 days, 19 patients (15.2%) suffered an AF/AT recurrence (mean time to recurrence: 252 ± 122 days). No patients with early recurrence also had recurrences after the 90-day blanking period. Figure 1 shows the survival curve from AF/AT recurrence during follow-up after the 90-day blanking period. After the blanking period, the 1-year freedom from atrial arrhythmia recurrence was 86.4%; 17 patients experienced at least one AF/AT recurrence: 10 (8%) had an AF recurrence, 6 (4.8%) had an AT occurrence and 1 (0.8%) suffered both events. One patient underwent a redo ablation (by mean of RF).
3.4 Clinical and procedural parameters associated with atrial arrhythmia recurrences
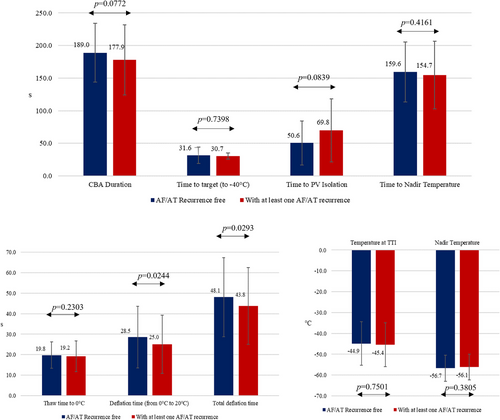
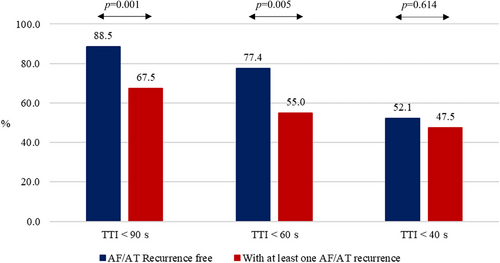
Univariate analysis of clinical parameters associated with a 1-year AF/AT recurrence is reported in Table S1. No parameter reached statistical significance. Concerning the procedural parameters (Figure 2), patients with atrial arrhythmia recurrences had a shorter deflation time and shorter total deflation time (28.5 s vs. 25.0 s for deflation time, p = .0244; 48.1 s vs. 43.8 s for total deflation time, p = .0293, respectively), moreover CB ablation with measured TTI < 90 s or TTI < 60 s was more frequent in the group of patients without AF/AT recurrence (231 out 261, 88.5% for TTI < 90 and 202 out 261, 77.4% for TTI < 60 s) than in the group of patients who experienced at least one AF/AT recurrence (27 out 40, 67.5%, p = .001 for TTI < 90 s; 22 out 40, 55.0%, p = .005 for TTI < 60) (Figure 3).
4 DISCUSSION
We assessed the medium-term procedural outcome of PV isolation by means of a novel CB technology in patients with paroxysmal AF. Our key findings were (1) one-year freedom from atrial arrhythmia recurrence was 86.4%, which is comparable to that achieved with AFA-Pro CB or RF ablation; (2) the recurrence rate during the blanking period was low (3.2%); and (3) patients with atrial arrhythmia recurrences had a shorter deflation time and total deflation time and displayed a lower presence of TTI < 90 s and TTI < 60 s.
4.1 One-year freedom from arrhythmias after catheter ablation
Catheter ablation is a well-established treatment in patients with both paroxysmal and persistent AF. The development of new technologies has enabled high percentages (over 80%) of 1-year freedom from arrhythmias to be achieved, with a low incidence of complications. Similar data have been reported with both RF and lesion quality markers11-13 and AFA-Pro CB.14 Recently, a new CB technology (POLARx) has been introduced into clinical practice. Although the acute safety and efficacy of the POLARx CB seem to be similar to those of the AFA-Pro CB, data on medium- and long-term efficacy are required. Yap et al.15 enrolled 110 patients (POLARx: n = 57; AFA-Pro: n = 53), mainly (75%) with paroxysmal AF. The 1-year freedom from atrial arrhythmias was 82% and 87% for the POLARx and AFA-Pro group, respectively (log-rank p = .60). Knecht S et al.9 enrolled 80 consecutive patients with AF (64% paroxysmal) undergoing PV isolation. After a single procedure and a follow-up of 12 months, 68% in the POLARx CB group and 70% in the AFA-Pro CB group showed no recurrence of AF/atrial tachycardias (p = .422). Tanese N et al.8 studied 267 consecutive patients undergoing a first CB procedure for paroxysmal AF (137 POLARx, 130 AFA-Pro). After a mean follow-up of 15 ± 5 months, 20 patients (15%) had recurrences in AFA-Pro group and 27 patients (19%) in POLARx group (p = .35). Based on survival analysis, no significant difference was observed between both groups with a 12-month free of recurrence survival of 91.2% (85.1−95.4%) vs. 83.7% (76.0%−89.1%) (log-rank test p = .11). Our data, which were collected in a larger, multicenter registry, seem to confirm these preliminary experiences.
4.2 Recurrence during the blanking period
Early recurrences of atrial fibrillation (ERAF) have been reported in up to 50% of patients within the first 3 months after AF ablation.16-18 Although these arrhythmias do not indicate therapy failure, they are associated with a higher AF recurrence rate over the long term. The new technologies have significantly reduced the incidence of ERAF after point-by-point RF PV isolation to less than 10%.12, 13 Recently, in a large multicenter registry,19 using the AFA-Pro CB in 3681 patients with both paroxysmal (74.3%) and persistent AF, ERAF occurred in only 8.6% of patients and was more frequent in patients with persistent AF, those who had tried at least 2 antiarrhythmic drugs, and patients on anti-arrhythmic drugs during the blanking period. In our series of patients treated with the new POLARx CB, we confirmed a low rate of ERAF, which occurred in only 4 (3.2%) patients.
4.3 Predictors of atrial arrhythmia recurrences
Despite the high rate of acute success in achieving PV isolation, the medium- and long-term outcome of both point-by-point RF and CB ablation still display a non-negligible number of cases of atrial arrhythmia recurrence, mainly due to PV reconnections1 although the mechanisms of AF induction and maintenance, including those involved in paroxysmal AF, are not completely known; this limits our ablation strategies and prevents us from understanding what we are actually doing when performing pulmonary vein isolation (PVI).20 In 112 consecutive patients who underwent a repeat procedure 14 ± 3 months after an index cryoablation of AF, Aryana A et al.21 found that a time-to-effect of ≤60 s and a thaw time to 0°C ≥10 s significantly predicted PV isolation durability. Mugnai G et al.22 studied 300 consecutive patients undergoing a repeat ablation 18.2 ± 10.8 months after the index CB ablation. They found that the rate of late PV reconnection after CB ablation was low (1.18 PVs/patient). Shorter time to isolation, colder nadir temperatures and achievement of -40°C within 60 s were associated with durable PV isolation. So far, no data are available on predictors of the durability of PV isolation when CB ablation is performed with the new POLARx CB. In our series, we did not find any clinical predictor of recurrence, although patients with atrial arrhythmia recurrences had a shorter deflation time and total deflation time and a lower presence of TTI < 90 s and TTI < 60 s, thus confirming that the freezing behavior plays a crucial role in the efficacy of CB ablation. A prolonged balloon thaw time is believed to signify a colder CB ablation with longer therapeutic ice crystallization. The latter is a process that occurs during the thawing phase of the CB and is believed to promote additional cellular injury.23 Therefore, longer thawing intervals may not only represent a marker of colder CB ablations but also more effective CB ablations.21 Similarly, a shorter TTI has been proved to be a marker of efficiency of CB ablations.21, 22
4.4 Limitations
The results of this study should be interpreted with caution, owing to a number of limitations. First, as this was a multicenter study, each center and operator maintained their usual clinical practice. Although all patients underwent the same ablation protocol, some aspects, such as pre-procedural imaging, oral anticoagulant management and timing of protocol-directed cryoablation were not standardized. Second, sinus rhythm maintenance was defined mainly on the basis of patients’ symptoms, ECG and scheduled 24-h Holter monitoring. Asymptomatic or short-lasting AF episodes may therefore have occurred unnoticed, and our success rate may have been over-estimated. Third, the length of follow-up was a little more than 1 year. Longer and randomized trials are warranted in order to confirm the long-term efficacy of the POLARx CB in PV isolation in patients with AF. Finally, we collected data only on patients with paroxysmal AF.
In addition, the patients were not followed with a continuous monitoring, so the rate of recurrences could have been underestimated. However, considering that our evaluation methods is comparable to those used in most similar literature, it looks rather acceptable for the conclusion of the study.
5 CONCLUSIONS
The novel POLARx cryo-balloon system is safe and effective for PV isolation. The recurrence rate during the blanking period was low (3.2%) and 1-year freedom from atrial arrhythmia recurrence was 86.4%, which is in line to that reported with AFA-Pro CB or RF ablation. Patients with atrial arrhythmia recurrences had a shorter deflation time and total deflation time and a lower presence of TTI < 90 s and < 60 s.
ACKNOWLEDGMENTS
No funding sources were involved in the research.
CONFLICT OF INTEREST STATEMENT
M.Ma. and M.M.B. are salaried employees of Boston Scientific. G.S. is a consultant for Medtronic. G.F. is a clinical proctor for Boston Scientific. F.M.C. received honorary from Abbott and Biosense Webster. C.T. received speaker's and proctor's fees from Boston Scientific, Medtronic, Abbott and Biosense Webster. He serves in the Advisory Board of Medtronic, Boston Scientific. M.Mo. received speaker's fees from Abbott, Boston Scientific and Medtronic. S.B. and P.R. received speaker's and proctor's fees from Boston Scientific and Medtronic. The other authors have no conflicts of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.