Therapeutic Role of Probiotics for the Treatment of Dyspepsia: A Review of the Literature
Funding: The authors received no specific funding for this work.
ABSTRACT
Background
Dyspepsia is a common condition with a high prevalence in the general population. Patients in whom traditional diagnostic procedures can detect no identifiable explanation for the symptoms are diagnosed as being affected by functional dyspepsia (FD). To date, no etiological therapy for FD is available, and the current management includes general measures, acid-suppressive drugs, prokinetic agents, fundus-relaxing drugs, antidepressants, and psychological interventions. Recent evidence suggests that microbiota imbalance is involved in the development of FD. As a consequence, the modulation of microbiota through the use of probiotics could represent an effective therapeutic strategy. Moreover, Helicobacter pylori (HP) infection is a frequent cause of dyspepsia, and patients diagnosed with HP-associated dyspepsia are treated with HP eradication. In this regard, probiotics supplementation may also be helpful for HP infection to increase the eradication success rate as well as to reduce gastrointestinal adverse events caused by antibiotics.
Purpose
This review of the literature aims to summarize and discuss the current evidence on the use of probiotics in the treatment of dyspepsia and as a supplement to HP eradication therapy.
Summary
- Recent studies suggest that microbiota imbalance plays a significant role in the development of functional dyspepsia (FD).
- Specifically, patients with FD have shown increased levels of Streptococcus and decreased levels of Prevotella, Veillonella, and Actinomyces.
- Several clinical trials have reported promising efficacy of probiotics in restoring microbiota balance and alleviating FD symptoms.
- Additionally, probiotics may enhance Helicobacter pylori (HP) eradication rates and reduce gastrointestinal side effects associated with antibiotics.
- Meta-analyses indicate that specific probiotic strains, such as Lactobacillus and Bifidobacterium, improve eradication success and patient compliance.
1 Introduction
Dyspepsia is defined according to the Rome IV criteria as any combination of the following 4 symptoms: postprandial fullness, early satiety, epigastric pain, and epigastric burning that are severe enough to interfere with the usual activities and occur at least 3 days per week over the last 3 months with an onset of at least 6 months in advance [1]. Dyspepsia can be initially classified into three groups depending on whether upper gastrointestinal endoscopy has been performed and, if so, whether relevant pathology is detected: uninvestigated dyspepsia, organic dyspepsia, and functional dyspepsia (FD). After an accurate history taking and physical exam, in the absence of alarm symptoms and signs, patients are diagnosed as being affected by uninvestigated dyspepsia and can be treated empirically on the basis of their clinical manifestations [1]. Uninvestigated dyspepsia prevalence varies between countries and ranges from 17.6% in studies defining uninvestigated dyspepsia according to Rome I criteria to 6.9% in those using Rome IV criteria [2]. If the response is unsatisfactory or early relapses occur, a test and treat approach for Helicobacter pylori (HP) infection is recommended [3]. A recent systematic review estimated that more than 4 billion individuals would be positive for HP [4]. Symptoms of patients diagnosed with HP-associated dyspepsia are treated by HP eradication [5]. Moreover, patients undergoing endoscopy for suspected organic dyspepsia are often diagnosed with pathological findings that may be responsible for the symptoms, such as peptic ulcer, gastritis, or many other gastrointestinal or systemic causes [6].
Patients in whom no identifiable explanation for the symptoms can be detected by traditional diagnostic procedures are diagnosed as being affected by FD. A recent global survey investigating the worldwide prevalence of dyspepsia showed that FD was the most prevalent gastroduodenal disorder, with a pooled prevalence rate between 4.8% and 7.2% [7]. Functional dyspepsia can be further classified into two subtypes: postprandial distress syndrome (PDS), which is characterized by postprandial fullness and early satiety, and epigastric pain syndrome (EPS), which is characterized by epigastric pain and epigastric burning [8]. Putative pathophysiological mechanisms underlying FD are dysregulation of the gut–brain axis and immune system dysfunction, delayed gastric emptying, impaired gastric accommodation to a meal, visceral hypersensitivity to gastric distension, altered duodenal sensitivity to lipids or acids, altered antro-duodenojejunal motility and gastric electrical rhythm, and unsuppressed postprandial phasic contractility in the proximal stomach [9]. Pathogenetic factors in FD are genetic predisposition, infection from HP or other organisms, inflammation, and psychosocial factors [9].
The current management of uninvestigated dyspepsia mostly includes general measures, acid-suppressive drugs, and prokinetic agents. First and subsequent lines of therapy for HP-related dyspepsia, instead, rely on a course of antibiotics based on local patterns of resistance, followed by the confirmation of HP eradication [5]. Once FD is diagnosed after ruling out HP infection and organic diseases, treatment primarily involves acid-suppressive therapy, prokinetics, fundus-relaxing agents, antidepressants, and psychological interventions, especially if these options have not been previously attempted [10]. Recent evidence suggests that microbiota imbalance is involved in the development of FD [11, 12]. Therefore, modulating the gut microbiota through the use of probiotics could represent an effective further therapeutic strategy. Probiotics are defined according to the World Health Organization as live microorganisms which, if administered in adequate amounts, can confer health benefits to the host [13]. Probiotics are able to escape the effects of gastric acid, bile, and pancreatic juice, thus colonizing the host's gastrointestinal tract and rebalance the intestinal microbiota [13]. This review of the literature aims to summarize and discuss the current evidence on the use of probiotics in the treatment of dyspepsia.
2 Probiotics and Helicobacter pylori
Helicobacter pylori infection provokes chronic mucosal inflammation in the stomach and duodenum, which, in turn, might lead to gastroduodenal motor and sensory dysfunction, and it has also been linked with dyspeptic symptoms [14] Its eradication is essential to solve the related symptoms, but probiotics have been shown to play a role. Since there has been a decrease in eradication rates due to increasing antibiotic resistance and more frequent side effects due to the addition of several antibiotics for a prolonged time [5] the use of probiotics has been studied together with alternative therapies. The first study on probiotics and HP was carried out in 1995, highlighting that Lactobacillus acidophilus and one strain of Lactobacillus rhamnosus were found to have in vitro inhibitory activities on HP growth [15].
Subsequently, several studies explored the role of probiotics as an additional therapy for HP infection [5, 16-18]. There is an open debate on the real impact of probiotic use in HP treatment in clinical practice, advising their use only for specific strains supported by a previously demonstrated clinical efficacy for both reducing gastrointestinal adverse events caused by antibiotics and improving the HP eradication rate. These effects seem to be achieved through enhancing compliance with the therapy [16, 17, 19]. Two recent meta-analyses [16, 19] reported significantly increased eradication rates (Odds ratio (OR) 2.07; 95% confidence interval (CI), 1.40–3.06) and reduced side effect rates (OR 0.31; 95% CI, 0.12–0.79) during concomitant probiotic treatment to antibiotics therapy for HP eradication. Of note, the evaluation of the microbial shifts after HP treatment highlighted variations in the gut microbiome of Bacteroides, Bifidobacterium, Clostridium, Enterobacteriaceae, and Lactobacillus [20] strains. Since this emerging evidence suggests that not all probiotics are useful against HP infection and only a few strains seem to play a positive role in eradication treatment, the most investigated probiotic strains in this setting were the Lactobacillus and Bifidobacterium species [21]. However, there are still many questions to be answered on the role of probiotic supplementation in HP treatments, including the optimal dose and the duration of the therapy.
2.1 Rationale for the Use of Probiotics During H. pylori Therapy
Probiotics act as antagonists to HP by food and binding sites competition [22]. Indeed, a previous ex vivo study demonstrated reduced colonization ability by HP according to the evaluation of rectal swabs, stomach homogenates, and luminal contents from ileum and colon in germ-free mice previously colonized by probiotics (L. rhamnosus, strain R0011, and L. acidophilus, strain R0052) [23]. Notably, in another study, certain probiotic strains such as Saccharomyces boulardii showed interference with HP adhesion to gastric and duodenal cells due to its neuraminidase activity [24] selective for a(2–3)-linked sialic acid, able to remove surface a(2–3)-linked sialic acid, which is the ligand for the sialic acid-binding HP adhesin. Moreover, probiotics can enhance mucus production by increasing the expression of MUC2 and MUC3 as shown for L. plantarum and L. rhamnosus strains [25] and epithelial junction proteins production [26]. These effects, together with the secretion of bioactive molecules and the production of IgA acting against pathogens, can strengthen the gastric mucosal layer and increase the resistance to HP colonization [25]. Moreover, probiotics themselves secrete antimicrobial agents derived from fermentation, such as acetic and lactic acid, which are able to lower the intragastric pH and inhibit urease activity [27].
2.2 Evidence for the Use of Probiotics Alone for H. pylori Eradication
Few studies evaluated the effect of probiotics alone in the eradication of HP, all using different probiotic strains, and only a few of them included an adequate sample size [28-31]. Moreover, most of them were conducted on children [32] since the use of triple therapy in pediatric patients is associated with overall lower efficacy and increased risk of antibiotic resistance in this population [33]. The first study [34] in adults evaluating the role of probiotics (L. acidophilus La1) in combination with proton pump inhibitor (PPI) therapy failed to achieve eradication of HP and reported only a diminished bacterial load after treatment.
Several meta-analyses aiming at the evaluation of the role of probiotics in HP eradication [16, 35-37] concluded that although probiotic therapy reduces the HP bacterial load, this evidence does not justify their use as monotherapy. Indeed, a recent meta-analysis on probiotic monotherapy [38], including 11 studies and 403 patients, reported a mean eradication rate of 14%, with minimal variations across different strains (i.e., 16% for Lactobacilli, 12% for Saccharomyces boulardii and 14% for multi-strain combinations).
2.3 Probiotics Concomitant Treatment With Antibiotics
Several studies and meta-analyses [16, 19, 35-37, 39-45] evaluated the adjuvant role of probiotics in addition to antibiotic therapies for HP (Table 1). Most of them evaluated the role of probiotics as a whole without distinguishing among differential bacterial strains or antibiotic regimens [19, 39]. Various regimens have been proposed over the years as first- and second-line therapy for HP.
| Author, year | Trials (patients) | Age | H. pylori eradicating regimens | Probiotics | Results |
|---|---|---|---|---|---|
| Tong 2006 | 14 RCTs (n = 1671) | Any age | Triple therapy | Several probiotic strategies |
Pooled eradication rates with and without probiotics 83.6% (95% CI 80.5%–86.7%) and 74.8% (95% CI 71.1%–78.5%). OR 1.84 (95% CI 1.34–2.54) |
| Zou 2009 | 8 RCTs (n = 1372) | Any age | Triple therapy | Lactobacillus |
Pooled eradication rates with and without probiotics were 82.26% (95% CI = 78.01%–86.51%) and 76.97% (95% CI = 73.11%–80.83%) OR 1.78 (95% CI = 1.21–2.62) |
| Szajewska 2010 | 5 RCTs (n = 1307) | Any age | Triple therapy | Saccharomyces boulardii | RR 1.13, 95% CI 1.05–1.21 |
| Zheng 2013 | 9 RCTs (n = 1163) | Any age | Triple and sequential therapy | Lactobacillus | RR 1.14, 95% CI 1.06–1.22 |
| Wang 2013 | 10 RCTs (n = 1469) | Any age | Triple therapy | Lactobacillus- and Bifidobacterium-containing probiotics | OR 2.066, 95% CI 1.398–3.055 |
| Li 2014 | 7 RCTs (n = 508) | Children | Triple therapy | Several probiotic strategies |
Probiotic use ITT OR 1.96 (95% CI 1.28–3.02) PP OR 2.25 (95% CI 1.41–3.57) |
| Dang 2014 | 33 RCTs (n = 4459) | Any age | First line treatment (triple, sequential, quadruple therapy) | Several probiotic strategies |
ITT RR 1.122, 95% CI 1.086–1.159 PP RR 1.114, 95% CI 1.070–1.159 |
| Zhu 2014 | 14 RCTs (n = 2259) | Adults | Triple therapy | Several probiotic strategies |
ITT OR 1.67 (95% CI 1.38–2.02) PP OR 1.68 (95% CI 1.35–2.08) |
| Zhang 2015 | 45 RCTs (n = 6997) | Any age | Any eradicating regimens | Several probiotic strategies |
Eradication rates with and without probiotics were 82.31% and 72.08%. PP RR 1.11, 95% CI: 1.08–1.15, ITT RR 1.13, 95% CI: 1.10–1.16 |
| Gong 2015 | 23 RCTs (n = 3900) | Subjects > 14 years old | Triple therapy | Several probiotic strategies |
Pooled eradication rates without and with probiotics were 1464/2026 (72.26%; 95% CI, 67.66%–74.13%) and 1513/1874 (80.74%; 95% CI, 74.68%–82.76%). OR = 0.58; 95% CI, 0.50–0.68. |
| Lv 2015 | 21 RCTs (n = 3814) | Any age | Triple therapy | Lactobacillus, Bifidobacterium, Saccharomyces or a mixture of the three |
The pooled eradication rates of the probiotic group were 80.3% (1.709/2.128) by ITT and 83.8% (1.709/2.039) by PP analyses. ITT RR 1.12, 95% CI 1.06–1.19. |
| Lu 2016 | 13 RCTs (n = 2306) | Adults | Any eradicating regimens | Several probiotic strategies |
Probiotic use RR 1.15, 95% CI 1.10–1.20 |
| Lau 2016 | 30 RCTs (n = 4515) | Any age | Triple therapy | Several probiotic strategies | RR 1.122, 95% CI 1.091–1.153 |
| Lu 2016 | 21 RCTs (n = 3520) | Adults | Triple therapy | Several probiotic strategies |
ITT OR 1.21, 95% CI: 0.86 1.69 PP OR 1.28, 95% CI: 0.88, 1.86 |
| McFarland 2016 | 19 RCTs (n = 2730) | Any age | Double, triple, or quadruple therapy | Multi-strain probiotics (mixture of 2 or more different strains of bacteria or fungi) | RR 1.12, 95% CI 1.08–1.17 |
| Wang 2017 | 140 studies (n = 20,215) | Adults | Triple, sequential, or quadruple therapy | 10 probiotic strategies | Eradication rate 84.1% in probiotic group versus 70.5% in control. |
| Wen 2017 | 17 RCTs (n = 1932) | Children | 14-days triple therapy | Several probiotics strategies |
Probiotic use RR: 1.16, 95% CI: 1.07–1.26 |
| Feng 2017 | 29 studies (n = 3122) | Children | Triple therapy | 17 probiotic regimens |
Probiotic use RR 1.19, 95% CI 1.13–1.25 |
| Losurdo 2018 | 11 studies (n = 517) | Any age | None |
Lactobacillus-containing probiotics, only 2 studies Saccharomyces boulardii and 3 multi-strain probiotics |
Mean weighted eradication rate 14%, 95% CI: 2%–25% |
| Shi 2019 | 40 studies (n = 8924) | Adults | First line treatment (triple, sequential, quadruple therapy) | Several probiotic strategies |
Probiotic use RR 1.140, 95% CI 1.101–1.180 |
| Fang 2019 | 5 RCTs (n = 484) | Children | Triple therapy | Lactobacillus |
Lactobacillus use RR 1.19, 95% CI 1.07–1.33 |
| Zhou 2019 | 18 RCTs (n = 3592) | Any age | First line treatment (triple, sequential, quadruple therapy) | Saccharomyces boulardii | RR 1.09, 95% CI: 1.05–1.13 |
| Yu 2019 | 11 RCTs (n = 724) | Any age | Triple therapy | Lactobacillus | RR 1.16, 95% CI 1.08–1.25 |
| Wang 2023 | 34 RCTs (n = 9004) | Any age | Triple therapy | 10 probiotic strategies (triple therapy alone, triple therapy added with Bacillus, Lactobacillus, Saccharomyces, Bifidobacterium-Lactobacillus, Bacillus-Streptococcus, Lactobacillus-Streptococcus, Lactobacillus-Propionibacterium, Bifidobacterium-Lactobacillus-Streptococcus, Bifidobacterium-Lactobacillus- Saccharomyces) |
Improvement in eradication rate (RR 1.14, 95% CI: 1.07–1.21, p < 0.01), reduction of the side effects rate (RR 0.61, 95% CI: 0.53–0.71, p < 0.01) Bifidobacterium-Lactobacillus and Bifidobacterium-Lactobacillus-Saccharomyces had the best comprehensive performance |
| Musazadeh 2023 | 18 meta-analyses (n = 47,278) | Any age | Triple and quadruple therapy | Several probiotic strategies |
Pooled ESRR: 1.13; 95% CI: 1.11, 1.14, p < 0.01 ESOR = 1.86, 95% CI: 1.70, 2.03, p < 0.01 |
Regarding the combination with triple therapy, a recent meta-analysis [16] including 140 studies on adults and a total of 20,215 patients evaluated the role of probiotics in addition to triple therapy of different durations. Taken together, antibiotic regimens irrespectively to the line of treatment, the supplementation of probiotics led to higher eradication rates compared to the control group, 84.1 versus 70.5, respectively (Risk ratio (RR) 1.17, 95% CI 1.15–1.18); the authors additionally reported that Lactobacillus acidophilus, Saccharomyces boulardii, Lactobacillus + Bifidobacterium + Enterococcus, and Lactobacillus + Bifidobacterium + Bacillus cereus showed higher efficacy in increasing eradication rates when added to 10-day triple therapy, whereas only the first 3 mentioned probiotic mixtures showed a benefit in 14-day triple therapy.
As regards quadruple therapy and probiotics supplementation, a network meta-analysis carried out in 2019 [46], including 40 studies with 8924 patients and comparing the addition of probiotics to triple and bismuth-containing quadruple therapy, showed that the addition of probiotics was able to increase the eradication rates in all settings (RR range 1.74 for quadruple with and without probiotics to 6.08 for quadruple with probiotics compared to triple therapy), except when triple therapy plus probiotics was compared to quadruple therapy alone.
Only one trial reported data on triple therapy with esomeprazole, levofloxacin, and amoxicillin with L. reuteri for 14 days and without probiotic supplementation for HP eradication [47]. The eradication rate was significantly influenced by probiotic supplementation with L. reuteri (80% vs. 62%; p < 0.05), and the incidence of side effects was significantly lower. Moxifloxacin-based regimens were investigated in addition to probiotics in two studies without reaching significantly higher eradication rates [48, 49].
A recent umbrella meta-analysis published in 2023 involving 18 eligible studies revealed that probiotics have beneficial impacts on HP eradication (RR 1.13, 95% CI: 1.11–1.14, p < 0.01, and OR 1.86, 95% CI 1.70–2.03, p < 0.01). Greater effects on HP eradication were observed when higher doses (∼1010 CFU) and mixed strains were supplemented [50].
Comparable results were shown in a network meta-analysis on probiotics supplementation in standard triple therapy including 9004 patients randomized to 10 kinds of therapies; when probiotics were added, the authors found better outcomes than triple therapy alone, with higher eradication rates [51].
2.4 Novel H. pylori Eradicating Therapies and Probiotics Formulations
Novel eradicating therapies have been used in combination with probiotic supplementation. Vonoprazan is a novel potassium-competitive acid inhibitor currently approved for administration in Japan, with advantages over traditional PPIs in acid suppression in terms of rapid efficacy and long-lasting effect duration [52]. Kakiuchi et al. [53] evaluated the additional effect of a multiple antibiotic-resistant lactic acid bacteria preparation of Enterococcus faecium 129 BIO 3B-R, in addition to triple therapy using Vonoprazan. The authors found that the eradication rate in the two groups was comparable (83.9% and 94.1%), without any significant difference either for the incidence rate of diarrhea (73.1% vs. 56.5% respectively; p = 0.361) or for stool consistency.
Probiotic supplementation is also achievable with other products beyond capsule/sachet-based bacteria-only preparations (CBOP). For example, fermented milk-based probiotics differ from CBOP for various components able to exert a greater inhibitory effect on the HP in vivo and in vitro. A systematic review with pooled data analysis [54] including 10 RCTs and 963 patients concluded that fermented milk-based probiotic preparations improved HP eradication by 5%–15%, without significantly influencing the rate of adverse effects.
Promising results have also been reported for other fermented foods such as Kefir [55], Glycyrrhiza glabra (liquorice) [56] and Thai Fermented Rice Noodles [57], which have been shown to produce bacteriocin-like substances inhibiting HP.
3 Functional Dyspepsia
3.1 Rationale for the Use of Probiotics in Functional Dyspepsia
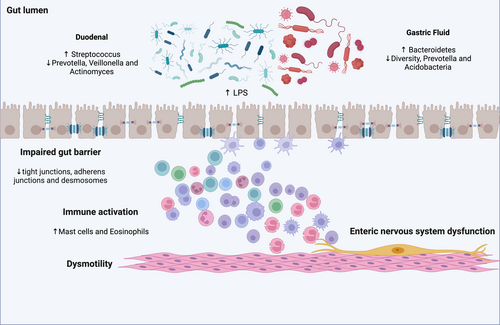
Definitive data about microbiota and FD are not available. Duodenal low-grade inflammation may be involved in the etiopathogenesis of FD, inducing mucosal immune activation, duodenal barrier dysfunction, and sensory-motor dysfunction (Figure 1). An altered duodenal microbiota, food antigens, or infection may precipitate duodenal microinflammation in a subset of FD patients [58]. Several authors analyzed FD patients' microbiota in order to evaluate its alpha diversity and the main species associated with dyspeptic symptoms. Fukui et al. showed that the relative abundance of mucosa-associated Streptococcus was positively correlated with upper gastrointestinal symptoms in FD patients [59]. In this line, Zhong et al. evaluated stool and duodenal microbiota in FD patients and healthy controls. The results showed an inverse relationship between the relative abundance of Streptococcus and the anaerobic genera Prevotella, Veillonella, and Actinomyces, which were significantly decreased in patients with FD [60]. Other studies directly assessing duodenal microbiota [61-65] confirmed that patients with FD had lower intra- and interspecies variability. Among others, increased relative abundances of bacteria from the Streptococcus genus were the most consistently reported alteration (Table 2). Other studies assessing microbiota in gastric fluid aspirate confirmed a significantly low average interindividual diversity and a significantly low abundance of the genus Prevotella in patients with FD, which, therefore, was considered to be involved in its pathogenesis. These changes were also restored by treatment with yogurt containing 109 colony-forming units of Lactobacillus gasseri [66]. In addition, the same research group found other alterations in the GF microbiota of FD patients. The GF microbiota had a greater Bacteroidetes abundance than Proteobacteria and an absence of Acidobacteria in the FD group. In contrast, the GF microbiota of the HC group had a greater Proteobacteria abundance than Bacteroidetes and the presence of Acidobacteria [67]. They also revealed that probiotic therapy with Lactobacillus gasseri in patients with FD shifted the composition of the GF microbiota to the one observed in the HC volunteers [67]. Since changes in the GF microbiota paralleled the improvement of symptoms, they were suggested to be involved in the pathophysiology underlying FD.
| Citation | Year | Microbial alteration | Duodenal microbiota assessment | Enrolled population |
|---|---|---|---|---|
| Zhong et al. [60] | 2017 | Increased relative abundance of Streptococcus. Decreased relative abundance of Prevotella, Veillonella and Actinomyces. | Duodenal biopsies | 9 FD vs. 9 healthy controls |
| Fukui et al. [59] | 2020 | Streptococcus abundance positively correlated with severe upper gastrointestinal symptoms. Reduced β-diversity. Increased relative abundance of Firmicutes | Mucosal brush sample of all sites in upper gut | 11 FD vs. 7 healthy controls |
| Wauters et al. [61] | 2021 | Lower relative abundance of Neisseria and Porphyromonas | Mucosal brush samples of duodenum | 28 FD vs. 30 healthy controls |
| Zheng et al. [64] | 2022 | Differences in ACE index, Shannon index and observed-species index. Increased relative abundance in Alloprevotella, Peptostreptococcus, Sutterella, Corynebacteriurn, Faecalibacterium, Staphylococcus, Eubacteriumnodatumgro-up, Lachnoclostridiurn and Lautropia Lower relative abundance of Catonella | Mucosal brush samples of duodenum | 20 FD vs. 5 healthy control |
| Higher activity of ureolysis and fumarate respiration in FD | ||||
| Shanahan et al. [62] | 2023 | Relative abundances of predominant members of the Firmicutes, Bacteroidota and Fusobacteriota phyla were linked to symptom burden in FD. | Duodenal biopsies | 56 FD vs. 30 healthy controls |
| Inverse relationships between the relative abundances of Streptococcus and Prevotella, and Veillonella spp. with gastric emptying time, were also observed. | ||||
| Tziatzios et al. [65] | 2024 | Lower ɑ-diversity in FD and IBS vs. healthy controls | Duodenal aspirate | 20 FD vs. 20 IBS vs. 10 healthy controls |
| Relative abundance of Chloroflexota, Rhodothermota and Thermotogota phyla were consistently lower in subjects with FD when compared to CG but similar to IBS | ||||
| Kim et al. [63] | 2024 | Increased relative abundance of duodenal Streptococcus and reductions in stool Butyricicoccus | Duodenal biopsy and brushing | 12 FD vs. 16 healthy controls |
Also, dysbiosis in FD may be linked to impaired intestinal permeability. Indeed, indirect evidence regarding the potential physio-pathological role of microbiota imbalances in FD comes from Vanheel et al., which reported an impaired intestinal barrier function in FD [68]. Duodenal biopsy specimens obtained from 15 patients with FD showed lower transepithelial electrical resistance (TEER) and increased paracellular passage compared with healthy controls, which is indicative of impaired mucosal integrity. In addition, abnormal expression of cell-to-cell adhesion proteins at the level of tight junctions, adherens junctions, and desmosomes was shown. Furthermore, an increased infiltration of mucosal mast cells and eosinophils showed the presence of low-grade inflammation, whose severity was related to the extent of increased permeability [68]. As a possible underlying mechanism, it is supposed that the toxic bacterial cellular components of the gut, such as lipopolysaccharides, could stimulate leukocytes to produce pro-inflammatory cytokines, triggering gastric inflammation and consequently increasing mucosal permeability, which may lead to gastric (enteric) nervous system dysfunction [69].
A recent study summarizes all the previous findings confirming the presence of duodenal mucosal inflammation and impaired expression of tight junction proteins in patients with FD. Moreover, the relative abundance of duodenal Streptococcus and reductions in stool Butyricicoccus were confirmed. These changes in the microbiota were both correlated with symptom severity. Therefore, the authors concluded that assessing changes in the abundance of stool Butyricicoccus may be an effective biomarker for enhancing FD diagnosis and monitoring [63].
3.2 Probiotic Effect in Functional Dyspepsia
Only a few studies have investigated probiotics' effects on FD (Table 3). In a preliminary randomized controlled trial (RCT) by Kim et al., 72 patients with functional gastrointestinal disorders, including FD, were assigned to one of five treatment groups containing probiotics or placebo, aiming to evaluate the efficacy and safety of commonly used probiotics. Results showed that the combination of probiotics had the most consistent pattern of gastrointestinal symptoms improvement assessed through the Gastrointestinal Quality of Life Index (GIQLI) and GI visual analogue scale (GIVAS), even if not statistically significant. The absence of conclusive evidence in this pilot trial was thought to be due to the small sample size [70].
| Study, year | Country | Method | Sample size, number of patients (% female) | Age (years) | Indication | Main findings | Criteria used to define symptom improvement following therapy |
|---|---|---|---|---|---|---|---|
| Kim L. et al. 2006 | USA |
IMPJ I: Probioticsa (Lactobacillus acidophilus, Bifidobacterium bifidum, Bacillus subtilis, Lactobacillus bulgaricus, Lactobacillus lactis, and Bacillus lichenformis), Barley grass and oat grass juice and ionic plant-based minerals. IMPJ II: Probioticsb (Lactobacillus acidophilus, Bifidobacterium bifidum, Lactobacillus bulgaricus, Lactobacillus lactis, Lactobacillus brevis, Lactobacillus caucasicus, Lactobacillus fermenti, Lactobacillus leichmannii, Lactobacillus casei, Lactobacillus plantarum, Lactobacillus helveticus, and Saccharomyces boulardii) No spore-forming probiotics, Barley grass and oat grass juice and ionic plant-based minerals IM: Probioticsa PJ: Barley grass and oat grass juice, Ionic plant-based minerals IMA: Probioticsc (Bacillus coagulans, Saccharomyces boulardii, Bacillus subtilis, Lactobacillus salivarius, and Lactobacillus plantarum), mushrooms and algae Placebo 12 weeks treatment |
72 (66.7%–75.0% females) | Range 21–72 years | FGID including FD (Rome II criteria) |
No significant differences among groups. Consistent pattern of GI symptoms improvement in the IMPJ I and IMPJ II groups. Less consistent changes in the IM, PJ and IMA groups. |
Gastrointestinal Quality of Life Index (GIQLI) and GI visual analogue scale (GIVAS) |
| Ianiro G. et al. 2013 | Italy |
Extra-virgin olive oil enriched with anti-oxidants (Oo/Ao) or probiotics (Oo/Pr) Oo/Ao: Two vials daily, each containing: Selenium methionine, Q10 coenzyme, ascorbyl palmitate, resveratrol, silicon dioxide. Oo/Pr: L. reuterii 100 billion/g, L. rhamnosus GG 350 billion/g, Saccharomyces boulardii 20 billion/g, vitamin B6 hydrochloride, inositol, silica; Q10 coenzyme. 7 days treatment |
8 | — | FD (Rome III criteria) | Amelioration of nausea and abdominal pain/discomfort in subjects receiving Oo/Pr compared to Oo or Oo/Ao (p = 0.04) | Validated questionnaire to evaluate GI symptoms |
| Nakae H. et al. 2016 | Japan |
118 g of yogurt containing 109 colony-forming units of L. gasseri (LG21 yogurt) every day 12 weeks treatment |
44 | Range 20–60 years | FD (Rome III criteria) |
Amelioration of EPS and PDS symptoms (p < 0.001). Abundance of Prevotella significantly correlated with PDS symptoms. |
Validated questionnaire |
| Igarashi M. et al. 2017 | Japan |
118 g of yogurt containing 109 colony-forming units of L. gasseri (LG21 yogurt) every day 12 weeks treatment |
24 21 healthy controls |
— | FD (Rome III criteria) | Probiotic therapy in patients with FD shifted the composition of the GF microbiota to that observed in the HC | Validated questionnaire |
| Ohtsu T. et al. 2017 | Japan |
L. gasseri group: 109 CFU of L. gasseri OLL2716/unit (85 g) of yogurt Placebo group: one unit (85 g) of yogurt made from a mixture of raw milk, dairy products, sugar, a sweetener (stevia), and raw water, fermented with Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophiles. 12 weeks treatment |
58 (75% females) patients in the L. gasseri group and 58 (74% females) in the placebo group | Mean age 42.8 ± 9.0 years | FD (Rome III criteria) | Not statistically significant amelioration of symptoms in the L. gasseri group | Validated questionnaire (4, 8, and 12 weeks assessment). |
| Rahmani P. et al. 2020 | Iran |
Placebo L. reuteri 108 CFU 4 weeks treatment |
125 | Average 7.3 ± 1.7 years old (case group) 7.7 ± 2.1 years old (control group) | FD (Rome III criteria) | 68% children treated with probiotic showed a significant (p < 0.001) response to probiotic treatment compared to placebo group in terms of duration of pain, severity, and frequency | Wang-Baker FACES Pain Rating Scale (WBFPRS) |
| Sun E. et al. 2021 | China |
Beverage containing 5 × 108 cfu/mL of Lactobacillus paracasei 28 days treatment |
26 (57% female) | Mean age 44.3 ± 11.7 years | FD (Rome IV criteria) |
Clinical symptom scores significantly decreased. of probiotics and relevant beneficial intestinal metabolites of harmful bacteria and intestinal metabolites |
Validated questionnaire |
| Wauters L. et al. 2021 | Belgium |
Spore-forming probiotics group: Bacillus coagulans MY01 and Bacillus subtilis MY02, 2·5 × 109 CFU per capsule, twice daily Placebo group: 350 mg maltodextrin per capsule, twice daily 8 weeks treatment |
68 (32 probiotics, 36 placebo, 75% female) | Mean age 40.1 ± 14.4 years | FD (Rome IV criteria) | Decrease in PDS score ≥ 0·7 was higher for probiotics than placebo (48% vs. 20%) | Leuven Postprandial Distress Scale (LPDS), patient assessment of upper gastrointestinal disorders symptom severity index (PAGI-SYM) and quality of life (PAGI–QOL) |
| Drago L. et al. 2021 | Italy |
L. rhamnosus, L. pentosus, L. plantarum and L. delbrueckii > 108 CFU/Active Fluorescence Units [AFU] 30 days treatment |
2676 (1357 PDS patients, 1319 EPS patients) | — | FD (Rome IV criteria) | Postprandial filling and early satiety improved after treatment. No difference in the EPS group. | Validated questionnaire |
| Zhang Q. et al. 2024 | China |
|
200 | Range 18–60 years old | FD (Rome IV criteria) | CRR in FD score for the BL-99_high group significantly higher than placebo (90% vs. 58%), than BL-99_low (74.0%) and PPI group (70.0%) | Clinical response rate (CRR) of FD score |
- a Fifty million CFU (six species): Lactobacillus acidophilus, Bifidobacterium bifidum, Bacillus subtilis, Lactobacillus bulgaricus, Lactobacillus lactis, and Bacillus lichenformis.
- b Fifty million CFU (twelve species): Lactobacillus acidophilus, Bifidobacterium bifidum, Lactobacillus bulgaricus, Lactobacillus lactis, Lactobacillus brevis, Lactobacillus caucasicus, Lactobacillus fermenti, Lactobacillus leichmannii, Lactobacillus caseii, Lactobacillus plantarum, Lactobacillus helveticus, and Saccharomyces boulardii. No spore-forming probiotics.
- c Fifty million CFU (five species): Bacillus coagulans, Saccharomyces boulardii, Bacillus subtilis, Lactobacillus salivarius, and Lactobacillus.
Also, L. gasseri showed promising results in ameliorating symptoms in FD patients [66, 67]. A RCT with L. gasseri OLL2716 in 116 patients with FD showed that symptom resolution was achieved in 17% and 35.5% in the placebo and L. gasseri OLL2716 (p = 0.048) arms, respectively [71]. A different trial showed that FD symptoms were relieved in participants after 28 days of treatment with a beverage containing Lactobacillus paracasei LC-37 (LC-37), with a response rate of about 90% for abdominal pain. Lactobacillus, Lactococcus, and Weissella significantly increased, and the abundance of harmful bacteria such as Lachnoclostridium significantly decreased [72]. However, results were severely limited by the open-label design, the low number of participants, and the lack of a control group [72].
Another RCT by Wauters et al. aimed to assess spore-forming probiotics' efficacy on reducing symptoms in functional dyspepsia as monotherapy or add-on therapy to long-term treatment with proton-pump inhibitor [61]. Functional dyspepsia patients and controls received 8 weeks of treatment with probiotics (Bacillus coagulans MY01 and Bacillus subtilis MY02, 2.5 × 109 colony-forming units per capsule) or placebo consumed twice per day, followed by an open-label extension phase of 8 weeks. Symptoms were assessed through the Leuven Postprandial Distress Scale, along with immune activation markers (high-sensitivity C reactive protein, lipopolysaccharide binding protein, systemic cytokines and peripheral blood mononuclear cells), and fecal microbiota. Among the 68 FD patients (50% of whom on proton-pump inhibitors), the proportion of clinical responders was higher with probiotics than with placebo (respectively 48% vs. 20%, RR 1.95 [95% CI 1.07–4.11]; p = 0.028). Finally, a recent well-conducted RCT assigned 200 FD patients to receive placebo, rabeprazole, or Bifidobacterium animalis subsp. lactis BL-99 (BL-99; low, high doses) for 8 weeks. The primary outcome was the clinical response rate (CRR) of FD score after 8-week treatment, which was significantly higher for the BL-99 high dose group than that for placebo (90% vs. 58%, p = 0.001), BL-99 low dose (74%, p = 0.044) and positive control (70%, p = 0.017) after 8-week treatment. This effect was sustained until 2 weeks after treatment but disappeared 8 weeks after treatment. Metagenomic and metabolomics revealed that BL-99 promoted the accumulation of SCFA-producing microbiota and the increase of SCFA levels in stool and serum, which may account for the increase of serum gastrin levels, supporting its use in FD [73].
As for multistrain formulations, a probiotic combination of Lacticaseibacillus rhamnosus LR04, Lactiplantibacillus pentosus LPS01, Lactiplantibacillus plantarum LP01, and Lactobacillus delbrueckii subsp. delbruekii LDD01, alone or in combination with prokinetics, antacids, or proton-pump-inhibitors, was evaluated in FD patients to assess whether it could contribute to improving symptoms over 30 days. Patients with FD were enrolled and divided into PDS and EPS groups. All patients showed significant improvements in dyspeptic symptoms following treatment. In patients with PDS, probiotics alone resulted in the lowest prevalence of symptoms following treatment, while patients with EPS showed no clear between-treatment differences [74].
Also, antioxidants have been proposed as a treatment for functional dyspepsia; extra-virgin olive oil, a common ingredient of the Mediterranean diet known for its antioxidant properties, was added to the common diet of 8 subjects with functional dyspepsia for 7 days to evaluate its effect when enriched with antioxidants or probiotics (L. reuterii, L. rhamnosus GG, Saccharomyces boulardii). All patients had a significant improvement of dyspeptic symptoms, with a greater effect observed for the probiotic-enriched group [75].
A systematic review included 6 RCTs, only 3 of which were included in the meta-analysis, reported that Lactobacillus strains showed potential positive effects in terms of improving upper gastrointestinal symptoms in patients with FD. Probiotic supplementation showed a trend of improving the global dyspepsia score (RR: 1.35, 95% CI 0.99–1.84; p = 0.061) and bacterial composition in the gastrointestinal tract, without any serious adverse events. The evidence, however, is insufficient to draw clear conclusions regarding efficacy [76]. In summary, dysbiosis is one of the main pathological mechanisms in the pathogenesis of FD. The use of probiotics in FD could be beneficial, but it is still debated due to the lack of strong evidence available at present for their use.
4 Conclusion
Probiotics alone have no meaningful influence on the eradication of HP infection, although a significant reduction in adverse events has been reported during probiotic supplementation, especially diarrhea and abdominal discomfort, which in turn may enhance patients' compliance with the therapy and increase eradication rates. A trend toward the efficacy of probiotics in ameliorating FD symptoms is supported by several pieces of evidence. The main pitfall of studies assessing the efficacy of probiotics in FD is a high variability in the strain that can be more beneficial, in the duration of the treatment, and in the criteria used to define symptom improvement following therapy. Nevertheless, data on probiotics remain promising, and given their safety and low rate of side effects, their use should be further investigated in order to determine the optimal probiotic strain, dosage, and therapy duration.
Author Contributions
G.M. and G.B. designed the review. G.M. and M.F. performed the literature search. All authors drafted the manuscript, critically revised it, and approved the final version of the manuscript. All authors approved the final version of the article, including the authorship list.
Acknowledgments
The authors have nothing to report. Open access publishing facilitated by Universita degli Studi di Bologna, as part of the Wiley - CRUI-CARE agreement.
Ethics Statement
Ethical review and approval were waived for this study due to the use of already available published data.
Consent
Informed consent was obtained from all subjects involved in the study by the investigator of each published study included in the present review.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data presented in this study are openly available in Medline and Embase.




