Diagnosis, pathophysiology, management and future issues of trigeminal surgical nerve injuries
Abstract
The trigeminal nerve constitutes the largest sensory cortex representation in the brain compared with other sensory nerves. This is likely due to the fact that the trigeminal nerve underpins our very existence, as it sensorially protects, our five senses including the organs that provide sight, smell, taste, hearing, speech and meninges protecting our brain. Thus, when trigeminal nerve injuries occur, which in the main are preventable and painful, the majority of patients experience mixed symptoms including altered sensation, numbness and ongoing or elicited neuropathic pain. These neuropathic features cause significant impact on the patients’ ability to function, for example cold allodynia prevents the patient enjoying cold foods and drinks and undertaking out-door activities or mechanical allodynia frequently interferes with eating, speaking, kissing and sleep. The resultant chronic symptoms and functional impedance result in significant psychological morbidity. Prevention of nerve injuries related to local anaesthesia (LA), endodontics, implants and third molar surgery is imperative as there is no magic bullet to repair these sensory nerve injuries with their related neuropathic pain. Some causes have higher levels of resolution (third molar surgery and LA) some lower levels of resolution (implant surgery and endodontics) and many patient factors will dictate the prevalence of chronic neuropathic pain. The patient must have appropriate consent and their expectations managed with understanding the potential benefits and risks for their chosen interventions. The authors have aimed to provide an up to date evidence base for diagnosis and management of trigeminal nerve injuries.
Background
Trigeminal nerve injury (TNI) and subsequent post-traumatic trigeminal neuropathic pain (PTNP), is a problematic consequence of dental or oromaxillofacial surgical procedures with major medico-legal implications.1 The incidence of lingual nerve injury has remained static in the UK over the last 30 years, but is increasing in the US, as is the incidence of inferior alveolar nerve (IAN) injury in the UK; the latter being due to implant surgery and endodontic therapy.2 Trigeminal nerve injuries are generally classified as temporary but can persist and become permanent (by definition after 3 months). Based upon the limited evidence base, nerve injuries caused by implant and endodontic treatments are mainly painful and permanent.3 Temporary nerve injuries are more likely related to local anaesthesia (LA) or third molar surgery, with mandibular related surgery patients are advised that the rate of permanent inferior alveolar or lingual nerve injuries occur between 0.1–2% of cases.4, 5 LA nerve injuries have a 75% likelihood of recovery.6, 7
The fifth cranial nerve divisions two and three are the most commonly damaged, caused by implants, endodontics and third molar surgery (or other high-risk extractions).8 Nerve damage from surgery can cause chronic postsurgical pain, however, PTNP and chronic postsurgical pain appears to be limited in dentistry and maxillofacial surgery (3–5%)9-11, likely as a result of local anaesthetic (LA) infiltration injections preventing peripheral and central sensitisation (Fig. 1). However, the true incidence of trigeminal nerve injuries is not known due to the fact that many procedures occur in private practices and incidents are underreported. Other common general surgical procedures cause chronic postsurgical pain in 20–45% of patients after surgical limb amputation, thoracotomy and breast surgery for example.12, 13 Many of these chronic postsurgical pain patients actually experience neuropathic pain.14, 15
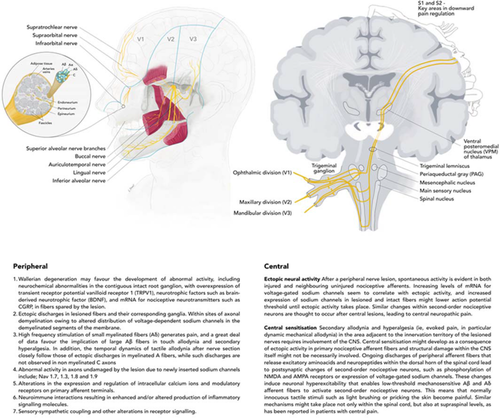
Iatrogenic (caused by surgery or medicine) trigeminal nerve injuries, result in pain in 70% of patients seen seeking treatment in our clinics.16 The ongoing or evoked pain results in interference with eating, speaking, sleeping, applying makeup, shaving, kissing, tooth brushing and drinking; just about every social interaction we take for granted. As a result, these injuries have a significant negative effect on the patient's self-image, quality of life and psychology.16
Risk factors
Risk factors for chronic postsurgical pain (not limited to PTNP) are many (Table 1), highlighting, the complexity of predisposition to persistent pain due to sensory nerve injury.17
| Preoperative factors |
| Pain, moderate to severe, lasting more than 1 month |
| Repeated surgery |
| Psychological vulnerability (e.g. catastrophising) |
| Preoperative anxiety |
| Female gender |
| Older age |
| Workers’ compensation |
| Genetic predisposition |
| Inefficient diffuse noxious inhibitory control (DNIC)—a descending pathway of pain inhibition |
| Intraoperative factors |
| Surgical approach with risk of nerve damage |
| Post-operative factors |
| High pain experience (severe) |
| Radiation therapy to area |
| Neurotoxic chemotherapy |
| Depression |
| Psychological vulnerability |
| Neuroticism anxiety |
Nerve damage is likely to result from a combination of poor risk assessment, poor technique and late recognition or management of intraoperative and post-operative signs of neuropathy. Risk assessment involves the patient selection, preoperative planning, both clinical and radiographic and suitable treatment protocol and follow up (Table 2 summarises the surgical risk factors associated with trigeminal nerve injuries and how to avoid them). It is important that the clinician is familiar with the nerve injury risk factors, specific for each of the type of invasive procedures. For example, in the case of protrusion through the inferior dental canal (IDC) and resultant direct IAN mechanical injury by implant drill a ‘sudden give’ or an ‘electric shock’ type feeling or high level sudden pain, even with working under LA, is reported by most of the patients seen on our clinic with LA, extraction, implant or endodontic related PTNP. This should result in the clinician stopping surgery, not reaching for another LA block injection and reassessing their surgical position (implant or endodontic) with regard the injured nerve. The problem with implant related nerve injuries is that they are entirely avoidable as this is elective surgery, and likely to be permanent and painful for the patient.16 However, we must state that even in the best hands, a nerve injury can still occur despite the world's best will to avoid it. This is inherent to the surgical specialty.
| Dental local anaesthesia (LA) |
|
|
Third molar surgery Lingual nerve |
|
| Third molar surgery Inferior alveolar nerve |
|
| Dental implants |
|
| Endodontics |
|
Pathophysiology
It is pertinent to recognise that the trigeminal neural pathways have important differences compared to the spinal nerves. The proprioceptive trigeminal afferents are the only first neuron fibres to have their cell bodies in the central nervous system (CNS). This not the only basic morphological difference where the fifth cranial nerve differs from other sensory nerves. The nuclei for the TN including motor, sensory and special sensory nuclei, are all embedded in the midbrain and not the spinal system. The trigeminocervical complex converging input from C2 and C3 likely explains the often comorbid head and neck pains or autonomic signs and symptoms seen in chronic trigeminal pain, including PTNP.18 In addition, these interactions as well as close anatomical relationship between the trigeminal sensory nuclei and other cranial nerves (7th, 8th and 9th) may relate to referred pain and symptoms in these nerve distributions as well. A well-known interconnection between the fifth and seventh cranial nerve is tested by performing the corneal reflex. Despite structural differences between the trigeminal somatosensory system and other spinal sensory nerves, there are many similarities with the somatosensory system of the rest of the body, for example using a common channel, the Transient Receptor Potential Cation Channel Subfamily M member 8 (TRPM8), for recognising cold sensations.19 A more in depth comparison of channels and transmitters is not within the scope of this article but we refer the readers to the included references.19-21
A normally functioning sensory system depends on the maintenance of equilibrium between the neurons and their environment.22 Sequence of events after nerve injury are described below.
Peripheral nervous system
- Changes in the equilibrium, as caused by nerve damage, leading to a cascade of events progressing from the peripheral to the CNS.23 During this stage, the presence of inflammatory mediators released during the tissue injury and from the recruited immune cells leads to increased sodium and calcium channel currents, which reduce the thresholds of the nociceptors in the peripheral nervous system (PNS).17 This increased sensitivity at the site of injury is called peripheral sensitisation (primary hyperalgesia and allodynia).23
- After peripheral injury adenosine triphosphate (ATP) signal transduction induces activation of both cell types further contributing in an inflammatory cascade.24 The vesicular nucleotide transporter regulates ATP release and could be a potential pharmacological target. Another channel, the subunit α2/δ-1 of the L-type channel of the dihydropyridine receptor, has shown to be highly selective for gabapentin and is abundantly present in the trigeminal neurons. Other key molecules in pain transmission are CGRP and nitric oxide that are released after inflammation occurs, causing upregulation of neurokinin 1 (NK1) receptors. This upregulation causes a higher excitability of the trigeminal neurons. The NK1 receptors are also present in the glial cells.
- Neuropeptides, neurotransmitters and channels
- ◦ Nerve growth factor (NGF) plays a role in neural navigation of axonal growth. NGF is also upregulated in the trigeminal ganglion (TG) and nucleus.25-27
- ◦ Calcitonin gene related peptide (CGRP), Substance P and Neuropeptide Y expression in TG cells increases in response to injury.28
- ◦ Sodium voltage gated channels related to pain including, Nav 1.8 and 1.9, are linked to severity of pain after lingual nerve injuries.29, 30 A study reported changes in the expression pattern of growth associated protein 43 in the IAN region of the TG. An increase in myelination and axon density of regenerated fibres was associated with the overall recovery process.31
- Paracrine effects cause simultaneous release of IL-1 β that in turn suppresses voltage-gated potassium channels through protein kinase C/G protein-coupled pathways, which ultimately increases the neural excitability. Studies showed the desirable effect of NK1 blockade at the TG to prevent central sensitisation. Eugenol is a potential inhibitor of the voltage-gated potassium, calcium and sodium channels as well as the hyperpolarisation-activated cyclic nucleotide-gated (HCN) channels. The HCN channels have been identified as key factors in mechanical allodynia.32
- An extensive review by Holland reports the morphological structural and electrophysiological post-injury changes after peripheral sensory nerve of the TG in cats.33 Crush injuries recovered faster with less central disruption than transection injury, chemical nerve injuries were not evaluated. All nerve injuries resulted in lower conduction velocities and sensory impairment. When immediate re-apposition of cut ends is performed no cell death occurred; however, proximal degeneration and distal Wallerian degeneration were seen as well as axonal sprouting. Associated degenerative changes of brainstem nuclei were observed. If neural gaps were needed to be covered, stretching the nerve after release from its connective tissues resulted in better functional results compared to neural grafting.
- Traumatic injury to a peripheral nerve, at the distal stump of the nerve fibre, causes Wallerian degeneration at the distal ends of the damaged nerve.34, 35 Schwann cells, responsible for providing trophic support to the nerve fibres, begin to degenerate and lose their myelin or encapsulation in cases of unmyelinated nerves.36
- Schwann cells and their recruited immune cells, clear the debris and release (neuro)-trophic factors that facilitate axonal growth.37
Central consequences—trigeminal ganglion and secondary/ tertiary neurons
- Membrane excitability changes with lower resting membrane potentials causing lower thresholds for transduction.
- Reduction of synaptic activity (with reduced release of inhibitory neurotransmitters), number, inhibition by inhibitory interneurons caused by less transmitter synthesis, and vesicular transport and postsynaptic lower receptor sensitivity.
- Descending tracts facilitate further in the release of postsynaptic potentials.
- Sprouting starts enhancing excitatory synapses further.
- Polysynaptic pathways start to form, causing epileptiform activity with burst-like discharges and synchronisation.
- Increased excitability and synaptic plasticity lead to central sensitisation causing hyperalgesia, allodynia, hyperpathia and aftersensations. This process initiates as early as two days after injury or inflammation and increases when the nociceptor input has halted. This altered pain perception and processing has been evaluated in other pain conditions such as fibromyalgia, migraine-type headache, temporomandibular disorders, rheumatoid arthritis and others.
- Neuropeptides. The chronic constriction sensory nerve injury model in rats revealed
- ◦ A decrease in substance P immunoreactivity at 60 days after injury in the spinal dorsal horn bilaterally.
- ◦ Neuropeptides changes were also observed up to 120 days after injury. Decline in GABA-immunoreactive neurons starts at 896 h after injury bilaterally, with recovery normal levels are resumed at 8 weeks.
- ◦ Glutamate decarboxylase immunoreactive cells also decline after injury, in combination with synaptic changes, for example, long-term potentiation (LTP), central sensitisation gradually becomes clinically apparent and reduces the chance for reversal. The electrophysiological pathway starting at the nociceptive fiber projecting to the TG after action potential firing.
- ◦ Excitatory postsynaptic potentials induce presynaptic transmitter release as well as an enhanced postsynaptic transmitter effect: LTP.
- The continued activity post injury may also lead to maladaptive plasticity in the CNS, that is, increase in the synaptic strength leading to easier activation of nociceptors with what were previously subthreshold stimuli and an enhancement of the receptive fields.39 Therefore, the uninjured area surrounding the site of damage (or even contralateral area in unilateral nerve damage) also becomes sensitised to mechanical inputs.22, 39 This uncoupling of the stimulus intensity and stimulus location due to the CNS plasticity is called central sensitisation.39
Diagnosis
Diagnostic criteria
- Facial and/or oral pain in the distribution(s) of one or both trigeminal nerve(s) and fulfilling criterion C
- History of an identifiable traumatic event to the trigeminal nerve(s), with clinically evident positive (hyperalgesia, allodynia) and/or negative (hypoaesthesia, hypoalgesia) signs of trigeminal nerve dysfunction
- Evidence of causation demonstrated by both of the following: a. pain is localised to the distribution(s) of the trigeminal nerve(s) affected by the traumatic event, b. pain has developed <6 months after the traumatic event (up to 6 months to allow for development of neuropathy after chemotherapy and radiation. Many surgical injuries have immediate onset, but it is possible that the pain only comes on after a few days or weeks.)
- Not better accounted for by another ICHD-3 diagnosis.
These criteria are very similar to newly suggested criteria proposed by an international collaborative group of orofacial pain and headache researchers under the name of International Classification of Orofacial Pain (ICOP) version 1.0 beta (draft for review) 2019. In the ICOP, PTTN has been slightly renamed to PTNP. Both the ICHD-3 and ICOP present diagnostic criteria for PTTN/PTNP, and the criteria B and D from above can be used for non-painful post-traumatic trigeminal neuropathy (nPTTN) for patients with trigeminal nerve damage without any associated neuropathic pain. A recent review has highlighted some limitations in diagnosis of postsurgical neuropathy and pain in the trigeminal system.42
Clinical assessment
When assessing patients with surgically induced nerve injuries we recommend a holistic approach.43, 44 We must treat the patient with the nerve injury not just the nerve injury. Many of these patients have experienced an unexpected traumatic event which demands a thorough history taking and examination including attention for sensory testing and psychological assessment. These elements are necessary both in diagnosis and in the choice of therapy.
- Focal sensory neuropathy (mostly present). There is almost always an area of sensory deficit (i.e. often with a mixture of pain, numbness and altered sensation). There may be positive (elicited pain = mechanical allodynia or hyperalgesia) or negative signs (numbness = anaesthesia, expanded two-point discrimination, reduced light touch). This is an important diagnostic feature for sensory nerve neuropathy.
- Pain, discomfort, altered sensation, numbness (anaesthesia). Neuropathic pain, a positive sign, commonly presents with mechanical allodynia (pain to a non-noxious stimuli), mechanical hyperalgesia (increased pain to a noxious stimuli) and hyperpathia (continued altered sensation or pain after stimulation ceases). Fifty to seventy percentage of patients report a combination of numbness, altered sensation and pain is experienced, the pain may be either spontaneous ongoing pain, which often had a burning character, and spontaneous shooting, electric shock-like sensations (neuralgia).16 Evoked pain due to touch (mechanical allodynia) or cold (thermal allodynia) often leads patients to having difficulties with daily function, such as eating, socialising, kissing, speaking, drinking and tooth brushing.16
Psychological factors (anxiety, stress, depression, post-traumatic stress disorder and anger) are related to PTNP45 and as a consequence, the patients are often anxious, tearful due to the psychological repercussions of surgery. The presence of anxiety or depression has been suggested to negatively affect treatment outcomes in other pain conditions.46 In striving for better outcomes, it is therefore advisable to also pay attention to the psychological impact. Psychological assessment requires the use of validated questionnaires exploring anxiety, depression, post-traumatic stress disorder, catastrophising and somatisation. These symptoms are often compounded by the lack of appropriate urgent or continued management by the treating clinician and informed consent, which is given by only 30% of patients, most of whom are not specifically warned about potential nerve injury.16
Mechanosensory assessment
The clinical phenotype of somatosensory function in trigeminal nerve damage includes both positive and negative symptoms, which may be associated with spontaneous or evoked pain. Clinical mechanosensory tests are summarised in Figure 2. These clinical mechanosensory tests have been shown to have a high specificity however they have a low sensitivity. Additional quantitative sensory testing (QST) or emerging imaging technologies such as magnetic resonance neurography or multimodal assessments can aid in further diagnostics.47, 48
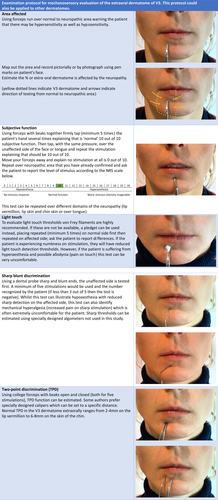
The positive symptoms may be represented by, for example, dysesthesias and negative symptoms, for example, numbness.49, 50 These symptoms are more pronounced and clinically detectable when a major nerve branch is involved.49 In PTNP, the pain is generally of moderate to severe intensity, continuous in nature, lasts for most of the day, and is present on most days.16, 51 Thus the patient generally presents with hyperaesthesia or hypoaesthesia, the latter is generally better tolerated.
Management
Decision on managing the patient with a nerve injury is based upon the holistic assessment of the patient. The clinician must assess the degree and impact of the nerve injury and the type of patient. Some patients may present with large painful neuropathies but are happy to continue as is with minimal life impact, whereas others may present with small areas of neuropathy with no pain but significant related functional and psychological impact. As with all decisions in life there are benefits and risks with any intervention. No reparative surgery or chronic pain medication is devoid of side effects or potential risks. The patient has to be made aware of the diagnosis, prognosis and possible interventions with associated risks and benefits. This is a long conversation and may need to take place over several consultations.
Many nerve injuries require therapeutic management, as surgical treatment is rarely indicated. The management may include: patient reassurance and education, medical, surgical and/or psychological treatments.
Urgent surgical intervention should be recommended for known or highly suspected nerve injury, and those related to endodontic or implant nerve injury. Later surgical intervention for hypoaesthetic nerve injuries does not return the patient to normality43, 44 and surgery for patients with pain and hyperaesthesia is not appropriate as the pain is not abated and patients are faced with long term antiepileptics or antidepressants for chronic pain.13
LA, orthognathic, trauma and non-traumatic (chemotherapy, radiation, secondary to systemic disease and neoplastic local disease) nerve injuries
- Homecheck – If you cause extreme (funny bone) pain during an intervention, for example an injection, root canal treatment, extraction or implant preparation in your patient do follow them up the next day and check they are OK. If the patient reports numbness, altered sensation and or pain, reassure them, acknowledge their complaints without minimising and arrange review.
- Continue to support and reassure your patient and advise them to visit to confirm the presence of neuropathy. If the neuropathy affects most of the dermatome +/- associated with severe neuropathic pain nerve injury must be suspected. Reassure your patient that 75% of these injuries resolve.
- Say sorry as this is not an admission of guilt.
- Initiate medical management (recommended for other peripheral sensory nerve injuries)
- ◦ High dose oral NSAIDs (400–800 mg Ibuprofen PO QDS) for 2 days only. Bandolier Oxford league table summarises the optimal analgesia for post-operative pain and combined ibuprofen and paracetamol have the smallest number needed to be treated.52
- ◦ GMP prescription for prednisolone 5-day step-down dose of 50-40-30-20-10 mg PO (not for patients with contraindications for steroids or NSAIDs).
- ◦ Vitamin B complex (Riboflavin 400 mg once daily for maximum of 3 months plus other vit B complex).53
- Arrange a review of the patient. All advice is summarised on the Trigeminalnerve.org.uk website.
- Long-term management of patients with non-resolving LA nerve injuries. The reality for these patients is that if they have persistent neuropathic pain, they have to be treated as such with psychological and medical management. Topical local anaesthetic (lidocaine 5%) patches may assist the patient in sleeping and playing sports in cold weather.54 Psychological interventions play a significant role in managing these patients and recommendations for treatment of trigeminal neuropathic pain are also well described by Renton and Zakzrewska.55
Summary of type and timing of management
Management of third molar related nerve injuries will depend upon the presentation of the patient (pain, functional and psychological implications), duration and cause of the nerve injury (Fig. 3).45, 56 It is recognised that neuropathic pain does not respond to surgical intervention thus prevention and early management are paramount in preventing chronic life-long pain after routine surgery in these patients. Advice is summarised on the Trigeminalnerve.org.uk website.
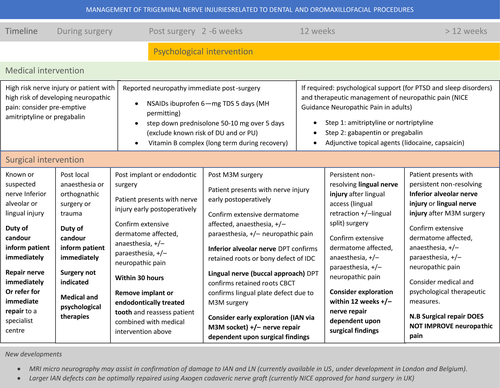
The patient with the nerve injury must be treated, not the nerve injury in isolation. The neuropathy, pain, numness or pareasthesia, with associated functional and psychological impact will be the driving force behind the patient seeking treatment.56 These factors must be assessed and the potential outcomes, good or bad, be discussed and agreed with the patient.
Patients sustaining LA, orthognathic, oncology and trauma related nerve injuries will mainly be managed therapeutically.57-61
Overall there is poor evidence to support late surgical intervention for trigeminal nerve injuries.62, 63 Most studies report on repair procedures undertaken too late and early repair is imperative to minimise central irreversible changes and possibly chronic pain. Generally surgical repair of the trigeminal nerves never returns the patient to preoperative neural function, in addition, there is a risk of making a numb patient (without pain) into one with PTNP (with neuropathic pain).43, 44 As with other post-trauma sensory neuropathies it is recognised that immediate repair is optimal,64, 65 however, this rarely is applied to dental nerve injuries with the misconception that we should sit and wait for resolution resulting in long delays before surgical intervention.66-68 Referral to a skilled microsurgeon is preferably done in time.
Some recent studies have highlighted immediate repair with cadaveric treated human nerve graft sucessful in managing various sized defects in planned resection of nerves related to benign tumour resection or trauma.68-70
Recent reports have also concluded, similar to other surgical sites, that neuropathic pain does not resolve with surgery, this being the main driver for surgical repair.43, 71
Many reports have recommended the use of conduits (venous, prosthetic), sural nerve grafts and other techniques without sufficient evidence and many with poor ouctomes including neuropathy and pain from the donor sites.72 The future may prove that nerve growth factors, other growth promoting chemical, anti neuropathic pain agents and specialised conduits may play a role in improving repair of trigeminal nerve injuries. The overall conclusions from reviews in this area, is that we have a lot of evidence base to harness. The singular consensus is that prevention of these nerve injuries is possible and optimal.
- Counselling is the most useful effective tool for managing patients with problematic permanent sensory nerve injuries.
- Medical intervention is indicated for patients with pain or discomfort or with anxiety and or depression in relation with chronic pain.73 However, due to the multiple noxious side effects of chronic pain medication, <18% of patients remain adherent with medication
- Acute (medical)
- Late (chronic pain management with psychological interventions)
- Surgical intervention is indicated for:
- Immediate surgical repair for suspected or known nerve injury or intended surgical defect after removal of benign tumour or recent trauma.60
- Immediate removal of the implant after an implant related injury with post-operative neuropathy with or without neuropathic pain.59, 74
- Explorative surgery within 36 h if related to development of neuropathy after overfill of root canal-treated tooth.75-77
- Within 2–4 weeks if clinical presentation of persistent neuropathy is paramount. Radiographic follow-up is not necessary however if there is cone beam computed tomography (CBCT) evidence of breach of lingual plate or IDC consider immediate action: nerve exploration+/- repair;
- ◦ Lingual nerve neuropathy patients with CBCT evidence of damage to lingual plate adjacent to third molar surgical site.
- ◦ Inferior alveolar nerve with retained roots or evidence of bone inclusions or compression of IDC.
- Within 3 months of injury;
- ◦ Non-resolving lingual or inferior dental nerve injuries. Exploratory surgery for lingual or inferior alveolar nerve injuries within 3 months post injury. Surgical intervention is not effective for neuropathic pain.43, 78 If this is the driving force behind seeking surgery, surgical intervention should be reconsidered.
Future directions
Exciting results were reported of allografting lingual and IAN injuries. Using a pre-prepared human treated cadaveric allograft the inferior alveolar and lingual nerve can be repaired with minimal tension. This is undertaken using microscopy and described in several publications by John Zuniga and Michael Miloro.66, 79, 80 This is likely to be the treatment of choice if repair is indicated and direct reanastomosis cannot be undertaken most commonly for the IAN. One of the main issues regarding nerve repair is the early identification of the neuroma relating to the patients’ symptoms, and the connectivity of the nerve itself, that is, is the nerve actually functioning. Recent developments with magnetic resonance neurography has availed the surgeon to identify the nerve lesion and neural functionality to facilitate appropriate and earlier nerve repair intervention.48
Conclusions
This article was intended to acknowledge and share some key issues around surgical trigeminal nerve injuries including pathophysiology, diagnosis and management. Unfortunately, many of the suggested treatment options do not ‘fix’ the patient but aim to manage their symptoms as best as possible, improve function and allow them time to accommodate to these unfortunate events, which is often not very satisfactory.
- Neuropathic pain as well as altered sensation and numbness is what most patients experience with iatrogenic sensory nerve injury. This has a significant and unpleasant effect on the patient (improve your consent).
- The majority of iatrogenic nerve injuries are avoidable.
- Owing to the significant problems following nerve injury, pre-operative strategies for minimising this risk of nerve damage need to be considered carefully. Peri-operative planning, operative execution and post-operative care needs improving to minimise and hopefully abolish these injuries.
- Several strategies are presented to assist in preventing nerve injuries.
- Inferior alveolar nerve injuries in relation to implant and endodontic dentistry are permanent and ‘unfixable’ unless treated quickly within 30 h.
- There is a need for a consensus and standardisation of risk assessment and management, a holistic approach in managing the pain, related effect on functionality and psychological implications caused to the patients affected by iatrogenic nerve injury.
Acknowledgements
We would like to thank Laure Baert for illustrating the figures.
Conflict of interest
TR and FVC: none to declare.




