Sexual dimorphism and morphometric relationships in pelvic bones of Commerson's dolphins (Cephalorhynchus c. commersonii) from Tierra del Fuego, Argentina
The hydrodynamic shape of the cetacean body and the reduction of the pelvic girdle leading to the loss of the pelvic limbs are the result of evolutionary adaptations to aquatic life; these changes are clearly represented in fossil remains (Thewissen et al. 2009). Only a few altered and reduced rudiments of pelvic bones (os coxae) remain in cetaceans (Berta et al. 2006). They consist of thin bony and/or cartilaginous structures, but without a symphysis or acetabular cavity, and are not articulated to the vertebral column (Bejder and Hall 2002). They are embedded within the abdominal wall muscles on both sides, cranial to the anus (Tajima et al. 2004). Additional pelvic limb bones corresponding to the femur, and rarely to the tibia, have been reported in some cetaceans (Omura 1978, 1980; Gingerich et al. 1990). It has been suggested that the first important reduction of the pelvic girdle occurred in the ilium (Gingerich et al. 1994, Bejder and Hall 2002). The identity and the establishment of homology among vestigial elements, found in the pelvic girdle, have not been recognized and remain under discussion (Bejder and Hall 2002). The vestigial pelvis is associated with the origin and attachment of muscles of the genital and anal region of both sexes (Adam 2002, Berta et al. 2006). A detailed anatomical study associated with the pelvic bones was made by Tajima et al. (2004) for the finless porpoise (Neophocaena phocaenoides). Relationships between such vestigial bones and the surrounding soft tissue are described as follows by these authors: the primary M. ischiocaudalis originates from the caudal portion of the pelvic bone and extends caudally to attach to the chevrons; the M. ischiocavernosus has its origin at the caudal portion of latero-dorsal surface of the pelvic bone and covers the M. bulbospongiosus, whose origin is more anteriorly than the former. Both latter muscles are larger in males than females (Tajima et al. 2004).
Several studies have shown the existence of clear sexual dimorphism in the pelvic bones of different species, such as the bottlenose dolphin (Rommel 1990), spotted and spinner dolphins (Stenella attenuata and S. longirostris) (Perrin 1975), harbor porpoise (Phocoena phocoena) (van Bree 1973), finless porpoise (Neophocaena phocaenoides) (Yoshida et al. 1994), common dolphin (Delphinus delphis) (Collet and Saint Girons 1984), Commerson's dolphin (Cephalorhynchus commersonii) (Collet and Robineau 1988, Goodall et al. 1988), and baleen whales (Heyerdahl 1973). In addition, morphometric analysis of the harbor porpoise's pelvic bones has shown positive correlation with sexual maturity (Andersen et al. 1992).
The Commerson's dolphin (C. c. commersonii) is found in shallow waters of the continental shelf off the eastern coast of South America between 40°S and 56°S (Goodall et al. 1988). It is probably the most abundant dolphin near shore, affected by incidental catch in Tierra del Fuego and southern Patagonia (Goodall 1978; Goodall et al. 1988, 1994, 2008) and vulnerable to other anthropogenic threats, such as pollution, habitat disturbances, and commercial shipping traffic throughout its range (Pimper et al. 2010, Cáceres-Saez et al. 2013a, b). Based on geographic, morphological, and genetic data, a new subspecies (C. c. kerguelensis) was determined for animals at the Kerguelen Islands in the Indian Ocean (Robineau et al. 2007). Externally, the sex of both subspecies of Commerson's dolphin can be determined from differences in the genital patch (Robineau 1984, Goodall et al. 1988). In decomposed carcasses, external pigmentation, internal reproductive organs and other soft tissue may be damaged or destroyed, hindering the ability to identify sex in those specimens. If the pelvic bones are present, they may contribute to the determination of sex. Although other studies have shown the existence of sexual dimorphism in this species (Collet and Robineau 1988, Goodall et al. 1988), they have failed to quantify morphometric relationships between the pelvic bones and age or total body length of individuals. The aim of this study was to determine sexual dimorphism across ontogeny in the Commerson's dolphins from southernmost South America.
We studied a total of 321 pelvic bones (139 pairs as well as 17 left and 26 right single bones) from 52 female and 130 male Commerson's dolphins from the Goodall collection (RNP) at the Museo Acatushún de Aves y Mamíferos Marinos Australes (AMMA), Tierra del Fuego, Argentina. Sex of the dolphins was determined by direct observation of the genital patch or genital organs during necropsies, or, when this region was damaged or decomposed, via molecular sexing (Pimper et al. 2009, 2010).
Three morphological measurements were used in the analyses: length and weight of the pelvic bone (LPB and WPB) and external total body length (TBL). The LPB was measured to the nearest mm along the bone's long axis from the cranial to the caudal tips (Fig. 1) using a caliper, and TBL as a straight line from the tip of the rostrum to the fluke notch of the dolphin (Norris 1961) taken at the time of encounter. For WPB each cleaned bone was weighed with a digital balance with an analytical scale to the nearest gram. During dolphin necropsies the pelvic bones and reproductive organs were carefully excised, the left and right bones were labeled and their cranial and caudal orientation evaluated. We adopted the use of standardized nomenclature in the Nomina Anatomica Veterinaria (NAV (Nomina Anatomica Veterinaria), 2012). Each pair of pelvic bones was cleaned by hand with a small knife, macerated in fresh water and dried at room temperature. Specimens were stored in plastic bags or mounted on cards tagged with their data, in a designated cupboard at the museum. Age was estimated following Myrick et al. (1983) and Hohn et al. (1989) for 40 females and 80 males, counting the number of growth layer groups (GLGs, Perrin and Myrick 1980) in dentine of the teeth. One GLG was assumed to represent one year (Lockyer et al. 1981, 1988; Dellabianca et al. 2012). In addition, 46 females and 119 males were examined and classified as follows for physical maturity based on fusion of vertebral body epiphyses (using visual examination of their periphery): Class 0, fetus or neonate in which the neural spine was still unfused to the body of the cervical vertebrae; Class 1, juvenile, with neural spines fused but no epiphyseal fusion; Class 2, subadult, with some epiphyses fused to their vertebral body, starting at head and tail; and Class 3, physically mature individuals with all vertebral epiphyses fused (Perrin 1975, Goodall et al. 1988). For a description of the group under study, the frequency distribution of external total body length (TBL) of both males and females was studied. Sexual dimorphism was analyzed by comparing the LPB and WPB between males and females.
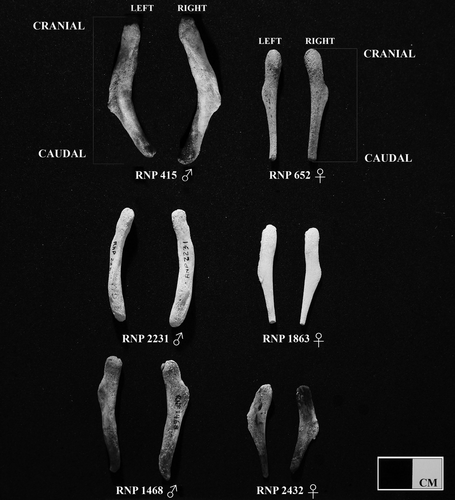
Morphometric Features
Differences (or asymmetry) in the length and weight between right and left pelvic bones for each sex were examined through a Student t-test for paired measures and ANCOVA (using the TBL as covariate). As we did not detect asymmetry in LPB or WPB (see below, but it should be pointed out that the mean was used in further statistical analyses), they were presented as a mean with standard deviation. Physical maturity of the specimens was used for a stratified analysis in classes. The Wilcoxon test was applied to evaluate sexual dimorphism within physical maturity classes on each morphometric measurement (LPB, WPB, and TBL). Data were tested for normal distribution and the homogeneity of variance was verified. The level of statistical significance was set at P < 0.05.
Fitting Growth Models
In order to analyze growth patterns in pelvic bones and compare them between sexes, allometric equations for each of the measurements of pelvic bones and total body length were analyzed. The relation of each variable (LPB and WPB) to TBL was examined with the equation of allometry: log(y) = log(a) + blog(x); where y is the measurement of the dependent variable, x is the total body length (TBL, independent variable) of the specimen, b is the growth coefficient of allometry (slope) and a is the y-intercept (Galatius 2005, Cassini et al. 2012). This model was fitted to the data using the standardized major axis (SMA) method, which is more appropriate for dealing with allometric approaches (Warton et al. 2006). The significance of allometry coefficients was evaluated by means of two-tailed t-tests. Deviations from isometry were assessed by comparing the allometric coefficient with that expected under geometric similarity (Alexander 1985). These were performed with F-tests setting the null coefficient at 1.0 in LPB (value expected under geometric similarity between two variables that grow linearly) and 3.0 in WPB (value expected under isometry between one variable that grows linearly [TBL] and the other which grows cubically [WPB]; Peters 1983). Following Read and Tolley's (1997) criteria, positive allometry was indicated when the growth coefficient was significantly higher (and negative when lower) than the expected isometrical values. Differences in the allometric coefficient (slope) between sexes were tested following the recommendations of Warton et al. (2006), using a likelihood ratio test for common SMA slope and comparing it to a chi-squared distribution (Warton and Weber 2002). All statistical analyses were performed using the smatr package in R software (Warton and Weber 2002).
 (1)
(1)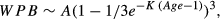 (2)
(2)Discriminant Function Analyses (DFA)
This analysis was performed to develop classification functions to assign the sex of the Commerson's dolphin by physical maturity classes. All DFA were cross-validated using the jackknife procedure and prior probabilities of belonging to each group were fixed to 50%. The cutting point for scores of males and females was calculated as the mean point between the weighted mean of the canonical variables for each group.
We found that the shape of pelvic bones (os coxae) was variable among specimens, although the main difference observed between sexes was that pelvic bones of males had a thicker and more rounded structure in cross-section. With increasing age, they become sturdier, developing a longitudinal torsion with a rounded knob at the cranial end (Fig. 1). In contrast, the pelvic bones of females were flat, narrower in the caudal section, this usually becoming more pronounced in older animals, forming a pointed tip (Fig. 1). The males under study ranged in TBL from about 85.6 to 148 cm (n = 110) and the females from 89.3 to 147.2 cm (n = 48), respectively (Fig. 2). Modal peak for males was at 121–130 cm and for females at 131–140 cm, suggesting that most of the specimens were adult.
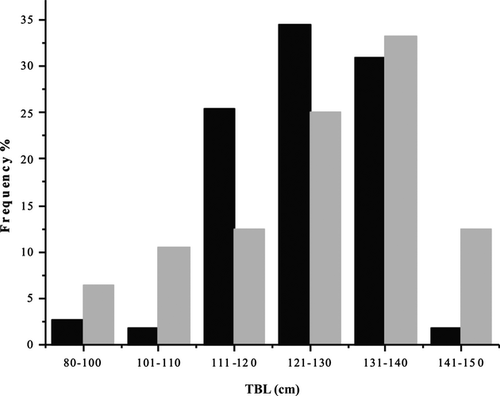
The LPB in males ranged between 18.35 mm and 66.80 mm, whereas in females between 15.35 mm and 47.13 mm. Through direct comparison between left and right LPB, no significant differences were found for males or females (t100 = 0.084 and t34 = 0.255, respectively). This is congruent with the ANCOVA results (F1,192 = 0.0529 males and F1,76 = 0.348 females). The pelvic bones weighed from 0.04 g to 2.27 g in males and from 0.03 g to 0.39 g in females. In addition, direct comparison between left and right WPB showed no significant differences for males and females (t99 = 0.601 and t35 = 1.194; respectively). This is also consistent with the ANCOVA results (F1,192 = 0.0395 males and F1,77 = 0.493 females).
Mean values and standard deviations for pelvic bone morphometric measurements and TBL by physical maturity classes (from Class 0 to III) and sex are presented in Table 1. Statistical analysis was not performed in Class 0 due to low sample size (only two specimens per sex). Significant differences associated with sex and physical maturity were found for measurements of the pelvic bones and TBL (Table 1). The WPB showed significant differences from Class I to III, while statistical differences in LPB were found for Class II and III. The TBL had differences only in Class III, with adult females being larger than adult males (136.26 ± 6.45 cm and 129.72 ± 5.44 cm, respectively).
| Physical maturity | Variable | Females | Males | Means difference (SE) | Wilcoxon test | ||
|---|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | ||||
| Class 0 | TBL | 2 | 89.15 ± 0.21 | 2 | 89.3 ± 5.23 | −0.15 (n.a.) | n.a. |
| LPB | 2 | 17.58 ± 3.15 | 2 | 21.53 ± 4.49 | −3.95 (n.a.) | n.a. | |
| WPB | 2 | 0.0375 ± 0.004 | 2 | 0.0875 ± 0.05 | −0.05 (n.a) | n.a. | |
| Class I | TBL | 6 | 114.36 ± 9.28 | 11 | 111.44 ± 5.37 | 2.921 (3.517) | 44.5 |
| LPB | 6 | 27.45 ± 3.35 | 13 | 29.61 ± 4.08 | −2.16 (1.92) | 26.0 | |
| WPB | 6 | 0.11 ± 0.04 | 13 | 0.20 ± 0.08 | −0.09 (0.04) | 12.0* | |
| Class II | TBL | 22 | 127.67 ± 11.01 | 52 | 126.32 ± 8.54 | 1.348 (2.374) | 636.0 |
| LPB | 26 | 32.97 ± 5.80 | 58 | 41.17 ± 6.67 | −8.19 (1.52) | 269.5*** | |
| WPB | 26 | 0.183 ± 0.08 | 58 | 0.617 ± 0.32 | −0.43 (0.04) | 73.0*** | |
| Class III | TBL | 10 | 136.26 ± 6.45 | 38 | 129.73 ± 5.44 | 6.531 (2.011) | 297.0** |
| LPB | 12 | 37.13 ± 5.59 | 46 | 49.54 ± 4.65 | −12.42 (1.57) | 18.0*** | |
| WPB | 12 | 0.229 ± 0.08 | 46 | 1.076 ± 0.30 | −0.85 (0.05) | 2.0*** | |
Note
- Statistical significance at *P < 0.05, **P < 0.01, ***P < 0.001, and n.a.: not applicable. Total body length (TBL) is given in cm, length of pelvic bone (LPB) in mm, and weight of pelvic bone (WPB) in g.
All regressions were statistically significant for both sexes, and showed correlation between LPB and WPB with TBL (Table 2). Relationships of LPB and WPB to the TBL in females had the highest determination coefficient (r2 = 0.65 and r2 = 0.66, respectively). In the allometric analysis, the slopes of variables differed significantly from the value expected for the isometric growth (Table 2). The LPB and WPB had positive allometric growth. For both variables, the slope of males was significantly higher than females (LPB: Likelihood ratio = 8.197, P = 0.004, df = 1; WPB: LR = 31.93, P < 0.0001, df = 1; Fig. 3A, B).
| Measurement | n | r 2 | Slope | 95% CI | Intercept | 95% CI | F-isometry | Tendency |
|---|---|---|---|---|---|---|---|---|
| LPB female | 48 | 0.65 | 1.89 | 1.59–2.25 | −4.35 | −5.38 to 3.33 | 61.74a | + |
| LPB male | 110 | 0.58 | 2.58 | 2.28–2.92 | −6.38 | −7.37 to 5.39 | 144.95a | + |
| WPB female | 48 | 0.66 | 4.35 | 3.67–5.17 | −14.29 | −16.61 to 11.96 | 19.73b | + |
| WPB male | 110 | 0.57 | 8.32 | 7.34–9.43 | −25.98 | −29.21 to 22.75 | 46.32b | + |
Note
- All regressions and F-test for isometry were significant (P < 0.0001).
- a Isometrical value = 1.
- b Isometrical value = 3.
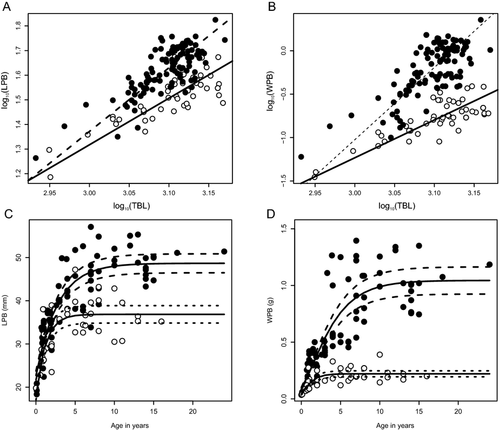
Significant correlation with age was found in the two variables studied (LPB and WPB) for both sexes. Males had a slightly higher correlation for length (ρ = 0.73) and weight (ρ = 0.84) of pelvic bones than females (ρ = 0.67 and ρ = 0.65) in relation to age. Growth parameters were obtained from the von Bertalanffy growth model in males and females of the Commerson's dolphin (Table 3) and curves were represented (Fig. 3). The LPB in females increased from birth until the age of ~6, or once the asymptotic value of 36.9 mm was achieved, while in males the LPB increased until the age of 10 and reached a higher asymptotic value of 48.7 mm (Fig. 3C, Table 3). Growth rate was also different between the sexes, and remained higher in females (Table 3). Regarding WPB, as with LPB, males reached higher asymptotic values than females (1.04 g and 0.22 g, respectively; Fig. 3D). The WPB in females increased from birth until the age of ~5, whereas in males it still grew until about 13 yr of age. The growth rate was also higher in females than in males (Table 3).
| Variable | Sex | n | Asymptotic measurement | Growth rate | Age at inflection | |||
|---|---|---|---|---|---|---|---|---|
| A | SE | K | SE | I | SE | |||
| LPB | F | 40 | 36.86 | 0.99 | 0.83 | 0.24 | −0.80 | 0.38 |
| LPB | M | 77 | 48.67 | 1.10 | 0.36 | 0.06 | −1.71 | 0.46 |
| WPB | F | 40 | 0.22 | 0.01 | 1.03 | 0.45 | 0.26a | 0.27 |
| WPB | M | 77 | 1.04 | 0.06 | 0.34 | 0.07 | 1.49 | 0.27 |
- a Value does not differ significantly from zero.
In all DFA functions the cutting point was zero, with the females taking negative values and males positive ones. Only the DFA function evaluated for physical maturity Class I has a nonsignificant Wilks' Lambda and had the higher total error rate from cross-validation analysis (Table 4). In all discriminant functions females had lower error rate than males. The best function was that constructed for physical maturity Class III, which had the lower total error rate (~2%).
| Physical maturity | Function | Wilks' λ | F (n df, d df) | P | Correct classification % | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reclassifications | Cross-validation | |||||||||
| Total | Female | Male | Total | Female | Male | |||||
| All | −0.0075 × TBL + 0.0574 × LPB + 2.3326 × WPB − 6.1929 | 0.5361 | 44.425 (3, 154) | <0.0001 | 83.54% | 93.8% | 79.1% | 81.64% | 89.6% | 78.2% |
| I | −0.0079 × TBL + −0.0529 × LPB + 16.5823 × WPB − 7.7565 | 0.5599 | 3.406 (3, 13) | 0.0502 | 82.35% | 100% | 72.7% | 70.59% | 83.3% | 63.6% |
| II | −0.0091 × TBL + 0.0768 × LPB + 3.2528 × WPB − 7.4645 | 0.5226 | 21.317 (3, 70) | <0.0001 | 85.13% | 90.9% | 82.7% | 85.13% | 90.9% | 82.7% |
| III | −0.0088 × TBL + −0.0219 × LPB + 3.9494 × WPB − 10.0336 | 0.2883 | 36.203 (3, 44) | <0.0001 | 97.92% | 100% | 97.4% | 97.92% | 100% | 97.4% |
The pelvic bones of the Commerson's dolphins off Tierra del Fuego differed between sexes in morphometric features. Male pelvic bones become thicker and sturdier, developing a longitudinal torsion with a rounded knob at the end of the cranial section (Goodall et al. 1988) (Fig. 1). Fagone et al. (2000) suggest that the thickening of pelvic bones in adult male manatees (Trichechus manatus latirostris) is due to sexual maturity and presence of testosterone. For a few species of the genus Stenella (Perrin 1975), the shape of the pelvic bones was remarkably different in males and females, even in juveniles. In other odontocetes, such as the finless porpoise, male pelvic bones were more elongated, robust, and larger than those of females (Tajima et al. 2004). Also, De Smet (1975) stated that sexual differences in the pelvic bones of cetaceans may be seen in relation to the presence of a large crus penis and the well developed ischiocavernosus muscle of males, for which the pelvic bone provides a good base for attachment. Collet and Robineau (1988) found sexual dimorphism in the size of pelvic bones in 11 sexually mature specimens of C. c. kerguelensis. Male pelvic bones of this subspecies also tend to be larger than those of females, ranging in length from 53.5 mm to 78 mm and 51 mm to 63.5 mm; respectively. We found sexual dimorphism in the LPB and WPB of the Tierra del Fuego Commerson's dolphins, especially in subadult and adult (physically mature) specimens. However, sexual dimorphism is more conspicuous and detected in early maturity stages on the WPB. Both variables grow at higher rates in males than in females with respect to TBL; with greater differences for the WPB than the LPB. As mentioned earlier, the pelvic bones are more robust in males than in females, and consequently heavier. This could explain why sexual dimorphism was detected in early stages for weight instead of length of the pelvic bones. Moreover, they grow with positive allometry, indicating that male adults have proportionately longer and heavier pelvic bones than females. This is in agreement with observations made in the harbor porpoise, where males show stronger positive allometry for length of pelvic bones (Galatius 2005).
The von Bertalanffy growth model results were coherent with both morphometric and allometric analyses. Male pelvic bones were larger than those of females, as was indicated by the asymptotic value reached in both LPB and WPB. We found that the mean TBL was different in the two sexes, according to the physical maturity classes. In this regard, differences were found in Class III, which would indicate that females reach a larger TBL than males; this difference is evident in adult specimens, reinforcing sexual dimorphism for length of pelvic bones. According to growth models, female pelvic bones grow faster, reaching their asymptote length at an earlier stage (~6 yr), than males that continue growing to at least age 10. Although Commerson's dolphins are sexually mature at about 5–6 yr of age, physical maturity, estimated from fusion of vertebral body epiphyses, is complete by 12 yr of age (Lockyer et al. 1988) and the continuity in growth becomes evident. Hence, male pelvic bones seem to continue to growth within an extended physical maturity. Physical maturity is attained after sexual maturity and is defined as when skeletal growth stops (Chivers 2002). Goodall et al. (1988) found that in adult animals (≥8 yr), the total body length of females was larger than in males and asymptotic length was also found to be greater in females than in males (Pedraza 2008; NAD, personal observation). The DFA was consistent with all the previous analyses discussed. Taking into account the coefficients of variables in the functions, pelvic bone weight has the higher contribution to the classification. This may be due to the fact that the differences became more conspicuous in weight from maturity of Class II. On the basis of our observations we conclude that the sex of Commerson's dolphins can be determined through morphometric assessment of their pelvic bones. As regards to LPB and WPB, we suggest that they might be employed to differentiate between sexes; particularly for specimens beginning at Class I and Class II (WPB and LPB, respectively).
Aspects of the specific musculature associated with pelvic bones in the Commerson's dolphin have not yet been described for Tierra del Fuego specimens. It is well known that the penis of terrestrial mammals is supported by the strong musculo-fibrous attachment of the crus penis to the ischial component at caudal end of the os coxae (Dyce et al. 1987, NAV 2012). Presumably the mechanical forces associated with these muscles influence the pelvic vestige size and shape (Adam 2002). Anatomical features indicate that in male cetaceans the pelvic bones still have a functional role in the act of copulation, while in the female such a role is limited (De Smet 1975), and probably also in support of the anus—via muscle attachment—and adjacent structures in both sexes. Hence, we hypothesize that larger and more robust pelvic bones in males (especially at the adult stage) might be related to the more highly developed muscles that attach to the penis. Nevertheless, further anatomical data regarding the associated musculature of the pelvic bones are needed to perform an extensive study; and to evaluate the functional hypothesis of sexual dimorphism related to mating behavior of this species.
Acknowledgments
Most of the research in Tierra del Fuego was carried out under grants from the Committee for Research and Exploration of the National Geographic Society to RNPG. We thank Total Austral SA, the Fundación RNP Goodall, and several other companies for their support of the Museo Acatushún de Aves y Mamíferos Marinos Australes (AMMA). We especially thank the AMMA assistants and volunteers who helped collect and clean specimens over the last 36 yr, P. Cuervo for assistance with the photographs, and S. Lucero for his comments. Research in Tierra del Fuego is carried out under permit from the local government. ICS thanks MO Cáceres for support and comments to improve an earlier version of this manuscript. At the time this paper was written ICS, NAD, LEP, FPB, and GHC were supported by Ph.D. fellowships from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) of Argentina. We are very grateful to the anonymous reviewers for their time and constructive remarks on the manuscript.




