Four years of experience with the Australian kidney paired donation programme
Abstract
New approaches to increase kidney transplantation rates through expansion of live donor kidney transplantation have become necessary due to ongoing shortage of deceased donor organs. These strategies include desensitization in antibody-incompatible transplants to overcome the barrier of blood group incompatibility or human leucocyte antigen antibodies between recipient and donor and kidney paired donation (KPD) programmes. In KPD, a kidney transplant candidate with an incompatible live donor joins a registry of other incompatible pairs in order to find potentially compatible transplant solutions. To match the largest possible number of donor–recipient pairs while minimizing immunologic risk, KPD programmes use sophisticated algorithms to identify suitable matches with simultaneous two-way or more complex multi-way exchanges as well as including non-directed anonymous donors to start a chain of compatible transplantations. Because of the significant immunologic barriers when fewer donor options are available, the optimal solution for difficult-to-match, highly sensitized patients is access to more potential donors using large multi-centre or national KPD registries. This review focuses on the first 4 years of experience with the Australian multi-centre KPD programme that was established in October 2010.
Kidney transplantation is the best treatment option for eligible patients with end-stage kidney disease (ESKD).1 The global shortage of deceased donor organs has led to increased reliance on living kidney donation programmes.2 Live donor kidney transplantation (LDKT) is associated shorter waiting time with less accrued comorbidity resulting in superior long-term patient and graft survivals.3 Up to 54% of otherwise appropriate live donor–recipient pairs are unable to advance to LDKT because of ABO blood group incompatibility or pre-existing donor specific antibodies (DSA) to human leucocyte antigen (HLA).4 These immunologic barriers may be overcome using desensitization strategies targeting removal and suppression of ABO antibodies or DSA using a combination of plasma exchange, intravenous immunoglobulin or B-cell/plasma cell-depleting agents5-8 however, long-term outcomes may be suboptimal.9 Kidney paired donation (KPD) is an alternative approach to overcome immunologic barriers that allows a medically suitable but incompatible pair to exchange kidneys with one or more other incompatible pairs so that all recipients receive compatible organs from strangers10-13 with comparable outcomes to unsensitized recipients if DSA are avoided.14 Following the initial report in 2009 of a successful regional pilot programme in Western Australia,15 the Australian Organ and Tissue Authority (OTA) decided to support the establishment of a national KPD registry as part of the initiative to increase organ donation rates in the country. The first match cycle of the national KPD programme, known as the Australian paired Kidney eXchange (AKX) programme, was performed on 14 October 2010, and the first interstate national live kidney exchange transplants were performed on 24 November 2010 between two pairs in New South Wales and one pair in Victoria. The AKX programme is now an integral part of the kidney transplantation practice in Australia and contributes to over 10% of all LDKT performed each year. Among the four existing national KPD registries, which include The Netherlands, United Kingdom, Canada and Australia, the AKX programme has proven to be the most effective in terms of proportion of patients receiving LDKT to registered transplant candidates.13 KPD is a relatively novel strategy in kidney transplantation, and as such, it is continuously evolving. This overview provides an update on the Australian KPD programme, discussing distinguishing features that make the AKX registry one of the most effective KPD programmes in terms of proportion of to registered transplant candidates to transplanted patients.13
Principles of KPD
KPD was initially proposed as an exchange of donors between two pairs (a two-way exchange), surmounting each other's ABO or HLA incompatibility problem by simply exchanging donors and thus completely avoiding immunologic barriers.10-12 Using sophisticated matching algorithms and software, KPD donations can be arranged involving multiple pairs per chain (n-way exchanges).13, 16-18 In ‘conventional’ KPD using these closed loops of two or more pairs, transplants must be simultaneously performed to guarantee all recipients receive a transplant and eliminate the risk of any donor reneging and leaving a recipient with no transplants after their co-registered donor has donated their kidney. Conventional KPD transplants require incompatible pairs to be matched to other pairs with reciprocal incompatibilities, which may be challenging when only few pairs are available.17, 19 Incorporation of non-directed anonymous donors (NDAD), also known as Good Samaritan or altruistic donors,20, 21 into a KPD registry can initiate a chain of transplants without obligatory matching of a paired recipient.22, 23 Such chains are usually terminated with the last paired registered donor donating to a waitlist recipient.24 In some KPD programmes, the last paired donor is left as a bridge donor to initiate a future NDAD chain at a later date.25 Because simultaneous arrangement of long chains is logistically challenging, NDAD chains may be performed non-simultaneously, despite a small risk of donor withdrawal resulting in premature chain breakdowns.22 This small risk of donor withdrawal within a chain is offset by the benefit of leveraging one NDAD to enable multiple transplants. Furthermore, NDAD chains can facilitate transplants with pairs who cannot otherwise be matched in closed n-way chains, as is the case in pairs where the patient is broadly HLA sensitized (low donor match potential) with their registered donor bearing common HLA antigens (low recipient match potential) with reduced match probability to other broadly sensitized recipients. While multi-way chains can represent a significant logistic challenge, logistics alone should not limit the ability to offer LDKT to KPD patients, some of whom have limited alternative kidney transplantation options.
Allocation Algorithms in KPD
The match probability in a KPD registry depends on the number of incompatible pairs in the pool,17, 19 the ratio of ABO-incompatible to HLA-incompatible pairs, the level of sensitization of transplant candidates and the algorithm-specific allocation rules. The matching algorithm for selection of donor–recipient pairs within the KPD pool is critical and must be optimized in order to maximize the potential number of suitable matched donor-recipient pairs in any match cycle. The two critical requirements of priority for matching are the blood group matching and the likelihood of the recipient finding a DSA or crossmatch negative donor in the pool.16, 18 Additional ranking factors can be used for prioritization but should not rule out possible transplants and limit the potential of the pool. The putative benefits of receiving a preferred donor (e.g. CMV negative into negative) are outweighed by the advantage of a timely living donor transplantation.3 All major KPD registries use a virtual crossmatch approach to allocate suitable donors in the pool to registered transplant candidates, although the extent of HLA antigens included in the virtual crossmatch algorithm varies among registries.13, 16, 18, 22, 26, 27 Donors are usually typed at a minimum for HLA-A, -B, -C, -DR and -DQ, and in some registries, -DQA, -DPA, -DPB and -DRB3/4/5 typing are also included. Acceptable mismatches are identified by excluding donors with HLA antigens to which a particular recipient has DSA. The set threshold for acceptable DSA can vary from serological methods11, 16 to highly sensitive single antigen beads technology.13, 18, 22, 26 With graft outcomes following ABO-incompatible transplants being comparable with ABO-compatible transplants,5, 6 KPD exchange may not be necessary in centres performing desensitization, thus diverting an important source of otherwise unsensitized recipients from KPD pools with a resultant higher proportion of highly sensitized registered pairs.27, 28 When the number of HLA-incompatible registered pairs is greater than those with ABO incompatibility, match probability decreases.13
The Australian KPD Programme
The Australian KPD registry is known as the AKX Programme and was established in 2010 following the initial experience of the Western Australia pilot programme.15 The AKX programme has a dedicated central support staff coordinating the work between transplanting units, tissue typing laboratories and the National Organ Matching System (NOMS), which developed and maintained the computer system that performs matching. Details on the specifications and ranking rules of the NOMS algorithm donor and recipient HLA typing and HLA antibody definition have been reported previously.18, 27 Match cycles occur every 3 months. After the computer-based ABO and virtual crossmatch (negative DSA) allocation, the final decision regarding transplantation is made after the cell-dependent cytotoxicity (CDC) and flow cytometry crossmatches of proposed pairs are performed. While in some KPD programmes regulations require donors to travel to the recipient centre for surgery, in Australia, the kidneys are transported between centres. Surgeries take place on the same day with simultaneous anaesthetic induction time within a chain of transplants.
From October 2010 until October 2014, the Australian KPD programme has facilitated 105 kidney transplants among the 215 registered pairs (49% of registered KPD candidates) as well as four waitlist recipients at the end of a NDAD chain (Fig. 1). By 4 December 2014, a total of 107 transplants had been completed. Of the KPD-facilitated transplants, 93 were achieved using n-way chains, and 16 were performed through NDAD chains (15%; Table 1). Only a small minority of transplant candidates joining the AKX registry have ABO incompatibility without anti-HLA DSA to their intended donors (15%), while the majority of patients are incompatible with their donors because of sensitization to HLA antigens (85%). Moreover, the pool of sensitized recipients consists primarily of highly sensitized, HLA-incompatible transplant candidates, as 35% of registered patient have calculated panel reactive antibody (cPRA) 95–100%. Considering the type of immunological incompatibility of registered recipients, the relatively high proportion of transplants achieved with the AKX programme is remarkable. Overall transplant rates have been excellent, and the proportion of patients with cPRA 50–96% being transplanted (62%) is fairly similar to the proportion of transplanted patients with cPRA 0–50% (73%), although, not surprisingly, the proportion of transplants among extremely highly sensitized candidates with cPRA ≥ 97% has been low (25%). Blood group O recipients are notoriously more difficult to match, and in AKX, they represent 57% of the referred transplant candidates. When the proportion of KPD transplants versus registered patients is analysed, the proportion for O recipients is only marginally lower (46%) compared with blood group B (53%), A (61%) and AB recipients (67%). NDAD chains have been a minor driver in the Australian programme due to the low number of NDADs included. Nevertheless, the power of NDAD chains in facilitating transplants with pairs who cannot otherwise be matched in closed n-way chains is demonstrated by the higher cPRA of the broadly HLA-sensitized patients transplanted (Fig. 2). Despite the still short follow-up after transplantation, outcomes are comparable with those from compatible live donor transplantation. At 1 year, patient survival is 97% and graft survival is 96%. Of three graft losses, one was an early failure due to surgical complications, and two were due to death of patient with functioning graft.
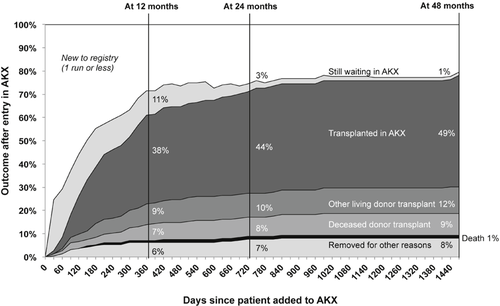
Time to outcome for candidates added to the Australian paired Kidney eXchange (AKX) programme. The cumulative percentage of candidates reaching each possible outcome by 12, 24 and 48 months is highlighted. Between October 2010 and October 2014, the AKX programme facilitated live donor kidney transplantation in 105 of the 215 registered patients.
| Pairs registered | 220 (51 in last MC) |
| Recipients registered | 215 (49 in last MC) |
| NDAD registered | 4 |
| Scheduled MC | 16 |
| Pairs per MC | 40–50 |
| Match rate per MC | 25% |
| Transplant rate per MC | 18% |
| Completion rate per MC | 70% |
| Transplants facilitated | 109 |
| Transplants completed | 107 |
| Registered recipient transplants | 103 |
| Waitlist recipient transplants | 4 |
- MC, match cycle; NDAD, non-directed anonymous donor.
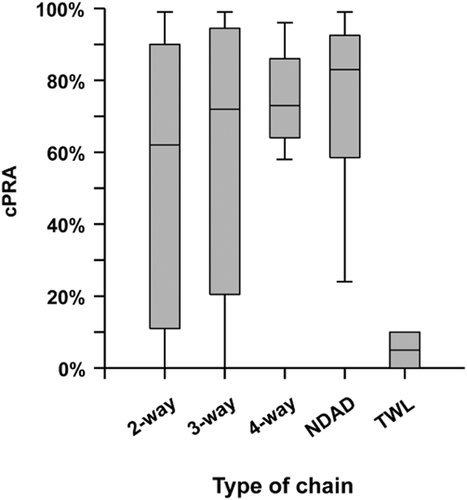
Calculated panel reactive antibody (cPRA) of patients transplanted through the Australian paired Kidney eXchange (AKX) programme by type of chain. NDAD, non-directed anonymous donor chain recipients; TWL, transplant waitlist recipient of the kidney from last paired registered donor in the NDAD chain.
Further outcomes for match cycle-eligible candidates are shown in Figure 1. Less than 2% of patients were removed due to death. Approximately 20% of patients left the AKX programme either because they received a deceased donor transplant (9%) or found an alternative living donor transplant option (12%), while others left the programme because either the donor or the recipient became medically unsuitable (8%) (Fig. 1). Unfortunately, no suitable transplant option can be found either through KPD, deceased donor transplantation or through desensitization for antibody-incompatible live donor transplantation for 11% of transplant candidates within 12 months of registering in the AKX, and 3% and 1% of them remain on dialysis after 24 and 48 months, respectively. A careful review of patients still waiting for a transplant after two match cycles demonstrates that half of them are so broadly sensitized to HLA antigens that they could only receive a kidney from an HLA identical donor (Fig. 3). For highly sensitized patients with cPRA ≥ 95%, the probability of receiving a blood group and HLA-compatible deceased donor kidney without prioritization rules would be less than 1%, and therefore, these patients would often wait in excess of 10 years on the transplant waitlist. While patients with cPRA ≥ 95% are clearly disadvantaged as a group because a lower proportion of that group gets a transplant; data from the Australian KPD registry indicate that the 95–96% group is transplanted at a proportion equivalent to their proportion in the registry, comparable with any other cPRA cohort, whereas it is those candidates with cPRA ≥ 97% that are driving the lower overall transplant rates for highly sensitized patients (traditionally defined as ≥95%) (Fig. 4). It is this ≥97% cohort therefore that truly represents the highly sensitized, difficult-to-transplant patient and which accumulates over time in the KPD registries. Additional strategies for these difficult-to-match pairs are required.
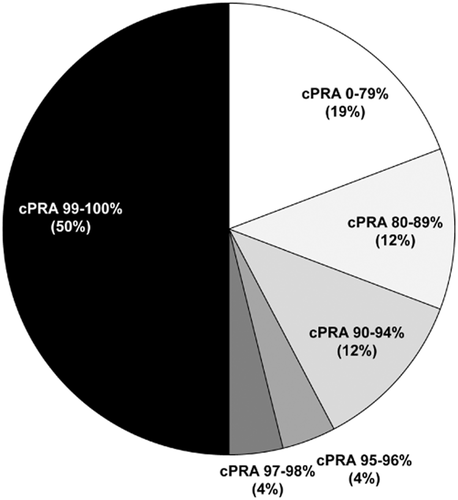
Level of sensitization (calculated panel reactive antibody (cPRA)) of 26 patients registered in the Australian paired Kidney eXchange (AKX) programme for at least two match cycles and who are still waiting for any kidney transplant. Nearly 60% of patients have cPRA of 95% or greater.
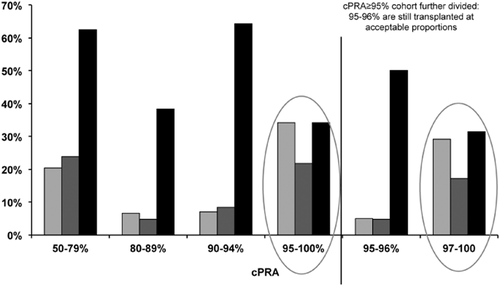
Transplant activity by calculated panel reactive antibody (cPRA) cohort: cPRA 97% is the true threshold for diminished access; the 95–96% groups are transplanted at a proportion equivalent to their proportion in the registry, comparable with any other cPRA cohort.  , % of programme registrants;
, % of programme registrants;  , % of registrants transplanted; ■, % of cPRA group transplanted.
, % of registrants transplanted; ■, % of cPRA group transplanted.
Because in the Australian KPD programme organs are transported, rather than donors moving to the recipient's centre, an important question is the cold ischaemia of locally used (within the same hospital and/or city) compared with couriered kidneys. Cold ischaemia time (CIT) of kidneys transplanted within the same state (n = 44) was 213 ± 61 min (same hospital: 155 ± 39 min). CIT for transplanted kidney transported interstate along the East Coast was 408 ± 63 min and East-to-West 629 ± 100 min. There was one single instance of delayed graft function requiring dialysis in a recipient from an interstate organ with a CIT of 400 min.
It is interesting to observe that participation in the KPD programme by region does not reflect the proportion of ESKD patients listed on the NOMS deceased donor waiting (Fig. 5). While 47% of patients listed in the NOMS deceased donor waitlist are transplant candidates from New South Wales and 33% are from Victoria, referral to AKX is the reverse (Victoria 51%, New South Wales 27%). Western Australia has a high proportion of AKX referrals compared with their deceased donor transplant waitlist, whereas Queensland and South Australia have equal share of AKX registrants and deceased donor wait listing. The reasons for this discrepancy are unclear; the programme does not collect data on pairs who should be entered but are not, and thus, it is difficult to speculate whether different practice patterns explain this regional variability in referrals.
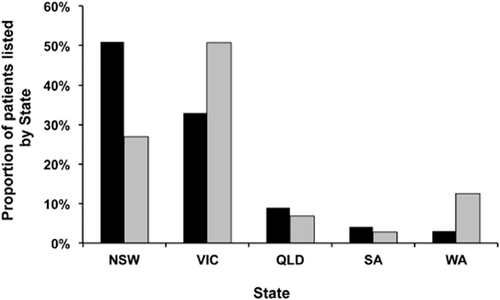
Proportion of transplant candidates registered on the National Organ Matching System (NOMS) deceased donor list and in the Australian Paired Kidney eXchange (AKX) programme by State (NSW, New South Wales; VIC, Victoria; QLD, Queensland; SA, South Australia; WA, Western Australia). ■, NOMS deceased donor list;  , AKX registry.
, AKX registry.
International Comparison
The AKX programme compares favourably with the other national programmes of the Netherlands, United Kingdom (UK) and Canada.13 In the Dutch programme, 655 registered pairs were enrolled between April 2004 and April 2014; 57% of pairs were included because of ABO incompatibility and 43% because of HAL incompatibility. Over the 10-year period, the Dutch KPD scheme facilitated 242 kidney transplants (transplant rate 37%) using two-way and multi-way chains.13 The AKX programme also outperforms the UK programme, in which between April 2007 and April 2014, 284 of the 991 registered patients (transplant rate 29%) proceeded to receive a kidney transplant through two-way and three-way loops and 36 through NDAD chains (3.6%),13 despite a higher proportion of unsensitized ABO-incompatible pairs (36%) in the UK KPD pool compared with the AKX registry. The Canadian programme active since 2009 shares many commonalities with the Australian programme: Its KPD pool is mainly composed of highly sensitized, HLA-incompatible pairs (88%), and the proportion of KPD transplant among registered transplant candidates is equally excellent (44%).13 A major driver of the success in Canadian registry is the inclusion of a large number of NDADs (n = 54 as of December 2013), facilitating 62% of the registered recipient transplants versus 38% in n-way exchanges.13 Comparison between the four national programmes suggests that match and transplant rates from N-way loops does not necessarily rely on the pool size of incompatible pairs in a match cycle.13 Indeed, transplant rate from loops is 41% in the Australian programme despite inclusion of only 40–50 patients per match cycle, whereas transplant rates from loops were only 25% in the UK programme despite inclusion of 160–180 patients in each allocation round. The most plausible explanation for this success is the unique feature of the Australian programme to allow ABO-incompatible donor matching for patients with anti-A and/or anti-B titres of 1:64 or less.27 On the other hand, the power of NDAD domino chains in KPD programme is clearly demonstrated by the Canadian programme, where 62% of all KPD transplants were facilitated by NDAD chains.13
KPD and the Difficult-to-Match Patient
When high-proportion pairs in a KPD programme are enrolled as a result of HLA incompatibility, recipients with broad sensitization and high cPRA compete for a very limited number of donors bearing uncommon HLA genotypes.29, 30 This leads to accumulation of patients in the KPD pool with high cPRA and specificities to common HLA antigens, demanding alternative allocation strategies to assist these patients. Additional strategies for difficult-to-match pairs are ABO-incompatible matching, inclusion of HLA-compatible pairs and combination of KPD with antibody removal strategies.
Given the excellent outcomes of ABO-incompatible transplantation in the absence of DSA,6, 31 an additional strategy to increase options for sensitized patients is to accept kidneys from ABO-incompatible but HLA-acceptable donors within an existing KPD pool. The acceptance of ABO-incompatible donors for some of the highly sensitized recipients in a KPD programme results in a virtual expansion of the donor pool. While the actual pool size is unchanged, by allowing ABO-incompatible matching, more exchanges within the same pool are possible thereby increasing the match probability. This strategy has already been applied successfully in the Australian programme,27 with 19% of patients being matched to an ABO-incompatible donor. The majority of these patients were highly sensitized (43% had cPRA >90%), 81% of them had DSA to their original donor, and by accepting ABO-incompatible matching, they avoided any DSA to the matched donor.
Another strategy to increase KPD pair numbers is inclusion of ABO/HLA compatible who may also benefit from receiving a transplant immunologically better suited for them.32, 33 Although compatible pairs participating in a KPD programme may be disadvantaged by waiting for a match, delaying transplant surgery that may otherwise have proceeded, unbalanced KPD can be attractive to otherwise compatible pairs who have a high degree of HLA mismatch, if the KPD allocation algorithm provides a better HLA match for the recipient in the compatible pair.34 The selection of a better matched donor may ultimately be important for those likely to require repeat transplantation, with lower potential for subsequent sensitization.35 There are other instances where it may be prudent considering for a compatible pair to be included in a KPD programme. One example is when a husband is considering donating to the spouse after previous pregnancies, even in the presence of low-level DSA and despite negative cell-based crossmatch. Without prior transplants, there is no screening history dating back to the time of exposure one could rely upon, and even if no strong DSA is currently detectable or in the absence of DSA, there could still be a memory response to past exposure. Another situation is that of a homozygous living donor who is fully mismatched with their recipient. In addition to the advantage for the co-registered recipients, if they were to gain a better match, the homozygous donor would be extremely valuable to highly sensitized recipients. The positive impact of including more compatible pairs in KPD programmes is mathematically substantiated36 and may be more acceptable if such pairs are seen to gain additional benefit. One of the risks of this principle to enhance KPD programmes is that it may induce some to consider enhancing the opportunities of a better donor for ABO-/HLA-compatible pairs who are otherwise sub-optimally matched for age37, 38 and or size.39 While this is likely to be seen as a trading, it should not be a priori discarded. Body size matching could be justified where a parent compatible to their infant child needing a kidney is too large and the donated kidney would not fit into the recipient. Constraints limiting trading should be carefully defined within policy in national programmes, and the matching algorithm would need to be refined to take into account these factors.
An additional option to help highly sensitized patients is to combine KPD with antibody removal strategies in which recipients are matched with donors against whom they have donor-specific HLA antibodies, but of lesser strength than their original donor.7, 30, 40, 41 KPD in the presence of DSA has been used in a small number of recipients with no or minimal desensitization (high-dose IVIG).41 Although these KPD recipients had a positive virtual crossmatch based on the DSA identified by SAB testing, they all had negative CDC, and many had a negative flow cytometric crossmatch.41 KPD combined with desensitization is a strategy employed in the majority of incompatible live donor transplants performed at Johns Hopkins.30, 40
Outlook
Although not the sole solution for diminished access to kidney transplantation, KPD programmes have significant advantages and fewer medical risks than desensitization for high-titre ABO or HLA incompatibility. KPD has become one of the fastest growing source of LDKTs. Facilitating immunologically compatible kidney transplants should remain the primary aim of a KPD programme, but for those extremely highly sensitized patients, KPD can enable living donor kidney transplantations with low immunological risk and longer graft survivals. By also including NDAD and compatible pairs, it is possible to achieve a sufficiently large pool of donors and recipients in order to find the best match for the most difficult-to-transplant recipients. KPD is also beneficial to unpaired patients because by removing all patients with potential living donor from the deceased donor waitlist, it will lower their waiting time. Finally, increased access to kidney transplantation will help reduce the demand for illegal commercial transplantation.
Acknowledgements
We would like to acknowledge the Australian OTA. The opinions expressed herein are those of the authors and not necessarily those of the OTA. The authors wish to thank the collaboration of all participating transplant centres and would also like to thank the patients and their donors for agreeing to participate in this programme. We express our gratitude to Mrs Jenni Wright and the NOMS team for their support throughout the programme, and Dr Peter Hughes, Dr Scott Campbell and Dr Kate Wyburn of the Renal Transplant Advisory Committee AKX Clinical Advisory Subcommittee for his advice and support.




