Acoustic recordings, biological observations, and genetic identification of a rare(?) beaked whale in the North Pacific: Mesoplodon carlhubbsi
Abstract
Although Hubbs' beaked whale (Mesoplodon carlhubbsi) was previously known from over 60 strandings on both sides of the North Pacific, it had been identified alive in the wild only once, off Oregon in 1994. In September 2021, we conducted a search effort for beaked whales off the coast of Oregon using a towed hydrophone array and a visual search team. Approximately 350 km off the Columbia River mouth, we detected the vocalizations of an unidentified mesoplodont whale; we stopped our vessel and waited in the area until two unidentified juvenile Mesoplodon surfaced and stayed near our vessel for almost 2 hr. During that time, we took numerous photographs and videos, made behavioral observations, and recorded their vocalizations. The DNA sequence from a biopsy sample identified them as M. carlhubbsi. In this paper, we discuss our biological observations, including color patterning and acquired markings, behavioral observations, and describe for the first time the acoustic characteristics of this species. We confirm that M. carlhubbsi is the source of a previously unidentified acoustic signal known as BW37V, and we update what is known about the at-sea distribution of this species based on previous recordings and observational records.
1 INTRODUCTION
In 1945, Carl Leavitt Hubbs, a preeminent American ichthyologist, documented a beaked whale (Family Ziphiidae) that stranded alive on a beach near his office in La Jolla, California (Hubbs, 1946). Although originally misidentified (as Andrews' beaked whale Mesoplodon bowdoini), the skull and color pattern were different from any ziphiid previously known to science, and it was eventually recognized as a new species—Hubbs' beaked whale, M. carlhubbsi (Moore, 1963). It would be almost 50 years, however, before this species would be identified alive in the wild. On July 26, 1994, during a marine mammal survey off Oregon, two groups of Hubbs' beaked whales were identified by the color pattern considered to be diagnostic of adult males (Yamada et al. 2012; R.L.P., personal observation). To our knowledge, there have been no other reported sightings of this species at sea, and it continues to be known almost entirely from specimens stranded on beaches off western North America and Japan.
More recently, cetacean acousticians have been cataloging distinctive and unique sets of calls from unidentified beaked whales in the eastern North Pacific. From these, Baumann-Pickering et al. (2014) suggested that call type “BW40” could be Hubbs' beaked whale. Later, Griffiths et al. (2019) suggested that a different call type, BW37V, recorded from various locations off Oregon and California, was more likely to be Hubbs' beaked whale because its distribution more accurately reflected the known range of this species based on stranding data.
During September 2021, we conducted a research cruise that focused on visual and acoustic detections of beaked whales within the Exclusive Economic Zone (i.e., within 200 nmi/370 km of the coast) of Oregon. Here we report on an extended encounter with a pair of juvenile Hubbs' beaked whales. Species identification was confirmed by DNA sequencing of a biopsy sample; we also confirmed the link with the BW37V acoustic signal, and provide new information on the acoustic features, appearance, behavior, and distribution of this poorly known species.
2 METHODS
2.1 Acoustic sampling and data analysis
Research was conducted while onboard the 25.6 m R/V Pacific Storm (Marine Mammal Institute, Oregon State University). A hydrophone array was towed behind the vessel at mean depth of 27.5 m ± 8.7 m, all hours of all days at sea. Analog signals from two HTI-96-min (High-Tech, Inc.; Long Beach, MS) hydrophone elements in an oil-filled tube were digitized at 400 kHz with an NI-USB-6356 data acquisition system (National Instruments; Austin, TX) and recorded to hard disk with PAMGuard software (v.2.01.05; Gillespie et al., 2008). The hydrophones have a flat frequency response (±3 dB) from 1 to 30 kHz and a usable frequency range up to 150 kHz (see “standard hydrophone” calibration curve in Wildlife Acoustics, 2016). Digitized signals were decimated to 200 kHz and monitored in real-time using the spectrogram and time-bearing window of the PAMGuard software platform on a laptop computer. Pulsed sounds were automatically classified based on peak frequencies and presence of a frequency-modulated (FM) upsweep, and these classifications were displayed as color-coded symbols in a click-time-bearing window. Bearing angles relative to the main axis of the array were calculated in PAMGuard from the difference in arrival times of pulsed signals at the two hydrophones. Analysts monitoring these displays could select pulsed signals and display plots of their power spectrum and a Wigner-Ville time-frequency representation of the pulse. Pulses that appeared to be typical of beaked whales were selected as “event clicks” and bearing angles were plotted in PAMGuard relative to the ship's track.
Drifting acoustic spar buoy recorders (DASBRs; Griffiths & Barlow, 2016) were also used to remotely monitor beaked whales. DASBRs were deployed from the vessel and later recovered to download recordings. Each DASBR recorded signals from HTI-92-WB and HTI-96-min hydrophones (High-Tech Inc., Long Beach, MS) at approximately 100 and 110 m depths, respectively, on a SoundTrap ST4300 recorder (Ocean Instruments, Auckland, NZ) at a sampling rate of 384 kHz. These hydrophones had flat frequency responses (± dB) of 20 Hz–50 kHz and 20 Hz–30 kHz, respectively (illustrated as the “low noise” and “standard” hydrophones in Wildlife Acoustics, 2016). The HTI-92-WB hydrophone has less low-frequency self-noise (SPL equivalent of 27 dB re: at 1 kHz) than the HTI-96-min hydrophone (42 dB re: at 1 kHz). For this reason, we used the higher signal-to-noise ratio (SNR) signals from the former in quantifying beaked whale sounds.
To quantify the acoustic signals from our one Hubbs' encounter in more detail, acoustic files from both the towed hydrophone array and the DASBR were postprocessed in PAMGuard using the same settings as the real-time monitoring of the towed hydrophone array. Additionally, six template signals from North Pacific beaked whales (Cuvier's; Stejneger's, M. stejnegeri; Baird's, Berardius bairdii; BW70; BW37V; and BW43) were added to the power spectrum display to assist in relating the new signals to previously cataloged beaked whale signals (Baumann-Pickering et al., 2013; Griffiths et al., 2019; Stimpert et al., 2014; Zimmer et al., 2005); only one characteristic template was displayed for each species. Pulses were labeled as PAMGuard “events,” with each event representing a single individual (as best as possible). Data from pulses in these identified events were extracted from PAMGuard databases and binary files using the R package PAMPal (https://CRAN.R-project.org/package=PAMpal). The extracted data were used to characterize the time-frequency characteristics of the pulses we recorded using custom R scripts. The frequency characteristics of the signals were calibrated based on frequency responses of the HTI-96-min (towed array) and HTI-92-WB (DASBR) hydrophones (Wildlife Acoustics, 2016). The end of a discreet, continuous echolocation series was considered the end of a foraging event, and three of these we recorded were designated F1, F2, and F3. Between events F1 and F2, four separate surfacing sequences were visually observed (see below) and designated V1-V4, and between each of the surfacing events there were three separate shallow dive events designated D1-D3 (Table 1).
| Event | Visual start time (UTC) | Visual end time (UTC) | Visual duration (min) | Acoustic signals present (Y/N) | Number of acoustic signals and type | Acoustic start time (UTC) | Acoustic end time (UTC) | Acoustic duration (min) | Recording platform |
|---|---|---|---|---|---|---|---|---|---|
| F1 | Y | 6 > 60 kHz FM upsweeps, 16 BW37V | 19:12:33 | 19:15:52 | 3.3 | TA | |||
| V1 | 20:35:29 | 20:39:44 | 4.2 | N | |||||
| D1 | 20:39:44 | 20:42:19 | 2.6 | N | |||||
| V2 | 20:42:19 | 20:45:05 | 2.8 | N | |||||
| D2 | 20:45:05 | 20:49:30 | 4.4 | N | |||||
| V3 | 20:49:30 | 21:02:52 | 13.4 | Y | 131 surface clicks | 20:50:02 | 20:50:13 | 0.2 | TA |
| D3 | 21:02:52 | 21:08:22 | 5.5 | N | |||||
| V4 | 21:08:22 | 21:13:46 | 5.4 | Y | 21 BW37V | 21:08:34 | 21:08:49 | 0.2 | TA |
| F2 | Y | 1233 BW37V | 22:01:28 | 22:36:11 | 34.7 | DASBR | |||
| — | Y | 51 BW37V | 22:56:00 | 22:59:00 | 3.0 | TA | |||
| F3 | Y | 2918 BW37V | 01:39:59 | 02:02:07 | 22.1 | DASBR |
2.2 Visual survey
Concurrent with the acoustic data collection, an independent visual survey was conducted using two pairs of 25 × 150 mm binoculars mounted above the vessel's wheelhouse (6.7 m ASL), using standard line-transect methods (Buckland et al., 2001). A team of four observers rotated through two binocular stations at 30-min intervals, during daylight hours, weather permitting (generally, Beaufort sea state <6 and visibility >1 km). Data (date, time, visibility, angle and distance to sightings, species identity, and group size estimate) were recorded using the program SeaScribe (https://briwildlife.org/seascribe/).
2.3 Genetic identification of species
We used a 150-lb. draw weight, recurve crossbow to collect a skin biopsy sample from one of the whales (Animal 2). In the laboratory, total genomic DNA was extracted from the single sample using standard methods adopted for small samples (Baker et al., 1994). An approximately 500 bp fragment of the mitochondrial (mt)DNA control region was amplified and sequenced using standard methods described by Dalebout et al. (2004). The sequences were edited by eye and aligned in the program Sequencher vs 5.4.6 (GeneCodes Corporation). The edited mtDNA control region sequence was then submitted to GenBank (OQ567713) and compared to a curated reference database of all known species of beaked whales, using the web-based program DNA-Surveillance (Ross et al., 2003). The sex of the whale was determined based on amplification and agarose gel visualization of sex-specific markers (X chromosome: Aasen & Medrano, 1990; Y chromosome: Gilson et al., 1998).
3 RESULTS
3.1 Narrative of events
At 19:12 UTC (12:12 PDT) on September 22, 2021, frequency-modulated (FM) pulses were detected on the towed hydrophone array and identified as likely being from a Mesoplodon beaked whale because of a higher peak frequency than echolocation pulses from the other two genera of ziphiids commonly found in the eastern North Pacific (i.e., Ziphius and Berardius). The location was 45°56′N, 128°34′W, approximately 350 km off the Columbia River mouth (Figure 1); the water depth was 2,509 m. Bearing angles to the sound source were plotted in PAMGuard, and a likely location of the whales was estimated for both the left and right sides of the transect line, as there is an inherent ambiguity with a two-hydrophone linear array. After 3.3 min, the whales stopped echolocating and were presumed to be at the end of a foraging dive (F1; Table 1). At this time, the ship was positioned to keep both left and right localizations within 2 km of the ship, to give observers on the binoculars an opportunity to visually detect and possibly confirm species identification of the whale(s) if they surfaced.
At 20:35 UTC (13:35 PDT), we sighted a pair of mesoplodont whales (V1), 200 m off the port beam, swimming in the direction of our vessel, which was pointed downwind and moving just fast enough to maintain a heading (speed over ground 2–3 km/hr). Hereafter, we refer to them as Animal 1 and Animal 2, respectively. The sighting was 1.43 km and 83 min after the initial acoustic detection, but we do not know if this was the first surfacing after the clicking stopped. For the next 38 min, the whales were observed during three additional surfacing sequences (V2, V3, and V4; Table 1); they remained within 500 m of our vessel and usually <200 m. During the surface sequences, they usually stayed within 50 m of each other and sometimes as close as 2–3 m. Impulsive signals were detected on the array at 20:50 when the whales were visible behind the ship, and at 21:08, several near-surface FM echolocation pulses were recorded as they came within 100 m of the towed hydrophone array (Table 1).
Our repeated encounters with the two whales at the surface allowed us to visually assess their physical features at close range and to collect thousands of photos and video. Despite this, because they were juveniles (see below), we were unable to identify them to species, and genetic analysis of a biopsy sample was necessary to confirm their identity. During a close approach to the bow, we fired a biopsy dart that hit Animal 2 below the dorsal fin; the whale slapped the surface with its fluke, and both whales quickly swam away from the vessel. At that time, we lost track of the whales as we pulled in our towed hydrophone array in preparation to retrieve the biopsy dart. The whales were not seen again.
At 21:34, a DASBR was deployed between the location of last sighting and the initial acoustic detection and was allowed to drift for 37 hr. A 37.4-min series of beaked whale echolocation pulses (F2; Table 1) was recorded by the drifting DASBR 169 min after the first foraging dive (F1) ended. Another 22.1-min series of beaked whale echolocation pulses (F3) was recorded by the drifting DASBR, 184 min after the end of F2. At the start of the F2 echolocation series the DASBR was 0.59 km away from the final sighting location and 1.35 km away at the start of F3. After the biopsy dart was picked up, the towed hydrophone array was redeployed at 21:44. A 3-min series of beaked whale echolocation pulses was recorded from the towed array starting at 22:56, at which time the vessel was 3.45 km away from the last sighting location. This series was 20 min after the end of F2 recorded on the DASBR and may have been produced by the same group between F2 and F3 or may have been produced by another group that was not seen. Sperm whale (Physeter macrocephalus) clicks were detected periodically throughout the encounter, but none were seen, and no other cetacean vocalizations (e.g., delphinids) were detected.
3.2 Genetic identification of species
From the mtDNA sequence, we identified the whale as a Hubbs' beaked whale using reference sequences in the program DNA Surveillance. A BLAST search of GenBank confirmed the sequence was an exact match to the published record AY579511, a voucher specimen of Hubbs' beaked whale taken as bycatch in a California pelagic gill-net fishery. The whale was further identified as a female based on sex-specific genetic markers.
3.3 Acoustic characterization of vocalizations
Griffiths et al. (2019) designated as “BW37V” the echolocation pulse that they thought might be from Hubbs' beaked whale because it has a distinctive valley (or notch) in the frequency spectrum at ~37–39 kHz, between two frequency peaks at ~36 and 48 kHz. The FM echolocation signals that we recorded on the towed hydrophone array, and on the DASBR, before, during, and after our encounter with two Hubbs' beaked whales closely match BW37V. Most signals exhibited two frequency peaks in their frequency spectra with a valley between them (Figure 2, Table 2), with the exception of some FM upsweeps that were detected abeam of the array and contained energy above 60 kHz with low SNR (Table 1). Using the mean power spectrum, the resulting values of the two dominant peaks and the valley are very similar to those measured for BW37V (Table 2). We also report the mean frequency measurements (and standard deviations) from the pulses themselves but find they do not describe the uniqueness of BW37V as well as the characteristics derived from the mean power spectrum (Table 2). Mean interpulse intervals (IPI) were slightly higher for the towed array recordings but are well within the distribution reported for BW37V (Table 2). These FM pulses more closely resemble those described for BW37V than any other previously described beaked whale echolocation pulse types recorded in the North Pacific (Baumann-Pickering et al., 2013) and confirms the previous speculation that BW37V is attributable to Hubbs' beaked whale.
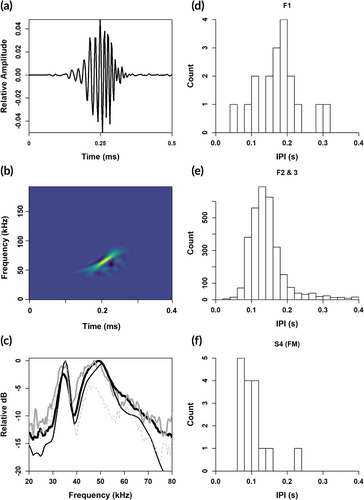
| Towed array (F1, this study) | DASBR (F2 and F3, this study) | DASBR (Griffiths et al. 2019) | |
|---|---|---|---|
| Taken from the mean power spectrum | |||
| Lower peak frequency (kHz) | 34.8 | 35.3 | 34.8 |
| Upper peak frequency (kHz) | 47.3 | 50.3 | 46.9 |
| Valley frequency (kHz) | 37.9 | 39.8 | 37.5 |
| Taken from the individual pulse level | |||
| −10 dB Center frequency (kHz) | 42.5 (29.1) | 41.8 (8.0) | 46.5 (9.1) |
| −10 dB lower endpoint (kHz) | 38.4 (27.4) | 36.8 (6.0) | 36.8 (4.9) |
| −10 dB bandwidth (kHz) | 8.1 (5.4) | 10.0 (6.2) | 19.3 (10.4) |
| Duration (μs) | 97.0 (39.8) | 137.5 (87.0) | 213.1 (87.7) |
| Interpulse interval, IPI (s) | 0.17 (0.06) | 0.16 (0.10) | 0.15 (0.06) |
| Sample size | 22 | 3,824a | 238 |
- a There were some outlier click durations that resulted in a large standard deviation. For the pulse level characteristics, the data have been truncated to durations ≤500 μs, resulting in a sample size of 3,515 pulses.
Additional pulsed signals were recorded from the towed array on two occasions when the whales were seen near the surface in the vicinity of the ship. During surface sequence V3 (Table 1), 131 low-frequency clicks were detected in four click trains at a bearing angle that was consistent with the location of the whales near the ship. These clicks contained no FM upsweep and had a median peak frequency of 4.4 kHz, a median duration of 130 ms, and median lower and upper 10 dB bounds of 3.0 and 6.6 kHz. ICI was variable and typically started around 0.032 s and increased in interval to 0.3 s, with a median ICI of 0.041 s (Figure 3). There were fewer clicks in the first click train than the subsequent three (n = 5, 52, 43, and 31, respectively). During surface sequence V4, 21 FM pulses were detected at the surface from both individuals (i.e., they were received from different bearing angles). These had spectral characteristics resembling echolocation click type BW37V (Figure 2), but with a much shorter IPI of 0.096 s. They also occurred in trains of 3–7 clicks. The visual team reported that the two individuals were oriented toward the array and approximately 100 m away when these clicks occurred.
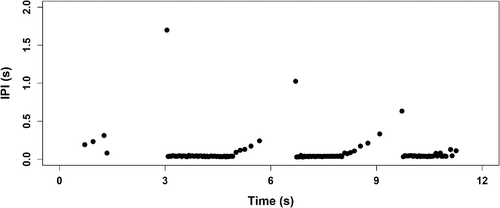
If the two different foraging events recorded by the drifting DASBRs (F2, F3) were made by the same pair of whales, the duration of a complete dive cycle, from the end of F1 to the end of F2 and from the end of F2 to the end of F3, was 200 min and 205 min, respectively.
3.4 Behavior, morphology, and color pattern
The whales seemed curious about the boat, initially swimming to within 10 m of our vessel and passing under the bow; at times they lifted their heads above the water and, in the photographs, appeared to be looking at us. Typically, they surfaced with their beaks projecting out of the water at an approximately 45° angle (Figure 4); at other times, their beaks remained low in the water when they surfaced (Figure 5a). They were small to average-sized mesoplodont whales with an estimated body length of 4.5–5 m and features typical for the genus: a spindle-shaped body, with a moderately sloping melon, and medium length beak (Figure 4). The gape was relatively straight but with a slight upward arch toward the rear; no erupted teeth were visible (Figure 4). The dorsal fin was located about two thirds of the way along the back; it was somewhat falcate and wide-based, low, and triangular (Figure 5b, see also Figure S2).


Both whales were presumed to be juveniles. Adult male and female M. carlhubbsi have distinctive, ontogenetically developed color patterns: males have a “brilliant white” beak and a white prominence in front of the blowhole; females also have a white beak, but the top of the head remains generally dark (Jefferson et al., 2015; Mead, 1989; Mead et al., 1982; see below). The whales had a nondescript, uniform gray, “juvenile color pattern” (Yamada et al., 2012) typical of young mesoplodont whales (Figure 5). Furthermore, because we did not see any other whales during the 120 min we spent with this pair (i.e., from first acoustic detection to the last visual observation), we inferred that they were independent of their mothers.
In good light, the sides of the face and melon were slightly paler than the rest of the head and body (Figure 4). The pale face of young M. carlhubbsi is framed somewhat by a dark longitudinal band that extends back, from the tip of the upper beak, most of the way to the blowhole; this feature is most pronounced in the fetus (fig. 7a,b in Mead et al., 1982), becoming less so in calves (Figure 6), and much reduced but still discernable in juveniles (Figures 4 and 5). The lips and tip of the beak were white in both whales (Figure 4). In contrast to the conspicuous all-white beak of adults, the beak of M. carlhubbsi calves is mostly dark (Figure 6) and lightens with age. It appears from Figure 4, that in maturing M. carlhubbsi the beak starts to lighten first at the tip and along the lips and spreads from there, a pattern that has recently been described for another white-beaked Mesoplodon: the strap-toothed beaked whale (M. layardii; Pitman et al., 2019).

Both whales had a conspicuous dark eyepatch that contrasted with the pale face (Figure 5a); a prominent feature in younger calves (Figure 6b). The trailing edge of the dark eyepatch merges with a dark gray band that extends dorsally up and over the back, behind the blowhole, and forms a vertical, posterior boundary to the pale face (Figures 4 and 5a,c); this band is also evident in younger calves (Figure 6a,b). Another color pattern feature on the calf is a thin, dark, eye-to-gape line that travels forward from the bottom of the dark eyepatch and meets the posterior end of the gape and possibly continuing onto the upper lip (Figure 6a,b). This latter feature occurs on many other young Mesoplodon (for examples, see M. densirostris, M. hectori, M. europaeus, etc., in Jefferson et al., 2015) and was still evident but obscured in the individuals that we photographed (Figure 5c). There was no other pigmentation patterning that we could discern on the back, sides, or head of either whale. Furthermore, except for the white lips and beak tip, these are subtle features, visible only in good light, and are largely absent in adult M. carlhubbsi, which, except for the white beak and melon, generally darken with age to a blackish color in adult males and females (Mead et al., 1982; Yamada, 2009).
Animal 2 was genetically confirmed to be a female. The beak of the adult female M. carlhubbsi has been described (and illustrated) as “distinctly lighter than the rest of the head” but showing less contrast than in adult males (Jefferson et al., 2015; Mead et al., 1982; Yamada et al., 2012). However, it now appears, from fresh-stranded individuals, that the beak of adult females can be just as white as that of adult males, although the top of the melon remains dark in females (photograph p. 144 in Jefferson et al., 2015; Figure 7).

3.5 Other markings
Both whales had acquired (i.e., adventitious) markings as well. Small, irregular patches of orangish-brown diatoms were scattered around the body, especially on Animal 1 (Figure 5a); diatom patches are common on beaked whales (e.g., Jefferson et al., 2015; Pitman et al., 2019; Ritter & Brederlau, 1999; Rosso et al., 2021). Both whales had a few superficial, short, linear scars, none of which appeared to be tooth-rake marks from conspecifics. They both also had a mottled appearance due to small, scattered, pale patches, which appeared to be due to sloughing skin (Figure 5a–c). Animal 1 also had at least two cookiecutter shark bites (Isistius spp., but see Grace et al., 2018, for other possible shark genera; Figure 5a), including a relatively fresh one with red, exposed flesh (not shown); Animal 2 also had at least two healed cookiecutter shark wounds (Figure 5b, c). The bite wounds that were largely healed were pale gray, and it appeared that they were going to heal the same color as the surrounding skin as it does in at least several species of Mesoplodon spp. (Pitman et al., 2019; Rosso et al., 2021). Animal 1 also appeared to have a damaged beak, perhaps the result of an injury: there was a prominent transverse crease on the rostrum, just forward of the base of the melon, and forward of the crease the rostrum had a slight upward bend (Figure 8).

4 DISCUSSION
4.1 Acoustic characteristics
Prior to this study, little was known about the acoustic signals produced by M. carlhubbsi. Previously, recordings were made from two young, captive individuals that had recently stranded (Lynn & Reiss, 1992; Marten, 2000; Figure 6). Both papers analyzed sounds recorded independently from the same individuals and described rapid, 0.3–2 kHz pulsed sounds that may have been burst pulses. Given the limitations of their equipment and methods, this frequency range may represent the pulse repetition rate. Lynn and Reiss (1992) also described 2.6–10.7 kHz whistles. None of the sounds described in these papers resemble the normal frequency-modulated (FM) echolocation pulses that are characteristic of other beaked whale species (Baumann-Pickering et al., 2013).
We recorded two different signal types on the towed array while the whales were at the surface: one containing FM pulses like those emitted while the whales were at depth, and another, which was lower in frequency and without the FM upsweep. Both types were emitted in discrete click trains. The FM pulses typically showed a distinctive valley (or notch) in the frequency spectrum at ~37–39 kHz which we suggest is the most distinctive and characteristic attribute of Hubbs' beaked whale echolocation signals. We believe that the low-amplitude FM pulses that did not show this characteristic were likely off-axis signals. Lynn and Weiss (1992) reported that two captive juvenile Hubbs' beaked whales emitted low frequency pulse sequences, and that these sequences occurred more often when humans were present. The number of FM pulses per sequence detected during surface event V4 was like those described by Lynn and Weiss (1992) but spanned a different frequency range (although their upper frequency limit was 40 kHz). These surface FM pulses had the same frequency content but a shorter ICI than those at depth, most likely due to the whales' proximity to the hydrophone array; we believe they were directing clicks at the array, thereby requiring a shorter two-way travel time than their typical foraging pulses.
Pulse sequences emitted by sperm whales are known as codas (e.g., Watkins & Schevill, 1977). The clicks recorded during V3 had more clicks per sequence (31–52 for three of the four sequences) than those previously described for either the captive Hubbs' beaked whales described above or for sperm whale codas in the Eastern Tropical Pacific (Weilgart & Whitehead, 1993, 1997). One study on Caribbean sperm whales reported coda sequences of up to 30 clicks (Moore et al., 1993), and another study off the east coast of Japan found more than 14 clicks per coda (Amano et al., 2014). Because we detected sperm whales on our recordings, we cannot rule out that those clicks could be an uncommon sperm whale coda from the eastern North Pacific, but they could also represent a previously undescribed form of beaked whale communication. There have been few reports of mesoplodont whale communication (Aguilar de Soto et al., 2012; Dunn et al., 2013), but our encounter was exceptional in that two, apparently unperturbed, juveniles stayed close to our vessel for approximately 2 hr, which may have provided a rare opportunity to record acoustic social communication within this genus.
Our estimate of dive-cycle duration (M = 204 min) for Hubbs' beaked whale is longer than the mean values for other beaked whale species based on tag data (summarized in Barlow & McCullough, 2023) but is within the range for Cuvier's and Blainville's (M. densirostris) beaked whales (Baird et al., 2006; Barlow et al., 2020; Schorr et al., 2014; Shearer et al., 2019). It is also longer than the median value (144 min) reported for Hubbs' beaked whale based on BW37V acoustic encounters, but again is within the range of observed values (Barlow & McCullough, 2023). It is also possible that interactions with our vessel could have affected their normal dive cycles.
4.2 Acquired markings
The relatively few cookiecutter shark bites present were likely due to the young age of the whales and because M. carlhubbsi is known to inhabit mainly cool temperate waters where cookiecutter sharks are less common (Ebert et al., 2013). Both whales also had a series of thin, dark, largely transverse lines over the top of the rostrum (Figures 4 and 8), and Animal 2 had similar-looking scars that also appeared to radiate down and back from the leading edge of the lower jaw arch (Figure 4b). Similar lines are often present on fresh specimens of Mesoplodon, including the stranded adult female M. carlhubbsi in Figure 7 (inset), and we suspect that these were acquired during prey capture (e.g., rake marks from squid beaks or tentacle hooks; see also Baird, 2016).
4.3 Juvenile pairing
The maximum length of M. carlhubbsi has been reported to be 5.3 m for both sexes (Yamada et al., 2012); based on our length estimates (4.5–5 m) and the juvenile color pattern described above, the animals we photographed were juveniles. In our experience, it is highly unusual for any Mesoplodon to exhibit the degree of interest in a vessel at sea that we observed (but see Barlow et al., 2022; Ritter & Brederlau, 1999; Rosso et al., 2021), and it is possible that the young age of these two whales explains their apparent curiosity. Our observation of two juveniles, seemingly about the same age based on similarity of color pattern development, traveling together, without adults present, is not unprecedented for M. carlhubbsi or perhaps for other ziphiids as well. A pair of young male M. carlhubbsi stranded alive at Ocean Beach in San Francisco on August 24, 1989 (Figure 6; lengths: 2.99 m and 2.87 m; California Academy of Sciences 23122 and 23751, respectively; Heyning & Mead, 1996, their figs. D and E; Lynn & Reiss, 1992), and two juvenile M. densirostris photographed swimming together in the Bahamas were reportedly unaccompanied by adults (Jefferson et al., 2015, p. 172, bottom right). In addition, pairs of unaccompanied juvenile Z. cavirostris have been observed in the Mediterranean Sea and Western North Atlantic (T.P., personal observation). Further observations will be necessary to confirm the prevalence and significance, if any, of pairing among juvenile beaked whales.
4.4 Rostrum injury
The apparent rostrum injury to Animal 1 (Figure 8) appeared to be relatively minor, but at least two M. carlhubbsi have stranded in California with damaged beaks that were suspected to be the cause of death. A 4.4-m female from San Simeon Bay, in April 1962, had a cracked lower jaw, and this “head injury” was the suggested cause of death (Roest, 1964; but see Mead et al., 1982 for corrected species identification). A 2.7 m male from Santa Cruz in May 2017 had the cause of death listed as “subacute maxillary and mandibular fracture, with secondary mixed bacterial infection from unknown source” (Long Marine Laboratory, Santa Cruz, CA).
Among 74 beaked whales that stranded in Western Australia between 1940 and 2010, six individuals of three species (one Blainville's beaked whale; four Gray's beaked whale, M. grayi; and one Shepherd's beaked whale, Tasmacetus shepherdi) had damaged rostrums and at least four of these were injured premortem (Groom et al., 2014). At least five of the six were immature, and Groom et al. (2014) suggested that younger individuals may be more susceptible to rostrum injury due to their bones not being fully ossified. Although the cause of death was not determined for any of these, Groom et al. (2014) stated that “presumably feeding would have been difficult due to the rostral injury.”
Dinis et al. (2017) reviewed rostrum damage in live ziphiids, describing examples from M. densirostris and Z. cavirostris. Among possible causes, they cited “trauma caused by intraspecific interactions including play, competition, or adult/juvenile interactions, interspecific interactions such as predation, or anthropogenic factors, including entanglement and ship strikes.” To that list, we would also add inadvertently swimming into objects (including the bottom) in the darkness of depths or at night while not echolocating, perhaps to avoid alerting killer whales. Although Dinis et al. (2017) concluded that beaked whales with major rostrum deformities could, at least in some cases, feed and reproduce normally, the prevalence of rostrum injuries among stranded beaked whales suggests that this may be an important and perhaps underrated source of mortality within this group (see also Groom et al., 2014).
4.5 Distribution and habitat
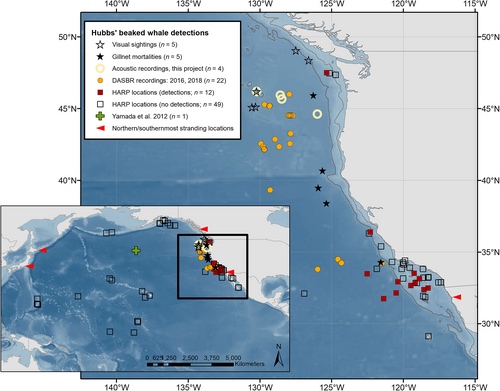
Nearly all prior information on the distribution of M. carlhubbsi comes from approximately 60 strandings in Japan and the west coast of North America (Yamada et al., 2012). The northernmost stranding reported from the eastern Pacific was Prince Rupert, British Columbia, Canada (Mead et al., 1982), and the southernmost was from Ensenada, Baja California, Mexico, in June 2011 (Heckel et al., 2020; Figure 1). The first live sightings at sea of which we are aware were of two separate groups, observed 57 min apart, off Oregon in July 1994 (Yamada et al., 2012; R.L.P., personal observation; Figure 1). In addition to the Oregon sightings, there are two previously unreported records that we have identified as M. carlhubbsi from photographs taken by B. Gisborne during Cetacean Research Program surveys by Fisheries and Oceans Canada. These included a group of 5–7 whales offshore of Vancouver Island, British Columbia, Canada, on March 4, 2015, at 48°22′N, 126°36′W (Figure S1), and a pair of whales, also off Vancouver Island, on July 12, 2016, at 49°04′N, 127°30′W (Figures 1 and S2). The second sighting was also confirmed based on acoustic recordings made at the time. To our knowledge, these were the first photographs of this species alive in the wild, although there have been photographs of living strandings (e.g., Nakajima et al., 2005).
With confirmation that M. carlhubbsi is the source of the BW37V vocalization, the at-sea distribution of Hubbs' beaked whale in the eastern North Pacific becomes clearer. Figure 1 shows the plotted locations of 47 at-sea detections of M. carlhubbsi, which includes the visual sightings described above (n = 5), gill-net mortalities reported by high seas fisheries observers (Griffiths et al., 2019; n = 5), previous acoustic detections of BW37V from free-floating DASBRs (Griffiths et al., 2019, n = 13; Simonis et al., 2020, n = 9), bottom-mounted HARPs (High-frequency Acoustic Recording Packages; Rice et al., 2021, n = 1 site; S.B.-P. & J.S.T., unpublished data, n = 11 sites), and our acoustic detections (n = 4, including 3 from the towed hydrophone array and 1 from a DASBR; Table S1). Also shown in Figure 1 are 50 HARP deployment locations in the North Pacific where there were no detections of BW37V (Rice et al., 2021; S.B.-P. & J.S.T., unpublished data). For purposes of this review, any DASBR detections recorded within 10 km of each other were considered duplicates and the location of only the first detection was plotted.
MacLeod et al. (2006) speculated that M. carlhubbsi might range continuously across the North Pacific between the latitudes of 30°N and 45°N (i.e., between the latitudes where nearly all the strandings have occurred), but they also acknowledged that there was no direct evidence to support their contention. Yamada et al. (2012) reported that a specimen of M. carlhubbsi had been collected in the mid-Pacific by a fishery observer at approximately 43°N, 163°W (Figure 1); from this they also inferred a trans-Pacific distribution, but we have not been able to locate this specimen or confirm the record.
Ziphiids are generally thought to preferentially associate with seamounts and continental slope areas (Groom et al., 2014, and references cited therein). However, as Griffiths and Barlow (2016) and Griffiths et al. (2019) point out, previous acoustic detections now confirmed to be M. carlhubbsi (BW37V) were regularly recorded in deep, oceanic waters over abyssal plains with no obvious topographic relief (Figure 1). This suggests a preference for habitat that could be defined as much or more by oceanography as bathymetry. This deep-water habitat extends across the North Pacific between the latitudes where all live detections and most strandings of M. carlhubbsi have occurred (Figure 1), which supports the idea of a continuous North Pacific range (MacLeod et al., 2006; Yamada et al., 2012), as is often depicted in range maps (Yamada, 2009; Jefferson et al., 2015). Furthermore, if M. carlhubbsi does occur across the entire North Pacific, it opens the possibility of a sizeable offshore population for what has historically been regarded as a rare whale with an uncertain population status, at least in the region of the California Current (Moore & Barlow, 2013, 2017). Identifying the acoustic signature of Hubbs' beaked whale can now allow for passive acoustic assessments of its distribution and relative abundance in the North Pacific and help determine its status there.
ACKNOWLEDGMENTS
We are grateful to our colleagues at NOAA Fisheries' Southwest, Northeast, and Pacific Islands Fisheries Science Centers, particularly Dave Weller, Annette Henry, Shannon Rankin, Jennifer McCullough, Sofie Van Parijs, and Sean Hayes for their support of this research, which included funding and equipment loans. We thank Robert L. Brownell, Jr. for comments that improved our manuscript, and The Marine Mammal Center (Sausalito, CA) and the Stranding Network Hokkaido, Japan, for providing important photographs. The talent and dedication of the captain (Ron “Yogi” Briggs) and crew (Ken Serven and Kevin Cool) of the R/V Pacific Storm contributed significantly to our success at sea. We received critical funding from Oregon State University's Marine Mammal Institute Gray Whale License Plate Program. Additional thanks go to John Ford, James Pilkington, Robert L. Brownell, Jr., and Jim Caretta for helping us locate records of M. carlhubbsi. The field research was carried out under MMPA/ESA permit #22306 issued to Southwest Fisheries Science Center, La Jolla, CA, and IACUC permit #SWPI2019-02 M issued to the Marine Mammal Institute, Newport, OR. The British Columbia field work was made possible by John Ford, Lisa Spaven, Robin Abernethy, and James Pilkington of Fisheries and Oceans Canada's Cetacean Research Program at the Pacific Biological Station, Nanaimo, BC; funding was provided by Fisheries and Oceans Canada's Species at Risk Program; data were collected under Marine Mammal Research License MML-01.
AUTHOR CONTRIBUTIONS
Lisa T. Ballance: Conceptualization; data curation; funding acquisition; investigation; project administration; resources; supervision; writing – original draft; writing – review and editing. Robert L. Pitman: Conceptualization; formal analysis; investigation; writing – original draft; writing – review and editing. Jay Barlow: Conceptualization; data curation; formal analysis; investigation; methodology; resources; software; supervision; validation; writing – original draft; writing – review and editing. Todd Pusser: Conceptualization; investigation; methodology; writing – original draft; writing – review and editing. Annamaria Izzi DeAngelis: Conceptualization; data curation; formal analysis; investigation; methodology; validation; visualization; writing – original draft. Craig Hayslip: Data curation; investigation; project administration; resources; writing – review and editing. Ladd M Irvine: Formal analysis; investigation; methodology; resources; visualization; writing – review and editing. Debbie Steel: Data curation; formal analysis; methodology; validation. Scott Baker: Data curation; formal analysis; methodology; resources; supervision; validation; writing – review and editing. Daniel Gillies: Investigation; resources; validation; writing – review and editing. Simone Baumann-Pickering: Conceptualization; investigation; writing – original draft; writing – review and editing. Jennifer S Trickey: Formal analysis; validation; writing – review and editing. Brian Gisborne: Data curation; funding acquisition; investigation; resources; writing – review and editing.




