Oral cancers preceded by proliferative verrucous leukoplakia exhibit distinctive molecular features
Alejandro Herreros-Pomares and David Hervás should both be considered as first authors.
Juan Sandoval and Jose Bagan, should both be considered as the last authors.
The manuscript has not been previously presented.
Abstract
Objective
Proliferative verrucous leukoplakia (PVL) has high rates of malignant transformation into oral squamous cell carcinoma (OSCC), but the clinical and evolutionary pattern of OSCC from PVL (PVL-OSCC) is more favorable than that of OSCC not preceded by PVL (OSCC). Here, we aimed to explore the pathophysiologic differences between PVL-OSCC and OSCC through transcriptomic and DNA methylation analyses.
Materials and methods
In this case-control study, oral biopsies from 8 PVL-OSCC and 10 OSCC patients were obtained for global sequencing using RNAseq and a genome-wide DNA methylation analysis via the Infinium EPIC Platform (graphical abstract).
Results
One hundred and thirty-three differentially expressed genes (DEGs) were detected, 94 of them upregulated in OSCC. Most of these genes were previously described in cancer and associated with prognosis. The integrative analysis revealed 26 DEGs, corresponding to 37 CpGs, whose promoters were regulated by DNA methylation. Twenty-nine of the CpGs were found as hypermethylated in PVL-OSCC. Only 5 of the genes that were aberrantly methylated and differentially expressed were upregulated in PVL-OSCC patients, whereas 21 were underexpressed.
Conclusions
PVL-OSCC patients presented lower expression of cancer-related genes. Hypermethylation of the promoter region of many genes was also noticed, indicating that DNA methylation could be a regulatory mechanism.
1 INTRODUCTION
Proliferative verrucous leucoplakia (PVL) is a rare oral disorder that initially manifests as a homogeneous leukoplakia, but progresses affecting multiple oral mucosa locations with a warty tendency (Hansen et al., 1985). PVL exhibits tremendous treatment resistance, a high rate of recurrences and development of new leukoplakias, and a very high risk of malignant transformation (MT) into oral cancer (Ramos-García et al., 2021). The overall incidence of MT ranges from 44% reported by Ramos-González-Moles et al. (2021) to 66% reported by Ibáñez et al. (2022), and 71% found by Villa et al. (2018). Second primary tumors and field cancerization are also frequent in PVL, with decreasing time intervals between the appearance of one tumor and the next one over time (Bagan et al., 2020). A previous study conducted by our group including 55 PVL patients concluded that patients who develop oral cancers are commonly non-smokers females and those who develop more than one oral squamous cell carcinoma (OSCC) are more likely to develop lesions of the gingiva (Bagan et al., 2011).
Recent meta-analyses have pointed out that patients who developed OSCC from the progression of proliferative leukoplakias have a better prognosis than OSCC patients that have not been preceded by proliferative leukoplakias (Faustino et al., 2022; González-Moles et al., 2021). The reason behind this prognostic difference for the same histopathological disease is unknown. However, deciphering the underlying molecular differences between OSCC derived from PVL and conventional OSCC could be essential to identify potential biomarkers for pathological diagnosis, prognosis, and treatment response and also for improved characterization of the molecular mechanism of the diseases.
Several authors have tried to identify the molecular mechanisms that govern the pathogenesis of PVL. For instance, molecular alterations in a specific genetic region, such as p16INK4a, and p14ARF aberrations (common in oral verrucous leukoplakia) in the locus 9p21 have been related to distinct histologic types of oral premalignancy (Kresty et al., 2008). However, there is no conclusive evidence of the correlation between the prevalence of PVL and DNA aneuploidy, loss of heterozygosity at locus 9p21, and the specific expression of Mcm (mini chromosome maintenance) protein (Okoturo et al., 2018). In a recent systematic review, Rintala et al. (2019) also found that aneuploidy might be a valuable structural biomarker for PVL diagnosis, but it should be further validated. In any case, genetic alterations only define small subsets of certain cancer types and a new focus lies on gene expression regulatory events such as epigenetic factors since tumor behavior does not depend exclusively on changes in gene expression. DNA methylation alterations have been linked to cancer initiation and progression in many cancers during the last years, including head and neck cancers (Ekanayake Weeramange et al., 2020; Flausino et al., 2021; Gaździcka et al., 2020; Romanowska et al., 2020). Previous findings in oral cancer revealed that DNA methylation analysis of a specific set of genes may serve to detect early-stage oral cancer (Morandi et al., 2017) and we recently reported the potential of several methylation markers detected in PVL to become novel OSCC diagnostic biomarkers (Herreros-Pomares et al., 2021). With all this in mind, we designed an integrative analysis of an RNAseq sequencing analysis with a genome-wide DNA methylation analysis to determine molecular differences between OSCC patients derived from PVL or not. Our findings helped us to understand the clinical, histological, and evolutionary differences between them.
2 MATERIALS AND METHODS
2.1 Patients and tissue samples
This case-control study included 18 OSCC patients visited and treated at the Stomatology and Maxillofacial Surgery Department of the General University Hospital of Valencia. Participants were distributed in two groups: the case study group, named PVL-OSCC, consisted of 8 patients with OSCC preceded by the evolution of PVL determined following the criteria provided by Villa et al. (2018). The control group, named OSCC, was composed of 10 patients without a prior medical history of PVL. For all patients, two representative biopsies were taken from the same area of the lesions, including epithelium and the underlying connective tissue between 2019 and 2021. One of each pair of specimens was formalin-fixed, paraffin-embedded (FFPE) for routine histopathological characterization. The other sample was fresh-frozen (FF) at −80°C until the RNAseq sequencing and DNA methylation analysis.
2.2 Library preparation and sequencing
Samples were extracted using a column-based DNA extraction method (E.Z.N.A. DNA kit and DNeasy Blood & Tissue Kit; Qiagen) following the manufacturer's instructions. All DNA samples were treated with RNaseA for 1 h at 45°C, quantified by the fluorometric method (Quant-iT PicoGreen dsDNA Assay, Life Technologies), and assessed for purity by NanoDrop 2000 (Thermo Scientific) 260/280 and 260/230 ratio measurements. DNA integrity of FF samples was checked by electrophoresis in a 1.3% agarose gel.
cDNA synthesis and library generation were performed using the Illumina TruSeq RNA Library Prep Kit with PolyA selection for ribo depletion, following the manufacturer's instructions. Libraries were sequenced on an Illumina NovaSeq 6000 with a paired-end design.
2.3 Genome-wide DNA methylation
Epigenomic studies were performed with the Infinium EPIC DNA methylation BeadChip platform (Illumina) used for the interrogation of over 850,000 CpG sites (dinucleotides that are the main target for methylation), which has been previously established as a reliable technology to detect epigenetic alteration in our and other laboratories (Moran et al., 2016). 600 ng of purified DNA were randomly distributed on a 96-well plate and processed using the EZ-96 DNA Methylation kit (Zymo Research Corp.) following the manufacturer's recommendations for Infinium assays. Bisulfite-converted DNA was processed as previously described (Sandoval et al., 2011). MethylationEPIC beadarray shares the Infinium HD chemistry Assay (Illumina Inc.) used to interrogate the cytosine markers with HumanMethylation450 beadchip. Thus, the applicable protocol for MethylationEPIC is the same as for HumanMethylation450, which is the Infinium HD Methylation Assay Protocol. 4 μL of tissue bisulfite-DNA were processed following the Illumina Infinium HD Methylation Assay Protocol (Sandoval et al., 2011).
2.4 Data pre-processing
A full description of the data pre-processing is available in Supplementary Materials and Methods. Briefly, quality control was assessed using fastqc, multiqc, and qualimap softwares for the RNAseq data. Trimmed reads were mapped to Homo sapiens genome assembly GRCh38 (hg38) using STAR software for alignment. For the DNA methylation analysis, the resulting raw data (IDATs) were normalized using the minfi R package and functional normalization. CpG markers present on MethylationEPIC were classified based on their chromosome location, the Infinium chemistry used to interrogate the marker (Infinium I, Infinium II), and the feature category gene region as per UCSC annotation (TSS200, TSS1500, 5'UTR, 1st Exon, Body, 3'UTR). Probes and sample filtering involved a two-step process in which unreliable betas with high detection p > 0.01 (1620 CpGs) and 2932 CpGs associated with SNPs were removed. Sex chromosome probes were also removed (19,681 CpGs). After the filtering, the remaining 842,179 CpGs were considered valid for the study.
2.5 Statistical analysis
Exploratory analysis of the RNAseq and methylation data was performed using principal component analysis (PCA) and heatmaps of clustered observations and variables. Differential gene expression analysis using the DESeq2 method was performed on the raw count matrix for comparing the two groups. For a more robust estimation, shrunken log2 fold changes (log2FC) were estimated from the raw log2FC estimates using the ashr procedure (Stephens, 2017). p-values were also adjusted for multiple comparisons by using False Discovery Rate (FDR). Genes were considered relevant when adjusted p < 0.05 and shrunken log2FC >1.5 or <−1.5.
For the methylation analysis, only the CpGs located in the promoter region of the relevant genes selected from the RNAseq results were included in the analysis. An elastic net penalized logistic regression model was adjusted to select the CpGs able to discriminate between both groups. A full description of the penalized logistic regression model is available in Supplementary Materials and Methods. All statistical analyses were performed using R (version 4.2.0) and R packages glmnet (version 4.1–4) and DESeq2 (version 1.30.1).
2.6 Ethics statement
This study was approved by the Ethics Committee for Human Research of the University of Valencia (Ref. H1523722754549). Informed written consent was obtained from all participants after an explanation of the nature of the study.
3 RESULTS
3.1 Patients characteristics
This study included 8 patients who developed OSCC from the progression of PVL (PVL-OSCC) and 10 OSCC patients without a prior medical history of PVL (OSCC). The clinicopathological and evolutionary characteristics of both groups of patients are described in Table 1. The median age in this cohort was 76.11 years and the median time to MT from the diagnosis of PVL to OSCC was 9.2 years. Most of the patients were non-smokers (72.2%), males (66.7%) with a tumor located in the gingiva (44.4%). 50% of the patients were diagnosed at stage IV of the disease.
| Characteristics | Total | PVL history | No PVL history | |||
|---|---|---|---|---|---|---|
| N = 18 | % | N = 8 | % | N = 10 | % | |
| Age at diagnosis | 76.11 ± 11.02 | 80.25 ± 12.42 | 72.80 ± 9.05 | |||
| Gender | ||||||
| Male | 12 | 66.7 | 6 | 75.0 | 6 | 60.0 |
| Female | 6 | 33.3 | 2 | 25.0 | 4 | 40.0 |
| Tobacco smoking | ||||||
| Yes | 5 | 27.8 | 1 | 12.5 | 4 | 40.0 |
| No | 13 | 72.2 | 7 | 87.5 | 6 | 60.0 |
| Alcoholism | ||||||
| Yes | 1 | 5.6 | 0 | 0.0 | 1 | 10.0 |
| No | 17 | 94.4 | 8 | 100.0 | 9 | 90.0 |
| Location of OSCC | ||||||
| Gingiva | 8 | 44.4 | 5 | 62.5 | 3 | 30.0 |
| Palate | 3 | 16.7 | 2 | 25.0 | 1 | 10.0 |
| Floor of mouth | 1 | 5.6 | 0 | 0.0 | 1 | 10.0 |
| Tongue | 4 | 22.2 | 0 | 0.0 | 4 | 40.0 |
| Buccal mucosa | 1 | 5.6 | 1 | 12.5 | 0 | 0.0 |
| Lips | 1 | 5.6 | 0 | 0.0 | 1 | 10.0 |
| Clinical form of the OSCC | ||||||
| Erythroplastic | 2 | 11.1 | 2 | 25.0 | 0 | 0.0 |
| Ulcerated | 10 | 55.6 | 1 | 12.5 | 9 | 90.0 |
| Exophytic | 3 | 16.7 | 3 | 37.5 | 0 | 0.0 |
| Mixed | 3 | 16.7 | 2 | 25.0 | 1 | 10.0 |
| Differentiation grade | ||||||
| G0 | 2 | 11.1 | 2 | 25.0 | 0 | 0.0 |
| G1 | 9 | 50.0 | 4 | 50.0 | 5 | 50.0 |
| G2 | 5 | 27.8 | 1 | 12.5 | 4 | 40.0 |
| G3 | 2 | 11.1 | 1 | 12.5 | 1 | 10.0 |
| Cancer infiltratation | ||||||
| Bone | 6 | 33.3 | 3 | 37.5 | 3 | 30.0 |
| Perineural | 9 | 50.0 | 2 | 25.0 | 7 | 70.0 |
| Lymphovascular | 4 | 22.2 | 2 | 25.0 | 2 | 20.0 |
| No | 1 | 5.6 | 1 | 10.0 | 0 | 0.0 |
| Lymph node metastasis | ||||||
| Yes | 4 | 22.2 | 1 | 12.5 | 3 | 30.0 |
| No | 14 | 77.8 | 7 | 87.5 | 7 | 70.0 |
| Distant metastasis | ||||||
| Yes | 1 | 5.6 | 0 | 0.0 | 1 | 10.0 |
| No | 17 | 94.4 | 8 | 100.0 | 9 | 90.0 |
| TNM staging | ||||||
| I | 3 | 16.7 | 1 | 12.5 | 2 | 20.0 |
| II | 5 | 27.8 | 3 | 37.5 | 2 | 20.0 |
| III | 1 | 5.6 | 1 | 12.5 | 0 | 0.0 |
| IV | 9 | 50.0 | 3 | 37.5 | 6 | 60.0 |
| Second primary tumors | ||||||
| Yes | 6 | 33.3 | 4 | 50.0 | 2 | 20.0 |
| No | 12 | 66.7 | 4 | 50.0 | 8 | 80.0 |
- a No significant differences were found for the clinicopathological characteristics of OSCC patients with prior history of PVL or not.
No significant differences were found between both groups for the clinicopathological characteristics, which could be due to the limited sample size of the study. However, a tendency to a better prognosis can be glimpsed for PVL-OSCC patients who showed a tendency to lower frequency of poorly differentiated tumors (G0/1: 75% vs. 50%), perineural infiltrations (25% vs. 70%), presence of lymph node metastasis in the neck (12.5% vs. 30%), and stage IV tumors (37.5% vs. 60%).
3.2 OSCC patients with prior history of PVL show lower expression of cancer-related genes
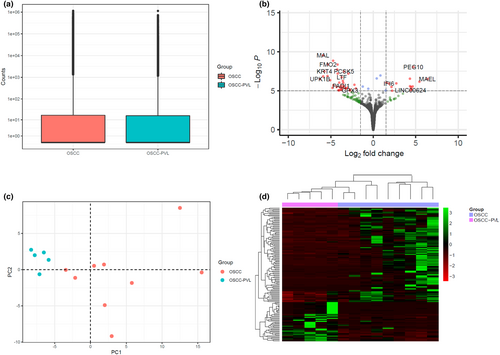
All RNAseq specimens were sequenced by Illumina Technology and used to carry out a differential expression (DE) analysis between the PVL-OSCC and OSCC patients. As shown in Figure 1a, the dispersion, i.e., the interlibrary variation between samples, and the median of the expression values were similar in both groups, therefore supporting the feasibility of the comparison of both groups in a DE analysis. The DE analysis revealed 133 differentially expressed genes (DEGs) after correcting for multiple comparisons under an FDR <0.05 (Figure 1b). Of them, 112 were protein-coding genes, 10 were long non-coding RNAs (lncRNAs), 6 were pseudogenes, 3 were genes to be experimentally confirmed (TEC) and 2 were antisense RNAs. Importantly, 94 out of the 133 DEGs were upregulated in the control group and 39 in PVL-OSCC patients. A full summary of the DE results for these genes is provided in Table S1. Unsupervised PCA showed individual over-dispersion between replicates due to their intrinsic differences, especially for OSCC patients (Figure 1c). Noticeably, samples presented good separation according to the prior history of PVL in this analysis uncovering biological variation differences among both groups. In consonance, individuals were classified into two major subgroups in an unsupervised hierarchical clustering analysis, showing a differentiated expression pattern between OSCC patients with a prior history of PVL or not (Figure 1d). Downstream analyses were performed with the STRING server to evaluate the functional profiles specifically associated with the set of DEGs revealed no significant enrichment of GO terms. However, additional literature searches carried out in the Human Protein Atlas confirmed that the set of 133 DEGs was particularly enriched in cancer-related genes and that 67 of them were previously associated with prognosis in cancer patients (Table 2). Importantly, 35 out of 94 DEGs upregulated in OSCC patients without a prior history of PVL (37.2%) had been previously associated with a favorable prognosis in contrast to the 22 genes (23.4%) linked to a bad prognosis. For PVL-OSCC patients, 11 out of 39 upregulated DEGs (28.2%) had been associated with good and 11 (28.2%) with bad prognosis.
| Genes upregulated in OSCC patients without prior history of PVL | ||||
|---|---|---|---|---|
| Cancer type | Good prognosis | N | Bad prognosis | N |
| Breast cancer | CDON | 1 | — | — |
| Cervical cancer | HS3ST6, SMPD3, SLC25A42 | 3 | — | — |
| Colorectal cancer | CLCA4, GIPC2 | 2 | TLX1 | 1 |
| Endometrial cancer | CRAT, GCNT3,SMPD3, UPK1B, PLLP, BDH2 | 6 | MAL, ACAA2, CERK, COG5 | 4 |
| Head and neck cancer | CLCA4, EPHX3, SLC44A4, TMPRSS11B, PITPNC1 | 5 | BCHE | 1 |
| Liver cancer | SLC51A | 1 | GCNT3, COG5 | 2 |
| Lung cancer | ZDHHC11B, SLC25A42, BDH2 | 3 | — | — |
| Ovarian cancer | AADAC | 1 | EMP1, KRT4 | 2 |
| Pancreatic cancer | CERK | 1 | EMP1, AADAC | 2 |
| Renal cancer | ATP6V0A4, C15orf59, CLDN8, CRAT, DBNDD1, FMO1, GIPC2, LTF, MAL, MPV17L, PHYHD1, SLC44A4, PLLP, ACAA2, NPNT, PPAP2B, HIBADH, BDH2 | 18 | NDN, PADI1, PAMR1, PCSK5,BHLHE41, CDON,TNFAIP8L1 | 7 |
| Stomach cancer | — | — | GPX3, PAMR1, UPK1B, AADAC | 4 |
| Testis cancer | SNX31 | 1 | — | — |
| Thyroid cancer | KLHDC8A, BDH2 | 2 | PEG3, COG5 | 2 |
| Urothelial cancer | SLC44A4, BHLHE41 | 2 | EMP1, KRT4, BARX2, GAS7, CERK | 5 |
| Genes upregulated in OSCC patients with prior history of PVL | ||||
|---|---|---|---|---|
| Cancer type | Good prognosis | N | Bad prognosis | N |
| Breast cancer | RCL1 | 1 | — | |
| Endometrial cancer | FOXA2, NCLN, MRPL23 | 3 | DHRS2, HOXB9, PEG10 | 3 |
| Head and neck cancer | — | — | HOXB9 | 1 |
| Liver cancer | RCL1, MPDZ | 2 | NCLN | 1 |
| Ovarian cancer | FOXA2, IFI27, | 2 | — | |
| Pancreatic cancer | FOXA2, ARMC5, MPDZ | 3 | HIST1H1C, OASL | 2 |
| Renal cancer | CDC37L1, RCL1 | 2 | DHRS2, ELFN2, IRF7, DRAP1, NCLN, HIST1H1C, HIST1H2BK, OASL, ADAM8 | 9 |
| Stomach cancer | NCLN | 1 | — | — |
| Thyroid cancer | DRAP1 | 1 | — | — |
| Urothelial cancer | DHRS2, HIST1H1C | 2 | — | — |
- a Prognostic values retrieved from Uhlen et al., 2017 and Human Protein Atlas (https://www.proteinatlas.org/).
3.3 Promoter DNA methylation constitutes a mechanism of suppression of gene expression in OSCC patients with a prior medical history of PVL
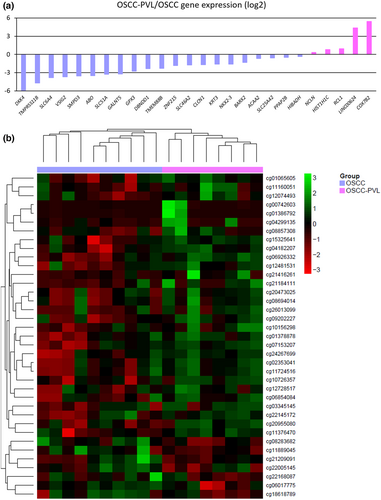
To further explore the regulation of DEGs between both groups, we performed an integrative analysis of the RNAseq results with a genome-wide DNA methylation analysis. A total of 842,179 CpGs were available after the pre-processing and quality filter of the raw methylation data, of which 472 were selected for being located in the promoters of the DEGs. We performed an elastic net penalized logistic regression and found that 26 of the genes (37 CpGs) were able to discriminate between both groups (Table 3). Only 5 of the genes (COX7B2, LINC00624, RCL1, HIST1H1C, and NCLN) that were aberrantly methylated and differentially expressed were upregulated in PVL-OSCC patients, whereas 21 (DKK4, TMPRSS11B, SLC6A4, VSIG2, SMPD3, ABO, SLC51A, GALNT5, GPX3, DBNDD1, TMEM88B, ZNF215, SLC46A2, CLCN1, KRT3, NKX2-3, BARX2, ACAA2, SLC25A42, PPAP2B, and HIBADH) were significantly underexpressed (Figure 2a). In consonance with these findings, 29 out of the 37 CpGs were found as hypermethylated in PVL-OSCC patients. Results of the selected CpGs are shown in a heatmap where both groups are separated by their methylation status (Figure 2b). Of note, 28 of the 37 CpGs (75%) were located in CpG islands or shores, high-density or flanking regions that play an essential role for fine-tuned regulation of gene expression patterns. The overrepresentation of these features in our identified promoters supports the idea that these genes are prone to be regulated by epigenetic mechanisms. In consonance, a negative correlation between the methylation status and the gene expression was found for most of the genes analyzed (Figure S1).
| OSCC without prior PVL | OSCC with prior PVL | ||||||
|---|---|---|---|---|---|---|---|
| CpG | Gene | log2FC | Adj. p-value | CpG | Gene | log2FC | Adj. p-value |
| cg21209091 | DKK4 | −5.902 | 0.0002 | cg18618789 | COX7B2 | 5.441 | 0.0007 |
| cg06017775 | TMPRSS11B | −4.688 | 0.0015 | cg11889045 | LINC00624 | 4.400 | 0.0035 |
| cg12074493 | SLC6A4 | −3.848 | 0.0031 | cg21184111 | RCL1 | 0.949 | 0.0443 |
| cg12481531 | VSIG2 | −3.713 | 0.0022 | cg08283682 | HIST1H1C | 0.808 | 0.0190 |
| cg09202227 | SMPD3 | −3.532 | 0.0008 | cg08857308 | NCLN | 0.355 | 0.0375 |
| cg20473025 | |||||||
| cg08694014 | |||||||
| cg26013099 | |||||||
| cg24267699 | ABO | −3.498 | 0.0016 | ||||
| cg20955080 | |||||||
| cg07153207 | SLC51A | −3.271 | 0.0024 | ||||
| cg06926332 | GALNT5 | −3.222 | 0.0025 | ||||
| cg01378878 | GPX3 | −2.741 | 0.0036 | ||||
| cg22005145 | |||||||
| cg00742603 | DBNDD1 | −2.361 | 0.0071 | ||||
| cg01386792 | |||||||
| cg10156298 | TMEM88B | −2.322 | 0.0132 | ||||
| cg12728517 | ZNF215 | −1.818 | 0.0333 | ||||
| cg02353041 | SLC46A2 | −1.724 | 0.0396 | ||||
| cg10726357 | |||||||
| cg11724516 | |||||||
| cg04182207 | CLCN1 | −1.710 | 0.0202 | ||||
| cg15325641 | KRT3 | −1.609 | 0.0328 | ||||
| cg06854084 | NKX2-3 | −1.591 | 0.0490 | ||||
| cg04299135 | BARX2 | −1.322 | 0.0336 | ||||
| cg03345145 | ACAA2 | −0.689 | 0.0419 | ||||
| cg21416261 | SLC25A42 | −0.587 | 0.0026 | ||||
| cg22145172 | |||||||
| cg11376470 | PPAP2B | −0.503 | 0.0198 | ||||
| cg11160051 | |||||||
| cg01065605 | HIBADH | −0.33 | 0.0181 | ||||
| cg22168087 | |||||||
4 DISCUSSION
Screening programs for the early detection of asymptomatic carcinoma are essential to reduce the mortality of OSCC, given that the majority of oral carcinomas are preceded by visible lesions, such as PVL (Brocklehurst et al., 2013). Importantly, there exists evidence pointing out that the clinical and evolutionary pattern of OSCC developed from the progression of oral leucoplakias differ from that of OSCC that were not preceded by them (Faustino et al., 2022; González-Moles et al., 2021). The reason behind these disparities remains undefined, although studying the molecular differences between OSCC derived from oral leukoplakias and conventional OSCC could offer new tools to develop a molecular classification of OSCC patients, better predict prognosis and facilitate the therapeutic decision-making of physicians.
In this study, clinical and molecular comparisons were performed between PVL-OSCC and conventional OSCC patients. No significant differences were found between both groups for the clinicopathological characteristics, but a tendency to a better prognosis can be glimpsed for PVL-OSCC patients who showed a lower frequency of poorly differentiated tumors, perineural infiltrations, presence of lymph node metastasis in the neck and stage IV tumors. These findings align with those reported by Faustino et al. (2022) and González-Moles et al. (2021) and support the idea that PVL-OSCC has a better prognosis than conventional OSCC. In particular, González-Moles et al. (2021) carried out a meta-analysis including 23 studies that concluded that the overall mortality rate of PVL-OSCC was around 21%, which is significantly lower than the 34.7–50% mortality rate reported for conventional OSCC. Additionally, in this study, tobacco smoking was less frequent in PVL-OSCC than in conventional OSCC patients. Accordingly, tobacco smoking seems to be linked to the development of conventional OSCC but its impact on PVL-OSCC is less clear (Bombeccari et al., 2018). In that sense, tobacco smoking has been associated with epigenetic changes, including DNA methylation, which has been suggested to serve a role in the carcinogenesis process and progression of oral and oropharyngeal cancers (Russo et al., 2018). Unfortunately, the limited sample size of this study hampered the clinical comparison of cases in terms of tumor grade and site and its clinical presentation, including depth of neoplastic and perineural infiltrations.
In addition to the clinical comparison, we performed a differential gene expression analysis to investigate and characterize the molecular differences between PVL-OSCC and OSCC patients. Results from this analysis showed that more than 70% of the DEGs were upregulated in OSCC patients not preceded by PVL. Importantly, most DEGs comprised cancer-related genes that had been linked to prognosis in different cancer types, suggesting that these genes could be responsible for the difference in prognosis reported in both groups. Zhang et al. (2016) investigated oral dysplasias, Farah and Fox (2019) evaluated a cohort of 24 patients with leukoplakia with or without dysplasia and our group (Llorens et al., 2021) compared the transcriptomic profile of PVL patients with that of healthy donors. Unfortunately, no studies have been carried out to date comparing the global expression status between PVL-OSCC and other OSCC patients. Thus, further confirmation of our findings in independent cohorts is required.
Epigenetic modifications regulate many physiological and pathological processes, including cell proliferation, apoptosis, inflammation, and carcinogenesis (Koch et al., 2018). In our study, by integrating promoter-associated DNA methylation levels from DEGs, we also identified 26 genes (21 of them downregulated in PVL-OSCC) whose deregulated expression correlated with epigenetic alterations. Importantly, the expression of most of these genes has been previously linked to cancer development and progression with a remarkable number of publications focusing on OSCC. For instance, DKK4 and SMPD3 promoters methylation seem to correlate with tumor grade in OSCC (Jabalee et al., 2020; Kheirandish et al., 2020), whereas polymorphisms of the promoter of SLC6A4 are not implicated in the OSCC development (Abdo et al., 2012). The role of ABO blood groups is more controversial. Verma and colleagues reported that A+ve individuals have a higher risk of developing OSCC (Verma et al., 2021), whereas Ramesh et al. (2017) concluded that people with blood groups B+ve and O+ve having tobacco chewing habits are more at risk of developing oral cancer. A different study concluded that GPX3 is downregulated in OSCC (Pedro et al., 2018) and Lee et al. (2015) reported that GPX3 may be silenced by cigarette exposure. CLCN1 was reported to predict survival in OSCC (Cao et al., 2019). Similarly, associations between NKX2-3 and prognosis have been reported (Zhu et al., 2020) and Abe et al. (2016) described that oral leucoplakias are associated with aberrant promoter methylation of NKX2-3. Importantly, KRT3 was also selected as a potential diagnostic biomarker associated with a cancerous change in oral leukoplakia (Li et al., 2022). Lin et al. (2016) reported that BARX2 expression is mostly downregulated in OSCC and that its expression suppresses the expansion of OSCC cells. Similarly, Vishwakarma et al. (2017) determined that PPAP2B is downregulated in the tumor tissues of 70% of OSCC patients and that its expression negatively correlates with tumor size.
Other genes have not been directly related to oral cancer, but publications can be found in other tumor types. For instance, the differential methylation status of VSIG2 can predict overall survival (OS) in lung and pancreatic cancers (Deng et al., 2021) and its expression has been used in prognostic signatures for bladder cancer, colon adenocarcinoma, and acute myeloid leukemia (Yan et al., 2019). Overexpression of TMPRSS11B has also been associated with poor survival in lung cancer and a prognostic model built for lung adenocarcinoma included SLC25A42 for OS prediction (Zhao et al., 2018). Importantly, the expression of GALNT5 was reported to mediate carcinogenesis and progression via activation of AKT/ERK signaling in cholangiocarcinoma and it was proposed as a candidate for hepatoblastoma tumorigenesis (Rodrigues et al., 2014). Moreover, its expression was associated with poor outcomes in hepatocellular carcinoma (HCC) and pancreatic ductal adenocarcinoma (Li et al., 2019). The hypermethylation of ZNF215 has been found an independent prognostic biomarker in prostate cancer (Angulo et al., 2016) and Maind and Raut (2019) reported its expression as a diagnostic and prognostic biomarker of basal-like breast cancer. Additionally, loss of ZNF215 imprinting has been also associated with poor 5-year survival (Yang et al., 2021). The role of SLC46A2 in cancer is less clear. Its expression has been found to diminish in lung cancer (Kim et al., 2015), but it seems to be involved in the genetic predisposition to cervical cancer (Engelmark et al., 2006). Lastly, DBNDD1 was identified as a novel putative melanoma susceptibility gene (Fang et al., 2020) and ACAA2 has been proposed as a prognostic factor in lower grade gliomas (Wu et al., 2020).
COX7B2, LINC00624, RCL1, HIST1H1C, and NCLN were the only aberrantly methylated DEGs upregulated in PVL-OSCC patients. A significant correlation has been reported between the overexpression of NCLN in OSCC and the presence of pathogenic bacteria associated with chronic inflammation (Moghimi et al., 2020). In addition, a rare polymorphism of the COX7B2 gene has been linked to nasopharyngeal carcinoma (Liang et al., 2004). RCL1 has been associated with a good prognosis in breast cancer (Ren et al., 2022), and its downregulation correlated with tumor progression in HCC patients (Jiaze et al., 2022). The overexpression of LINC00624 has been reported to enhance tumor growth and metastasis in HCC and prostate cancer (Zhou et al., 2022). Lastly, increased expression of HIST1H1C has been related to poor clinical outcomes in pancreatic cancer and uterine carcinosarcoma (An et al., 2020).
All these findings evidence the molecular differences existing between PVL-OSCC patients and conventional OSCC and could be linked to their differential behavior reported in the clinics. Further investigations in this sense will provide new insight into the role of these molecules in OSCC and could have major implications in future clinical practice, improving diagnosis, prognosis, and even treatment.
5 CONCLUSION
PVL-OSCC patients showed a general downregulation of genes previously associated with cancer prognosis that could explain the better prognosis reported for this subgroup of patients. Hypermethylation of the promotor of many of these genes was also detected in these patients, suggesting that DNA methylation could be one of the mechanisms regulating the expression of the genes. Further evaluation of these markers could be a powerful method to better understand the molecular events ongoing in both cases. Overall, our preliminary study indicates the potential use of genome-wide approaches in oral cancer prognosis. However, it shows limitations regarding the restricted sample size. As a result, further studies with larger sample sizes are needed to shed light on the clinical and molecular differences of PVL-OSCC patients.
AUTHOR CONTRIBUTIONS
Alejandro Herreros-Pomares: investigation; methodology; data curation; validation; writing – original draft; visualization. David Hervas: Data curation, methodology; validation; formal analysis; visualization. Leticia Bagan-Debon: Investigation; methodology; writing – review and editing; visualization. Alex Proaño: Data Curation. Diana Garcia: Investigation. Juan Sandoval: Investigation; methodology. Jose Bagan: Conceptualization; funding acquisition; investigation; methodology; writing – review and editing.
ACKNOWLEDGMENTS
This document is the result of a research line funded by Instituto de Salud Carlos III (Spain) through the project “PI19/00790” (Co-funded by European Regional Development Fund/European Social Fund “A way to make Europe/Investing in your future”). Principal Investigator: Jose Bagan. Pre-cancer and oral cancer research group of the University of Valencia.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Open Research
Data availability statement
RNA-seq and methylation data have been deposited in the ArrayExpress database at EMBL-EBI (www.ebi.ac.uk/arrayexpress) under accession numbers E-MTAB-12205 and E-MTAB-12202, respectively.




