The chemokine lymphotactin and its recombinant variants in oral cancer cell regulation
Abstract
Background
The expression of XCR1 receptor and its metamorphic ligand lymphotactin (hLtn) has been shown in cancers but their precise role in tumorigenesis is poorly understood including the significance of the physiologically existing hLtn monomeric (CC3) and dimeric (W55D) confirmations where the latter thought to function as the receptor antagonist. The aim of this study was to explore the functional role of bioengineered hLtn variants and the role of fibroblasts in XCR1/hLtn expression regulation in oral cancer cells (OCCL).
Material and methods
qRT-PCR and flow cytometry were performed to evaluate mRNA and protein expression of XCR1 and hLtn. Recombinant hLtn variants (wild-type, CC3 and W55D mutant) were designed, expressed, purified and evaluated using proliferation, adhesion and chemotaxis assays. XCR1 and hLtn expression regulation by fibroblasts was determined using indirect co-culture. XCR1 and hLtn expression in primary and metastatic OSCC tissue was assessed using immunohistochemistry.
Results
hLtn caused a significant decrease in OCCL XCR1 surface protein expression. hLtn CC3 mutant was highly functional facilitating proliferation and migration. Conditioned media from primary cancer-associated and senescent fibroblasts significantly upregulated XCR1 and hLtn mRNA expression in OCCL. Immunohistochemistry revealed higher XCR1 and hLtn expression in metastatic tumour deposits and surrounding stroma compared to primary OSCC tissue.
Conclusions
The development of hLtn biological mutants, regulation of XCR1 expression by its ligand hLtn and crosstalk with fibroblasts are novel findings suggesting an important role for the XCR1/hLtn axis within the OSCC tumour microenvironment. These discoveries build upon previous studies and suggest that the hLtn/XCR1 axis has a significant role in stromal crosstalk and OSCC progression.
1 INTRODUCTION
Chemokines are chemo-attractive cytokines with four subfamilies classified by the arrangement of the N-terminal cysteine residues (C, CC, CXC and CXXXC). Chemokines function primarily in mediating immune cell navigation and inflammatory responses (Sokol & Luster, 2015). Chemokines and their receptors have been shown to play a key role in tumour metastasis and reported to be overexpressed in the tumour microenvironment (TME) facilitating cancer progression (Nagarsheth, Wicha, & Zou, 2017). In particular, stromal fibroblasts (i.e. cancer-associated fibroblasts, CAF) have been shown to promote tumour growth, progression and migration through paracrine signalling (Liu et al., 2019).
XCR1 is the sole member of the C-chemokine family, and its ligand is lymphotactin (hLtn) (Hughes & Nibbs, 2018). Recently, it has become apparent that chemokines influence survival and progression in a range of tumours including oral squamous cell carcinoma (OSCC; Panda, Padhiary, & Routray, 2016). Expression of XCR1 and hLtn outside the immune system and their involvement in cancer cell regulation was first shown by our previous work in OSCC (Khurram et al., 2010). Since then, further studies have shown a role for the XCR1 receptor in breast, lung and ovarian carcinoma revealing a wider role than what was first envisaged (Kim & Wu, 2012; Wang et al., 2015; Yang, Qi, Lin, & Ou, 2017).
The ligand lymphotactin (hLtn) is a metamorphic protein, adopting two distinct conformations with canonical (CC3) and non-canonical (W55D) chemokine protein folds (Volkman, Liu, & Peterson, 2009). The structure is salt and temperature dependent with the monomeric (CC3) form predominately seen at higher salt concentrations and at 10°C, whereas the dimeric structure (W55D) is present at lower salt concentrations and at a temperature of 40°C (Kuloglu, McCaslin, Markley, & Volkman, 2002). Interestingly, both forms are distributed equally in physiological conditions with a conversion rate of ~1/s (Volkman, Liu, & Peterson, 2009). Currently, there are no studies investigating the biological role of each conformation.
Increased proliferation, adhesion and migration are a hallmark of cancer cells and chemokine–chemokine receptor interactions have been shown to influence these characteristics. hLtn has been shown to mediate cell proliferation in oral cancer cell lines (OCCL) through its receptor XCR1 (Khurram et al., 2010). However, it is not known whether this interaction is associated with the canonical chemokine fold only. Another key characteristic of cancer cells is the ability to adhere to extracellular matrix (ECM) components facilitating migration and invasion. Whilst it has been previously reported that hLtn with a canonical fold can activate the receptor and mediate adhesion to ECM, migration and invasion, the exact contribution of different hLtn conformations in cancer is still unknown.
In the current study, we further evaluate the expression and role of XCR1 receptor and its ligand in oral cancer cell regulation as well as the influence of fibroblasts.
2 MATERIALS AND METHODS
2.1 Cell culture
Human primary normal oral fibroblast (NOF) (origin buccal) and CAF (origin: floor of the mouth and lateral tongue) were obtained from excess tissue removed during biopsy (South Sheffield Ethics Approval Committee Ref: 09/H1308/66; Ref: 13/NS/0120, STH17021) (Elmusrati, Pilborough, Khurram, & Lambert, 2017). OCCL, H357 and SCC4 (ATCC) and primary fibroblasts were grown in appropriate media as described previously (Khurram et al., 2010). The conditioned media (CM) were prepared by culturing the fibroblasts with serum-free media for 24-hr, filtering (0.22 µM cut-off) and storing at −20°C until further use. OCCL were seeded in six-well plates (2.5—5 × 104 cells) and allowed to adhere overnight, followed by serum starvation for 24 hr prior to exposure to the CM.
2.2 RNA extraction and qRT-PCR
Total RNA was extracted from the cultured cells using a RNeasy Mini Kit (Qiagen) and reverse transcribed to complementary-DNA using a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher). Subsequently, the samples were subjected to TaqMan qPCR analysis using 7900HT Fast Real-Time PCR System (Thermo Fisher). The TaqMan probes used for amplification were as follows: XCR1 (I.D. Hs00245540_s1) and hLtn (I.D. Hs00751481_s1). Amplification was normalised to human B2M expression (I.D. Hs00187842_m1) in all samples using the method as previously described (Elmusrati et al., 2017).
2.3 Surface protein expression using flow cytometry
OCCL suspensions were prepared using a cell dissociation buffer (Sigma-Aldrich) and resuspended at a density of 1 × 106 cells in a cold flow buffer (PBS containing 10% [v/v] FCS). Cells were incubated with XCR1 antibody (20 µg/ml) for one-hour on ice. Cells were washed three times with buffer before the addition of Alexa Fluor® 488-conjugated secondary antibodies (2 µg/ml, Thermo Fisher) for 30 min on ice in the dark. Data were acquired using a FACSCalibur (Becton Dickinson) and analysed using FlowJo 10 software. Negative control samples were incubated with rabbit serum.
2.4 Site-directed mutagenesis, protein expression and purification
Mutagenesis was performed on the pET24a vector containing the HLTEV WT-XCL1 fusion protein using pairs of complementary primers (Supplementary S1) and the QuikChange II site-directed mutagenesis kit (Agilent) to create W55D and CC3 (V21C, V29C) mutants (Warburton, Omar Ali, & Choon Liong, 2015). The resulting plasmid was transformed into Escherichia coli strain BL21(DE3) (New England Biolab) and overexpression induced using auto-induction media (Studier, 2014) at 30°C for 24-hr. Plasmid validation was performed by gene sequencing while the mutant proteins were confirmed by their 280-nm absorbance and using gel electrophoresis. The subsequent purification scheme for the hLtn variants is described in the Supplementary S1.
2.5 Proliferation assay
These assays were performed as previously described (Khurram et al., 2010). OCCL were treated hLtn variants (100 ng/ml) in SFM. 2 × 103 cells per well were seeded in a 96-well plate and incubated at 37°C in a 5% CO2 incubator for 48 and 72-hr. Cell proliferation was measured using the CellTiter 96® Aqueous One Solution Cell Proliferation assay, a tetrazolium compound [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS] (Promega) following a 1-hr incubation and absorbance measured at 492 nm. Cell numbers were calculated using a standard curve for each assay.
2.6 Adhesion assay
Adhesion assays were performed as described previously (Khurram et al., 2010) using collagen type 1 solution from rat tail, collagen IV from human cell culture and plasma fibronectin (1–10 µg/ml, Sigma-Aldrich). The plate was coated for 1-hr and non-specific binding was blocked using 1% (w/v) BSA in PBS. Cells were exposed to hLtn (100 ng/ml) for 24-hr prior to seeding in a 96 well plate (4 × 104 cell/well). Adhered cells were quantified indirectly using an MTS assay.
2.7 Chemotaxis assay
Chemotaxis was assessed using 8 µm Transwell ® polycarbonate membrane cell culture inserts (Sigma-Aldrich) and performed as previously described (Khurram et al., 2010). The upper chamber was seeded with 1 × 105 cells/100 µl with 500 µl hLtn (100 ng/ml in SFM) added to the bottom well. Inserts were fixed in 10% (v/v) formalin after 4-hr of incubation, and migrated cells were stained with 0.5% (w/v) crystal violet (in 10% [v/v] ethanol) for 10 min followed by a wash in distilled water. Migrated cells were counted in five random fields per membrane (100× magnification) using a light microscope.
2.8 Myofibroblast differentiation
Cells were seeded into six-well plates (5 × 104 cells/well) and allowed to adhere overnight at 37°C. Following 24-hr serum starvation, exposure to TGF-β1 for 24-hr (5 ng/ml; R&D system) was performed and phenotypes were identified as previously described (Elmusrati et al., 2017). The images were obtained using a fluorescence microscope (Carl Zeiss Ltd.).
2.9 Senescence induction by genotoxic stimuli
NOFs were grown to approximately 70% confluence before treatment with 500 µM H2O2 in SFM for 2-hr. The H2O2 was then removed, and the cells were left in growth media for 14 days to facilitate senescence. Media were changed every 2–3 days. Successful induction of senescence was determined using the senescence-associated β-galactosidase detection kit (Abcam) according to the manufacturer's instructions. Bright-field microscopy was used to estimate the percentage of blue-stained cells per microscopic field.
2.10 Immunohistochemical analyses
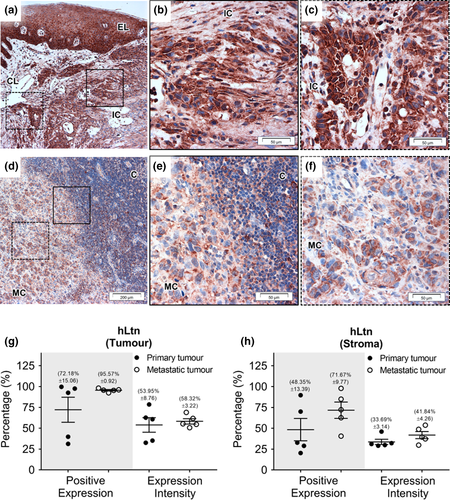
Immunohistochemistry was carried out on primary OSCC (n = 5) with matched metastatic lymph nodes (n = 5), and reactive lymph nodes (n = 5). Immunohistochemistry for XCR1 and hLtn (both 10 µg/ml, LS-Bio) was performed as previously described (Khurram et al., 2010). Sections were scanned using the TissueFAXS SL 120 Histo High-throughput System (Wien, Austria). Percentage of positive cells and staining intensity was quantified in six random regions (~0.3 mm2) of interest (tumour and adjacent stroma) using the HistoQuest Analysis Software (Tissue Gnostics Imaging Solution).
2.11 Statistical data analysis
Data were expressed as mean and standard error of mean. Paired Student's t test was used to determine the statistical significance of results, and a p < .05 was considered statistically significant. All assays were performed in triplicate.
3 RESULTS
3.1 XCR1 surface expression in OCCL is downregulated following exposure to hLtn whilst adhesion to ECM components is increased
Flow cytometry showed variable XCR1 surface expression in OCCL with greater expression in SCC4 cells (88.67%) compared to H357 cells (36.67%; p < .0001) (Figure 1a,b). Following 24-hr exposure to hLtn, a significant elevation in SCC4 XCR1 mRNA expression was detected (Figure 1b, p < .01). Significant downregulation of surface XCR1 expression was seen compared to the control with SCC4 cells showing a greater reduction in median fluorescence intensity (16% reduction; p < .05) compared to H357 (8% reduction, p < .05) (Figure 1c).
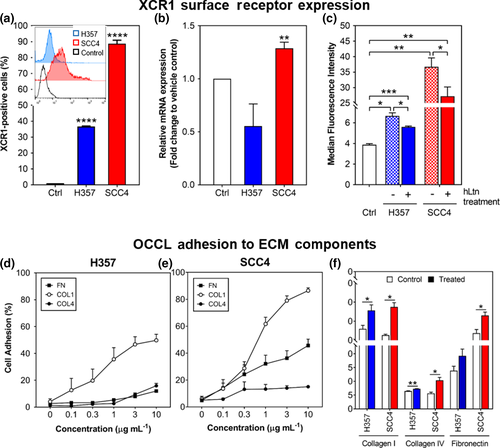
OCCL adhesion to collagen I, fibronectin and collagen IV was investigated since attachment to these is a key step in OSCC migration, invasion and progression (Figure 1d,e). Adhesion to collagen I was observed for both H357 and SCC4 cells with the highest seen to fibronectin and the lowest to collagen IV. hLtn exposure significantly increased SCC4 and H357 adhesion to collagen I (15% and 10%, respectively (p < .05), compared to control. Only SCC4 cells showed significant adhesion to collagen IV and fibronectin with 5% and 9% increase, respectively (p < .05), postexposure.
3.2 hLtn variants influence OCCL proliferation while the canonical hLtn induces chemotaxis
OCCL proliferation, adhesion to ECM components and chemotaxis have been shown previously to be influenced by wild-type hLtn. CC3 is a mutant with a canonical chemokine fold while W55D exists in a dimer form (Liu et al., 2019). Exposure of H357 cells to wild-type, rCC3 and rW55D variants significantly increased proliferation (p < .05) compared to controls with rCC3 inducing the highest increase of 2.3-fold (p = .01). No significant differences were seen between the other hLtn mutant variants (Figure 2a). Exposure to all hLtn variants also significantly increased the proliferation of SCC4 cells for WT, rWT, rCC3 and rW55D (p < .05) (Figure 2b).
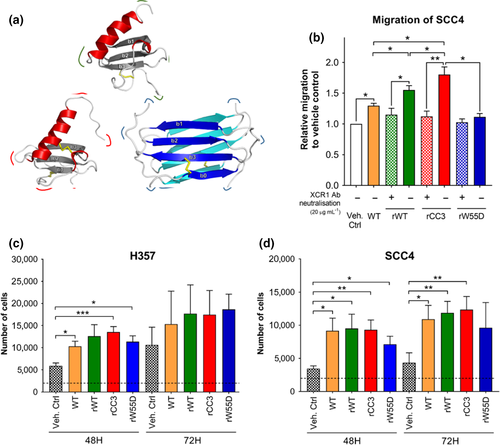
Canonical chemokine fold hLtn variants (WT, rWT, rCC3) also significantly increased OCCL migration compared to control (p = .019, p = .016 and p = .023, respectively) (Figure 2c). rCC3 induced higher migration compared to the WT, rWT and rW55D variants (p = .028, p = .046 and p = .03, respectively). Interestingly, the rWT stimulated migration significantly more than the commercially available WT (p = .014). No migration towards the rW55D was observed.
3.3 Conditioned media from CAF upregulate whereas senescent fibroblasts downregulate mRNA expression of XCR1 and hLtn in OCCL
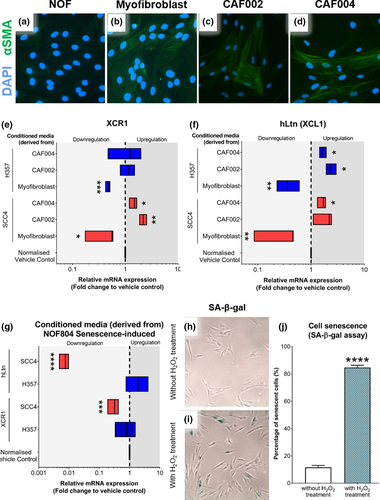
Fibroblasts are the predominant cells in OSCC microenvironment. To investigate the role of fibroblasts in modulating XCR1 and hLtn expression in OSCC, CM derived from fibroblasts was used to treat H357 and SCC4 OCCLs. Different fibroblast phenotypes were employed: primary CAF, TGF-β1-induced myofibroblasts and hydrogen peroxide-induced senescent fibroblasts (s-NOF). CAF phenotype was assessed through α-smooth muscle actin (α-SMA) expression, and expression was seen in TGF-β1-induced fibroblasts and primary CAF (Figure 3b–e). CAF-derived CM significantly increased mRNA expression of XCR1 and hLtn in SCC4 but not in H357 cells compared to control (CAF004: p < .05, CAF002: p < .01) (Figure 3f–g). In contrast, CM from experimentally induced myofibroblasts downregulated XCR1 and hLtn mRNA expression, ranging from 2.3- to 3.3-fold in both cell lines (SCC4: p < .05, H357: p < .001). Following the assessment of the senescence phenotype (Figure 3h–j), the OCCLs were similarly treated with senescent-NOF CM. The results showed a significant downregulation in both XCR1 (p < .001) and hLtn (p < .0001) mRNA transcripts in SCC4 cells compared to the normalised vehicle control (Figure 3k). No significant changes in H357 cells were observed.
3.4 Stromal expression of XCR1 is higher in metastatic deposits compared to the primary site
XCR1 and hLtn expression have been shown previously in OSCC tissue both in primary and metastatic tumours (Elmusrati et al., 2017), and our findings confirmed this. Diffuse expression of XCR1 was seen within the primary tumour, with greater staining in the basal layers of dysplastic surface epithelium and the invasive component (Figure 4).
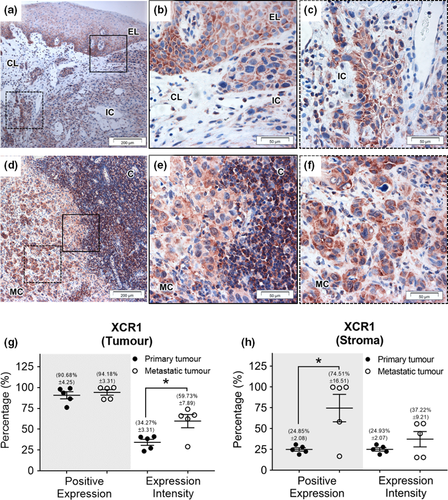
Strong staining for XCR1 was seen in both the primary and metastatic OSCC tissue (90%–95% positive expression) with significantly higher expression intensity seen in metastatic deposits (59.73% ± 7.89) compared to the primary tumour (34.27% ± 3.66) (p < .05) (Figure 4g). The metastatic tumours also showed significantly higher XCR1 percentage positivity (p < .05) than primary tumours in the surrounding stroma (Figure 4h). Positive expression of hLtn was identified in primary and metastatic tumours (72.18% ± 15.06 and 95.57% ± 0.92, respectively) as well as stroma but no significant differences were detected (Figure 5).
4 DISCUSSION
OSCC spreads from the primary site in the oral cavity to neck lymph nodes due to their proximity resulting in poor prognosis (Irani, 2016). In this study, we aimed to build on our previous work and further elucidate the role of XCR1 and hLtn in oral cancer, the relationship between their expression and the role of fibroblasts, the predominant cell type in OSCC microenvironment. There is a significant volume of literature showing the role of chemokines in cancer but a significant gap in knowledge relating to the C-chemokine family particularly in the context of oral cancer.
Chemokine receptors have been shown to be regulated by their ligands in homeostasis and disease, mediated through autocrine or paracrine signalling (Chen et al., 2018). Our results showed a decrease in surface XCR1 receptor after 24-hr exposure to its ligand and previous work has shown cytoplasmic hLtn within the OCCL (Khurram et al., 2010) raising the possibility of XCR1/hLtn interaction further suggesting possible autocrine regulation through receptor internalisation (Bennett, Fox, & Signoret, 2011). Autocrine regulation has been reported for other chemokine receptors in skin after wounding (Kroeze et al., 2012) and in systemic sclerosis by dermal fibroblasts (Carulli et al., 2005).
The hLtn variants developed and employed in this study modulated the behaviour of OCCLs. The key difference between the CC3 and W55D mutant is that the former is the monomer form while the latter is the dimer form of hLtn. Functionally, the dimer is thought to act as a receptor antagonist and does not induce chemotaxis and calcium flux in XCR1-transfected HEK cell (Fox et al., 2015). Proliferation assays showed hLtn/XCR1 involvement in the process, where the canonical fold (WT and CC3 mutant) appeared to influence OCCL growth as previously described (Khurram et al., 2010). However, the dimeric form still induced proliferation of OCCLs comparable to other variant forms. Due to the complexity of chemokine receptor activation and mechanism of action, a possible explanation is that receptor activation initiates signal transduction through homo- or hetero-dimerisation of chemokine receptors allowing dimeric ligand anchorage (Kleist et al., 2016), or the receptor activation allows production of intracellular hLtn by OCCL which has been shown to stimulate receptor activity (Khurram et al., 2010).
Cancer progression is a dynamic process involving changes in cancer cells and the TME through secretion of pro-tumour factors by CAF. In ovarian cancer, CAF have been shown to secrete numerous chemokines such as CCL5, CXCL1, CXCL 11, CXCL12, cytokines and soluble factors facilitating its progression (Thuwajit et al., 2017). CM from CAF have shown to influence pro-cancerous behaviour in gastric cancer cells (Hu et al., 2013), endometrial cancer (Teng et al., 2016), cervical cancer (Chu, Yang, Huang, & Liu, 2014) and oral cancer (Elmusrati et al., 2017). Our results show that mRNA transcripts of both XCR1 and hLtn are upregulated in OCCL when exposed to CAF-CM. In contrast, myofibroblast conditioned medium causes downregulation of both transcripts compared to control. Our initial assumption was that myofibroblasts would behave in a similar way to CAF due to phenotype similarities although it is not unusual to find heterogeneous fibroblast subpopulations in tissues (Sriram, Bigliardi, & Bigliardi-Qi, 2015). The difference in behaviour could perhaps be attributed to variability between primary CAF and experimentally induced cells following stimulation. Fibroblasts have been shown to express different markers between subtypes further adding to fibroblast heterogeneity (Vaheri, Enzerink, Räsänen, & Salmenperä, 2009). CAF-CM have also been shown to induce proliferation and angiogenesis in ovarian cancer cells in vitro through TGF-β1, VEGF and PCNA (Xu, Xu, Cai, Yang, & Lin, 2013) and promote carcinogenesis in hepatocellular carcinoma (Ding et al., 2015). Moreover, the result showed significantly higher XCR1 and hLtn mRNA expression in SCC4 cells compared to H357 by CAF-CM, suggesting the influence is XCR1-dependent as SCC4 cells express higher levels of the receptor and ligand. Additionally, senescence in cancer can be either pro- or anti-tumorigenic in association with chemokine. CCL5 from senescent fibroblast CM has been shown to promote growth and angiogenesis in prostate cancer (Eyman, Damodarasamy, Plymate, & Reed, 2009). In our case, the mRNA expression of both XCL1 and XCR1 is reduced in SCC4 cell line. CAFs contain mix population with senescent fibroblast, where this in line with the result with myofibroblast. This study is the first to demonstrate regulation of XCR1 and hLtn in oral cancer by secreted factors from CAF.
Expression of XCR1 and hLtn in reactive lymph nodes as well as primary and metastatic OSCC is consistent with our previous findings; however, in the current study, we have used matched controls and quantitative analysis to show that XCR1 and hLtn staining are different between primary and metastatic tumours as well as analysing staining within the stroma (Khurram et al., 2010). The quantitative evaluation showed a high number of XCR1-expressing cells in both primary and metastatic OSCC with increased staining intensity in metastatic deposits and surrounding stromal fibroblasts was seen compared to primary OSCC. Crosstalk between tumour and stroma has been shown to play a key role in cancer dissemination suggesting that XCR1 and hLtn may influence cancer growth and dissemination through tumour/stromal crosstalk (Yang et al., 2017), possibly through stimulation of cell migration and invasion which would facilitate tumour invasion and metastasis to lymph nodes in vivo (Khurram et al., 2010).
In conclusion, our novel findings show that hLtn can regulate the expression of its receptor XCR1 through a possible autocrine mechanism. Our hLtn constructs are functional with the non-canonical hLtn W55D promoting OCCL proliferation but not migration. Additionally, CM from CAF increase XCR1 and hLtn mRNA expression in OCCL. Moreover, XCR1 and hLtn are expressed in both primary and metastatic OSCC tissue with higher tumour and stromal expression in metastatic deposits. This suggests an important role for XCR1 and hLtn in OSCC progression through interaction with the microenvironment and needs to be elucidated further.
ACKNOWLEDGEMENT
This study was supported by the Malaysian Government Agency Majlis Amanah Rakyat (MARA). We thank Dr Helen Colley and Amy Harding for providing the primary cells for the project.
CONFLICT OF INTEREST
The authors listed in this paper certify that the authors have no conflict of interest and have no affiliations or involvement with any organisation with any financial interest. We thank Dr Helen Colley and Amy Harding for providing the primary cells for the project.
AUTHOR CONTRIBUTIONS
Amir Zaki Abdullah Zubir: data curation; formal analysis; funding acquisition; investigation; Methodology; Project administration; Validation; Writing-original draft. Simon Whawell: conceptualization; methodology; project administration; resources; supervision; writing-original draft; writing-review and editing. Tuck Seng Wong: conceptualization; investigation; methodology; project administration; resources; software; supervision; writing-original draft. Syed A Khurram: conceptualization; investigation; methodology; project administration; resources; supervision; validation; writing-original draft; writing-review and editing.




