Hypotensive Anesthesia Combined with Tranexamic Acid Reduces Perioperative Blood Loss in Simultaneous Bilateral Total Hip Arthroplasty: A Retrospective Cohort Study
Disclosure: The authors had no conflict of interest. All authors have contributed significantly to this manuscript and approved the submission. We thank Dr. Yi-Qi Du for the technical assistance.
Abstract
Objective
To assess the efficacy and safety of hypotensive anesthesia (HA) combined with tranexamic acid (TXA) for reducing perioperative blood loss in simultaneous bilateral total hip arthroplasty (SBTHA).
Methods
In this retrospective cohort study, a total of 183 eligible patients (15 females and 168 males, 44.01 ± 9.29 years old) who underwent SBTHA from January 2015 to September 2020 at our medical center were enrolled for analysis. Fifty-nine patients received standard general anesthesia (Std-GA group), the other 85 and 39 patients received HA with an intraoperative mean arterial pressure between 70 and 80 mmHg (70–80 HA group) and below 70 mmHg (<70 HA group), respectively. TXA was administrated to all patients. Perioperative blood loss (total, dominant, and hidden), transfusion rate and volume, hemoglobin and hematocrit reduction, duration of operation and anesthesia, length of hospitalization, range of hip motion as well as postoperative complications were collected from hospital's electronic records and compared between groups.
Results
All patients were followed for more than 3 months. Total blood loss in the two HA groups (1390.25 ± 595.67 ml and 1377.74 ± 423.46 ml, respectively) was significantly reduced compared with that in Std-GA group (1850.83 ± 800.73 ml, P < 0.001). Both dominant and hidden blood loss were dramatically decreased when HA was applied (both P < 0.001). Accordingly, the transfusion rate along with volume in 70–80 HA group (14.1%, 425.00 ± 128.81 ml) and <70 HA group (12.8%, 340.00 ± 134.16 ml) were reduced in comparison with those in Std-GA group (37.3%, 690.91 ± 370.21ml; P = 0.001 and P = 0.014, respectively). The maximal hemoglobin and hematocrit reduction in both HA groups were significantly less than those in Std-GA group (both P < 0.001). Of note, 70–80 and <70 HA groups exhibited comparable efficacy with no significant differences between them. Besides, significant difference in duration of surgery was found among groups (P = 0.044 and P < 0.001), while no differences in anesthesia time and postoperative range of hip motion were observed. Regarding complications, the incidence of both acute kidney injury and postoperative hypotension in <70 HA group was significantly higher than that in 70–80 HA and Std-GA groups (P = 0.014 and P < 0.001). Incidence of acute myocardial injury was similar among groups (P = 0.099) and no other severe complications or mortality were recorded.
Conclusion
The combination of HA with a mean arterial pressure (MAP) of 70–80 mmHg and TXA could significantly reduce blood loss and transfusion during SBTHA, in addition to shortening operation time and length of hospitalization, and with no increase in complications.
Introduction
Total hip arthroplasty (THA) is recognized as one of the most successful surgical interventions to relieve pain and improve function for patients with end-stage hip diseases. The demand of THA in China exceeds 400,000 and will be increasing by 25%–30% per year1. As a matter of fact, a substantial portion of patients undergo bilateral progression and eventually require bilateral THA. For those patients, simultaneous bilateral total hip arthroplasty (SBTHA) may be an alternative choice as the simultaneous procedures can dramatically improve the cost-effectiveness by reducing overall anesthetic time, length of hospitalization, and rehabilitation, and it would not impair the functional outcome or increase the risks of revision, reoperation, or postoperative complications when compared to staged surgeries2-4.
Nevertheless, the hemorrhage during SBTHA can be enormous. It is estimated that postoperative anemia develops in about 90% of patients after SBTHA, and the overall allogeneic transfusion rate can be up to 49.3%–72.7%, leading to a significant increase in morbidity, transfusion-related complications, and economic burden5-7. In an attempt to minimize blood loss and the need for transfusion, multiple blood managements have been developed to either reduce or salvage perioperative blood loss, including acute normovolemic hemodilution, tourniquet, hypotensive anesthesia, intraoperative blood salvage system, hypotensive anesthesia (HA), and antifibrinolytic agents8-13. Even though these strategies have resulted in some satisfying achievements, a considerable percentage of patients still suffer from postoperative anemia and require addition treatments14, 15. Therefore, finding an effective and safe blood management method still remains a top priority.
Hypotensive anesthesia is a widely used blood management strategy that makes use of the anesthesia techniques and/or cardiovascular agents to reduce blood pressure with the preservation of the perfusion of vital organs and distal extremities, thereby limiting intraoperative blood loss during surgery16. The administration of HA can effectively control bleeding of the soft tissue and bone surface of acetabulum and femur after osteotomy. Besides, HA could offer additional benefits, such as shortening the operation time, providing a dry surgical field, reducing the risk of tissue edema, improving myocardial performance, and reducing risk of embolism events17. However, excessive or prolonged hypotension during surgery would inevitably cause adverse effects which impact recovery after surgery, like postoperative delirium, acute kidney injuries (AKI), or acute myocardial injury (AMI), and so on. At the meantime, tranexamic acid (TXA) is another generally accepted antifibrinolytic agent used in orthopaedic surgeries. Differently, TXA is a synthetic amino acid analogous to lysine. It can effectively compete for the lysine binding site on plasminogen, thus inhibiting the activation of plasmin and promoting the retention of blood clots18. Empirical evidence has suggested that TXA could significantly reduce bleeding and the need for transfusion in the perioperative period of arthroplasty without increasing the risk of venous thrombotic events19, 20. Now that HA and TXA take effects through distinct principles as we mentioned above, we are wondering that if their combination would further reduce the blood loss and blood use in the perioperative period of SBTHA. However, to the best of our knowledge, no study has reported their combined efficacy. Whether their combination would produce additive effects, or even bring extra benefits, remains to be determined. What's more, the safety of such a combination is also a major concern.
In the present study, we constructed this retrospective cohort of patients who underwent SBTHA in our institute. The main aims of this study were to explore: (i) whether HA combined with TXA could further reduce perioperative blood loss and transfusion than TXA alone; (ii) how this combination would influence rehabilitation and postoperative complications after SBTHA; and (iii) what is the optimal range of blood pressure during SBTHA.
Methods
Study Design and Cohort Selection
A retrospective, observational cohort study was conducted. A prospectively maintained institutional database was retrieved to identify all patients who underwent surgical treatments for bilateral end-stage hip diseases between June 2015 and September 2020. Inclusion criteria included: (i) adult patients who underwent primary SBTHA; (ii) American Society of Anesthesiologists (ASA) physical status I-III; (iii) a follow-up time of over 3 months. Exclusive criteria included: (i) staged THA or revision surgeries; (ii) patients with severe cardiovascular or cerebrovascular disorder, chronic kidney disease (glomerular filtration rate <60 ml/(min·1.73 m2) or liver dysfunction (jaundice, sign of portal hypertension, or hepatic encephalopathy); (iii) patients with moderate-to-severe anemia (Hemoglobin, Hb < 90 g/L) and coagulation defects (platelet < 75 × 109/L; activated partial thromboplastin time >50 s or prothrombin time >15 s); (iv) patients allergic to TXA. According to the inclusion and exclusion criteria, a total of 183 patients were enrolled and divided into three groups according to whether HA was administered and the levels of intraoperative MAP during the surgery. Fifty-nine patients received standard general anesthesia (Std-GA group), and the other 85 and 39 patients received HA with a mean arterial pressure (MAP) between 70 and 80 mmHg (70–80 HA group) and below 70 mmHg (<70 HA group), respectively. The selection process is summarized in Figure 1.
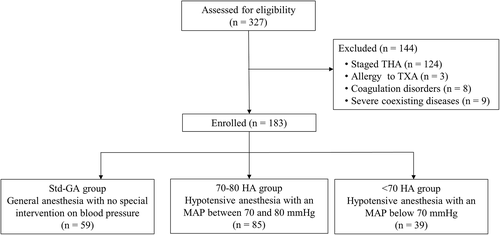
This study was approved by the Ethics Committee of West China Hospital and registered in the Chinese Clinical Trial Registry (ChiCTR2100047184). And because this was a retrospective study, permission for exemption of informed consent was obtained.
Anesthesia Protocol
All SBTHA were performed under general anesthesia. Briefly, sufentanil (0.15–0.4 μg/kg)/oxycodone (0.1–0.15 mg/kg) + propofol (1–2 mg/kg) + cisatracurium (0.1–0.2 mg/kg)/rocuronium (0.6–0.9 mg/kg) were used for anesthesia induction. Then, remifentanil (0.1–0.3 μg/kg/min) + sevoflurane (1%–3%)/desflurane (4%–6%) or propofol (2–6 mg/kg/min) was used to maintain anesthesia during surgery. For HA, expect the standard procedure, a rapid dose of sufentanil (50–100 μg) and propofol (50–100 mg)/increasing the concentration of inhaled anesthetic drugs were used to reduce arterial pressure to predetermined value. If necessary, nicardipine, nitroglycerin, or other drugs were used to assist in maintaining hypotensive status. When arterial pressure decreased below 60 mmHg, metaraminol or ephedrine was used to raise the blood pressure.
Tranexamic Acid Protocol
All patients received intravenous 15 mg/kg TXA diluted in 100 ml of saline 10 min prior to the initial incision of the first hip. Besides, subsequent doses were administrated 24 h after surgery according to the joint swelling and Hb level.
Surgical Methods
All SBTHA were performed using standard posterolateral approach (PLA) or direct anterior approach (DAA) as we described in detail before by surgeons who had completed fellowships in reconstructive surgery on adult patients21. Before suturing, 0.25% ropivacaine (40–60 ml) was injected all around the incision, layer by layer, to manage pain. The intraoperative blood loss was measured and recorded carefully.
Postoperative Treatment
Cephalosporin antibiotics were routinely prescribed within 24 h postoperatively to prevent infection. All patients received thromboembolic prophylaxis consisting of subcutaneous injection of 4000 IU low-molecular-weight heparin beginning 8 h after the operation and then 2000 IU at 24-h intervals. In addition, rivaroxaban was prescribed for another 10 days after discharge. Lower extremity strength training started on the day after surgery. Besides, all patients received mechanical thromboprophylaxis by means of a portable intermittent inflatable calf pump. Clinical symptoms of deep vein thrombosis (DVT) including limb swelling, superficial venous engorgement or calf pain were examined daily during hospitalization. Both lower limbs were examined using diagnostic Doppler ultrasound on the second day after surgery, or earlier if a patient had symptoms or signs suggestive of DVT. If patients suddenly experienced chest discomfort, difficulty breathing, or even coughing up pink foamy sputum, an emergency CT angiography was performed to assist the diagnosis of pulmonary embolism (PE). CT scan was also performed in the diagnosis of stroke when exhibiting relevant symptoms.
Outcome Measurements
Data Collection
Data were collected from electronic medical records. The collected information included demographic information (age, sex, body mass index [BMI]), diagnosis, coexisting diseases, physical examination (baseline blood pressure, range of hip motion), ASA grade, operative approach, laboratory measurements (Hb, hematocrit (HCT), platelet, activated partial thromboplastin time, prothrombin time, troponin T (cTnT), creatine kinase-MB (CK-MB) and serum creatinine (Scr)), length of hospital stay, and complications information.
Blood Pressure Measurements
Real-time blood pressure recordings were extracted from the hospital anesthesia information management system. Intraoperative MAP was calculated from the arterial blood pressure during surgery at 5-min intervals.
Perioperative Blood Loss
Perioperative total blood loss was calculated with Nadler and Gross formula22, 23. In addition, if blood transfusion was performed, the volume transfused was added to the total blood loss. Dominant blood loss was calculated as the sum of the intraoperative blood loss and the drainage volume after surgery. Hidden blood loss was defined as total blood loss minus dominant blood loss.
Allogeneic Blood Transfusion
A standard blood transfusion protocol was followed for all patients, whereby Hb <70 g/L in asymptomatic patients or <100 g/L in patients who developed any anemia-related organ dysfunction, intolerable symptoms of anemia, or ongoing blood loss triggered a transfusion24. The number of transfusion cases and volume were also obtained from the medical record information.
Hemoglobin and Hematocrit Reduction
The maximal Hb and HCT reduction referred to the patient's Hb and HCT value before surgery minus the lowest value after surgery, which indirectly reflected the amount of blood loss.
Duration of Anesthesia and Surgery
The duration of anesthesia was specified as the documented start of anesthesia until the departure from the operating room, while the duration of surgery was defined from the time of initial incision to the time of wound closure.
Postoperative Complications
The main postoperative complications we were concerned about included: AMI, AKI, postoperative hypotension, deep vein thrombosis (DVT), pulmonary embolism (PE), stroke along with 90-day mortality.
Acute myocardial injury was defined as at least one increased postoperative value of either CK-MB or cTNT above the upper limit of normal (0.03 ng/ml for cTNT and 8.8 ng/ml for CK-MB) in the 7 days after operation25. Similarly, AKI was considered if postoperative Ser was either more than 1.5-fold or more than 0.3 mg/dl before surgery25. Postoperative hypotension was defined as arterial pressure lower than 70 mmHg after recovery from anesthesia26. Diagnosis of DVT, PE, and stroke were based on the results of auxiliary examinations as we mentioned before. Data on 90-day mortality was concluded according to patients' follow-up records.
Statistical Analysis
Categorical data, such as sex, ASA grade, diagnosis, co-existing disease, surgical approach, transfusion rate, and complications incidence, are shown as the number (percentage). Chi-square or Fisher's exact tests were used to determine the differences among groups with the Bonferroni test for post hoc multiple comparisons. For the other continuous variables, the data are presented as the mean ± standard deviation (SD) and were compared by one-way analysis of variance (one-way ANOVA) with a post hoc Tukey HSD test for pairwise analysis when meeting the requirements of normality and homoscedasticity; otherwise, the data are presented as the median (interquartile range) and were compared using Kruskal–Wallis analysis with a post hoc Nemenyi test for pairwise comparison. Specifically, the perioperative levels of Hb and HCT, which were analyzed with a mixed linear model, and a post hoc Tukey HSD test was conducted for pairwise comparison between groups at each time point. Meanwhile, subgroup analysis based on surgical approaches were conducted. All analyses were performed using R software (version 4.0.3), and significance was taken at the 5% level.
Results
Demographics and General Characters
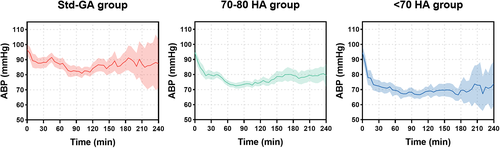
All patients were followed for more than 3 months. There were no significant differences regarding demographic information among the three groups. Meanwhile, the baseline characters including ASA grade, blood pressure at admission, diagnosis, co-existing disease, preoperative Hb and HCT levels, coagulation function, as well as surgical approach were comparable between groups. Details are shown in Table 1. The intraoperative MAP significantly differed among the groups, with 84.71 ± 5.56 mmHg, 74.39 ± 2.42 mmHg and 67.03 ± 2.65 mmHg in Std-GA group, 70–80 HA group, and < 70 HA group (P < 0.001; the trend of arterial pressure is shown in Figure 2).
| Variable | Std-GA group (n = 59) | 70–80 HA group (n = 85) | <70 HA group (n = 39) | Statistic |
|---|---|---|---|---|
| Age (year)† | 44.27 ± 8.89 | 44.20 ± 9.70 | 43.18 ± 9.16 | F = 0.195 |
| P = 0.832 | ||||
| BMI (kg/m2)† | 23.88 ± 2.77 | 23.19 ± 3.35 | 23.28 ± 3.55 | F = 1.137 |
| P = 0.425 | ||||
| Sex (male/female)‡ | 55/4 | 80/5 | 33/6 | P = 0.189 |
| Baseline ABP (mmHg)† | 97.93 ± 10.95 | 96.14 ± 9.92 | 94.72 ± 11.38 | F = 1.137 |
| P = 0.323 | ||||
| ASA grade‡ | ||||
| II | 52 (88.1%) | 79 (92.9%) | 34 (87.2%) | P = 0.473 |
| III | 7 (11.9%) | 110 (7.1%) | 40 (12.8%) | |
| Diagnosis‡ | ||||
| ONFH | 48 (81.4%) | 68 (80.0%) | 28 (71.8%) | P = 0.757 |
| AS | 4 (6.8%) | 4 (4.7%) | 4 (10.3%) | |
| OA | 2 (3.4%) | 7 (8.2%) | 4 (10.3%) | |
| DDH | 4 (6.8%) | 3 (3.5%) | 2 (5.1%) | |
| RA | 1 (1.7%) | 3 (3.5%) | 1 (2.6%) | |
| Co-existing diseases‡ | ||||
| Hypertension | 6 (10.2%) | 7 (8.2%) | 2 (5.1%) | P = 0.687 |
| Heart diseases | 2 (3.4%) | 1 (1.2%) | 1 (2.6%) | P = 0.554 |
| COPD | 0 (0.0%) | 1 (1.2%) | 1 (2.6%) | P = 0.485 |
| Diabetes | 0 (0.0%) | 5 (5.9%) | 1 (2.6%) | P = 0.101 |
| Range of motion (°)§ | ||||
| Flexion-left | 90 (90–100) | 90 (80–100) | 95 (80–100) | χ2 = 0.063 |
| P = 0.969 | ||||
| Flexion-right | 90 (80–100) | 90 (70–100) | 90 (80–100) | χ2 = 0.244 |
| P = 0.885 | ||||
| Abduction-left | 20 (13.75–25) | 20 (10–25) | 20 (10–30) | χ2 = 0.082 |
| P = 0.960 | ||||
| Abduction-right | 20 (10–30) | 15 (10–25) | 20 (10–27.5) | χ2 = 0.386 |
| P = 0.825 | ||||
| TXA (g)† | 3.61 ± 2.12 | 3.99 ± 2.72 | 4.18 ± 1.75 | F = 0.788 |
| P = 0.456 | ||||
| Surgical approach¶ | ||||
| PLA | 37 (62.7%) | 60 (70.6%) | 29 (74.4%) | χ2 = 1.708 |
| DAA | 22 (37.3%) | 25 (29.4%) | 10 (25.6%) | P = 0.426 |
| Hb (g/L)† | 147.83 ± 11.78 | 145.96 ± 14.23 | 148.72 ± 14.23 | F = 0.666 |
| P = 0.515 | ||||
| HCT (%)† | 44.63 ± 3.54 | 43.46 ± 3.44 | 44.67 ± 3.99 | F = 2.471 |
| P = 0.087 | ||||
| Plt (×109/L)† | 202.15 ± 55.65 | 201.46 ± 55.28 | 205.05 ± 57.99 | F = 0.056 |
| P = 0.945 | ||||
| APTT (s)† | 28.57 ± 3.41 | 28.78 ± 3.78 | 28.59 ± 3.54 | F = 0.067 |
| P = 0.936 | ||||
| PT (s)† | 11.01 ± 0.92 | 11.11 ± 0.83 | 10.91 ± 0.91 | F = 0.738 |
| P = 0.480 |
- Abbreviations: ABP, arterial blood pressure; APTT, activated partial thromboplastin time; AS, ankylosing spondylitis; ASA, American Society of Anesthesiologists; BMI, body mass index; COPD, chronic obstructive pulmonary disease; DDH, developmental dysplasia of hip; DAA, direct anterior approach; Hb, hemoglobin; HCT, hematocrit; OA, osteoarthritis; ONFH, osteonecrosis of the femoral head; PLA, posterolateral approach; Plt, platelet; PT, prothrombin time; RA, rheumatoid arthritis; TXA, tranexamic acid.
- † Data are presented as the mean ± standard deviation (SD) and compared by one-way analysis of variance (one-way ANOVA).
- ‡ Data are shown as the number (percentage) and compared using Fisher's exact tests.
- ¶ Data are shown as the number (percentage) and compared using chi-square tests.
- § Data are presented as the median (interquartile range) and were compared using Kruskal–Wallis analysis.
TXA administration
The total dose of TXA in Std-GA group, 70–80 HA group, and <70 HA group was 3.61 ± 2.12 g, 3.99 ± 2.72 g, and 4.18 ± 1.75 g, respectively. No significant difference was observed among groups (P = 0.456). Details are shown in Table 1.
Perioperative Blood Loss
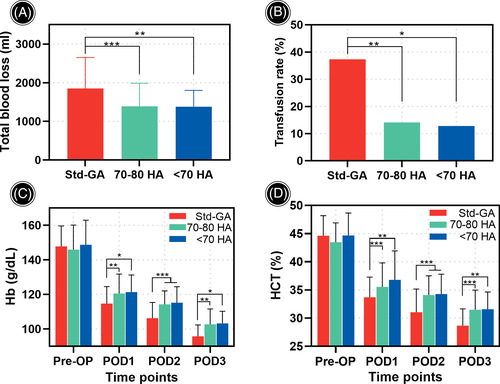
Perioperative total blood loss in 70–80 HA and <70 HA groups (1390.25 ± 595.67 ml and 1377.74 ± 423.46 ml) was significantly less than that in Std-GA group (1850.83 ± 800.73 ml, P < 0.001 and P = 0.001, respectively, Figure 3A). Specifically, the dominant blood loss was reduced from 541.53 ± 213.75 ml in Std-GA group to 350.18 ± 145.79 ml in 70–80 HA group (P < 0.001) and 338.36 ± 130.25 ml in <70 HA group (P < 0.001). Meanwhile, the hidden blood loss was reduced from 1309.31 ± 667.02 ml in Std-GA group to 1040.07 ± 484.88 ml in 70–80 HA group (P = 0.006) and 1039.38 ± 336.57 ml in <70 HA group (P = 0.008). Nevertheless, no difference was detected between the two HA groups regarding perioperative blood loss (P = 0.994, P = 0.930 and P = 1.000 for total, dominant and hidden blood loss, respectively). Details are shown in Table 2.
| Variables | Std-GA group (n = 59) | 70–80 HA group (n = 85) | <70 HA group (n = 39) | Statistic | Adjusted p. Value | ||
|---|---|---|---|---|---|---|---|
| Std-GA vs 70–80 HA vs <70 HA | Std-GA vs 70–80 HA | Std-GA vs <70 HA | 70–80 HA vs <70 HA | ||||
| Intraoperative MAP (mmHg)† | 84.71 ± 5.56 | 74.39 ± 2.42 | 67.03 ± 2.65 | F = 276.636 P < 0.001 |
P < 0.001 | P < 0.001 | P < 0.001 |
| Total blood loss (ml)† | 1850.83 ± 800.73 | 1390.25 ± 595.67 | 1377.74 ± 423.46 | F = 10.524 P < 0.001 |
P < 0.001 | P = 0.001 | P = 0.994 |
| Dominant blood loss (ml)† | 541.53 ± 213.75 | 350.18 ± 145.79 | 338.36 ± 130.25 | F = 27.016 P < 0.001 |
P < 0.001 | P < 0.001 | P = 0.930 |
| Hidden blood loss (ml)† | 1309.31 ± 667.02 | 1040.07 ± 484.88 | 1039.38 ± 336.57 | F = 5.240 P = 0.006 |
P = 0.008 | P = 0.037 | P = 1.000 |
| Transfusion¶ | 22 (37.3%) | 12 (14.1%) | 5 (12.8%) | χ2 = 13.280 P = 0.001 |
P = 0.007 | P = 0.032 | P = 1.000 |
| Transfusion volume (ml)† | 690.91 ± 370.21 | 425.00 ± 128.81 | 340.00 ± 134.16 | F = 4.810 P = 0.014 |
P = 0.043 | P = 0.055 | P = 0.852 |
| Maximal Hb reduction (g/L) † | 47.80 ± 13.10 | 39.24 ± 11.96 | 38.11 ± 9.02 | F = 8.915 P < 0.001 |
P < 0.001 | P = 0.003 | P = 0.903 |
| Maximal HCT reduction (%)† | 14.75 ± 4.39 | 11.11 ± 4.26 | 11.83 ± 2.76 | F = 12.321 P < 0.001 |
P < 0.001 | P = 0.006 | P = 0.665 |
- † Data are presented as the mean ± standard deviation (SD) and were compared by one-way analysis of variance with a post hoc Tukey HSD test for pairwise analysis.
- ¶ Data are shown as the number (percentage). Chi-square tests were used to determine the differences among groups with a post hoc Bonferroni test for multiple comparisons. MAP, mean arterial blood pressure.
Allogenic Transfusion Rate and Volume
The transfusion rate in 70–80 HA group (14.1%) and <70 HA group (12.8%) was similar (P = 1.000), both significantly lower than that in Std-GA group (37.3%, P = 0.007 and P = 0.032, respectively, Figure 3B). The mean volume of transfusion was also dramatically reduced in 70–80 HA group and <70 HA group (425.00 ± 128.81 ml and 340.00 ± 134.16 ml) compared with that in Std-GA group (690.91 ± 370.21 ml, P = 0.001 and P = 0.007). The details are shown in Table 2.
Hemoglobin and Hematocrit Reduction
The postoperative Hb and HCT levels in the two HA group were consistently higher than those in Std-GA group (Figure 3C, D). The maximal Hb reduction in Std-GA group was 47.80 ± 13.10 g/L, significantly more than that in 70–80 HA group (39.24 ± 11.96 g/L, P < 0.001) and <70 HA group (38.11 ± 9.02 g/L, P = 0.003). Similarly, the maximal HCT reduction in Std-GA group (14.75% ± 4.39%) was more than that in 70–80 HA group (11.11% ± 4.26%, P < 0.001) and <70 HA group (11.83% ± 2.76%, P = 0.006). No difference was detected between 70–80 HA group and <70 HA group in terms of postoperative Hb and HCT levels (P > 0.05). The details are presented in Table 2.
Subgroup Analysis
Subgroup analysis showed that the perioperative total blood loss, dominant and hidden blood loss, transfusion rate and volume, as well as the maximal Hb and HCT reduction were significantly reduced when HA was combined with TXA in both PLA and DAA surgical approaches. The details are presented in Table S1.
Duration of Surgery and Anesthesia
A slight difference with statistical significance was observed in duration of surgery among groups (154.75 ± 37.65 min in Std-GA group, 142.60 ± 35.34 min in 70–80 HA group and 137.55 ± 34.32 min in <70 HA group, P = 0.044), while there was no difference in terms of the duration of anesthesia among the three groups (P = 0.945). The details are presented in Table 3.
| Variables | Std-GA group (n = 59) | 70–80 HA group (n = 85) | <70 HA group (n = 39) | Statistic | Adjusted p. Value | ||
|---|---|---|---|---|---|---|---|
| Std-GA vs 70–80 HA vs <70 HA | Std-GA vs 70–80 HA | Std-GA vs <70 HA | 70–80 HA vs <70 HA | ||||
| Duration of surgery (min)† | 154.75 ± 37.65 | 142.60 ± 35.34 | 137.55 ± 34.32 | F = 3.191 P = 0.043 |
P = 0.116 | P = 0.055 | P = 0.747 |
| Duration of anesthesia (min)† | 205.78 ± 56.25 | 202.89 ± 45.66 | 203.51 ± 55.85 | F = 0.057 P = 0.945 |
P = 0.941 | P = 0.975 | P = 0.998 |
| Range of motion (°)§ | |||||||
| Flexion-left | 100 (100–110) | 110 (100–110) | 100 (100–100) | χ2 = 4.116 P = 0.128 |
P = 0.310 | P = 0.812 | P = 0.213 |
| Flexion-right | 100 (100–110) | 110 (100–110) | 100 (100–110) | χ2 = 3.388 P = 0.184 |
P = 0.187 | P = 0.862 | P = 0.750 |
| Abduction-left | 30 (30–40) | 30 (30–35) | 30 (30–40) | χ2 = 1.662 P = 0.436 |
P = 0.999 | P = 0.501 | P = 0.470 |
| Abduction-right | 30 (30–35) | 30 (30–35) | 30 (30–37.5) | χ2 = 2.471 P = 0.291 |
P = 0.960 | P = 0.306 | P = 0.394 |
| Length of hospital stay (day)† | 9.81 ± 4.63 | 6.72 ± 2.48 | 7.97 ± 2.99 | F = 14.325 P < 0.001 |
P < 0.001 | P = 0.026 | P = 0.141 |
- † Data are presented as the mean ± standard deviation (SD) and were compared by one-way analysis of variance with a post hoc Tukey HSD test for pairwise analysis.
- § Data are presented as the median (interquartile range) and were compared using Kruskal–Wallis analysis with a post hoc Nemenyi test for pairwise comparison.
Range of Hip Motion
The range of hip motion of each group had substantially improved compared with the preoperative status, and no significant intergroup difference was observed (P > 0.05). The details are presented in Table 3.
Length of Hospitalization
The mean length of hospitalization in 70–80 HA group and <70 HA group was similar (6.72 ± 2.48 and 7.97 ± 2.99 days, P = 0.141), and both were significantly shorter than that in Std-GA group (9.81 ± 4.63 days for P < 0.001 and P = 0.026, respectively). The details are presented in Table 3.
Postoperative Complications
The incidence of postoperative hypotension in <70 HA group (23.1%) was obviously higher than that in Std-GA group and 70–80 HA group (0% and 4.7%, P < 0.001, respectively). Besides, in <70 HA group, 15.4% patients developed AKI, which was dramatically higher than that in Std-GA HA group and 70–80 HA group (3.4% and 2.4%, P = 0.014 for A vs B vs C). The same tendency was observed regarding AMI, however, with no statistical significance (1.7%, 1.2% and 7.7% in Std-GA group, 70–80 HA group and < 70 HA group, P = 0.099). No DVT, PE, or stroke occurred in any group during hospitalization. All patients were discharged safely, and no patients died in the 90-day follow-up. The details are presented in Table 4.
| Variables | Std-GA group (n = 59) | 70–80 HA group (n = 85) | <70 HA group (n = 39) | Statistic | Adjusted p Value | ||
|---|---|---|---|---|---|---|---|
| Std-GA vs 70–80 HA vs <70 HA | Std-GA vs 70–80 HA | Std-GA vs <70 HA | 70–80 HA vs <70 HA | ||||
| Acute myocardial injury‡ | 1 (1.7%) | 1 (1.2%) | 3 (7.7%) | P = 0.099 | P = 1.000 | P = 0.894 | P = 0.275 |
| Acute kidney injury‡ | 2 (3.4%) | 2 (2.4%) | 6 (15.4%) | P = 0.014 | P = 1.000 | P = 0.167 | P = 0.035 |
| Postoperative hypotension‡ | 0 (0%) | 4 (4.7%) | 9 (23.1%) | P < 0.001 | P = 0.432 | P < 0.001 | P = 0.011 |
| Deep vein thrombosis‡ | 0 (0%) | 0 (0%) | 0 (0%) | - | |||
| Stroke | 0 (0%) | 0 (0%) | 0 (0%) | - | |||
| Pulmonary embolism | 0 (0%) | 0 (0%) | 0 (0%) | - | |||
| 90-day mortality | 0 (0%) | 0 (0%) | 0 (0%) | - | |||
- ‡ Data are shown as the number (percentage). Fisher's exact tests were used to determine the differences among groups with the post hoc Bonferroni test for multiple comparisons.
Discussion
Efficacy of the Combination of HA and TXA in SBTHA
Reducing massive blood loss and transfusion during SBTHA remains a major challenge in orthopaedics. Unlike most previous trials focusing on either TXA or HA alone, in the present study we evaluated the efficacy of their combination in reducing perioperative blood loss and blood use. We observed that the total blood loss was significantly reduced by approximately 30%, and the transfusion rate decreased from 37.3% to 12.8%–14.1% when HA was combined with TXA. Laboratory examinations also revealed better preservation of Hb and HCT after surgery when HA was administered with TXA.
In contrast to the antiplasmin ability of TXA, HA reduces hemorrhage by altering regional tissue perfusion during surgery27. It has been well-established that HA could significantly reduce intraoperative blood loss by up to 30–50%28, 29. Consistent with previous studies, we found that the dominant blood loss was decreased by 35.3%–37.5% when HA was applied, suggesting that the effects of HA could be incorporated to that of TXA directly. In addition, as the hypotensive status can be sustained for several hours after anesthesia recovery, so rebound bleeding can be obviated when the spillover hypotensive effects fade away30. The bleeding from the bone surface and muscles is continuously attenuated, further reducing the dominant blood loss after surgery.
Intriguingly, it is normally believed that TXA effectively inhibits the fibrinolysis induced by surgical trauma, while HA mainly has effects during surgery, and only few studies have focused on its role in hidden blood loss31, 32. In our study, we surprisingly noted that hidden blood loss was also reduced by approximately 20% when HA was combined with TXA. Banerjee and Issa et al. maintained that this was attributed to the fact that hypotension status provided relatively mild hemodynamic micro-environment, which may promote the clot formation and stabilization, thereby facilitating the effects of TXA30. However, the detailed mechanism still needs further study and discussion.
With the improvement in postoperative Hb and HCT levels, patients underwent a faster recovery after surgery, as evidenced by the length of hospital stay, which was reduced in the two HA groups. In some other aspects, although the postoperative hip range of motion was all within the expected scopes and was comparable among groups, it should not be ignored that the administration of HA significantly improved the surgical fields and brought great convenience for operation. Therefore, the operation time reduced accordingly. However, the administration of HA occupied additional time for anesthesia induction and recovery, thus the overall anesthesia time was similar among groups.
Safety of the Combination of HA and TXA in SBTHA
After fully verifying their effectiveness in reducing blood loss and transfusion, the safety of their combination became our primary concern, especially regarding the choice of the optimal hypotension range since the simultaneous modality inevitably introduces additional risks for patients. It's commonly acknowledged that the effects of hemostasis are predominantly determined by the degree of hypotension, that is lower blood pressure, less bleeding33. So early in the 1990s, an MAP of 50–65 mmHg was widely adopted to minimize bleeding34. Moreover, Sharrock and colleagues further investigated permanent adverse effects in a wide range of patients with certain high-risk factors, such as being elderly, low cardiac output, chronic renal dysfunction, and being hypertensive, and concluded that such level of hypotension was safe and could be tolerated well35-39.
Nevertheless, to date, an increasing body of evidence suggests that such a level of hypotension is closely associated with severe end-organ injuries. Salmasi and colleagues suggested in their report that MAP below 65 mmHg was related to both AKI and AMI25. When MAP is further reduced below 55 mmHg, even for only 1–5 min, the incidence of AKI and AMI significantly soars40. Meanwhile in another independent study, Sun et al. also drew the consistent conclusion that MAP <60 mmHg for more than 20 min and <55 mmHg for more than 10 min would increase the risk of AKI41. In fact, intraoperative hypotension of a more than 20 mmHg decrease or a 20% change in MAP for an hour could triple the risk of major adverse cardiac and cerebrovascular events42. More than that, overall mortality also significantly increased as the blood pressure became progressively lower43-45. All these results suggested that the former target of hypotension was not actually safe, and the optimal range of hypotension should be seriously reconsidered.
In line with previous studies, we also found that the incidence of AKI significantly increased from 2.4%–3.4% to 15.4% when intraoperative MAP decreased below 70 mmHg. In addition, although statistically nonsignificant, we still observed an ~5-fold increase in AMI. More remarkably, the postoperative hypotension soared from 0%–4.7% to 23.1%, aggravating the risks of neurological and other end-organ complications after surgery46-48. Therefore, excessively reducing blood pressure in pursuit of minimizing blood loss and transfusion is strongly not advocated. Based on our result, an MAP of 70 mmHg may be considered as the lower limit when HA was administrated during SBTHA.
The Optimal Range of MAP During SBTHA
Finding the balance between efficacy and safety of HA is of vital importance. However as aforementioned, the efficacy of HA is mainly determined by the degree of hypotension while the excessive hypotension would inevitably cause severe adverse effects. That means to guarantee the safety of patients during HA, the efficacy must be sacrificed based on the experience of previous clinical experience. While exciting, in our study we found that in the setting of TXA, an MAP between 70 and 80 mmHg did not attenuate its efficacy. Both outcomes regarding blood loss and transfusion were comparable between 70–80 HA group and <70 HA group. Besides, this consistency is also evident in Hb and HCT reduction. We assumed that this might be attributed to the application of TXA compensating for the effects of vacuum, which requires extreme hypotension, and thus assists HA in achieving maximum safe effects. However differently, the incidence of postoperative complications in 70–80 HA group was dramatically lower than that in <70 HA group and in line with that in Std-GA group. Besides, it should be noted that the incidence of AMI dramatically increased from 1.2% in 70–80 HA group to 7.7% IN <70 HA group, though the statistical difference was not significant. In the meantime, as considerable portion of patients in <70 HA group developed postoperative hypotension (some of them could even last for 2–3 days) and needed additional treatment, such as ephedrine to maintain their blood pressure, the overall length of hospital stay could accordingly be prolonged by about 1 day when compared with 70–80 HA group; again, albeit, the difference was not statistically significant due to the limitation of the involved patients. Taken together, we recommend HA with an MAP of 70–80 mmHg when combined with TXA during SBTHA, which minimizes perioperative blood loss and transfusion and with no increase in postoperative complications.
Limitations
This study had several limitations. First, the retrospective nature of our study limited our chance for randomization. Although selection bias was addressed by assembling groups with similar demographic characteristics and perioperative measurements, we could not rule out the possibility that patients with good conditions were more likely to accept the hypotension technique. Besides, the observed associations could result from residual confounding factors, such as perioperative medication effects. Residual confounding may constitute part of the observed associations, even though there is a strong biologic plausibility for the effect, and it was consistent across all analyses. Second, for ethical reasons, TXA was administrated to all patients except very few with contraindication in this cohort. Therefore, data on patients treated with HA only were missing. As solid evidence has demonstrated the relationship between hypotension and blood loss, we believe our results indicating that co-administration of HA and TXA improves both efficacy and safety are still convincing. Thirdly, hemodynamic parameters were lacking in our study. Even though we have tried to indirectly assess the safety of the combination of HA and TXA according to data from electronic medical records, this limitation must be acknowledged as a flaw in the study. And finally, the number of patients involved was relatively limited. Some certain outcomes were clinically significant, yet statistically were not. Given the SBTHA case itself is relatively seldom, multicenter cohort studies are still in consistent demand.
Conclusion
In conclusion, we found that a combination of TXA and HA could effectively reduce perioperative blood loss and transfusion throughout SBTHA. An MAP of 70–80 mmHg is the optimal range for SBTHA, which minimizes bleeding and blood product, in addition to shortening operation time and length of hospitalization, and without an apparent increase in serious complications. Based on our encouraging results, we strongly suggest conducting randomized controlled trials to systematically evaluate the efficacy and safety of hypotensive anesthesia in SBTHA.
Acknowledgements
This work was supported by National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (Z20191008), International Science and Technology Cooperation Project of Chengdu Science and Technology Bureau (2019-GH02-00076-HZ), Key Project of Health Commission of Sichuan Provincial (18ZD016) and 1.3.5 Project for Disciplines of Excellence of West China Hospital, Sichuan University (ZYJC18002). Meanwhile, this study was approved by the Ethics Committee of West China Hospital and registered in the Chinese Clinical Trial Registry (ChiCTR2100047184).




