Liver fat as risk factor of hepatic and cardiometabolic diseases
Summary
Non-alcoholic fatty liver disease (NAFLD) is a disorder characterized by excessive accumulation of fat in the liver that can progress to liver inflammation (non-alcoholic steatohepatitis [NASH]), liver fibrosis, and cirrhosis. Although most efforts for drug development are focusing on the treatment of the latest stages of NAFLD, where significant fibrosis and NASH are present, findings from studies suggest that the amount of liver fat may be an important independent risk factor and/or predictor of development and progression of NAFLD and metabolic diseases. In this review, we first describe the current tools available for quantification of liver fat in humans and then present the clinical and pathophysiological evidence that link liver fat with NAFLD progression as well as with cardiometabolic diseases. Finally, we discuss current pharmacological and non-pharmacological approaches to reduce liver fat and present open questions that have to be addressed in future studies.
1 INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is characterized by the presence of liver fat accumulation (NAFL or steatosis) that can progress to liver inflammation (non-alcoholic steatohepatitis [NASH]), liver fibrosis, and cirrhosis.1 The prevalence of the disease is very high and is continuously rising, affecting 32% of the general population worldwide.2 Importantly, NAFLD prevalence is even higher (40–70%) among patients with obesity and diabetes, or conversely, patients with NAFLD suffer often by more than one metabolic diseases (obesity, diabetes, hyperlipidemia, and metabolic syndrome).3, 4 NAFLD is also increasingly recognized as an independent risk factor for cardiovascular diseases.5
The gold standard for the diagnosis and staging of NAFLD remains liver biopsy, which is an invasive, time- and cost-consuming method, that is operator dependent showing moderate reproducibility.6 These important limitations make liver biopsy a suboptimal screening method for NAFLD—or for monitoring disease progression and treatment response.1, 6-8 Furthermore, no approved treatment for NAFLD exists to date, and several efforts for the development of drugs that can improve liver fibrosis have failed.1, 6-8 Thus, there is an urgent need for non-invasive tools for diagnosing and staging NAFLD, for identifying patients at high risk for disease progression, and for monitoring treatment response.6, 9-11 Additionally, therapeutic approaches targeting earlier stages of the disease may prove to be more fruitful.
For the diagnosis of NAFLD, the presence of ≥5% of fat in the liver is necessary. Accumulation of liver fat is thus the first and most crucial event for NAFLD development. In this review, we will first briefly present the current tools (non-invasive imaging modalities or blood-based tests) that are available for the quantitative or semi-quantitative assessment of fat amount in the liver. Second, we will discuss how increased liver fat accumulation may promote liver inflammation and fibrosis, and we will summarize the evidence showing the value of non-invasive liver fat quantification for predicting histologic changes in NAFLD. Third, we will present the findings linking liver fat with metabolic diseases and its value as independent predictor of their development and progression. Finally, we will discuss the impact of lifestyle interventions, bariatric operations, and medications on reducing liver fat and improving NAFLD.
2 METHODS OF DETECTION OF LIVER FAT
Hepatic steatosis can be quantified histologically by classifying liver fat into four grades, which represent the percentage of fatty hepatocytes: Steatosis (S) 0: 0–5%, S1: 5–33%, S2: 33–66%, S3: >66%.12 However, because of its invasiveness, liver biopsy is not an appropriate tool for the diagnosis of liver steatosis. Non-invasive methods are more suitable for this purpose and can be divided into imaging methods and blood-based tests (see Table 1).
| Test (reference) | Features/compounds | Application | Performance | Comments |
|---|---|---|---|---|
| Imaging modalities | ||||
| Abdominal ultrasound13 | Increased liver echogenicity compared with renal cortex | Recommended as first-line tool for diagnosis of steatosis | Moderate-to-severe steatosis vs no steatosis: Sn: 85% (80–89%); Sp: 93% (87–97%) | Detection of steatosis with >10–20% liver fat content; Limitations in obesity, fat quantification not possible, operator dependent |
| Controlled attenuation parameter (CAP),14 | Reflects attenuation of ultrasound signal in steatotic liver tissue in dB/m | Measurement within Fibroscan® examination |
Cut-off values (AUROC): S ≥ 1: 302 dB/m (87%); S ≥ 2: 331 dB/m (77%), S ≥ 3 of 337 dB/m (70%) |
New software SmartExam: continuous measurements of CAP captures ~200 CAP values - > lower measurement variability |
| MRI-PDFF15 | Non-invasive imaging biomarker for quantification of liver fat in the entire liver | Most accurate method to quantify liver steatosis | Detection of any grade steatosis (AUROC 0.99; Sn: 96%, Sp:100%) | high costs, limited availability |
| Blood-based tests | ||||
| Fatty liver index (FLI),16 | BMI, WC, GGT, TG | Most widely used non-commercial test | Cut-offs: <30 (Sn: 87%, Sp: 64%, AUROC: 0.84); ≥60 (Sn: 61%, Sp: 86%) | Recommended as a NAFLD screening tool in primary care; Fat quantification not possible; Gray zone where result is inconclusive |
| SteatoTest™17, 18 | Haptoglobin, total bilirubin, ALT, A2M, ApoA1, GGT, total cholesterol, TG, age, Glucose, gender, BMI | Commercial test | Cut-offs: ≥0.3 (Sn: 90%, Sp: 54%, AUROC: 0.80), ≥0.7 (Sn: 46%, Sp: 88%) | Consists of non-routine laboratory parameters; high costs |
| Hepatic steatosis index (HSI),19 | BMI, diabetes, AST/ALT | HSI < 30 rules out and >36 rules in fatty liver | Cut-offs: <30 (Sn: 93%, Sp: 40%, AUROC: 0.81), >36 (Sn: 46%, Sp: 92%) | Based on routinely available parameters |
| Index of NASH (ION),20 | female: TG, HOMA and ALT; male: waist-to-hip ratio, ALT, TG, HOMA | ION <11 rules out and ≥22 rules in fatty liver | Cut-offs: <11 (Sn: 81%, Sp: 56%, AUROC: 0.77), ≥22 (Sn: 60%, Sp: 82%) | Moderate performance |
| NAFLD liver fat score (NAFLD-LFS),21 | AST/ALT, diabetes, MetS, insulin | cut-off of −0.640 predicts increased liver fat content | cut-off −0.640 (Sn: 86%, Sp: 71%, AUROC: 0.87) | Developed with MRS as reference |
| Lipid accumulation product22 | WC, TG, gender | cut-offs not provided for ruling in or out steatosis | AUROC 0.79 for diagnosing steatosis >30% |
Moderate accuracy for steatosis >5% |
- Abbreviations: Sn (sensitivity), Sp (specificity), S (steatosis), AUROC (area under receiver operating characteristic curve), MRI-PDFF (magnetic resonance imaging – proton density fat fraction), BMI (body mass index), WC (waist circumference), GGT (gamma-glutamyl transferase), TG (triglycerides), ALT (alanine aminotransferase), A2M (a2-macroglobulin), ApoA1 (apolipoprotein A1), AST (aspartate aminotransferase), HOMA (Homeostasis Model Assessment), MetS (metabolic syndrome), MRS (magnetic resonance spectroscopy).
2.1 Imaging methods
To assess hepatic steatosis, different imaging modalities are available, including ultrasound-based techniques, such as abdominal ultrasonography and controlled attenuation parameter (CAP), and magnetic resonance-based techniques such as MRI-estimated proton density fat fraction (MRI-PDFF).
2.1.1 Ultrasonography
Transabdominal ultrasound is the most commonly used imaging method for the diagnosis of hepatic steatosis, as it is widely available and inexpensive. It is recommended as the first-line diagnosis and/or screening tool for liver fat23, 24; however, it can only detect steatosis with >10–20% liver fat content.25 Taking liver biopsy as a reference, abdominal ultrasound has shown pooled sensitivities and specificities to distinguish moderate-to-severe steatosis from its absence of 85% (80–89%) and 93% (87–97%), respectively.13 Limitations of the ultrasonography include that it is operator-dependent and that it cannot provide an absolute quantification of liver fat. Apart from establishing the diagnosis of steatosis, ultrasonography has been occasionally used to assess the severity of steatosis.26 However, it demonstrates low sensitivity especially at detecting mild steatosis, and its accuracy is reduced in patients with obesity and renal disease.25-27 Thus, it is considered a suboptimal method for monitoring disease progression or treatment response.
2.1.2 Controlled attenuation parameter (CAP)
CAP is a tool which is integrated into the vibration-controlled transient elastography (VCTE) device Fibroscan®. CAP reflects the attenuation of the ultrasound signal in steatotic liver tissue quantitatively in dB/m and allows a non-invasive quantification of liver fat.28 A recent study of 450 patients with NAFLD determined cut-off values for S ≥ grade 1 of 302 dB/m (AUROC [area under receiver operating characteristic curve] 87%), for S ≥ grade 2 of 331 dB/m (AUROC 77%), and for S ≥ grade 3 of 337 dB/m (AUROC 70%).14 However, previous studies have described different cut-off values for grading of steatosis. This may be because of heterogeneous study populations with different body mass index (BMI) of patients, small number of cases, variable prevalence of type 2 diabetes mellitus, and different stages of fibrosis. Factors such as male sex, age, BMI, presence of metabolic syndrome and of alcohol abuse are positively associated with CAP levels as well as with failures in CAP measurement, which are estimated to be about 8%.29 Further studies are needed for precise differentiation of contiguous degrees of fatty liver. By comparing CAP and liver ultrasound head-to-head, it could be shown that the performance of CAP for detecting and grading liver steatosis was higher than that of liver ultrasound; however, the rate of overestimation was significantly higher for CAP than for ultrasonography (30.5% vs 12.4%; p < 0.05).30 SmartExam is a recently developed software that allows continuous measurements of CAP during the entire examination and captures roughly 200 CAP values. Preliminary data suggest that continuous CAP has a lower measurement variability than the original method with using the median of 10 measurements.31
2.1.3 MRI-PDFF (proton density fat fraction)
MRI-PDFF is considered one of the most accurate methods to detect and quantify liver steatosis. It has the advantage to assess steatosis across the whole liver. In a head-to-head comparison with CAP, MRI-PDFF could show a higher accuracy in identifying all grades of liver steatosis (AUROC 0.99).15 Furthermore, it has been shown that a reduction in MRI-PDFF values results in histologic improvement in NAFLD, including resolution of NASH, and fibrosis improvement.32, 33 However, because of its high costs and limited availability, MRI-PDFF is used almost exclusively in clinical trials but not in routine clinical practice.
2.1.4 CT and magnetic resonance spectroscopy (MRS)
CT has been previously used for liver fat quantification by using the Hounsfield scale (HU).34 Development of conversion equations based on very high linear correlations allows the conversion of the HU measurements to PDFF.34 However, because of the risk of ionized radiation and the low accuracy of the method in mild steatosis, CT is considered not appropriate for diagnosing and monitoring liver fat. MRS was the first method developed for liver fat quantification based on MRI. MRS has a sensitivity of 73–89% and specificity of 92–96% for accurately detecting different cut-off percentages of liver fat.35 1H-MRS is considered as the most accurate method to detect and quantify non-invasively liver steatosis.36 Main disadvantage of the method is the limited reproducibility, because it demands focusing on single voxels instead of volumetric assessments followed in MRI. Additionally, it requires expertise for data acquisition and processing and specific software systems, thus limiting its use.34
2.2 Blood-based tests
There are also various blood-based tests for the detection of liver fat, including fatty liver index (FLI),16 SteatoTest™,17 hepatic steatosis index (HSI),19 index of NASH (ION),20 NAFLD liver fat score (NAFLD-LFS),21 and lipid accumulation product (LAP).22 The features and performances of these tests are summarized in Table 1. As they have been validated against different reference methods, they are not easily comparable.
2.2.1 Fatty liver index (FLI)
Among the blood-based tests, the FLI is the most widely used non-commercial test consisting of the parameters BMI, waist circumference, gamma-glutamyl transferase (GGT), and triglyceride levels. The score was developed in 280 patients with NAFLD and showed a diagnostic accuracy of 84% for detecting liver steatosis on ultrasound. At a score <30, fatty liver could be excluded with a negative likelihood ratio (LR) of 0.2 and a sensitivity of 87%, and at a score >60, it could be diagnosed with an LR of 4.3 and specificity of 86%.16 In a larger study of 2652 patients with NAFLD, a similar diagnostic accuracy could be confirmed for the presence of steatosis (AUROC 81%).37 When using MRS as reference, AUROC for the prediction of liver fat >5.5% was 0.79 (IQR = 0.74, 0.84), without being able to quantify liver fat content.38 When using liver biopsy as reference standard, FLI could detect steatosis in a cohort of 324 patients with NAFLD with an AUROC of 0.83, however, again without ability to quantify liver fat content.39 Recently, the FLI has been recommended in the updated German NAFLD guidelines as a screening tool in primary care for patients with a high risk for NAFLD.40
2.2.2 SteatoTest™
SteatoTestTM (Biopredictive, Paris, France) was the first non-invasive blood-based score developed for the prediction of hepatic steatosis. In a cohort of 844 subjects with various chronic liver diseases, including chronic hepatitis B and C and alcohol-related liver disease, the AUROC for the presence of steatosis >5% was 0.80 using liver biopsy as reference. At a cut-off of 0.3, sensitivity was 90% and at a cut-off of 0.7 specificity was 88%.18 In a cohort of 600 subjects with available liver biopsy, nonbinary ROC (NonBinAUROC) for SteatoTest and steatosis grade according to the histologic SAF (steatosis, activity, and fibrosis) score was 0.822 (0.804–0.840; p < 0.0001), indicating a good correlation with steatosis grade on liver biopsy.41 When using MR-spectroscopy as reference standard in a cohort of 220 patients with T2DM, the AUROC for prediction of liver steatosis and the agreement with spectroscopy were low (0.674 and 0.042, respectively).42 In a meta-analysis of three studies with 494 patients with severe or morbid obesity undergoing bariatric surgery, the AUROC for prediction of steatosis >33% was 0.80.17
2.2.3 Hepatic steatosis index (HSI)
HSI has been developed in 10,724 health check-up subjects (5362 cases with NAFLD versus age- and sex-matched controls) in Korea and consists of three routinely available parameters (AST/ALT ratio, BMI, and diabetes). Using ultrasound as reference, HSI had an AUROC of 0.812 (95% CI, 0.801–0.824). At values of <30.0 or >36.0, HSI ruled out NAFLD with a sensitivity of 93.1% or detected NAFLD with a specificity of 92.4%, respectively. Of 2692 subjects with HSI < 30.0 or >36.0 in the derivation cohort, 2305 (85.6%) were correctly classified.19 In an external validation in 6927 Japanese health checkup subjects using ultrasound as reference, AUROC for HIS was 0.874 with a sensitivity of 88.8% at the low cut-off and a specificity of 98.5% at the high cut-off.43 In another population based study with two cohorts from Germany (SHIP; n = 4222 and EMIL, n = 2177) using ultrasound as reference, HSI showed an AUROC of 0.782 in the SHIP and of 0.841 in the EMIL cohort, respectively.44
2.2.4 Index of NASH (ION)
ION has been developed in a cohort of 4458 NAFLD patients from the National Health and Nutrition Examination Survey (NHANES III). Independent validation has been performed in a cohort of 152 patients with NAFLD on liver biopsy. The ION model is different for female and male: TG, ALT, and HOMA in female and waist-to-hip ratio, TG, ALT, and HOMA in male. The AUROC for ION was 0.77, specificity for ruling in steatosis at a cut-off >22 was 82%, and sensitivity for ruling out steatosis at a cut-off <11 was 81%.20 External validations in independent cohorts for detection of steatosis have not been performed widely. A validation for the noninvasive diagnosis of NASH has been performed in an Italian cohort with biopsy-proven NAFLD. However, diagnostic accuracy was relatively poor (AUROC 0.687; 95% CI = 0.62–0.75).45
2.2.5 NAFLD liver fat score (NAFLD-LFS)
The NAFLD-LFS consists of five variables (fasting AST level and the AST/ALT ratio, fasting insulin level, metabolic syndrome, and type 2 diabetes) and has been developed with MRS as reference method. AUROCs were 0.87 in the estimation group and 0.86 in the validation group. At a cut-off point of −0.640, increased liver fat content could be predicted with a sensitivity of 86% and specificity of 71%.21 In an external validation study with a total of 1301 community-based health checkup subjects who underwent liver fat quantification with MRI, NAFLD-LFS showed an AUROC of 0.72. The diagnostic agreement was 72.7% between NAFLD-LFS and 70.9% between ultrasound and MRI, showing that the diagnostic performance for detection of steatosis was comparable to that of ultrasonography.46 In another external validation study for the presence of steatosis with 324 subjects and liver biopsy as reference, the diagnostic accuracy was good with an AUROC of 0.80; however, the AUROC for predicting steatosis >33% and with that distinguishing between moderate and severe steatosis was only 0.72.39
2.2.6 Lipid accumulation product (LAP)
The LAP score consists of the three variables waist circumference, gender, and triglycerides and has been developed in a cohort of 588 patients (305 with suspected liver disease and 283 age- and sex-matched controls). For diagnosing steatosis >30%, the AUROC was 0.79.22 An external validation study using MRS as reference showed an AUROC of 0.78 (IQR 0.72, 0.83) for the detection of >5.5% steatosis. At a cut-off ≥20, steatosis could be ruled out with a sensitivity of 99% and at a cut-off ≥80, steatosis could be ruled in with a specificity of 94%. However, LAP was not able to quantitatively predict liver fat content.38 Another validation study from Italy with a total of 40,459 subjects aged ≥18 years using ultrasound as reference showed good diagnostic accuracies for the detection of steatosis with 0.843 (95% CI 0.837, 0.849) in male and 0.887 (95% CI 0.882, 0.892) in female, respectively.47
2.2.7 Comparisons between tests
In a head-to-head comparison among MRI-PDFF, CAP, FLI, HSI, and SteatoTest in 152 bariatric surgery candidates undergoing liver biopsy, MRI-PDFF demonstrated AUROCs >0.9 and sensitivities and specificities >80% for detecting >5%, >33%, and >66% steatosis. CAP demonstrated an AUROC of 0.83 for detecting >5% steatosis and below <0.80 for detecting >33% and >66% steatosis. Blood-based tests demonstrated in all steatosis grades AUROCs <0.80 with low specificities. In a between-tests comparison, MRI-PDFF outperformed CAP and all blood-based tests, whereas the performance of CAP was not significantly superior to the blood based-tests.48 In another study, FLI score ≥60 demonstrated a sensitivity of 60% and specificity of 80% and NAFLD FLS ≥ −0.640 a sensitivity of 68% and specificity of 78% to detect steatosis diagnosed with MRI-PDFF, indicating that both tests are suboptimal for diagnosing steatosis.49 The main limitation of the available blood-based tests is that they cannot provide an accurate assessment of liver fat % and consequently cannot be used for monitoring disease progression. Furthermore, they include gray zones, where steatosis can neither be confirmed nor be excluded. Moreover, they did not find their way into routine clinical practice as they do not add more information to the existing clinical standard with clinical, laboratory, and imaging examinations in suspected fatty liver.
3 LIVER FAT AS RISK FACTOR FOR NAFLD PROGRESSION
3.1 Pathophysiological mechanisms linking the amount of hepatic fat with NAFLD progression (Figure 1)
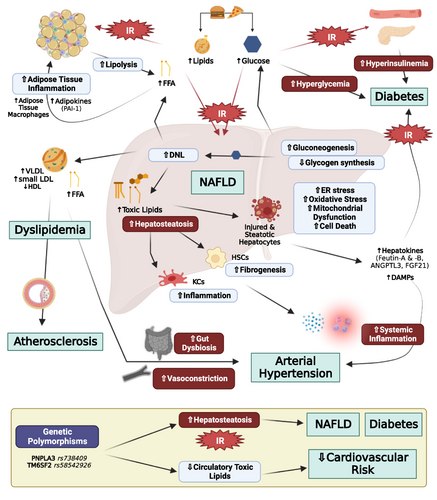
Liver fat is stored in the form of triacylglycerols (TGs) in patients with NAFLD. Around 60% of the hepatic TGs were derive from non-esterified free fatty acids (NEFAs) from adipose tissue after lipolysis, 25% from de novo hepatic lipogenesis by using 2-carbon precursors from glucose, fructose, and amino acids, and 15% from dietary fat that escapes storage in adipose tissue by spill over to NEFA pool or chylomicron remnant formation and uptake in the liver.50 High caloric intake increases both the dietary fat available for uptake by the liver as well as the amount of carbohydrates that can be converted to fat through de novo lipogenesis.1, 7, 8 Moreover, as body weight, body fat, and intracellular fat in muscle and adipose tissue increases, hepatic and peripheral insulin resistance develops.1, 7, 8 Specifically, insulin resistance in skeletal muscle reduces glucose uptake in muscle resulting in higher concentrations of glucose arriving in the liver, thus further stimulating hepatic de novo lipogenesis. The increased hepatic de novo lipogenesis impairs insulin action (hepatic insulin resistance), thus reducing hepatic glycogen synthesis and stimulating gluconeogenesis, consequently further aggravating hyperglycemia and hyperinsulinemia. Finally, insulin resistance in adipose tissue results in impaired suppression of lipolysis, consequently leading to increased fatty acid release from adipose tissue and high flux of NEFAs to the liver.1, 7, 8 Additionally, insulin resistance reduces lipid uptake by adipose tissue, thus redirecting chylomicron remnants to the liver.1, 7, 8
Accumulation of macrovesicular fat in hepatocytes acts probably in the beginning protectively by delaying hepatocyte injury. As the accumulation of fat though continues, lipid species (i.e., ceramides, lysophospatidylcholines, and saturated fatty acids) act toxically by promoting hepatocyte damage and cell death through multiple mechanisms.1, 7-9 Specifically, excess lipid accumulation induces endoplasmic reticulum stress, oxidative stress, and increases β oxidation in the mitochondria, which results in the formation of reactive oxygen species by depleting antioxidant reserves as well as mitochondrial dysfunction by impairment of mitochondrial membrane potential and electron transport.1, 7-9 Hepatocellular stress might lead to cell death and to the subsequent release of factors (cytokines, lipids, hormones, extracellular vesicles, and damage-associated molecular patterns) that promote inflammation by recruitment and activation of resident (Kupffer cells) and peripheral macrophages and subsequently fibrogenesis by activation of hepatic stellate cells.1, 7-10
Hormones secreted by adipose tissue, that is, adipokines, may further affect hepatic function and thus may contribute to NAFLD development or progression. Specifically, adiponectin reduces fatty acid synthesis by downregulating SREB1-C and increases fatty acid oxidation by stimulating PPAR-α4, 51-53 in the liver. Furthermore, it reduces gluconeogenesis by decreasing PEPCK/G6Pase and acts anti-inflammatory by inhibiting NF-kB. In obesity, adiponectin levels are reduced, thus depriving liver of adiponectin's hepatoprotective effects.4, 51-53 Leptin is another adipokine and major regulator of energy homeostasis.54, 55 Leptin seems to exert dual effects on hepatic function. It acts anti-steatotic by stimulating hepatic VLDL-triglyceride export and free fatty acid oxidation and by inhibiting hepatic de novo lipogenesis.51, 52, 56 On the other hand, leptin acts also pro-inflammatory and pro-fibrogenic by sensitizing Kupffer cells and activating hepatic stellate cells to express transforming growth factor β1.51, 52, 56 The elevated leptin levels observed in obesity may thus initially prevent lipid accumulation in non-adipose tissues but, as disease progresses, may act detrimentally by promoting NASH and liver fibrosis.51
Important evidence supporting the causal relationship of liver fat accumulation with NAFLD progression is provided by genetic studies. Specifically, the presence of several variants in certain genes, such as the rs738409 C > G single nucleotide polymorphism of PNPLA3, the TM6SF2 E167K variant, and the GCKR rs780094, has been associated with increased liver fat accumulation because of repression of lipase activity,57 impairment of VLDL secretion,58 and increased glucose uptake and de novo lipogenesis, respectively.59 All these variants are associated at the same time with a profound increase of the risk for development of NASH and liver fibrosis as well as with liver-related events and all-cause mortality.60-63
3.2 Liver fat amount as marker of NAFLD status and progression in clinical studies
Because of accumulation of liver fat is the first step in a lengthy process that may lead to liver inflammation and fibrosis, it is reasonable to ask whether quantifying liver fat may serve as a prognostic marker of risk for development of NASH or liver fibrosis as well as whether treatments that aim to reduce liver fat can prevent disease progression. Specifically, identifying patients at high risk for NAFLD progression is extremely important because of the very high prevalence of the disease that makes general screening of the population and planning of regular follow ups in all patients with NAFLD challenging. Additionally, no approved treatment for NAFLD exists to date, and most efforts that aimed to improve advanced liver fibrosis because of NAFLD have failed so far. This indicates that probably interventions at an earlier stage of NAFLD and especially in high-risk populations for developing advanced liver fibrosis in the future may be needed. Furthermore, in contrast to assessment of liver inflammation, the accurate quantification of liver fat is possible with non-invasive imaging modalities, which supports the use of fat amount as a marker of disease state or treatment response; if indeed, it is strongly related to disease progression.
3.2.1 Liver fat amount as marker of biopsy-proven steatosis, inflammation, and fibrosis
Several clinical studies have demonstrated so far that changes in liver fat content assessed by MRI-PDFF correlate strongly with histologic changes of NAFLD. Specifically, a decline or increase of liver fat in MRI-PDFF of approximately 5–6% identified an improvement or worsening in steatosis grade in liver histology with 90% specificity and almost 60% sensitivity.64 In another study, the patients with improvement in steatosis grade in liver histology had a mean reduction of liver fat in MRI-PDFF of approximately 20% compared to 0.8% reduction in patients without improvement of steatosis grade.65 Similar findings have been reported also in children with NAFLD, where improvement or worsening of steatosis grade was detected with 90% specificity when fat percent in MRI PDFF was reduced by 11% or increased by 5.5% from the baseline, respectively.66 Importantly, apart from liver steatosis grade, changes in liver fat content correlate also strongly with alterations in NAFLD activity score and fibrosis stage in histology. Specifically, a meta-analysis including seven studies showed that reduction of more than 30% in liver fat was associated with 7 times higher probability for histologic response, defined as a 2-point improvement in NAFLD activity score with at least 1-point improvement in lobular inflammation or ballooning and 5.45 times higher probability for NASH resolution.32 In another study, more than 30% decline in liver fat assessed by MRI-PDFF was associated in 40% of the cases with fibrosis regression, 25% with no changes and 13% with fibrosis progression. The adjusted OR for fibrosis regression by more than 30% reduction in liver fat was 6.46.33 These findings are very important, because they justify the use of liver fat amount assessed by MRI-PDFF as a non-invasive marker of treatment response, especially in early-phase NASH clinical trials.32, 67, 68
3.2.2 Liver fat amount as marker of liver cirrhosis, failure, hepatocellular carcinoma, and liver-related mortality
Because liver fat accumulation is a crucial step for development and progression of liver fibrosis and reduction of liver fat correlates strongly with histologic improvement of steatosis, inflammation, and fibrosis, it would have been expected that liver fat amount will be also positively associated with liver-related outcomes, such as liver cirrhosis, liver failure, hepatocellular carcinoma, and mortality. However, studies have reported contradictory results. For example, in an important study that followed 95 patients with NAFLD prospectively for 1.75 years with paired liver biopsies, patients in the higher liver fat group (≥15.7% in MRI-PDFF) at the baseline had much more often fibrosis progression compared to subjects in the lower liver fat group (38.1% vs 11.8%). After adjusting for age, sex, ethnicity, and BMI, the higher liver fat group had a 6.7 times higher risk of fibrosis progression, suggesting that indeed higher liver fat content can predict progression of liver fibrosis.69 Similarly, liver fat was positively associated with overall mortality in another study including 129 patients with biopsy-proven NAFLD that were followed prospectively for more than 20 years.70 In contrast, several cross-sectional as well as longitudinal studies that assessed liver fat either histologically or with CAP reported that fat amount was either not associated with liver-related events and mortality or was even lower in patients with HCC, decompensated liver cirrhosis, or portal hypertension.71-75 This paradox is explained by the fact that the importance of liver fat for NAFLD progression may vary between the different stages of the disease. Initially, liver fat is an important contributor for development of hepatic inflammation and activation of fibrotic mechanisms. As disease progresses though to advanced fibrosis and cirrhosis, liver fat is reduced. This phenomenon has been described as “burned-out” NASH, and the mechanisms leading to it remain still unclear. It is suspected that an increase in adiponectin, which downregulates fatty acid synthesis and increases β-oxidation may lead to a reduction of liver fat in later stages of NAFLD.76 However, robust evidence that can explain this phenomenon is currently lacking. Nevertheless, this observation indicates that liver fat amount and its changes can be used as marker of NAFLD status and treatment response in the early or middle stages of the disease and not in advanced fibrosis or cirrhosis.
4 LIVER FAT AMOUNT AS RISK FACTOR OF CARDIOMETABOLIC DISEASES
Several studies have suggested that not only the presence of liver fat but also the amount of liver fat is associated with the development and progression of metabolic diseases and their complications.
4.1 Diabetes
Evidence from studies investigating NAFLD pathophysiology support the presence of an association between liver fat and diabetes. Specifically, increased liver fat accumulation through hepatic de novo lipogenesis as well as because of high caloric intake and increased lipolysis promotes hepatic resistance to insulin actions, thus leading to higher production of glucose and VLDL. This leads subsequently to mild hyperglycemia and compensatory hyperinsulinemia and hypertriglyceridemia, thus further aggravating insulin resistance and hyperglycemia.77, 78 Additionally, liver fat has been linked with proinflammatory changes in adipose tissue. Specifically, liver fat amount is positively associated with the percentage of macrophages and the protein levels of the proinflammatory adipokine plasminogen activator inhibitor-1 (PAI-1) in subcutaneous adipose tissue.79 The presence of a proinflammatory status is considered a hallmark for the development of insulin resistance that can progress to diabetes.
Hormones secreted predominantly or exclusively from the liver, that is, hepatokines, may serve as important mediators of the effects of fatty liver on insulin sensitivity, glucose, and lipid homeostasis (reviewed in Stefan et al.80). Among the numerous different hepatokines that have been investigated in NAFLD-diabetes context, more robust evidence exist for fetuins (fetuin-A and fetuin-B), angiopoietin-like proteins (especially ANGPTL3), fibroblast growth factor 21 (FGF-21), sex hormone-binding globulin (SHBG), and follistatin.80 These hormones may regulate hepatic or adipose-tissue insulin sensitivity by modulating inflammation (e.g., fetuin-A) and insulin signaling (e.g., fetuin-B, FGF-21, and follistatin).81-84 Furthermore, they may affect beta cell maturation or function (e.g., fetuin-A),82, 85 and they may regulate lipid metabolism by altering lipases activity (e.g., ANGPTL3) and hepatic lipogenesis (e.g., SHBG).86, 87 The levels of these hormones correlate in humans significantly not only with NAFLD stage but also with parameters of adiposity, insulin sensitivity, glucose, and lipid metabolism.81, 88-94
Multiple observational studies further reported an association not only of NAFLD but specifically of liver fat amount with risk of incident prediabetes or diabetes. Specifically, in a German cohort, the patients with hepatic steatosis assessed by MRI had 1.6, 3.3, 2.5, and 4.8 relative risk ratio to be in the impaired fasting glucose (IFG) group, in the impaired glucose tolerance group (IGT), in the combined IFG + IGT group, or to have undiagnosed T2DM, respectively.95 In a large meta-analysis including 19 observational studies with almost 300,000 individuals, the presence of NAFLD was associated with 1.8-fold higher risk of incident diabetes, which was further increased up to 2.6-fold in patients with more severe steatosis according to ultrasonography.96 Similarly, an FLI ≥ 60 was associated with twofold higher risk of prediabetes and ninefold to tenfold higher risk for T2DM in a population of individuals with overweight/obesity.97 Nasr et al. showed that each steatosis grade increase in liver biopsy was associated with a HR of 1.6 for development of T2DM after adjusting for age, BMI, and fibrosis stage.70 People with grade 3 steatosis demonstrated additionally higher mortality risk. Importantly, the elevated risk for development of diabetes was decreased in subjects in whom the liver fat amount was reduced in subsequent biopsies.70 In another recent study, liver fat amount assessed with MRI-PDFF (and not fibrosis markers) was associated with hepatic insulin sensitivity among patients with T2DM and obesity.98 An important question though is still whether the association between liver fat and development of diabetes is a causal one or whether liver fat is simply reflecting the overall metabolic health. In a recently published study that used over 30,000 MRI scans of UK Biobank participants and performed a mendelian randomization, each standard deviation (SD) increase of liver fat was associated with 27% higher risk of T2DM, thus supporting the presence of a causal role of ectopic fat in the liver with development of T2DM.99
4.2 Hyperlipidemia/dyslipidemia
Liver fat accumulation enhances VLDL oversecretion in NAFLD.100-102 Subsequently, VLDL oversecretion combined with reduced lipoprotein clearance leads to elevated plasma triglycerides.100-102 Extensive exchange between VLDL and LDL particles further supported by lipolysis of triglyceride-rich particles results in the increased formation of dense small LDL particles.100-102 These pathophysiological links between altered systemic lipid-lipoprotein profile and liver fat content have been confirmed in clinical studies.
Specifically, the prevalence of hypertriglyceridemia or dyslipidemia (high LDL-C with low HDL-C) is estimated to be approximately 70% in patients with NAFLD.103 Lipoprotein subfraction analyses suggested that NAFLD is characterized by high ratios of total cholesterol and triglyceride to HDL-cholesterol, by high ratio of apolipoprotein B to apolipoprotein A1, and by large VLDL particle size and decreased LDL and HDL particle size.104-106 Importantly, these changes are associated with increased risk of atherogenesis; they are observed regardless of BMI and they are mainly driven by higher liver fat content and not by the presence of liver inflammation.105 Finally, the relation of liver fat with hyperlipidemia/dyslipidemia has been also observed in children and adolescents.107 Specifically, higher liver fat, even within the normal range and after adjusting for BMI, was associated with higher insulin resistance, total cholesterol, and triglycerides.107 Similarly, liver fat was independently associated with triglycerides and insulin resistance in adolescents with obesity.108
4.3 Arterial hypertension
Multiple mechanisms have been suggested to be involved in the promotion of hypertension by NAFLD. These include the induction of (a) systemic inflammation by NAFLD through the release of cytokines and damage-associated molecular patterns (DAMPs) by injured hepatocytes9, 109; (b) insulin resistance by altered release profiles of glucose, bile acids, VLDL, and hormones (e.g., fetuin A and RBP4) in NAFLD; (c) gut dysbiosis in NAFLD which can regulate the differentiation and maturation of immune cells and reduce the production of short-chain fatty acids that have vasodilatory function110; and (d) directly increased vasoconstriction and decreased vasodilatation by increased ADMA release, as well as by the effects in the renin-angiotensin system.109
Many longitudinal studies have shown that the presence of NAFLD is associated prospectively with incident hypertension.111-113 Of note, most of the studies have used either surrogate markers (such as FLI, GGT, or transaminases), liver ultrasound, or both for diagnosing NAFLD. In a large prospective cohort including 1521 individuals that were followed for 2.6 years and after adjusting for insulin resistance, systemic inflammation, and adiponectin levels, FLI was associated in a graded manner with increased incident hypertension (<30 vs 30–59 vs ≥ 60 = 1 vs 1.83 vs 2.09, respectively).111 In another study that included 1051 subjects that were followed for 6.2 years, one SD increase of liver fat assessed by liver computed tomography was associated with 42% increased odds of incident hypertension.114 This increased risk was maintained after adjusting for different factors including visceral adipose tissue mass and BMI.114 Thus, not only the presence of NAFLD but also the amount of liver fat is related to the risk of arterial hypertension. Nevertheless, it is important here to mention that studies that have evaluated prospectively and in large cohorts the relationship between liver fat amount assessed by MRI-PDFF or liver histology and incident arterial hypertension are currently lacking.
4.4 Cardiovascular risk
Because there is a close pathophysiologic and epidemiologic link between components of metabolic syndrome and NAFLD, multiple studies have also assessed whether NAFLD increases cardiovascular risk. A recent meta-analysis of 36 longitudinal studies that included data on almost 6 million middle-aged individuals and almost 100 thousand cases of fatal and non-fatal CVD with a median follow up of 6.5 years have shown that NAFLD was associated with a 45% increased risk of CVD events after adjustment for multiple factors, such as BMI, age, sex, adiposity measures, hypertension, dyslipidemia, and pre-existing diabetes. This risk was especially high for patients with higher fibrosis stage.115 Other studies have suggested that the ratios of aspartate/alanine transaminase116 representing a marker of liver inflammation or FLI score, representing liver fat content, are predictors of cardiovascular events.117, 118 In this context, a large prospective analysis based on UK Biobank has reported that FLI between 30 and 59 is associated with 16% higher risk and FLI ≥ 60 with 25% increased risk for major cardiovascular events at a median follow-up of 11.62 years after adjusting for multiple factors including transaminases and presence of diabetes, hypertension, and statin therapy.117 FLI seems also to have an independent prognostic value for cardiovascular events in newly diagnosed, treatment-naïve hypertensive patients.119 Furthermore, in an analysis including more than 5 million young adults aged 20 to 39 years, FLI between 30 and 59 was associated with 28% increased risk of myocardial infarction and 18% of stroke, whereas FLI above 60 was associated with 73% and 41% increased risk, respectively.120
Apart from the association of liver fat content with components of the metabolic syndrome, several studies have linked liver fat with other surrogate markers of cardiovascular health. Specifically, liver fat has been associated with the calcification in the thoracic aorta and celiac trunk,121 with the descending and infrarenal aortic diameter,122 with plaque presence,122 as well as with the echogenicity and thickness of carotid intima-media.122-124 Additionally, liver fat assessed by FLI has been associated with 10-year Framingham risk score beyond other cardiovascular risk factors.125, 126 Moreover, larger amount of liver fat is correlated with larger volumes of epicardial fat and coronary artery calcification.127 Additionally, in another study, a decrease in liver fat was associated with a reduction in carotid intima-media progression.122
4.5 Dissociation between fatty liver and cardiometabolic diseases
Although the majority of epidemiologic and experimental evidence supports the presence of a detrimental relationship between liver fat and cardiometabolic diseases, there is a significant heterogeneity in the findings as well as reports for inverse associations, most probably reflecting the complex pathophysiology of NAFLD.80, 128 There are many reasons explaining the discrepancies between studies. First of all, quantification of liver fat relies primarily on triglyceride concentrations (in MRI and MRS) or percentage of cells containing intracellular lipid droplets (histology).67, 129 However, it is now accepted that triglyceride accumulation in the liver acts most probably protectively and not detrimentally.8, 80, 128, 130 Specifically, it may protect the liver from increased availability of fatty acyl-CoAs and formation of toxic lipids (lipotoxicity) that will stimulate inflammatory processes both locally (in the liver) and systemically.128 Furthermore, it seems that not only the amount but also the type of accumulated lipid species plays a role in liver inflammation, insulin resistance, and cardiometabolic risk. For example mono- or poly-unsaturated fatty acids act beneficially by reducing oxidative stress and inflammation, whereas saturated fatty acids, lysophosphatidylcholines, and ceramides act detrimentally by promoting cellular damage.131 Diacylglycerols (DAGs) and ceramides affect also insulin signaling leading to increased glucose production. According to other studies, not the abundance but the compartmentation especially of diacylglycerols in the membrane is an important contributing factor to hepatic insulin resistance.117 Similarly, not only the concentrations of lipid droplets but their protein and lipid composition, their intracellular localization, and their distribution–zonation in the liver affect their functional properties.132 Consequently, it may not be triglyceride accumulation per se, but rather the exhaustion of “detoxification” and fat storage capacities in the liver that contributes to inflammation, insulin resistance, and elevated cardiovascular risk. In that case, liver fat assessment serves only as a proxy marker of the on-going pathophysiologic processes. This may explain why many but not all patients with NAFLD demonstrate insulin resistance and increased cardiovascular risk.
The most representative examples of dissociation or inverse association between liver fat and cardiometabolic risk are the cases of hepatic-genetically driven fatty liver.133-135 Patatin-like phospholipase domain-containing 3 (PNPLA3) is a protein involved in triglyceride hydrolysis. The rs738409 variant (I148M) of PNPLA3 is the strongest genetic determinant of fatty liver disease. However, this polymorphism is associated with insulin resistance and type 2 diabetes only in obese and not in normal-weight populations.136 Moreover, the polymorphism was not causally associated to ischemic heart disease in a mendelian randomization study137 or it was even linked to lower risk for coronary artery disease in another large genetic study.138 These heterogeneous findings have been attributed to different pathophysiologic mechanisms, such as to less de novo lipogenesis and ceramide accumulation and higher concentrations of polyunsaturated triglycerides in people with this polymorphism.139, 140 Similarly, TM6SF2 protein is involved in triglyceride and lipoprotein secretion. The rs58542926 TM6SF2 C > T variant is associated with fatty liver because of increased intracellular lipid retention as well as with insulin resistance and diabetes.136, 138, 141 However, the increased retention of the lipids in the liver results in lower circulating total cholesterol levels and reduced risk for cardiovascular complications.138, 142 Finally, the rs1260326 of glucokinase regulator (GCKR) leads to increased glucose uptake in the liver and consequently to increased hepatic de novo lipogenesis. However, this polymorphism is associated with lower insulin resistance and reduced risk of type 2 diabetes, especially in European and Asian populations.143
Another example showing the dissociation between liver fat and cardiometabolic risk is the burned-out NASH. Specifically, as NAFLD progresses to its late stages of advanced fibrosis or cirrhosis, liver fat content decreases. However, these patients with advanced fibrosis or cirrhosis seem to demonstrate the highest cardiovascular risk.76, 115 This increased risk may though reflect the accumulated negative impact of chronic long-term exposure of the cardiovascular system to hepatic lipotoxicity and inflammation. Liver fat quantification in these advanced stages of NAFLD will thus not capture disease temporal dynamics and may not serve as a reliable proxy marker linking fatty liver with cardiovascular risk.
Apart from genetically related NAFLD and cases of advanced liver fibrosis/cirrhosis, significant variation in the association between NAFLD and cardiometabolic state further exists. This has been attributed to different mechanisms involved in NAFLD development. Specifically, some recent approaches focus on dissecting fatty liver from visceral obesity-associated insulin resistance.80 In a recent analysis, fetuin-A and adiponectin levels have been suggested to serve as main distinguishers between these two conditions.80 In other approaches, anthropometric and routine biochemical parameters have been used to identify clusters among patients with NAFLD that differ in cardiometabolic risk.144-146 Sex, age, obesity, dyslipidemia, and insulin resistance seem to be the most important factors for clustering, but it still remains unclear whether the parameters themselves and not liver phenotype are the main drivers of altered cardiovascular risk in these patients.
4.6 Limitations of current studies
Several limitations should be recognized. First, clinical studies that focus on hard outcomes (e.g., major cardiovascular events) and they have assessed liver fat either with MRI-PDFF or with liver histology are currently lacking. Second, it is difficult to conclude whether the observed associations imply also causality, as well as to dissect the contribution of liver fat in cardiometabolic derangement, because patients with higher liver fat content often suffer simultaneously from more than one cardiometabolic diseases. Furthermore, most studies so far have not acknowledged the heterogeneity of NAFLD population, which is particularly important given the robust differences of genetically versus non-genetically driven NAFLD in their pathophysiology, clinical course, and association with cardiometabolic risk factors. Here clustering approaches which aim to identify populations with different cardiometabolic risk profiles based on both clinical/anthropometric parameters as well as biochemical measurements (e.g., hepatokines) are very useful, but more and larger studies are needed. The same also applies for assessing whether insulin resistance induced by fatty liver demonstrates different cardiometabolic disease trajectories compared to insulin resistance deriving from visceral obesity.
Independently of the findings from clinical studies, there is also very limited information about the role of NAFLD in cardiovascular function from experimental animal studies. This is probably related to the lack of animal models in which NAFLD and severe atherosclerosis or heart insufficiency coexist. Mice demonstrate important differences in lipid metabolism compared to humans, including the lack of cholesteryl ester transfer protein (CETP) that transfers cholesteryl esters from HDL to LDL and VLDL. Thus, lipid transport is predominantly performed by HDL and not LDL in mice. Diet-induced mouse models of obesity and NAFLD do not develop severe atherosclerosis or severe heart insufficiency, whereas genetic models of atherosclerosis do not necessarily develop NAFLD with inflammatory and fibrotic components.
Altogether, numerous observational studies support the presence of a strong association between liver fat presence and amount with the development and progression of metabolic diseases. The strongest associations that imply also causality through mendelian randomization studies are observed between liver fat amount and the development of diabetes. Studies investigating the association of liver fat with hard cardiovascular outcomes, such as major cardiovascular events as well as prospective studies assessing how improvement of liver fat content may affect cardiovascular risk, are currently lacking and are thus needed.
5 PHARMACOLOGIC AND NON-PHARMACOLOGIC INTERVENTIONS FOR REDUCING LIVER FAT
Non-pharmacologic interventions for the reduction of liver fat and/or treatment of NAFLD include lifestyle modifications and bariatric procedures.
5.1 Lifestyle modification
The cornerstone of NAFLD therapy is lifestyle modification with weight loss and enhanced physical activity. A biopsy-based randomized controlled clinical trial over 52 weeks has shown that calorie reduction and physical activity improved steatosis in 65% of patients after 1 year with a weight loss of 5% to 7%.147 A weight loss of 7% to 9%, resulted in NASH resolution in 64%, and a weight loss of ≥10% led to a regression of fibrosis by at least one stage in 45% and stabilization of fibrosis in the remaining 55%.147
Clinically significant weight loss usually requires a reduction of 500–1000 kcal/d from the baseline or a hypocaloric diet with a target of 1200 kcal/d for women and 1400–1500 kcal/d for men. Several hypocaloric diets may be appropriate for weight loss in subjects with liver fat and/or NAFLD.148
The Mediterranean diet is the best studied diet in NAFLD. In contrast to the Western diet, which is rich in highly processed foods with a high content of saturated fatty acids and carbohydrates, the Mediterranean diet is rich in dietary fibers, monounsaturated and omega-3 fatty acids, and phytosterols.149 It is thought to reduce the risk and progression of NAFLD through an antioxidant and anti-inflammatory effect. Even in the absence of weight loss, a Mediterranean diet reduced hepatic steatosis compared with a low-fat, high-carbohydrate diet.150 In contrast to the Mediterranean diet, other hypocaloric diets, such as low-carbohydrate and high-protein diets, meal replacement protocols, or intermittent fasting, are not specifically recommended in NAFLD guidelines, as sufficient data on the effects on histologic NAFLD/NASH endpoints are still lacking.40
The beneficial effect of a hypocaloric diet on liver steatosis and NAFLD may be enhanced by physical activity. Furthermore, it may improve NAFLD independently from achieving weight loss by reducing hepatic fat content and leading to a reduction of lipolysis in adipocytes, free fatty acid delivery to the liver, hepatic de novo lipogenesis, and an improvement in peripheral insulin sensitivity.151, 152 A systematic review and meta-analysis demonstrated that predominantly aerobic exercise resulted in a significant reduction in liver fat content even in the absence of dietary interventions.153 This has been challenged in a recent study which aimed to assess whether alternate day fasting and exercise alone or in combination are more efficient at reducing intrahepatic triglyceride content. Exercise alone provided rather modest reduction in liver fat (−1.3%), compared to alternate-day fasting alone (−2.25%) or to their combination (−5.48%).154 Nevertheless, it is recommended to perform 150–300 min of moderate-intensity exercise or 75–150 min of high-intensity exercise per week.152 However, lifestyle modifications aiming at weight loss remain challenging in clinical practice. Data from the non-interventional FLAG registry could show that less than 50% of patients with a mean BMI of 30 kg/m2 who received lifestyle advice achieved any weight loss after 12 months, and only 17.1% achieved more than 5%.155
5.2 Bariatric procedures
In patients with grade III obesity (BMI ≥ 40 kg/m2) or grade II obesity (BMI ≥ 35 kg/m2) with metabolic comorbidities where lifestyle modifications fail, bariatric surgery should be considered.156 The most commonly performed procedures are laparoscopic Roux-Y gastric bypass surgery and laparoscopic sleeve gastrectomy.156 A meta-analysis of 15 studies with 766 paired liver biopsies showed a pooled proportion of patients with improvement or resolution of steatosis in 91.6% (95% confidence interval [CI], 82.4–97.6%), in steatohepatitis in 81.3% (95% CI, 61.9–94.9%), and in fibrosis in 65.5% (95% CI, 38.2–88.1%).157 A recent meta-analysis of 37 studies with liver biopsy before and after bariatric surgery confirmed these observations. Resolution of steatosis was seen in 56% of patients with NAFLD, ballooning degeneration in 49%, inflammation in 45%, and fibrosis in 25%. Although the effects of bariatric surgery on histologic features of NAFLD (e.g., steatosis) are very promising, not all patients seem to benefit. Twelve percent of patients (95% CI, 5–20%) showed new or worsening features of NAFLD, such as fibrosis.158
Because of the invasiveness of bariatric surgery, endoscopic bariatric therapies in NAFLD have gained interest in the recent years. A prospective study with 21 patients with NASH and early hepatic fibrosis using endoscopically placed gastric balloons as part of an intensive lifestyle program showed a weight loss of 11.7% and significant improvements in histologic NASH activity after 6 months. At the baseline, liver steatosis grades 0/1/2 were present in 5%/67%/28%, respectively, and improved significantly after 6 months (grade 0: 55% and grade 1: 45%). Fibrosis stage improved in 15%, remained stable in 60%, and worsened in 25%.159
A recent meta-analysis including 18 studies with 863 patients on the effects of various endoscopic bariatric and metabolic therapies on NAFLD observed an average weight loss of 14.5% after 6 month of follow-up. Liver fat (measured non-invasively with different methods) and fibrosis significantly reduced by standardized mean difference of 1.0 (CI, −1.2, −0.8, p < 0.0001) and 0.7 (95% CI, 0.1,1.3, p = 0.02), respectively.160 To support weight reduction and improvement of steatosis in the short-term, endoscopic bariatric therapies seem promising; however, studies on the long-term effect and safety are currently lacking.
5.3 Pharmacologic intervention
Currently, there are no approved pharmacologic interventions for the reduction of liver fat and/or the treatment of NAFLD. However, numerous drug therapy approaches are in advanced phases of clinical trials.161 A selection of current phase III trials can be found in Table 2. Regulatory endpoints for NAFLD/NASH clinical trials in advanced stage development focus on the histological endpoints of NASH resolution without worsening of fibrosis or fibrosis improvement of at least one stage without worsening of NASH. Thus, the effects on steatosis alone as a marker of early-stage disease are not the primary goal. In the following, we will highlight the most promising classes of medications for the treatment of NAFLD and of steatosis.
| Drug; study name; (ClinicalTrials.gov identifier) | Study population | Mode of action; effects | Primary endpoint |
|---|---|---|---|
|
Obeticholic acid (OCA); REGENERATE [NCT02548351] REVERSE [NCT03439254] |
NASH, F1–3 Compensated cirrhosis |
FXR agonist; Bile acid synthesis ↓, Lipogenesis ↓, Fatty acid oxidation ↑, Gluconeogenesis ↓ | Resolution of NASH without worsening of fibrosis or fibrosis improvement without worsening of NASH; all-cause mortality and liver-related outcome (HCC, LTx). |
|
Resmetirom; MAESTRO-NASH [NCT03900429] MAESTRO-NAFLD [NCT04197479] |
NASH, F2/3 presumed NASH, F1–F4 (non-invasively measured, recruitment only USA) |
β-selective thyroid hormone receptor (THR) agonist; Lipogenesis ↓, Fatty acid oxidation ↑ | Resolution of NASH without worsening of fibrosis; all-cause mortality and liver-related outcome (HCC, LTx) |
|
Semaglutide; ESSENCE [NCT04822181] |
NASH F2/3 | GLP-1R agonist; Blood glucose control, weight ↓↓, cardiovascular events ↓ | Resolution of NASH and fibrosis improvement ≥1 stage by week 72 |
|
Lanifibranor; NATiV3 [NCT04849728] |
NASH F2/3 | Pan-PPAR (α/δ/γ) agonist; Steatosis ↓ (PPAR α), Inflammation ↓ (PPAR δ), Fibrosis ↓ (PPAR γ) | Resolution of NASH and fibrosis improvement ≥1 stage by week 72 |
|
Aramchol; ARMOR [NCT04104321] |
NASH F2/3 | Fatty acid-bile acid conjugate; Steatosis ↓, Collagen production in hepatic stellate cells (HSC) ↓ | Resolution of NASH without worsening of fibrosis or fibrosis improvement without worsening of NASH; all-cause mortality and liver-related outcome (HCC, LTx) |
|
Belapectin; NAVIGATE (IIb/III) [NCT04365868] |
Compensated cirrhosis without esophageal varices | Galectin-3 inhibitor; Inflammation ↓ Fibrosis ↓ | Emergence of varices, Safety |
- Abbreviations: NASH (non-alcoholic steatohepatitis), NAFLD (non-alcoholic fatty liver disease), FXR (farnesoid X receptor), HCC (hepatocellular carcinoma), LTx (liver transplantation), PPAR (peroxisome proliferator-activated receptor).
5.3.1 GLP-1 receptor agonists (GLP-1RA)
GLP-1RA are routinely being used for the treatment of T2DM and obesity162-165 Treatments with liraglutide, dulaglutide, or semaglutide have shown beneficial hepatic effects in mouse models of NAFLD, in which they have profoundly reduced hepatic fat and inflammation and modestly liver fibrosis.166-168 GLP-1RA have been shown to facilitate glycogen utilization turnover in the liver and affect inflammatory and fibrogenic markers of Kupffer and hepatic stellate cell activation.167, 168 The effects of GLP-1RA are most likely indirect, because GLP-1 receptors have not been convincingly identified in the liver, and are mediated by the profound weight loss and improvement of insulin sensitivity observed with GLP-1RA treatment. In humans, GLP-1RA (mostly liraglutide and semaglutide) improved steatosis, ballooning, and lobular inflammation, and achieved resolution of NASH without worsening of fibrosis.10, 169 Specifically for semaglutide, in a NASH phase II trial over 72 weeks, resolution of NASH with no worsening of fibrosis was observed in 56% of patients on 0.4 mg/d compared with 20% on placebo. The mean percent weight loss was 13% in the 0.4 mg group and 1% in the placebo group. The odds ratio for having a lower steatosis score in histological assessment after 72 weeks was 8.55 (4.49; 16.30) in the semaglutide 0.4 mg group compared to placebo, indicating significant effects of semaglutide on steatosis regression.170 Semaglutide is currently tested in a phase III NASH trial.
5.3.2 Dual or triple agonists (GLP-1, GIP, and glucagon agonists)
Dual or triple agonists of GLP-1, GIP, and/or glucagon receptor are currently being developed for the treatment of obesity and diabetes. Consequently, these medications are also tested regarding their efficacy in NAFLD. GLP-1/GIP co-agonists are in more advanced stages of clinical development, followed by GLP-1/Glucagon co-agonists, whereas triple agonists are in earlier stages. Tirzepatide, which is a GLP-1/GIP co-agonist, has been recently approved by the Food and Drug Administration (FDA) for the treatment of T2DM. Tirzepatide has been reported to be superior to semaglutide in reducing HbA1c and weight.171 In patients with T2DM, Tirzepatide reduces significantly liver transaminases.172 Additionally, it reduces significantly liver fat content (assessed with MRI) compared to insulin degludec.173 A current phase II trial is evaluating the effect of Tirzepatide versus placebo in liver histology in patients with NASH. Hepatic glucagon receptor signaling is important for fatty acid oxidation, triglyceride synthesis, and secretion. Treatment with glucagon receptor agonists reduces de novo lipogenesis, enhances mitochondrial biogenesis, and restores basal respiratory rates in hepatocytes in mice with NASH.174, 175 Several medications are under development with cotadutide showing beneficial effects in reducing liver transaminases and fibrosis markers in patients with overweight/obesity and T2DM.176
5.3.3 SGLT2 inhibitors (SGLT2i)
SGLT2i are established treatments for T2DM as well as for cardiac insufficiency.177 In mouse models of NASH, several SGLT2i have shown beneficial effects on reducing liver steatosis and inflammation by altering hepatic lipid composition (i.e., reducing the concentrations of pro-inflammatory lactosylceramides and increasing the concentrations of anti-inflammatory polyunsaturated triglycerides),178 by activating autophagy and reducing ER stress and apoptosis,179 by reducing de novo lipogenesis, and by inhibiting inflammatory response by blocking the NF-kB pathway.180 A meta-analysis of randomized clinical trials has shown that SGLT2i reduce liver transaminases. Additionally, they reduce by 2.05% the absolute percent of liver fat content assessed with MRI.181 An ongoing phase III clinical trial is evaluating the effect of dapagliflozin in patients with NASH and T2DM. Similarly, another trial is assessing the effects of empagliflozin alone or in combination with semaglutide versus placebo in patients with T2DM, NASH, and liver fibrosis.
5.3.4 PPAR agonists
PPARs are ligand-activated transcription factors of nuclear hormone receptor superfamily with three subtypes: PPARα, PPARγ, and PPARβ/δ. In a highly simplified description regarding the effects on NASH in mouse models, activation of PPARα acts anti-steatotic, of PPARγ anti-fibrotic, and of PPARβ/δ anti-inflammatory.182, 183 PPARγ agonists (e.g., Pioglitazone) were originally developed as treatments of T2DM because of their insulin-sensitizing effects, but they were also the first that were tested in humans with NAFLD. Pioglitazone in several studies managed to improve steatosis grade, inflammation, and ballooning, whereas it had mild effects on liver fibrosis.184 Because of side effects, pioglitazone has though not been extensively used in the daily clinical routine. Novel selective PPARγ agonists that are equally potent but have less side effects are under development and have shown beneficial hepatic effects in mouse models of NASH.183 On the other hand, the PPARα/δ agonist elafibranor has failed to demonstrate beneficial effects on NASH in a phase III clinical trial. Thus, current treatments that target all the PPAR subtypes to maximize hepatic benefit are currently under evaluation. The most promising one is lanifibranor, a pan-peroxisome proliferator-activated receptor (PPAR) agonist. In a phase IIb trial over 24 weeks, (n = 247) lanifibranor met its primary endpoint of a reduction of two points or more on the steatosis-activity-fibrosis (SAF) score, with no increase in fibrosis, in 49% of patients on 1200 mg/d compared with 27% on placebo.185 Improvement of steatosis of at least ≥1 point decrease between baseline and week 24 was seen in 55.4% (95% CI 44.1%; 66.3%) on 800 mg, in 65.1% (95% CI 53.8%; 75.2%) on 1200 mg, and in 25.9% (95% CI 16.8%; 36.9%) on placebo, indicating promising effects on steatosis with a short-term treatment.185 A phase III trial on NASH is ongoing.
5.3.5 Farnesoid X receptor agonists (Obeticholic acid)
Obeticholic acid (OCA) is a selective farnesoid X receptor (FXR) agonist. FXR is a ligand-activated transcription factor involved in the control of bile acid synthesis and is also central to a number of pathways in the liver, affecting glucose and lipid metabolism as well as inflammation and fibrosis.186 In an ongoing phase III trial in NASH, an 18-month interim analysis has shown that OCA met the endpoint of improvement by at least one stage in fibrosis with no worsening of NASH but did not meet the endpoint of NASH resolution. A ≥ 1-point improvement in steatosis in liver biopsy was seen in placebo versus OCA 25 mg in 38% and 41% (p = 0.40) in the ITT population (n = 931) and in 43% versus 52% (p = 0·072) in the per-protocol population (n = 668), respectively.187 Although OCA seems to have effects on steatosis regression, they appear to be relatively small and not significant. Additionally, OCA increases total cholesterol and LDL cholesterol and decreases HDL cholesterol by altering LDL and HDL particles concentrations.188, 189 The unfavorable increase in circulating cholesterol results from the inhibition of conversion of cholesterol to bile acids by OCA and consequently from cholesterols' increased availability. The approval of OCA for the treatment of NASH is awaited in the near future.
5.3.6 Thyroid hormone receptor beta agonists (Resmetirom)
Thyroid hormone receptor beta (THR-β) is crucial for liver homoeostasis, through multiple metabolic actions and has been shown to improve lipid metabolism. Resmetirom (MGL-3196) is a liver-directed, orally active, selective THR-β agonist designed to improve NASH by increasing hepatic fat metabolism and reducing lipotoxicity.190 In a phase II NASH trial over 36 weeks, resmetirom-treated patients showed a significant reduction of hepatic fat in MRI-PDFF compared with placebo at week 12 (−32·9% resmetirom vs – 10·4% placebo; p < 0·0001) and week 36 (−37·3% resmetirom vs – 8·5 placebo; p < 0·0001). Resmetirom has shown significant effects on steatosis resolution and is currently tested in two phase III clinical trials (MAESTRO-NASH and MAESTRO-NAFLD). An interim analysis from the MAESTRO-NAFLD trial over 52 weeks in NASH with compensated cirrhosis (n = 105), which recruited only in the United States, has recently been presented at the International Liver Congress 2022. At week 52, resmetirom lowered Fibroscan® CAP by −42 dB/m (p < 0.0001), liver stiffness by −7.6 kPa (p = 0.02), MR elastography by 0.68 kPa, baseline MRI-PDFF >5% by 37% (p < 0.0057), LDL-cholesterol by −20%, and ApoB by −20%.191 Similarly, positive results from an interim analysis of the MAESTRO-NASH study have been recently announced in a press release by the pharmaceutical company developing resmetirom.192 Resmetirom at 100 mg led to NASH resolution without worsening of fibrosis in 30% of patients (compared to 10% in placebo group) and to improvement of at least one stage in fibrosis without worsening of NAS in 26% of patients (compared to 14% in placebo group). The MAESTRO-NAFLD phase III trial has two important features: it is the first NASH phase III trial with data on compensated liver cirrhosis and primary as well as key secondary endpoints were measured non-invasively. If the latter will be accepted by regulatory authorities for future phase III approval studies, performing of NASH clinical trials will be greatly simplified, as liver biopsy will no longer be needed.
5.3.7 Aramchol
Aramchol, a partial inhibitor of hepatic stearoyl-CoA desaturase (SCD1), improved steatohepatitis and fibrosis in rodents and reduced steatosis in an early clinical trial. It has been tested in a phase IIb trial over 52 weeks including 247 patients with NASH. Aramchol 600 mg/d demonstrated a placebo-corrected decrease in liver triglycerides measured by MR spectroscopy of −3.1 (95% CI −6.4 to 0.2, p = 0.066); however, it did not meet the prespecified significance level.193 Nevertheless, the observed safety and changes in liver histology and enzymes provided a rationale for evaluating aramchol in an ongoing phase 3 program.
6 CONCLUSIONS – PERSPECTIVES
Multiple evidence support that the presence and amount of liver fat are relevant factors for the development and progression of NAFLD and of metabolic diseases. From pathophysiological point of view, liver fat accumulation is the first step for the impairment of hepatic function that can lead to liver inflammation and fibrosis. Changes in liver fat amount correlate strongly with NASH progression or regression and can thus be used for assessing drug efficacy in early-phase NASH clinical trials. Moreover, liver fat amount is strongly and (based on Mendelian randomization studies) causally related with the risk of diabetes. Liver fat amount is also associated with several cardiovascular and cardiometabolic markers, albeit causality has to be further assessed in future studies. Regarding the available diagnostic tools, blood-based tests can be useful for excluding the presence of steatosis. Ultrasound is sensitive at detecting moderate to severe steatosis, and MRI-PDFF is very accurate at quantifying liver fat amount. Bariatric procedures are highly effective at reducing liver fat as well as liver inflammation and fibrosis. Lifestyle interventions and especially Mediterranean diet and exercise are effective at reducing liver fat even at the absence of severe weight loss. Finally, several medications currently under evaluation in Phase III clinical trials have shown beneficial effects in reducing liver fat amount, inflammation, and, in some cases, fibrosis.
Points that we consider as highly relevant for further investigation in future studies include: (a) the development of blood-based tests that will be able to quantify accurately liver fat and thus being used for rapid diagnosis and monitoring disease progression or treatment response especially in primary care setting; (b) the investigation of the association of liver fat and NAFLD status with hard cardiovascular outcomes, such as major cardiovascular events as well as the assessment of the effect of liver fat content decrease with reduction of cardiovascular risk prospectively; (c) identification of possible independent pathways linking liver fat accumulation with cardiovascular and metabolic function through mechanistic studies. For this purpose, development of animal models with combined presence of NAFLD, atherosclerosis, and/or heart insufficiency are needed.
ACKNOWLEDGMENTS
The current study was funded by transCampus Dresden – King's College London Science to Business Initiative funded from Deutsche Forschungsgemeinschaft (DFG) [C6] and by Bundesministerium für Bildung und Forschung (BMBF) – Deutsches Zentrum für Diabetesforschung (DZD e.V.). Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST STATEMENT
DM reports research grant from Intercept, payment for lectures from Intercept, Gilead, MSD, Dr. Falk, support for attending meetings from Gilead, participation in the advisory board of Gilead and Echosens. SRB reports participation in the advisory board of Boehringer Ingelheim and speaker honoraria from Novo Nordisk, Boehringer Ingelheim. NP reports consulting fees and participation in the advisory board from Bayer Vital GmbH and speaker honoraria from Novo Nordisk and GWT-Dresden outside the submitted work. CSM reports grants through his institution from Merck, Massachusetts Life Sciences Center, and Boehringer Ingelheim. He has been a shareholder of and has received grants through his Institution and personal consulting fees from Coherus Inc. and AltrixBio. He reports personal consulting fees from Novo Nordisk; reports personal consulting fees and support with research reagents from Ansh Inc., collaborative research support from LabCorp Inc.; reports personal consulting fees from Genfit, Lumos, Amgen, Corcept, Intercept, 89 Bio, Madrigal, and Regeneron; reports educational activity meals through his institution or national conferences from Esperion, Merck, Boehringer Ingelheim, and travel support and fees from TMIOA, Elsevier, and the Cardio Metabolic Health Conference. None is related to the work presented herein.




