Branched-chain amino acid metabolism: Pathophysiological mechanism and therapeutic intervention in metabolic diseases
Summary
Branched-chain amino acids (BCAAs), including leucine, isoleucine, and valine, are essential for maintaining physiological functions and metabolic homeostasis. However, chronic elevation of BCAAs causes metabolic diseases such as obesity, type 2 diabetes (T2D), and metabolic-associated fatty liver disease (MAFLD). Adipose tissue, skeletal muscle, and the liver are the three major metabolic tissues not only responsible for controlling glucose, lipid, and energy balance but also for maintaining BCAA homeostasis. Under obese and diabetic conditions, different pathogenic factors like pro-inflammatory cytokines, lipotoxicity, and reduction of adiponectin and peroxisome proliferator-activated receptors γ (PPARγ) disrupt BCAA metabolism, leading to excessive accumulation of BCAAs and their downstream metabolites in metabolic tissues and circulation. Mechanistically, BCAAs and/or their downstream metabolites, such as branched-chain ketoacids (BCKAs) and 3-hydroxyisobutyrate (3-HIB), impair insulin signaling, inhibit adipogenesis, induce inflammatory responses, and cause lipotoxicity in the metabolic tissues, resulting in multiple metabolic disorders. In this review, we summarize the latest studies on the metabolic regulation of BCAA homeostasis by the three major metabolic tissues—adipose tissue, skeletal muscle, and liver—and how dysregulated BCAA metabolism affects glucose, lipid, and energy balance in these active metabolic tissues. We also summarize therapeutic approaches to restore normal BCAA metabolism as a treatment for metabolic diseases.
1 INTRODUCTION
Branched-chain amino acids (BCAAs), leucine, isoleucine, and valine are essential amino acids and constitute approximately 20% of the incorporated amino acids into proteins.1, 2 BCAAs perform a plethora of functions including lipid metabolism, gluconeogenesis, and serving as nitrogen donors in different biological processes. Their actions mainly involve several key metabolic pathways such as the mammalian target of rapamycin complex 1 (mTORC1), AMP-activated protein kinase (AMPK), and general control non-derepressible (GCN) 2, as well as insulin signaling.3-7 Despite their essential roles in maintaining physiological functions, chronic elevations of BCAAs and/or their downstream metabolites are associated with a higher risk of cardiometabolic diseases including type 2 diabetes (T2D), heart attack, stroke, and non-alcoholic fatty liver disease (NAFLD; also now known as metabolic-associated fatty liver diseases [MAFLD]).3, 8-12 The pathogenic events of these cardiometabolic diseases are intertwined and some of them are overlapped, which include insulin resistance, chronic sterile low-grade inflammation, aberrant metabolic and lipid pathways, etc. Interestingly, BCAAs and their downstream metabolites are known to participate in and exacerbate these pathogenic events in metabolic diseases. For instance, increased levels of BCAAs and/or their immediate catabolic metabolites, such as branched-chain keto acids (BCKAs), or incomplete oxidation of BCAA-derived metabolites (such as 3-hyroxyisobutyrate) can induce insulin resistance, inflammation, and lipotoxicity in the muscle and liver.,12, 13 as well as enhance lipolysis in adipocytes.14 In contrast, restriction of BCAA consumption improves glucose metabolism, reduces fat mass, and extends lifespan in C57BL/6 J natural aging and obese rodents.15-17 The beneficial effects of BCAA restriction on glucose metabolism are also observed in humans and rodents with obesity and diabetes.18-20 Therefore, BCAAs and their downstream metabolites not only serve as biomarkers but also as mediators of metabolic diseases and thus represent important therapeutic targets.
In this review, we discuss how BCAAs are metabolized in the major metabolic organs, and how their dysmetabolism contributes to obesity and its associated metabolic diseases. Furthermore, we will summarize the strategies for modifying BCAA metabolism and their therapeutic efficacy against the metabolic diseases that afflict millions of people worldwide.
2 OVERVIEW OF BCAA METABOLISM
BCAAs represent three out of nine essential amino acids. In the human diet, 20% of total protein intake is derived from BCAAs.1, 2 Contrary to the oxidation of other amino acids that occurs primarily in the liver, BCAAs uptake, and oxidation take place differentially in numerous tissues because of the low branched-chain amino acid transaminase (BCAT) activity in hepatocytes.21 However, due to the presence of BCAT in the non-parenchymal liver cells, some transamination may also happen in the liver.22
The primary sites for BCAA metabolism include skeletal muscle, adipose tissue, liver, kidney, pancreas, and heart.23 Once inside the cells, BCAAs can be used for protein synthesis, mitochondrial oxidation, and adding reserve to the amino acid pool.2 Additionally, BCAAs can act as key nitrogen donors in the brain for the production of neurotransmitters such as aminobutyric acid (GABA) and glutamate.4 BCAAs can also be utilized as the biosynthetic precursors of sterol and glucose.
The BCAA catabolism initiates with the reversible transamination of BCAAs into their respective branched-chain ketoacids (BCKAs) by BCAT1/2. Global disruption of Bcat2 but not Bcat1 leads to excessive accumulation of BCAAs and BCKAs in the mouse model.24 BCKAs are decarboxylated by the multi-subunit enzyme complex alpha-keto acid dehydrogenase (BCKDH) and generate the corresponding acyl-CoA derivatives and nicotinamide adenine dinucleotide (NADH).25 The acyl-CoA derivatives undergo a series of biochemical reactions to produce acetyl-CoA and succinyl-CoA and eventually integrate into the tricarboxylic acid (TCA) cycle (Figure 1).23, 26 The end products, acetyl-CoA and succinyl-CoA, can serve as substrates for anaplerotic reactions, and acetyl-CoA can also be utilized for lipogenesis in some cell types.
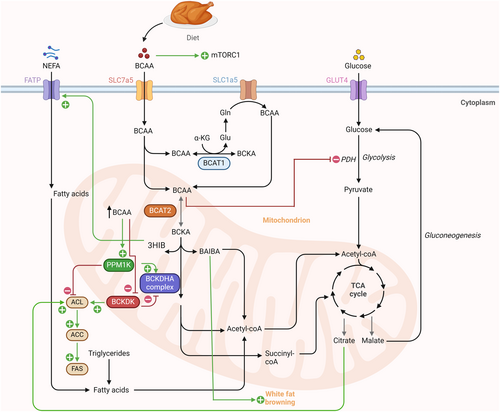
The BCKDH complex is a rate-limiting enzyme and comprises three subunits, including E1, E2, and E3.27 The activity of BCKDH is regulated by post-translational modification, i.e., phosphorylation and dephosphorylation at serine 293 (Ser293) on the E1α subunit, positively by the protein phosphatase 1 K, mitochondrial (PPM1K)28 and negatively by the branched-chain ketoacid dehydrogenase kinase (BCKDK).29 Under low BCAA conditions, the BCKDH complex is phosphorylated and inactivated by BCKDK, whereas an increase in the BCAAs/BCKAs ratio allows the activation of BCKDHA by PPM1K-mediated dephosphorylation at Ser293.29
The pioneer in-vivo 13C-isotope study shed light on the quantitative framework of whole-body BCAA disposal in mouse model.23 Quantitative analysis revealed that skeletal muscle (59%), adipose tissue (19%), and liver (8%) are mainly responsible for BCAA catabolism. On the other hand, the liver, and skeletal muscle both contribute largely to BCAA disposal into proteins.23 Different conditions can alter the distribution of BCAA oxidation among different tissues. Insulin shifts BCAA oxidation more toward skeletal muscles. Under diabetic conditions, a shift of BCAA oxidation from adipose tissue and liver to skeletal muscle was observed as evidenced by a reduction in the contribution of 13C-valine to succinate by ~60% and ~20% in adipose tissues and liver, respectively.23 This increase of BCAA catabolism in skeletal muscle may be due to attenuated oxidation in adipose tissue and liver, given that muscle has a higher capacity for BCAA oxidation. These findings suggest inter-tissue crosstalk under diabetic conditions, leading to a relocation of BCAA oxidation to tissues with higher efficiency (muscle), to adapt to the metabolic state. A recent BCAA flux study showed that BCAA-derived nitrogen is important in synthesizing non-essential amino acids such as alanine, glutamate, proline, and other metabolites including N-acetyl-Glu, N-acetyl-Asp, and glutathione in BAT via the mitochondrial BCAA carrier (MBC) dependent manner.30 Interestingly, mitochondrial dysfunction-induced blockage of BCAA-derived metabolites generation in BAT led to higher oxidative stress and attenuated insulin signaling in the liver.30 Again, this study indicates the crosstalk between BAT and the liver through BCAA metabolism. Despite these promising data, further exploration of BCAA flux among the metabolic tissues, longitudinal studies on BCAA metabolism in metabolic disease progression, and investigation into spatial and cell-specific BCAA metabolism in key metabolic tissues would deepen our understanding of the interaction/proportional contribution of metabolic tissues in control of BCAA homeostasis.
3 ASSOCIATION STUDIES OF BCAAS AND THEIR DOWNSTREAM METABOLITES WITH METABOLIC DISEASES
BCAAs can serve as a predictive risk biomarker for a variety of disorders, including obesity, T2D,8, 31 and CVD.9, 10 Accumulating evidence shows a positive association between plasma BCAA levels and obesity/T2D in human subjects from different ethnic groups and various diabetic rodent models, including diet-induced obesity (DIO) rats, ob/ob mice, and Zucker rats.32-34 The dietary BCAAs intake is also shown to be associated with the risk of developing dyslipidemia in the Chinese population.35 A positive association has been reported between dietary isoleucine and body mass index (BMI) in humans.36 In addition, single nucleotide polymorphisms (SNPs) in different BCAA catabolic genes, including PPM1K, BCAT2, and BCKDHA, are linked with higher serum BCAAs and increased risk of metabolic diseases.37-40 On the other hand, the genetic variants linked with insulin resistance are strongly associated with higher circulating BCAA levels in humans.41, 42 Plasma BCKA levels are also reported to be associated with obesity/T2D and nonalcoholic steatohepatitis (NASH).11, 32, 43 The circulatory level of 3-HIB (a catabolic intermediate of valine) is positively associated with the incidence of T2D and insulin resistance in human subjects in the EPIC-Norfolk study.12, 44 and insulin levels and HOMA-IR in the CARBFUNC cohort.45 Altogether, human studies show a positive association between BCAAs and their downstream metabolites with obesity/T2D and MAFLD.
4 THE UNDERLYING CAUSES OF DEFECTIVE BCAA METABOLISM IN OBESITY/T2D
In this section, we will further discuss how aberrant BCAA catabolism in the metabolic tissues contributes to the chronic elevation of BCAAs in metabolic diseases.
The proteins facilitating BCAA catabolism, such as BCKDHA and BCAT2, are reduced in adipose tissue, skeletal muscles, and the liver under obese or diabetic conditions, whereas the negative regulators of BCAA catabolism (such as BCKDK and phospho-BCKDHA at ser 293) are induced, suggesting the blockage of BCAA catabolism.46-49 In addition, the increased level of BCAAs during obesity is partly because of the BCAA catabolic rate drop in adipose tissue and the liver.49, 50 Further investigation is warranted into monitoring BCAA flux and their metabolic fates (such as to the TCA cycle or protein synthesis) in the metabolic tissues during the development and progression of metabolic diseases.
5 BCAA METABOLISM IN DIFFERENT TISSUES UNDER PHYSIOLOGICAL AND PATHOPHYSIOLOGICAL CONDITIONS
In the following sections, we will discuss how adipose tissue, skeletal muscle, and liver maintain BCAA homeostasis, and how their aberrant metabolism causes obesity and its related metabolic disorders.
5.1 BCAA metabolism in the adipose tissue
In addition to lipids, recent studies showed white adipose tissue (WAT)/BAT also plays a crucial role in maintaining multiple nutrient homeostasis, including glucose, retinol, amino acids, irons, etc.51 WAT acts as a caloric reservoir to store energy in the form of triglycerides via lipogenesis in the feeding state and releases energy as free fatty acids via lipolysis,52 whereas BAT is responsible for adaptive thermogenesis mainly via the action of uncoupling protein 1 (UCP1).
In vitro and ex vivo studies indicated adipose tissue can metabolize a large amount of BCAAs.50, 53 The uptake of BCAAs into adipocytes is mediated by the transporters Slc1a5, Slc3a2, and Slc7a5.54, 55 The direct transportation of BCAAs is facilitated by the Slc1a5 transporter. Simultaneously, a heterodimeric plasma membrane complex, formed by Slc3a2 and Slc7a5, allows for the exchange of internal glutamine and asparagine for external BCAAs.55, 56 The BCAA intermediate metabolites such as isovaleryl-CoA and 2-methylbutyryl-CoA (acyl-CoA) can be utilized to synthesize monomethyl branched-chain fatty acids (mmBCFA). The acyl-CoA precursors are transported from the mitochondria to the cytosol by carnitine acetyltransferase and elongated by the enzyme fatty acid synthase (FASN) in adipose tissue.57 Thus, mmBCFA serves as a substrate for de novo lipogenesis in adipose tissue (Figure 2).57 In 2016, Green et al, reported that BCAA catabolism is important for adipogenesis, as evidenced by the association between upregulated levels of BCAA catabolic enzymes and peroxisome proliferator-activated receptors (PPAR) γ during early adipogenesis.58 BCAAs promote adipocyte differentiation via the regulation of the mTORC1/ribosomal protein S6 kinase (S6K1) and mTORC1/eukaryotic mRNA translation initiation factor 4E-binding protein 1 (4E-BP1)/PPARγ axis.59 Further, the mitochondrial protein sirtuin 4 (SIRT4) regulates BCAA catabolism in pre-mature adipocytes by promoting the expression of PPARγ during early adipogenesis.60
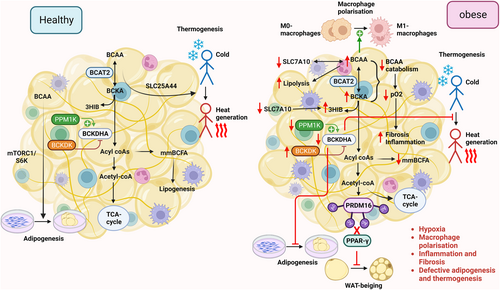
BAT is the second largest contributor to BCAA oxidation.23 Fatty acids and glucose are traditionally regarded as major substrates for thermogenesis in BAT,61 but a recent study by Yoneshiro et al, unprecedentedly reported the usage of BCAAs as thermogenic substrates.62 BCAA clearance from the bloodstream by BAT is stimulated in response to cold exposure and is facilitated by SLC25A44, a newly characterized BCAA transporter in brown adipocytes.62 These findings provided a new perspective on the role of BCAA catabolism in driving thermogenesis in BAT.
Several factors are known to regulate BCAA catabolism in adipose tissue, including adiponectin and PPARγ expression. Adiponectin, the most abundant adipokine, possesses multiple salutary properties, including insulin-sensitizing and anti-inflammatory effects.63-65 Adiponectin negatively associates with the risk of obesity/T2D.63, 66, 67 The activity of BCKDH is increased by adiponectin via upregulation of PPM1K expression and AMPK signaling in adipose tissue.68 Hypoadiponectinaemia could lead to higher BCAA levels in obesity and T2D, as adiponectin directly regulates the rate-limiting enzyme BCKDH. PPARγ, which belongs to the family of nuclear hormone receptors, plays an indispensable role in adipose tissue functions.69 Pharmacological activation of PPARγ using thiazolidinediones (an anti-diabetic drug) increases insulin sensitivity and adipogenesis in adipose tissue.70 In obese Zucker rats, the administration of PPARγ agonists upregulated the expression of genes related to BCAA catabolism (22 genes, such as Bckdhb, Dbt, Dld, Acca2, Pcca, etc.).71 Moreover, the ability of PPAR ligands to sensitize insulin is strongly associated with changes in the BCAA metabolic pathway in the adipose tissue of obese rats.71 Indeed, adipocyte-specific deletion of PPARγ caused higher serum BCAAs and decreased incorporation of BCAAs into triacylglycerol in WAT and BAT in mice, accompanied with glucose intolerance in mouse models. Treatment with rosiglitazone increased BCKDH activity in WAT and BAT and reversed the defects in adipocyte-specific PPARγ knockout mice.72
5.1.1 BCAA metabolism in the adipose tissue under pathophysiological conditions
Disruption of BCAA catabolism induces adipose tissue dysfunction. For instance, knockdown of Bckdha in pre-adipocytes impairs adipocyte differentiation, as shown by reduced expression of adipogenic genes, including Pparg, Plin4, Adipoq, and Leptin.58 Under obese conditions, the BCAA flux to mmBCFA is diminished, leading to a reduction of de novo lipogenesis (Figure 2).57 On the other hand, the reduced enzymatic activity of BCKDHA (probably due to attenuated protein expression of PPM1K and increased BCKDK expression) was observed in adipose tissues of genetically inherited or dietary-induced obese and diabetic mouse models.68 Bariatric surgery significantly reduces serum BCAA levels along with an increase in adipose tissue expression of BCAT2 and BCKDHA proteins in obese individuals.48 Consistently, transplantation of normal epididymal WAT to the mice with Bcat2 deletion, restores the BCAA levels to a normal level.50 The studies support the key role of adipose tissue in systemic BCAA homeostasis.
Under obesity, WAT undergoes hypertrophy and/or hyperplasia to store additional lipids. The deleterious hypertrophic effects are closely associated with exacerbated hypoxia, chronic low-grade inflammation, necrosis, and fibrosis.52 As continuous inflammatory activities proceed in hypertrophic adipocytes, WAT loses its plasticity.52 Consequently, it fails to remodel in response to nutritional and hormonal signals. Cifarelli et al, reported a negative correlation between adipose tissue oxygenation and plasma BCAA levels in healthy and obese human subjects.73 Interestingly, adipose tissue oxygenation showed a positive association with the BCAA catabolic genes, including BCKDHB, HIBDH, HADHA, MMUT, HMGCL, MCC2, and ACAT1, and a negative association with the genes involved in extracellular matrix (ECM) remodeling or fibrosis.73 In obese adipose tissue, hypoxia suppresses BCAA catabolism, reduces BCAA uptake in adipocytes, and channels BCAAs toward lipogenesis.57 This association study indicates that obesity-induced hypoxia may attenuate BCAA catabolism, potentially affecting adipose tissue remodeling and lipogenesis (Figure 2). It is currently unknown whether defective BCAA catabolism in adipocytes or other cell types contributes to adipose tissue fibrosis. However, defective BCAA metabolism has been shown to induce cardiac tissue fibrosis by activating the mTOR pathway in myocardial infarction (MI) mouse models.74
Adipose tissue is a heterogeneous tissue containing not only adipocytes but also immune cells, stem cells, endothelial cells, etc.75 M1 pro-inflammatory and lipid-laden macrophages impair adipose tissue functions, including induction of inflammation, insulin resistance, and promoting lipolysis, whereas M2 anti-inflammatory macrophages exert the opposite effects.76, 77 Aberrant BCAA metabolism has been reported to control macrophage polarization, therefore potentially involving in adipose tissue-resident macrophage functions (Figure 2). Zhao et al, reported that the rise in BCAA levels promotes the secretion of high mobility group box 1 (HMGB1) nuclear protein, an accelerator of M1 macrophage.78, 79 In db/db diabetic mice, BCAA supplementation led to an increase in the levels of BCKAs, resulting in macrophage hyperactivation. In addition, treatment with BCKAs resulted in increased production of pro-inflammatory cytokines like monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor-α (TNF-α), and interleukin-1β (IL-1β) in bone marrow-derived macrophages (BMDM) through induction of mitochondrial oxidative stress.80 Thus, local inflammation is exacerbated in adipose tissue when BCAA catabolism is disrupted. Consistently, TNF-α and IL-6 expressions were suppressed upon boosting BCAA catabolism using 3,6-dichlorobrenzo(b)thiophene-2-carboxylic acid (BT2) treatment in the peritoneal macrophages isolated from db/db diabetic mice. Interestingly, TNF-α and IL-1β have been shown to block leucine catabolism to the lipogenic substrate, but exert no obvious effect on its conversion to proteins in adipocytes.81 Further, treatment with TNF-α selectively attenuated the expression of BCAA transporter Slc1a5 in 3T3-L1 adipocytes. Such effects of TNF-α have also been observed in the epididymal fat of HFD mice.81 These findings suggest a reciprocal relationship between inflammation and BCAA metabolism in adipose tissue. On one hand, the changes in BCAA metabolism promote M1 macrophage polarization. On the other hand, the inflammatory conditions negatively affect BCAA metabolism. This reciprocal interaction forms a viscous cycle that exacerbates obesity-induced adipose tissue inflammation and metabolic dysfunction.
Defective BCAA catabolism also alters WAT browning and thermogenesis. For instance, genetic deletion of Bcat2 has been shown to prevent obesity and increase energy expenditure by promoting browning in WAT. BCKAs repress WAT browning by inhibiting the master regulator of beiging, PR domain-containing protein 16 (PRDM16).82 At the molecular level, BCKA-derived acetyl-CoA leads to the acetylation of PRDM16 and hence blocks its binding with PPARγ (Figure 2).83 Moreover, leucine deprivation has been shown to not only reduce inflammation but also promote WAT browning in C57BL/6 mice.84 Mechanistically, leucine deprivation activates GCN2 in macrophages. This, in turn, decreases monoamine oxidase A (MAOA) expression, resulting in a higher secretion of norepinephrine from macrophages to adipocytes, hence triggering WAT browning.84 In addition, knockout of Bckdha weakens fuel oxidation and thermogenesis, resulting in higher body weight gain and glucose intolerance.62 Thus, the collective findings indicated a close reciprocal relationship between BCAA metabolism and adipose tissue functions.
5.2 BCAA metabolism in the skeletal muscle
Skeletal muscle is a major site for glucose uptake, glycogen storage, thermogenesis, and acts as a pool of amino acids. The reserved pool of amino acids can be utilized for protein synthesis or catabolic processes to support the energy demand.85 Skeletal muscle is responsible for about 70% of whole-body glucose uptake via the glucose transporter (GLUT4) in muscle cells through the AKT-TBC1D4 and APPL2-TBC1D1 regulatory axes.86-88
In vivo isotope tracing analysis showed skeletal muscle contributes to approximately 59% of whole-body BCAA catabolism. The highest percentage of BCAA oxidation in skeletal muscle might be due to high transamination activity (~65%).89 BCAA catabolism in muscle regulates amino acid homeostasis, as glutamate derived from the BCAT2 reaction can either combine with pyruvate to form alanine or with ammonia to form glutamine (Figure 3).90
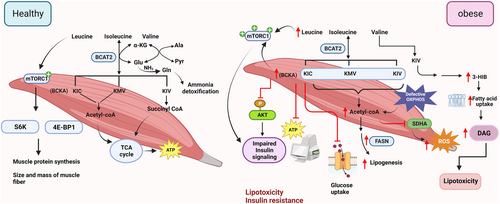
In healthy mice, acute BCKDK inhibition with BT2 promotes whole-body BCAA oxidation, primarily driven by the upsurge in BCAA oxidation in skeletal muscle.23 This highlights the significance of skeletal muscle in whole-body BCAA oxidation. BCAAs, particularly leucine, activate the mTORC1 pathway in skeletal muscle.91 Upon activation, mTORC1 phosphorylates downstream targets such as S6K1 and 4E-BP1, resulting in increased protein synthesis, an increment in both the size and mass of skeletal muscle fiber, and inhibition of skeletal muscle proteolysis (Figure 3).92 In addition, supplementation of BCAAs promotes mitochondrial biogenesis and expression of Sirt1 (the anti-aging molecule) in mice.93
Several conditions regulate BCAA metabolism in skeletal muscle. Endurance exercise enhances BCAA oxidation in skeletal muscle94 by activating the BCKDH complex.95 Overexpression of PGC-1α, a master mediator of exercise effects96 induces BCAA catabolism by upregulating the expression of Bcat2 and Bckdh.97 This induction is mediated through the activation of estrogen-related receptor-α (ERR-α).98 Like in adipose tissue, adiponectin also regulates BCAA catabolism in skeletal muscle. Adiponectin knockout mice exhibited higher Bckdk expression along with higher BCAA and BCKA levels in the skeletal muscle, indicating impaired BCAA oxidation.68 Conversely, treatment with adiponectin rescued HFD-induced elevation of BCAAs in the muscle tissue.99
5.2.1 BCAA metabolism in the skeletal muscle under pathophysiological conditions
BCAAs are required for protein synthesis and growth of skeletal muscle under physiological conditions.100 However, an increase in BCAA and BCKA levels in skeletal muscles is reported in both early and late stages of T2D, potentially due to the attenuated expression of BCAA catabolic genes.32, 47, 98 Interestingly, hyperglycemia further diminished the catabolic ability of skeletal muscle on BCAA in T2D patients.98 In addition, GSEA analysis revealed a positive correlation between insulin sensitivity and the BCAA catabolic pathway in skeletal muscle in human subjects with or without diabetes.101 Defective BCAA catabolism and subsequent elevation of BCAAs lead to persistent activation of the mTORC1/S6K1 pathway thereby blocking insulin signaling in skeletal muscle.102, 103
Although the physiological concentration of BCAAs enhances mitochondrial biogenesis,93, 104 high concentrations (e.g., 20mM) of BCAAs attenuate the expression of mitochondrial biogenesis and oxidation genes such as Pgc-1α, Cox5a, and Atp5, and glycolytic enzymes (Ldha, Ldhb, and Pdh) in insulin-resistant C2C12 myotubes.105 In addition, myocyte-specific inhibition of mitochondrial oxidative phosphorylation (OXPHOS) accelerates BCAA catabolism, resulting in increased acetyl-CoA production for lipogenesis. Consequently, the buildup of acetyl-CoA leads to acetylation-mediated inhibition of mitochondrial respiration and subsequent reactive oxygen species (ROS) production and inflammation in the skeletal muscle (Figure 3).106
The downstream metabolites of BCAAs are also emerging as mediators of insulin resistance. BCKAs hamper insulin signaling in skeletal muscle by impairing insulin-induced phosphorylation of AKT1 at serine residue 473 and insulin receptor substrate 1 (IRS1) at tyrosine residue 612.107 BCKAs also dampen mitochondrial oxygen consumption and glucose uptake in C2C12 myotubes.107 The valine metabolic intermediate 3-HIB is increased in the skeletal muscles of db/db diabetic mice and diabetic human subjects.13 3-HIB promotes trans-endothelial transport of fatty acids and the build-up of lipotoxic intermediates such as diacylglycerol (DAG), and hence blocks insulin signaling in skeletal muscle.13 Ketoisocarproic acid (KIC) generated from leucine, reduces insulin-driven glucose uptake in myotubes in a BCAT2-dependent manner (Figure 3).108
Diabetic animals also exhibit elevated levels of more distal BCAA downstream metabolites C3 and C5 acylcarnitines in skeletal muscle3, 109 potentially resulting from a shift in BCAA oxidation from adipose tissue and the liver to the skeletal muscle. In 2009, Newgard et al, demonstrated that supplementation of BCAAs with HFD induces insulin resistance by activating the mTOR and JNK pathways in the skeletal muscle of rats, perhaps due to excessive accumulation of acylcarnitines.3 Unlike in the rat model, supplementation of BCAAs had no obvious effects on glucose tolerance and insulin sensitivity in mice fed with an HFD or high-fat/high-sucrose diet.110 The discrepancy regarding the effects of BCAAs on rat and mouse models remains elusive.
Histone deacetylase 3 (HDAC3) is an epigenome modifier that regulates circadian rhythms. Muscle-specific deletion of HDAC3 leads to insulin resistance and reduced glucose utilization but increases exercise endurance and resistance in mouse models.111 HDAC3 deficiency upregulates BCAA catabolic genes, such as Bcat2, Dbt, and Ivd, and facilitates the utilization of BCAA catabolic machinery for anaplerotic reactions, increased TCA flux, and oxidative metabolism for muscle contraction, but this increase in BCAA may also cause insulin resistance in skeletal muscle.111 Taken together, these studies suggest that BCAA metabolism controls both lipid and glucose homeostasis as well as exercise endurance in skeletal muscle.
5.3 BCAA metabolism in the liver
The liver controls energy metabolism and serves as a metabolic hub for protein, lipid, and glucose homeostasis. This endocrine organ can coordinate with other tissues like adipose tissue and skeletal muscle via the production of hepatokines (liver-derived hormones) in control of whole-body homeostasis.112 The liver can convert carbohydrates into triglycerides via de novo lipogenesis, and it's regulated by carbohydrate responsive-element binding protein-ß (ChREBP) and sterol regulatory element-binding proteins (SREBP) family members (SREBP-1c).112, 113 Owing to the role of the liver in protein metabolism, it participates in various processes including plasma protein formation, deamination of amino acids, amino acid interconversion, and synthesis of urea (Figure 4).114
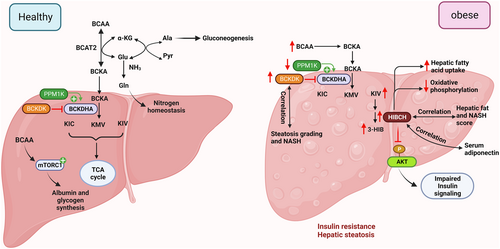
Unlike other essential amino acids, BCAAs bypass the first-phase metabolism due to the low BCAT2 activity in hepatocytes.21 The transamination of BCAAs to their respective BCKAs occurs in extrahepatic tissues, followed by oxidation in the liver, where BCKDH activity is high.2 The isotope-tracing experiment showed the liver is the largest contributor to the disposal of BCAAs into protein synthesis.2 The balance of BCAAs between the liver and blood is regulated by the hepatic transporter.115 Specifically, the transporter SLC43A1 facilitates BCAA efflux from the liver into the bloodstream.116 Glutamate generated by the BCAT2 reaction is converted into glutamine and transported back to the liver and participates in nitrogen homeostasis. Additionally, the transfer of α-amino nitrogen from glutamate to pyruvate leads to the formation of alanine, which is utilized in hepatic gluconeogenesis. In this way, the shuttle system involving glutamine and alanine enables the transfer of BCAA nitrogen from muscle to the liver for urea synthesis.117
BCKAs derived from extrahepatic tissue undergo complete oxidation in the liver. The liver removes ammonia generated from BCAA catabolism via urea synthesis. BCAA catabolic enzymes BCKDK and PPM1K positively and negatively regulate the activity of ATP citrate lyase (ACLY), thereby controlling hepatic de novo lipogenesis.49 BCAAs promote the synthesis of albumin and glycogen by activating the mTOR signaling pathway (Figure 4).118 Thus, BCAA metabolism regulates nitrogen homeostasis, de novo lipogenesis, and protein synthesis in the liver.
5.3.1 BCAA metabolism in liver tissue under pathophysiological conditions
Elevated levels of plasma BCAAs are associated with different statuses of MAFLD, ranging from steatosis, non-alcoholic steatohepatitis (NASH), and cirrhosis, as well as liver cancer (as shown in Table 1).
| Study population (sample size, study type, and ethnicity) | Key findings | References |
|---|---|---|
| Healthy controls and nondiabetic subjects with steatosis or NASH (using untargeted global metabolomic analysis) (n = 60) cross-sectional study, not defined | Higher plasma BCAA levels in NASH | 119 |
| Genome-scale metabolic model of hepatocytes from patients with NASH (n = 45), cross-sectional study, not defined | Participation of valine in the appearance of NASH | 120 |
| Frozen liver tissue samples from healthy, steatosis, and NASH (with or without fatty liver) subjects (n = 45), cross-sectional study, not defined | Elevation in hepatic valine (147% of normal), leucine (127%), and isoleucine (139%) during the transition from steatosis to NASH | 115 |
| Patients with NASH (both male and females) (undergone liver biopsy) (n = 137), cross-sectional study, Japanese population | The plasma BCAA levels in NASH patients decreased with the progression of fibrosis stages | 121 |
| MAFLD (with or without obesity) and control subjects (n = 64), cross-sectional study, Italian population | Subjects with MAFLD have elevated plasma levels of isoleucine and valine | 122 |
| A cohort study with subjects without MAFLD at baseline (n = 1302), prospective cohort study, Chinese population | At baseline, the total BCAA levels and individual BCAA were higher in subjects with MAFLD as compared to controls | 123 |
| Patients with different grades of steatosis with or without NASH (n = 288), cross-sectional study, European population | Positive association of BCKA/BCAA ratio α-ketoisovalerate with the grade of steatosis and NASH. | 11 |
| Patients with hepatocellular carcinoma (HCC) (n = 89) cross-sectional study, Egyptians population | Higher plasma BCAAs in patients with HCC | 124 |
During obesity, the hepatic activity of the BCKDH complex is compromised by the hyper-phosphorylation of Ser293 at the E1 alpha subunit, possibly resulting from the altered expression of Ppm1k (decreased) and Bckdk (increased).68 These changes might cause higher BCAA levels during obesity.
A positive correlation has been observed between plasma BCAAs and liver steatosis stages in subjects with severe obesity and MAFLD in children and adolescents.125 In the Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE), a positive association of BCAAs with hepatic steatosis was also identified.126 The metabolomic and transcriptomic data showed higher hepatic BCAA levels and reduced expression of BCKDHA and BCAA transporters in NASH patients with or without fatty liver.115 While hepatic BCAA levels remain unchanged between healthy subjects and those with steatosis (except for valine), a significant increase in BCAAs (% of normal) was observed in NASH, specifically valine (147%), leucine (127%), and isoleucine (137%).115 The association between BCAA levels and hepatic steatosis can be explained by a dose-dependent increase in SREBP1 expression and lipogenic markers, along with reduced β-oxidation following valine treatment in HepG2 hepatoma cells.127 Interestingly, a study in patients with severe obesity found that among BCKAs, α-ketoisovalerate (KIV) and the ratio of BCKA/BCAA showed a positive association with both the steatosis grade and NASH11 indicating the ratio of BCKA/BCAA could serve as a robust biomarker for the liver disease instead of relying on either BCAAs or BCKAs, and defective BCAA catabolism might causatively contribute to the development of MAFLD. Future longitudinal monitoring of BCAAs and BCKAs in the progression of steatosis to NASH and HCC in different populations might indicate the usefulness of BCAA/BCKA for predictive and diagnostic biomarkers for MAFLD.
The hepatic expression of BCAA catabolic genes also showed a strong association with steatosis and the NASH score during obesity. A human study showed hepatic mRNA expression of BCKDK and HIBCH (the enzyme that produces 3-HIB) is positively associated with steatosis and NASH score, and negatively associated with serum adiponectin levels (Figure 4).11, 45 Overexpression of HIBCH or treatment with 3-HIB leads to increased fatty acid uptake and ROS production in human Huh7 hepatocytes, accompanied by upregulation of genes involved in fatty acid metabolism or lipid storage45 siRNA-mediated knockdown of HIBCH expression sensitizes insulin action in the hepatocytes, which is similar to the observation of reduced AKT phosphorylation in myocytes and adipocytes treated with 3-HIB.12, 13 These findings explain the positive association between HIBCH expression/3-HIB levels, and hepatic steatosis and NASH in humans. On the other hand, HIBCH knockdown also resulted in the upregulation of genes related to TNF-α signaling and hypoxia45 yet the underlying causes and their effects on hepatocyte functions remain unknown.
Elevated plasma BCAA levels have been observed in patients with HCC and utilized as a biomarker for the progression to HCC development.124 Consistent with this finding, the BCAA catabolic pathway was identified as the most significantly downregulated pathway in the HCC cohort.128 The dramatic changes in BCAA catabolic genes are not due to changes in the promotor but somatic copy number variation (CNV) in tumor tissue.128 The accumulation of BCAAs, resulting from reduced hepatic BCAA catabolism, leads to the hyperactivation of the mTORC1 pathway in liver tumors. Additionally, sh-RNA-mediated knockdown of Bckdha also produces a similar effect on mTORC1 activation, which can be mitigated by treatment with the mTOR inhibitor rapamycin in the AML12 cell line.128 Consistent with these findings, BCAA dietary supplementation exacerbates tumor growth in vivo.128
Despite the higher levels of BCAA/BCKA observed in liver diseases (Table 1), BCAA supplementation yields several beneficial effects on liver functions, including increased protein synthesis, maintenance of nitrogen balance, and an improved survival rate in cirrhosis patients.129 However, different pre-clinical studies have reported conflicting findings regarding the effect of BCAA supplementation.130 For instance, in obese/diabetic mouse models, BCAAs supplementation demonstrated beneficial metabolic effects such as reduced body weight and repressed hepatic de novo lipogenesis. However, it can also lead to detrimental effects such as abnormal lipolysis in adipocytes and hepatic inflammation.14 In the choline-deficient, L-amino acid-defined high-fat diet (CDAHFD)-induced NASH mouse model, BCAA supplementation improved hepatic steatosis via a reduction of Fasn expression.131 In the HFD rat model, the anti-hepatic steatosis effect of BCAA is partly due to changes in gut microbiota. Notably, an increase in Ruminococcus flavefaciens (linked to portal acetic acid production) was found.132 These effects are negated under cellulose deficiency, as R. flavefaciens primarily uses cellulose to produce acetic acid.132
5.4 BCAA metabolism in heart
The heart is another tissue heavily influenced by BCAA metabolism. Carbohydrates, amino acids, lipids, and ketone bodies can be used as energy substrates to generate ATP in the mitochondria of the heart. The preference of substrate utilization in the heart is context-dependent (such as fatty acid and amino acids contribute to energy production during fasting and lactate during exercise) and pathological condition (glycogen metabolism is essential in the fetal heart).133, 134 In a healthy heart, the rate of BCAA oxidation within the TCA cycle is relatively low (4%) when compared to other primary sites of BCAA oxidation such as muscle, liver, and adipose tissue.23 Numerous studies showed a defective BCAA catabolism in heart diseases. Elevated BCAA levels blunted glucose oxidation through inhibition of the PDH complex via O-linked N-acetylglucosamine (O-GlcNAc) modification, thus rendering the heart more susceptible to ischemic injury.135 BCKA perfusion in the heart resulted in the inhibition of insulin-induced glucose oxidation with reduced AKT and PDH activity.136 This implied the regulatory role of BCAA metabolism in glucose oxidation and insulin signaling in cardiac tissue. Strikingly, defective BCAA catabolism alone can induce contractile dysfunction in the heart without external pathological stressors. The echocardiogram data indicated a decrease in cardiac function in 3-month-old PPM1K KO mice, which progressively worsened as the mice aged to 18 months.9 In addition, global PPM1K deletion also increased susceptibility of heart failure in mice under pathological stress, yet whether the effect is autonomous in cardiomyocytes or systemic elevation of BCAA/BCKA requires further investigation.9 Consistently, human patients with dilated cardiomyopathy also showed defects in BCAA catabolic machinery and diminished insulin signaling.9, 137 A study using 13C-labeled KIV flux revealed the fate of BCKA in heart tissue. The findings indicated that under pathological conditions, BCKA is preferentially reaminated back to BCAA, resulting in increased protein synthesis rates and leading to cardiac hypertrophy, rather than being oxidized.138 The low expression of BCAA transporter SLC25A44 in the heart contributes to the accumulation of BCAA derived from reamination.138 Taken together, intact BCAA catabolic machinery is vital for heart functions.
6 THERAPEUTIC APPROACHES AGAINST METABOLIC DISEASES BY TARGETING BCAA METABOLISM
Since high circulating BCAA levels and defective BCAA catabolism in the metabolic tissues are causatively linked with diabetes and MAFLD, restoring these defects could offer a feasible and novel approach for these non-communicable and uncurable diseases. In this section, we summarize the approaches, including pharmacological drugs, modulation of gut microbiota, and lifestyle interventions to modulate BCAA metabolism, and discuss their efficacy against metabolic diseases.
6.1 Use of pharmacological compounds to promote BCAA catabolism
6.1.1 Pharmacological compounds targeting BCKDK
6.1.1.1 BT2
BT2, an allosteric inhibitor of BCKDK, enhances the activity of the BCKDH complex, thereby promoting BCAA catabolism.139 In ob/ob mice, BT2 has been reported to significantly increase BCKDH activity in skeletal muscle, liver, and WAT, leading to a reduction in plasma BCAAs and BCKAs.46 Furthermore, BT2 treatment restored BCAA catabolism and improved insulin sensitivity and glucose tolerance in the DIO mouse model.46 Notably, BT2 enhances the efficacy of the anti-diabetic drug metformin in ob/ob and DIO mice.140 BT2 treatment also reduced hepatic triglyceride levels in insulin-resistant Zucker fatty rats.49 In the MAFLD mouse model fed with a CDAHFD, BT2 treatment alleviated hepatic inflammation and fibrosis (Figure 5).141 Likewise, BT2 treatment attenuated liver fibrosis, steatosis, and inflammation in low-density lipoprotein receptor knockout (Ldlr−/−) mice fed with a western diet.141 Thus, these preclinical studies showed that BT2 treatment is effective in alleviating metabolic diseases, but its use in humans has not been tested till date.
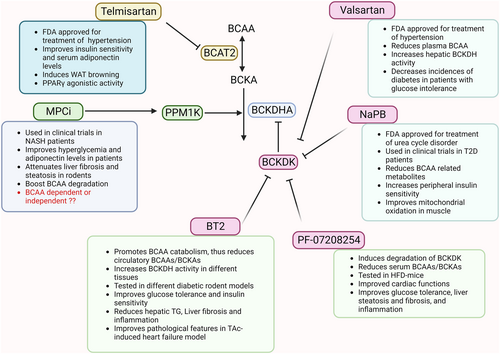
6.1.1.2 Pf-07208254
A new potent BCKDK inhibitor is identified by Flach et al in 2023.142 PF-07208254 induces degradation of BCKDK by reducing the proximity between BCKDK and the E2 subunit of the BCKDH enzyme complex.142 Chronic treatment with PF-07208254 showed improvement in metabolic parameters (glucose tolerance, live steatosis, fibrosis, and inflammation) through reduction in BCAAs, BCKAs, and phospho-BCKDHA levels in HFD-fed mice (Figure 5). PF-07208254 showed similar efficacy as BT2 for the treatment of metabolic diseases, however, it exhibits higher potency (lower half maximal inhibitory concentration [IC50]), due to better protein interaction with non-polar side chains within the BCKDK binding pocket.142
6.1.1.3 Valsartan
It is an angiotensin II receptor blocker used for the treatment of hypertension. It reduces the incidence of diabetes among patients with glucose intolerance and cardiovascular diseases.143 Interestingly, valsartan is reported to inhibit BCKDK in a concentration-dependent and ATP-competitive manner.144 The treatment with valsartan resulted in a reduction in plasma BCAA levels and increased activation of the hepatic BCKDH complex in rats (Figure 5).144 However, whether the anti-hypertension effects of valsartan are partially mediated by its modulating actions on BCKDK requires further investigation.
6.1.1.4 Sodium phenylbutyrate (NaPB)
NaPB has been utilized as a pharmacological activator to boost BCAA catabolism in a clinical trial for T2D patients (NetherlandsTrialRegister: NTR7426).145 NaPB, an FDA-approved drug commonly used for the treatment of urea cycle disorders, acts on the same binding site as BCKDK. Clinical trial data showed that NaPB treatment led to a decrease in BCAA catabolic metabolites, accompanied by improvements in peripheral insulin sensitivity, mitochondrial oxidation in muscle, and whole-body carbohydrate oxidation (Figure 5).145
6.1.2 Pharmacological compounds acting on other BCAA enzymes
6.1.2.1 Mitochondrial pyruvate carrier (MPC) inhibitor
Mitochondrial pyruvate carrier (MPC) is responsible for importing cytosolic pyruvate to the mitochondrial matrix for oxidative phosphorylation. In recent years, MPC has emerged as a promising therapeutic target for the treatment of metabolic diseases. Inhibition or genetic deletion of MPC has demonstrated notable improvements in liver fibrosis, NASH, and BCAA degradation in rodent models.146, 147 In vitro, MPC inhibition boosts BCAA catabolism by reducing phospho-BCKDHA levels through PPM1K. The effect of MPC inhibitor on BCKDH activity is mediated via the activation of mTOR and AMPK signaling pathways.147 Treatment with MPC inhibitor ameliorated hyperglycemia, hepatic steatosis, and HOMA-IR in Zucker diabetic fatty (ZDF) rats (Figure 5). Although MPC inhibitors do not significantly affect BCAA levels, they reduce circulatory 3-HIB levels, which might contribute to the improvement of insulin sensitivity and lipotoxicity in the skeletal muscle and liver.13, 45, 147
6.1.2.2 Telmisartan
Telmisartan is an FDA-approved drug for the treatment of hypertension, and it works by blocking angiotensin receptor II. Telmisartan has been shown to improve metabolic parameters including insulin sensitivity, triglyceride levels, adiponectin levels, and the expression of Ucp1 in obese mice.148 In addition, telmisartan blocks BCAA catabolism by inhibiting the BCAT2 enzyme and inducing browning of WAT, and protecting against diet-induced obesity.83 The induction of WAT browning has emerged as one of the approaches for the treatment of obesity/diabetes (Figure 5). Due to its pleiotropic beneficial effects, telmisartan may be utilized as a therapeutic against obesity/T2D, pending further evaluation of its efficacy in large clinical trials.
Given their approval by the FDA, re-purposing of Valsartan, Telmisartan, and NaPB for the treatment of T2D holds enormous potential. Nonetheless, large-scale clinical trials are needed to further validate their potential in the treatment of T2D. Testing the newly identified BCKDK inhibitor PF-07208254 on metabolic diseases in an independent laboratory is also urgent.
6.2 Gut microbiota modulation
The gut microbiota dysbiosis is one of the contributors to high plasma BCAAs in obesity/T2D.149 The gut microbes Prevotella copri and Bacteroides vulgatus with BCAA biosynthesis capacity are positively correlated with circulating BCAA levels in human subjects with insulin resistance. Metagenomic analysis showed that the expression of genes related to the synthesis and transport of BCAAs in the gut microbe were increased and decreased, respectively, in subjects with insulin resistance.149 In line with these observations, the higher abundance of genes involved in BCAA biosynthesis in the gut microbe is also found in HFD- and streptozotocin (STZ)-induced diabetic rats.150 In a cohort study consisting of morbidly obese women, lower microbial gene richness (MGR) was found in subjects with steatosis, thus pinpointing the role of the gut microbiome in hepatic steatosis. The gut microbiota of obese patients with hepatic steatosis also showed an increased potential for BCAA biosynthesis.151 In addition, phenylacetic acid, a microbial metabolite derived from aromatic amino acid metabolism, enhanced BCAA utilization and lipogenesis in hepatocytes.151 These studies altogether indicate that changes in gut microbiota affect BCAA levels during obesity/T2D. Thus, modulating the gut microbiota can represent as therapeutic approach using phytochemicals (Figure 6).
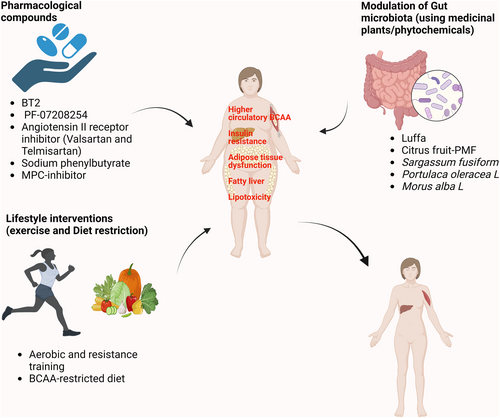
The phytochemical berberine is reported to improve insulin resistance by reducing circulating BCAA levels in HFD-fed mice. This effect is mediated through the modulation of the gut microbiota, specifically by decreasing the abundance of gut microbes involved in BCAAs biosynthesis.152 Additional examples of phytochemicals are discussed below.
The vegetable luffa belongs to the family Cucurbitaceae with medicinal value and reduces circulating BCAAs by upregulating BCAA catabolic enzymes in adipose tissue and the liver via gut microbiota modulation in the DIO mouse model.153 Luffa treatment suppressed the gut bacteria that are positively associated with serum BCAAs, thereby inhibiting the biosynthesis ability of BCAAs in DIO mice. Furthermore, the beneficial effects of luffa in promoting BCAA catabolism and improving metabolic parameters were abolished when gut microbiota was depleted by the antibiotic treatment.153
Citrus peel-derived phytochemical polymethoxyflavones (PMF) extract has been shown to alleviate hepatic steatosis and improve various metabolic parameters, including glucose tolerance, insulin sensitivity, serum cholesterol, and triglyceride level in HFD-fed mice. These effects are mediated through the suppression of the mTOR/P70S6K/SREBP pathway in the liver tissue.154, 155 Notably, the BCAA biosynthesis pathway in the gut microbiota was identified as the most downregulated pathway, resulting in lower serum BCAAs in DIO mice treated with PMF-rich extract (PMFE). The protective effects of PMFE were abolished upon antibiotic treatment. Additionally, the fecal transplantation from PMFE-treated DIO mice to DIO mice resulted in improved metabolic parameters and significantly lower serum BCAA levels,155 indicating the direct modulation of gut microbiota by PMFE.
Sargassum fusiform (SF-Alg), a type of brown algae, is a traditional Chinese medicine.156 Administration of SF-Alg results in lower fasting glucose, triglyceride, and total cholesterol levels, as well as increased levels of HDL-cholesterol in diabetic mouse models. Further, histological analysis showed smaller adipocyte size and reduced inflammation in the liver.157 Mechanistically, the modulation of gut microbiota (increase in Bacteroidetes to Firmicutes ratio), and reduced colon BCAA levels may be responsible for the beneficial effect of SF-Alg in diabetic mice, yet further investigation is needed.157
Portulaca oleracea L. (PE) is shown to have an anti-diabetic effect. PE influences gut microbiota composition, specifically the Firmicutes to Bacteroidetes ratio, inhibits bacterial BCAA biosynthesis, and promotes the expression of BCAA catabolic genes in liver and muscle tissue.158 Morus alba L. (mulberry leaf) extract has been found to possess anti-obesity effects and the ability to modulate the gut microbiota.159 Administration of M. alba L. water extracts (MLE) results in improved metabolic parameters, and reduced inflammation, by altering the abundance of gut bacteria related to BCAA biosynthesis in high-fat, high-sucrose (HFHS) diet-fed mice. Furthermore, MLE increased the expression of BCAA catabolic genes in the liver, skeletal muscle, and epididymal fat, thus promoting whole-body BCAA catabolism.160
The aforementioned medicinal herbs or plant-based extracts demonstrate the ability to modify BCAA metabolism by altering the gut microbiota and activating distinct signaling pathways, highlighting their therapeutic potential against metabolic diseases.
6.3 Lifestyle interventions
Lifestyle interventions, including exercise and diet, are crucial in the management of metabolic diseases (Figure 6).
6.3.1 Exercise
It is one of the promising approaches in the management of metabolic diseases. Aerobic exercise and resistance training have been shown to have favorable outcomes in T2D management.161 Exercise has long been reported to induce BCAA oxidation. Additionally, endurance exercise can lead to the activation of the BCKDH complex in skeletal muscle and the liver.95, 162 Strenuous exercise was shown to significantly reduce the serum BCAA levels as compared to the non-exercising control group.163 Surprisingly, different individuals respond differently to exercise (response variability) in the context of improvement in glucose tolerance and insulin sensitivity, thus being categorized into two categories: non-responders (ranging from 7% to 69%) and responders.164 The cause of the response variability to exercise lies in differential modulation in the gut microbiota and its metabolites. For instance, the responders group showed an increase in the biosynthesis of gut-derived short-chain fatty acids (SCFA) and GABA and a decrease in BCAA levels. However, the opposite was observed in the non-responder group.165 The combination of both exercise and modulation of gut microbiota using phytochemicals might show beneficial outcomes in non-responder groups. Interestingly, the beneficial effect of exercise on insulin sensitivity and inhibition of lipogenesis in sWAT was abolished by BCAA supplementation in obese mice.166
6.3.2 Diet restriction
Restriction of BCAAs consumption exerts beneficial effects on metabolic health, including better glycaemic control, body composition, and efficient mitochondrial functions in adipose tissue in rodent models.16, 19, 20 Deprivation of leucine alone for seven days resulted in several metabolic changes in adipose tissue such as higher energy expenditure, suppression of lipogenesis, an increase in fatty acid oxidation in WAT, and a higher Ucp1 expression in BAT in a mouse model.167 The effect of leucine on adipose tissue browning can be indirect via neuronal activation through GCN2 and the activating transcription factor 4 (ATF4) switch.168 It is worth mentioning that BCAA restriction increases the efficacy of metformin in ob/ob and DIO mice.140
In 2019, a randomized controlled trial was performed to assess the effects of a short-term (4-week) BCAAs-reduced (isocaloric) diet on T2D patients. It showed that short-term BCAAs dietary restriction resulted in better postprandial insulin sensitivity, attenuated activation of mTORC1 in adipose tissue, and changes in gut microbiota composition.18 While the abundance of intestinal Bacteroidetes declines and the percentage of Firmicutes rises in obesity169 such changes could be reversed by short-term BCAAs dietary restriction18 Strikingly, the beneficial effect of the BCAAs restriction is mediated by isoleucine or valine (not by leucine) partially through the activation of the hepatokine fibroblast growth factor 21 (FGF21).36, 170 The low-protein diet has been shown to upregulate FGF21, which increases energy expenditure and insulin sensitivity by targeting adipose tissue. The beneficial effects of a protein restriction diet can be credited, at least in part, to the reduction of BCAAs intake.17, 171
While BCAAs restrictions have shown multiple beneficial effects on metabolic health, it is crucial to acknowledge their vital role in protein synthesis and the maintenance of muscle mass in skeletal muscle.91 This raises concerns that limiting BCAAs might potentially impact muscle mass, particularly in aging populations, which already suffer from reduced muscle mass and strength, and increased frailty.172 Moreover, approximately one-third of the amino group in the neurotransmitter glutamate, crucial for brain development and cognitive functions, is derived from BCAAs.173 Also, lower plasma valine levels are correlated with accelerated cognitive decline in human subjects.174 Thus, BCAA deprivation might potentially affect cognitive functions. Furthermore, BCAA restriction has been shown to impair immune function and enhance the susceptibility to pathogens in mice.175 Of note, obese/diabetic patients display aberrant immunity (i.e. chronic inflammation) and are more susceptible to infection.176 Considering these potential side effects, future implementation of BCAA restriction in diabetic and obese patients needs to be personalized, with its efficacy and potential side effects closely monitored.
7 CONTROVERSIAL EFFECTS OF BCAAS AND/OR COMPONENT OF BCAAS
BCAAs restriction exerts multiple salutary effects on aging- and obesity-induced metabolic disorders. Paradoxically, supplementation of leucine also improves metabolic health in obese conditions.177 For example, leucine supplementation enhances insulin signaling, promotes mitochondrial biogenesis, and reduces lipid accumulation through the activation of PCG1α via SIRT1-mediated deacetylation in the liver, skeletal muscle, and adipose tissues of male C57BL6/J mice fed with a HFD (45% kcal fat).178 In addition, leucine supplementation increases sensitivity and secretion of insulin as well as reduces body weight and fat mass in HFD-fed adult mice (60% kcal from fat).179 Consistently with the notion that leucine might be beneficial, a more recent study revealed that the dietary restriction of valine and isoleucine but not leucine induces metabolic benefits via induction of the hepatokine fibroblast growth factor 21 (FGF21) and its action on adipose tissue “beiging” and thermogenesis as well as hepatic insulin sensitivity in C57BL6/J mice fed with a western diet (42% kcal from fat and 0.2% total cholesterol and 34% sucrose by weight).16, 36 The differential effects of isoleucine, valine, and leucine on metabolic health remain unknown. This may be attributed to their distinct activities on mTOR and their unique downstream catabolic pathways and metabolites180, 181 However, some studies have shown beneficial effects of leucine deprivation, such as reduced fat mass through induction of UCP1 in BAT and lipolysis in WAT, as well as modification of gut microbiota composition in the mice.167, 182
Recent studies suggest that the therapeutic outcomes of protein restriction, including BCAA restriction, are influenced by factors such as sex, the genetic background of the animals, duration of treatment, age for starting treatment, and ratio of BCAA to tryptophan and threonine in the diet.17, 183, 184 Additionally, different methodologies have been used in studies on leucine supplementation and BCAA restriction. For instance, one study used an isocaloric chow diet with reduced content of BCAA (67% of original concentration), while another study supplemented 1.5% leucine in drinking water under HFD conditions without altering the calorie content or levels of valine and isoleucine.36, 179 More rigorous experimental designs are needed to compare individual and combination effects of isoleucine, leucine, and valine in deprivation and supplementation in animal studies.
8 CONCLUSION
As essential nutrients, BCAAs play a vital role in different physiological processes and mediate crosstalk between various metabolic organs. Not only higher plasma BCAAs and downstream metabolites but dysregulated BCAA catabolism in various metabolic tissues is associated with the risk and development of metabolic diseases. However, the individual contribution of defective BCAA catabolism to these tissues in the development of metabolic disease still needs to be explored.
Utilizing the therapeutic approaches that help in boosting the BCAA catabolism or attenuation of BCAA biosynthesis could represent a promising strategy in the future for the management of metabolic diseases. The majority of previous studies focused on the investigation of the first few steps of BCAA catabolism (such as BCAA to BCKA), further investigation of the distal BCAA metabolism and their metabolites, as well as their crosstalk with other metabolic pathways such as the TCA cycle and lipogenesis in different metabolically active cells are attractive topics. A tissue-specific approach for promoting BCAA catabolism in adipose tissues and the liver might not only exert beneficial effects on metabolic diseases but might minimize its potential negative effects on protein synthesis and muscle mass in the skeletal muscle. However, more mechanistic insights are further needed to fully utilize the therapeutic potential of BCAAs.
Important take-to-home messages and open questions are listed below.
- Maintenance of the physiological concentration of BCAAs is regulated by the tight coordination between multiple metabolic tissues including adipose tissues, the liver, and the skeletal muscle.
- The disruption of the BCAA catabolic machinery and altered BCAA metabolic fates in the metabolic tissues lead to elevated circulating BCAA and altered profile of their downstream metabolites, which contribute to the development of metabolic diseases including obesity, diabetes, and the MAFLD.
- Targeting aberrant BCAA catabolism or BCAA restriction improves metabolic health in rodents and humans.
- What are the metabolic fates of BCAA in different metabolic tissues under physiological (such as fasting, feeding, and cold environment) and pathological (such as obesity and diabetes) conditions?
- Apart from the host tissues, aberrant BCAA metabolism in gut microbiota also contributes to systemic BCAA balance and metabolic disorders. Does BCAA metabolism in gut microbiota interact/intertwine with the host's BCAA metabolic pathways? If yes, what are the underlying regulatory mechanisms?
- Given that BCAA is important for protein synthesis in the skeletal muscle and muscle mass. Is a tissue-specific approach for promoting BCAA catabolism in adipose tissues and the liver sufficient to exert beneficial effects on metabolic diseases? Since this approach avoids the potential deleterious effects on skeletal muscle, particularly in aging population.
- Both pharmacological and dietary approaches targeting BCAA metabolism show multiple salutary effects on metabolic health in rodents. A recent clinical study showed that BCAA catabolic activator NaBP improves insulin resistance and reduces glucose levels in human diabetic subjects. Further human clinical trials involving a larger sample size, and more diverse populations will provide more evidence about the efficacy, generalization, and safety issues of BCAA-targeting approaches.
AUTHOR CONTRIBUTIONS
Shama Mansoori: Writing- conceptualization, drafting of original manuscript and editing, Kelvin Kwun Wang Ng: Writing, Melody Yuen Man Ho: revision, Kenneth K.Y. Cheng: Writing- review and editing, conceptualization, funding acquisition, and supervision. All authors approved the manuscript for the submission.
ACKNOWLEDGMENTS
We would like to acknowledge the financial support from the National Natural Science Foundation of China (NSFC; grants 81970675 and 92357305 to KKY Cheng), the Research Grant Council for the Collaborative Research Fund (C5044-23G), and the General Research Fund (GRF), Hong Kong Research Grant Council (RGC) (15101221 to KKY Cheng). Additionally, we appreciate the support from the Shenzhen Municipal Science and Technology Innovation Commission (grant JCYJ20210324130202006 to KKY Cheng) and the PolyU-Project of Strategic Importance (P0036848 to KKY Cheng) and PolyU-Project of RCMI (P0040979 to KKY Cheng).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.




