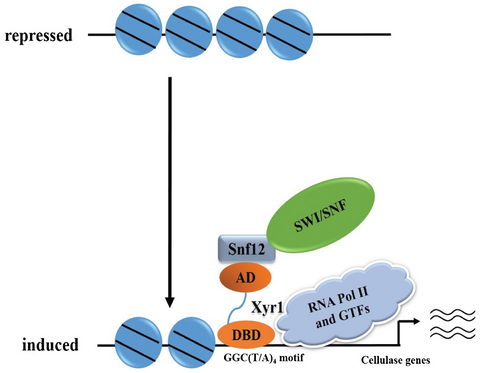
Trichoderma reesei XYR1 recruits SWI/SNF to facilitate cellulase gene expression
Summary
Cellulase gene expression in Trichoderma reesei is highly responsive to environmental cues and is under stringent regulation by multiple transcription factors. XYR1 (Xylanase regulator 1) has been identified as the most important transcriptional activator of cellulase/hemicellulase gene expression although the precise transactivating mechanism remains largely elusive. Here we show that the activation domain of XYR1 interacts with the T. reesei homolog of the TrSNF12 subunit of SWI/SNF complex. Deletion of Trsnf12 markedly impaired the induced cellulase gene expression. Individual loss of other SWI/SNF subunits including the catalytic subunit also severely compromised cellulase gene expression and interfered with loss of histone H4 in the cbh1 and eg1 promoters upon cellulose induction. In addition, we find that the SWI/SNF occupancy on cellulase gene promoters strictly required XYR1 and TrSNF12 but TrSNF12 was dispensable for the XYR1 binding to these promoters. These data suggest a model in which XYR1 recruits SWI/SNF through direct interactions with TrSNF12 to remodel chromatin at cellulase gene promoters, thereby activating cellulase gene expression to initiate the cellulolytic response in T. reesei.
Graphical Abstract
The mechanism by which the key transcriptional activator XYR1 activates cellulase gene expression in Trichoderma reesei remains largely unknown. We show that XYR1 interacts with the SWI/SNF homologous subunit TrSNF12 and potentially recruits this chromatin remodeling complex to alter the relevant chromatin environment, which then contribute to the induced cellulase gene expression.
Introduction
The saprophytic filamentous fungus Trichoderma reesei is one of the most prolific cellulase producers in industry. The enzyme cocktail secreted under biotechnological conditions by T. reesei is used in a number of applications including the pulp and paper, textile, food and biofuel industries (Lynd et al., 2002; Seiboth et al., 2012a). Energy efficient production of cellulases and hemicellulases in T. reesei is, however, achieved by tight gene regulation governed by both inducer-dependent expression and fast metabolized carbon source-mediated repression of related genes (Kubicek et al., 2009; Adav et al., 2011). A variety of other environmental and physiological factors have also been shown to affect T. reesei enzyme production (Pakula et al., 2005; Adav et al., 2011; Stappler et al., 2017). Several transcriptional factors have thus been identified to be involved in the regulation of the expression of cellulase genes in T. reesei. Among others, the xylanase regulator 1 (XYR1) is the most important transactivator, which is absolutely essential for expression of both xylanase and cellulase-encoding genes (Stricker et al., 2006). While cellulase gene expression has been shown to strictly follow the expression pattern of xyr1 (Mach-Aigner et al., 2008; Seiboth et al., 2012b), and XYR1 overexpression results in a full expression of cellulases even under non-inducing conditions (Lv et al., 2015), the exact mechanism by which XYR1 activates cellulase gene transcription remains elusive.
Eukaryotic cells tightly package their DNA with histones into chromatin. Successful access to the underlying DNA sequence by specific trans-acting factors and the general transcriptional machinery is thus critical for gene transcription (Struhl, 1999; Vignali et al., 2000). To overcome nucleosomal barriers in transcription, chromatin structure has to be dynamically modulated, a process that is dependent on chromatin-modifying enzymes working within large macromolecular complexes to either post-translationally modify histones or use ATP to slide or even evict histone octamers (Flaus and Owen-Hughes, 2001; Narlikar et al., 2013). Among the ATP-dependent chromatin remodelers, the SWI/SNF (SWItch/Sucrose NonFermentable) complex conserved from yeast to humans is the founding member made up of 12 subunits in yeast and 11-15 subunits in human including a highly conserved ATPase, SWI2/SNF2 (Peterson and Logie, 2000; Neely et al., 2002). Using ATP hydrolysis by the catalytic subunit, SWI/SNF disrupts histone-DNA contacts, resulting in sliding of the histone octamer on DNA or the eviction of histones (Kingston and Narlikar, 1999; Peterson and Workman, 2000; Sudarsanam and Winston, 2000). Recent studies have revealed a modular organization of these subunits within the yeast SWI/SNF complex with some subunits being crucial for the integrity and therefore the function of the complex whereas others being peripheral (Dutta et al., 2017). Together with the ARP module, composed of ARP7, ARP9 and RTT102, the catalytic module consisting of SNF2 and SNF11, is thought to form the catalytic core of SWI/SNF and harbors more than 90% of the catalytic activity of intact SWI/SNF (Yang et al., 2007). The SNF5/SWI3 module is made up of two submodules: a SNF6-SNF12-SWI3 submodule and a SNF5-SWP82-TAF14 submodule. Loss of SNF5, SNF6 and SNF12 in this module results in dissociation of the SWI/SNF complex (Dutta et al., 2017). While SWI3 has been shown to be important for scaffolding and assembling the entire SWI/SNF complex (Yang et al., 2007), SNF5 is thought to play a vital role not only as a bridge between SNF2 and the rest of the SWI/SNF complex including the independent SWI subunit, but also in promoting binding of the SNF2 ATPase domain to nucleosomal DNA and enhances the catalytic and nucleosome remodeling activities of SWI/SNF (Sen et al., 2017). This SNF5/SWI3 regulatory module is thus critical for the integrity of SWI/SNF and its loss differentially affects SNF2 occupancy at gene promoters (Dutta et al., 2017).
SWI/SNF is able to bind to both DNA and nucleosomes; however, it does so without sequence specificity (Cote et al., 1994; Quinn et al., 1996). Although different mechanisms have been suggested that might contribute to promoter recruitment of SWI/SNF, direct interactions between sequence-specific transcription activators and SWI/SNF have been considered as the primary mechanism of targeting the complex to specific genes (Yudkovsky et al., 1999; Prochasson et al., 2003). Studies in yeast have shown that acidic activation domains of the herpes virus VP16 protein, the yeast GCN4, HAP4, GAL4 and PHO4 activators can all directly interact with SWI/SNF through multiple contacts with the SNF5, SWI1 and SWI2/SNF2 subunits within the complex (Yudkovsky et al., 1999; Prochasson et al., 2003). In another model cellulolytic filamentous fungus Neurospora crassa, transcriptional factor WC-1 has been demonstrated to recruit SWI/SNF to remodel and loop chromatin at the circadian pacemaker gene frequency (frq) to initiate a circadian cycle (Wang et al., 2014). Therefore, promoter-specific recruitment seems to be a common mechanism for activation or even repression of SWI/SNF-regulated genes (Jackson et al., 1996).
Chromatin status changes have been recently shown to occur in T. reesei for cellulase genes in context to the applied conditions (repressing/inducing), which are probably mediated by specific transcription factors including XYR1 and the catabolite repressor CRE1 (Portnoy et al., 2011; Mello-de-Sousa et al., 2014; Mello-de-Sousa et al., 2015). Whereas changes in nucleosome positioning flanking a CAE (cbh2 activating element) region of the cbh2 promoter have been observed in response to applied conditions (Zeilinger et al., 1998), chromatin opening was found to occur in two prominent cellulase-encoding genes, cbh1 and cbh2, during cellulase induction compared to repression (Mello-de-Sousa et al., 2015). This opening is, however, strongly reduced in the absence of XYR1, suggesting that cellulase expression in T. reesei is clearly related to changes in the chromatin packaging although evidence supporting the direct involvement of XYR1 in mediating the chromatin status change is still lacking.
In this study, we identify a T. reesei TrSNF12 homolog as an interacting partner for XYR1. TrSNF12 also interacts with the homologous core subunit TrSWI1. Deletion of the relevant TrSWI/SNF subunit encoding genes severely compromises the induced cellulase gene expression. We further provide evidence that the SWI/SNF occupancy on cellulase gene promoters strictly requires XYR1 but TrSNF12 was dispensable for the XYR1 binding to these promoters. Moreover, the absence of TrSNF12 or TrSWI1 interfered with loss of histone H4 in cellulase gene promoters upon cellulose induction.
Results
TrSNF12 interacts with XYR1 in vitro
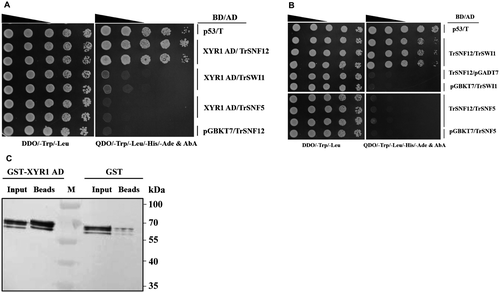
To explore the mechanism of XYR1 mediating the transcriptional activation of cellulase genes in T. reesei, a yeast two-hybrid (Y2H) screen was performed to isolate proteins interacting with XYR1 using its activation domain (AD, 776–860 aa) as a bait (Zheng et al., 2017b). One positive clone was retrieved revealing a sequence match for a full-length open reading frame of 501 amino acids annotated as a homolog of Saccharomyces cerevisiae SNF12 (GenBank: CAA96302.1), which hereafter was named TrSNF12 (jgi: Trire2:80835) (Fig. 1A). Further in silico analysis revealed that the T. reesei genome also contains predicted orthologs for other major components of the SWI/SNF complex (Table 1). While TrSNF12 also interacted with TrSWI1 but not TrSNF5, no interaction was found between XYR1 AD and these two core subunits (Fig. 1A and B). Together, these analyses suggest that an evolutionarily conserved SWI/SNF exists in T. reesei which may be similarly involved in chromatin modification and gene expression regulation. To further verify the interaction between TrSNF12 and XYR1 AD, we performed a pull-down assay. In accordance with the results of Y2H, His-tagged TrSNF12 was efficiently retained by the recombinant GST-XYR1 AD whereas only an insignificant amount of them could be detected in the GST coupled beads (Fig. 1C). Altogether, these results indicate that TrSNF12 directly interacts with XYR1 in vitro.
| Yeast subunits | N. crassa orthologs | T. reesei orthologs | Plate/liquid growth with deletion or down regulation | Cellulase gene expression with deletion/over expression |
|---|---|---|---|---|
| SWI1 | NCU05891 (28.57%a) | Tr77609 (26.63%a, 49.32%b) | Severely compromised/no effect | Abolished/no effect |
| SWI2 (SNF2)c | NCU06488 (56.29%a) | Tr57935 (48.96%a, 67.72%b) | No effect/no effect | Reduced (50%)/reduced (30%) |
| SWI3c | NCU08003 (53.12%a) | Tr21557 (53.12%a, 61.72%b) | No effect/no effect | Reduced (60%)/reduced (50%) |
| SNF5 | NCU00421 (35.95%a) | Tr54670 (32.71%a, 57.41%b) | Compromised/no effect | Abolished/no effect |
| SWP73(SNF12) | NCU03572 (22.13%a) | Tr80835 (23.09%a,56.49%b) | No effect/no effect | Reduced (50%~90%)/no effect |
| SWP82 | NCU03064 (30.99%a) | Tr36727 (31.88%a, 61.98%b) | nd. | nd. |
| SWP29 (TAF14) | NCU00444 (39.66%a) | Tr76330 (40.57%a, 58.67%b) | nd. | nd. |
| ARP7 | un. | Tr122701 (24%a) | nd. | nd. |
| SWP59 (ARP9) | NCU08840 (22.49%a) | Tr55381 (27.46%a, 56.45%b) | nd. | nd. |
| SNF6 | un. | un. | ||
| SNF11 | un. | un. | ||
| RTT102 | un. | un. |
- T. reesei and N. crassa homologs were listed based on their evolutionary similarity to the corresponding S. cerevisiae SWI/SNF subunits.
- un. = not identified. nd. = not determined.
- a Their amino acid identity to the S. cerevisiae counterparts was given in parenthesis.
- b The amino acid identity between orthologs of T. reesei and N. crassa.
- c Gene knock-down by Ptcu1 replacement.
TrSNF12 is a nuclear protein that contributes to the induced cellulase expression
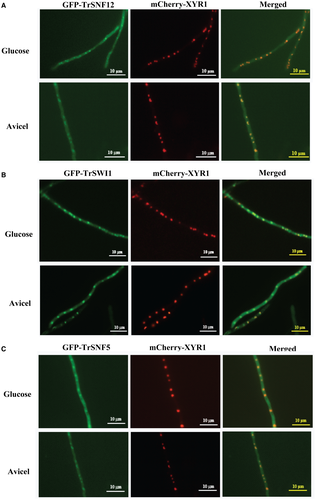
Considering the detected interaction between TrSNF12 and XYR1, the role of TrSNF12 in cellulase gene expression was investigated. Subcellular localization of TrSNF12 as well as two other core subunits, TrSWI1 and TrSNF5, was first investigated by constructing recombinant strains that simultaneously expressed the corresponding EGFP-tagged subunits and mCherry-XYR1 under the control of a copper responsive promoter Ptcu1 (Lv et al., 2015). When the conidia were germinated under cellulase repressing (glucose) and inducing (Avicel) conditions, all the GFP fluorescent signals were found to be predominant in the nucleus and well merged with the XYR1 fluorescence (Fig. 2). These results thus indicate that TrSNF12 as well as the two major core subunits are mainly nuclear proteins without a substantial change in its subcellular localization under different culture conditions.
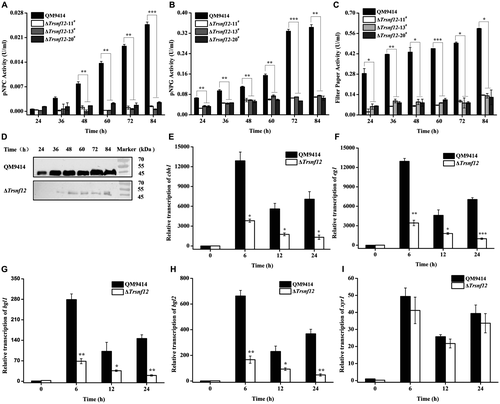
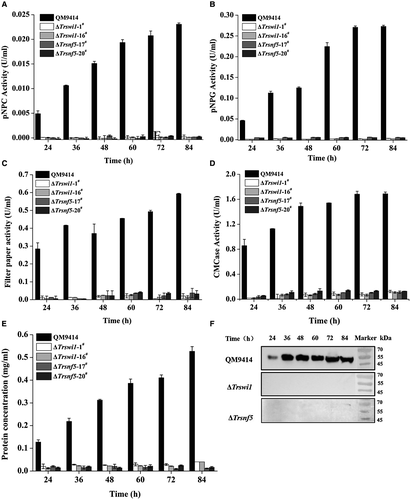
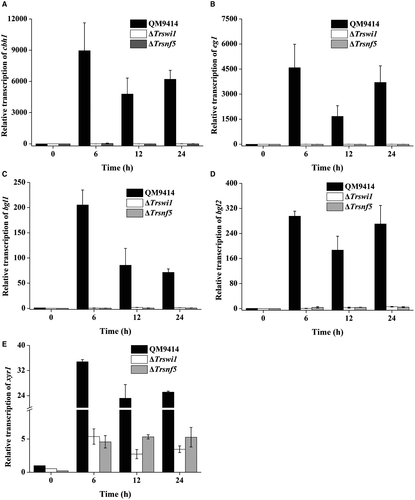
A Trsnf12 null mutant (ΔTrsnf12) was further generated by replacing the Trsnf12 coding region with the orotidine-5-decarboxylase gene pyr4 in QM9414Δpyr4 strain (Fig. S1). Deletion of Trsnf12 had hardly any effect on mycelia growth compared to QM9414 (Fig. S2). Analysis of the extracellular pNPC, pNPG and filter paper hydrolytic activities (FPA) from the ΔTrsnf12 culture supernatant on cellulose revealed that the absence of TrSNF12 resulted in an up to 20-fold reduction in extracellular cellobiohydrolase and a 5-fold reduction in β-glucosidase activities as well as FPA (Fig. 3A–C). Western blot analysis for the major cellulase component, CBH1, confirmed that 15~20 times less CBH1 was secreted in the Trsnf12 deletion strain than QM9414 (Fig. 3D). Further examination of endogenous cbh1, eg1, bgl1 and bgl2 mRNA levels by RT-qPCR demonstrated that the decreased cellulase activities as observed in the deletion strain resulted from a down-regulation in the steady state transcripts of these cellulase genes (Fig. 3E–H). Interestingly, disruption of Trsnf12 had no significant effect on xyr1 expression (Fig. 3I). Altogether, the data indicate that Trsnf12 plays an important role in modulating the induced cellulase gene expression in T. reesei.
Cellulase gene expression is compromised by the absence of other SWI/SNF subunits
To verify the potential role of TrSWI/SNF in cellulase gene expression, two other mutants with deletion of Trswi1 or Trsnf5 according to their homology with yeast SWI/SNF subunits were obtained (Fig. S3). Both subunits have been recently shown to be representative of two major SWI/SNF modules more directly associated with the TrSNF2 catalytic module (Dutta et al., 2017). Unlike Trsnf12, deletion of Trswi1 or Trsnf5 led to severely compromised vegetative growth and conidiation on agar plate although the final biomass of both deletion mutants was comparable to WT when cultured in liquid MA medium with glucose as the sole carbon source, suggesting that TrSWI/SNF also plays a role in hyphal growth and asexual spore formation on solid media (Fig. 4A). The slowed growth and reduced conidiation phenotypes of ΔTrswi1 and ΔTrsnf5 are consistent with those of yeast (Abrams et al., 1986; Laurent et al., 1990; Peterson and Herskowitz, 1992; Zhao et al., 2005). Unlike growth on plate, there was hardly any difference in biomass accumulation between the mutants and QM9414 in liquid culture with glucose as carbon source (Fig. 4B). To check the cellulolytic effect of Trswi1 and Trsnf5, the extracellular hydrolytic activities toward various substrates were determined using the ΔTrswi1 and ΔTrsnf5 culture supernatant on cellulose. In accordance with the ΔTrsnf12 phenotype, the absence of TrSWI1 or TrSNF5 abolished cellulase production (Fig. 5). Transcriptional analysis revealed that the impaired cellulase gene expression occurred at the transcription level since the deletion strains completely lost induced transcription of cbh1, eg1, bgl1 and bgl2 (Fig. 6A–D). Of particular note is that the transcription of xyr1 though not abolished, was significantly reduced on cellulose in mutant strains compared to QM9414, raising the possibility that the abolished cellulase gene expression in ΔTrswi1 and ΔTrsnf5 was partly resultant from the interference of the mutations with xyr1 expression (Fig. 6E).
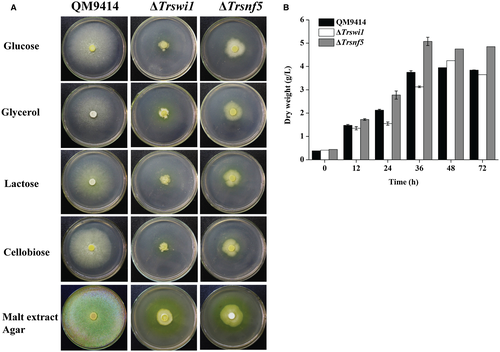
The growth and conidiation defect displayed by ΔTrswi1 and ΔTrsnf5 made it difficult to complement the respective deletion strain. We thus applied the promoter substitution strategy to achieve one-step replacement of the endogenous Trswi1 or Trsnf5 promoter with the Ptcu1 promoter (Zheng et al., 2017a). Consistent with the knock-out mutants, cellulase expression in the resultant Ptcu1-Trswi1 and Ptcu1-Trsnf5 strains was abolished in the presence of copper wherein the expression of Trswi1 or Trsnf5 was repressed, but remained almost the same as that of the parental strain without copper (Table 1 and Figs S4 and S5), suggesting that overexpression of Trswi1 or Trsnf5 otherwise does not facilitate cellulase expression. Similarly, Ptcu1 replacement of the promoter of Trsnf2 or Trswi3, which encode the catalytic subunit and another core subunit of the SNF5/SWI3 regulatory module including SNF12 respectively (Dutta et al., 2017), revealed that cellulase expression in the resultant strains was significantly reduced in the presence of copper. In contrast to Trswi1 and Trsnf5, overexpression of these two subunits without addition of copper somehow also interfered with cellulase expression (Table 1 and Fig. S6). Importantly, the impaired cellulase gene expression in the presence of copper was restored by complementation of the promoter-substituted strains with Trswi1 and Trsnf5 expressed under the Pgpd promoter respectively (Fig. S7A–D). The same strategy also rescued the cellulase expression of the Trsnf12 deletion mutant (Fig. S7E and F). Taken together, these data indicate that, similar to TrSNF12, other modular subunits and thus a functional SWI/SNF complex seem to contribute significantly to the induced cellulase gene transcription.
TrSWI1 binding to cellulase gene promoters relies on XYR1 and TrSNF12
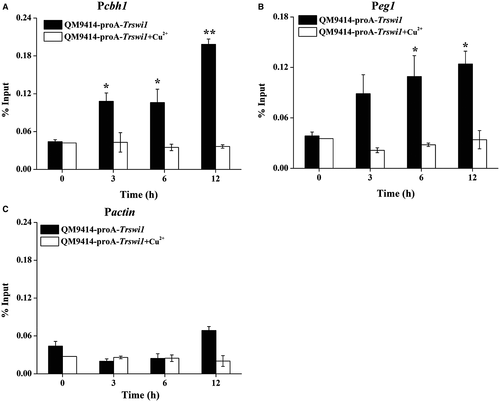
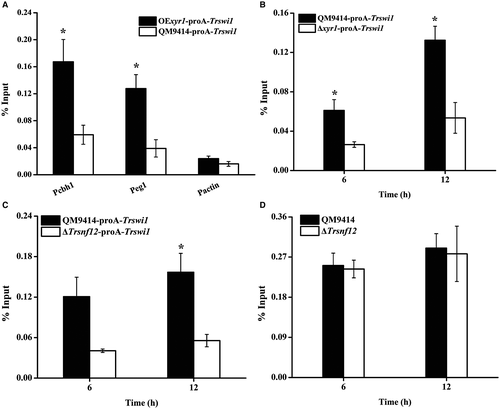
To test whether TrSWI/SNF (using TrSWI1 as a proxy) is recruited by XYR1 to the cellulase gene promoter, chromatin immunoprecipitation (ChIP) was performed in QM9414 expressing a protein A-tagged TrSWI1 under the control of Ptcu1. Fusion of protein A with TrSWI1 hardly affected its normal function (Fig. S8). TrSWI1, when expressed, bound strongly to cellulase gene promoters only with cellulose induction whereas no significant binding was observed if the strain was cultured in the presence of copper wherein the ProA-TrSWI1 expression was repressed (Fig. 7A and B). As expected, significant enrichment of TrSWI1 was not detected on the actin promoter regardless of the culture conditions (Fig. 7C). To determine whether SWI/SNF recruitment depends on TrSNF12 and XYR1, ChIP assays were performed either in QM9414 overexpressing XYR1 or in the Δxyr1 and ΔTrsnf12 strains, all of which simultaneously expressed TrSWI1 in the absence of copper. Comparable Trswi1 transcription in these strains was confirmed by quantitative RT-PCR (Fig. S9). XYR1 overexpression has been reported to lead to high-level cellulase gene expression even under repressing conditions (Lv et al., 2015). In accordance with this, TrSWI1 binding was found to occur even when cultured with glucose as the sole carbon source wherein XYR1 was expressed simultaneously (Fig. 8A). In contrast, no significant TrSWI1 binding to cellulase gene promoters was observed in the absence of XYR1 or TrSNF12 under cellulase inducing conditions (Fig. 8B and C). Similar analyses of the XYR1 binding to the same DNA sequence revealed no significant difference between the WT and Trsnf12 deletion strains (Fig. 8D). Together, these data suggest that SWI/SNF recruitment to cellulase gene promoters strictly depends on the expression of XYR1, most probably through its direct interactions with XYR1.
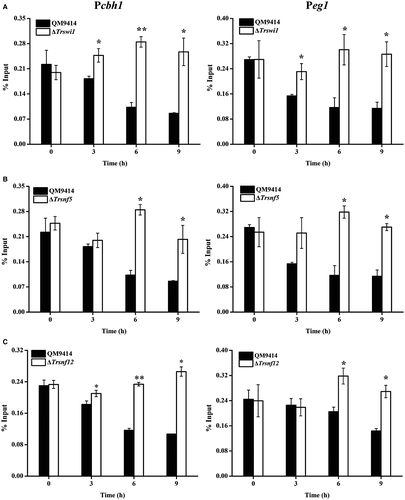
To further test whether SWI/SNF recruitment is associated with chromatin status change in cellulase gene promoters, loss of nucleosome components from the indicated promoter regions was tracked by ChIP to assay the loss of histone H4 (Fig. 9). Compared to non-inducing conditions, a gradual but significant loss of histone H4 was observed with cellulose induction in WT strain. In contrast, H4 occupancy on cellulase gene promoters appeared to be continuously present in ΔTrswi1, ΔTrsnf5 and ΔTrsnf12 background respectively. In all these data suggest that SWI/SNF is involved in remodeling nucleosomes positioned in cellulase gene promoters to activate their transcription.
Discussion
In this study, we showed that the major transactivator XYR1 recruits SWI/SNF to cellulase gene promoters to aid in remodeling the nucleosome environment, which thereby contributes to the efficient cellulase gene transcription. A search for interacting partners for XYR1 identified TrSNF12, a regulatory submodular component of SWI/SNF, which interacts directly with XYR1 in vitro. TrSNF12 as well as other modular subunits including the catalytic TrSNF2 of SWI/SNF was demonstrated to play critical roles in cellulase expression in T. reesei. In addition, TrSWI/SNF binding to cellulase gene promoters strictly relied on the expression of XYR1 and TrSNF12.
Although several transcriptional factors have been identified to be involved in regulating the expression of cellulase genes, only limited data are available on the role of chromatin in regulating transcription from cellulase gene promoters in T. reesei. Nonetheless, nucleosome repositioning or loss of nucleosomes upon cellulose induction has been shown to occur on cbh1 and cbh2 promoters (Zeilinger et al., 1998; Zeilinger et al., 2003; Mello-de-Sousa et al., 2014). Moreover, changes in the chromatin status of the regulatory regions of cellulase genes have been demonstrated both in context to the applied conditions (repressing/inducing) and to the presence of XYR1 (Mello-de-Sousa et al., 2015). However, so far it has not been investigated how exactly XYR1 additionally influences the chromatin packaging. In our present research, genes encoding components of SWI/SNF were identified in T. reesei with considerable identities to their orthologs in yeast and another cellulolytic model filamentous fungus N. crassa, suggesting that an evolutionarily conserved SWI/SNF exists in T. reesei. Although yeast SWI/SNF has been reported to consist of 12 identified subunits, only 9 ortholog encoding genes were identified in T. reesei. Whereas the missing orthologs of RTT102 and SNF11 does not contribute to the integrity of the complex in yeast, loss of SNF6 has been reported to decrease the association of other SWI/SNF subunits with the catalytic module (Dutta et al., 2017). Possibility thus exists that orthologs distantly related to these yeast counterparts do exist in T. reesei which awaits further identification.
A modular SWI/SNF complex architecture has been proposed and this modularity has been also reflected in gene expression profiles, in which changes clustered similarly upon deletion of subunits in same module (Dutta et al., 2017). Regardless of this, loss of subunits within closely related modules can have different effects in the regulation of specific genes (Dutta et al., 2017). In the present study, we found that, in contrast with Trsnf12 deletion, the absence of TrSWI1 or TrSNF5 displayed serious growth defect and produced much fewer conidia, which are consistent with those of yeast (Abrams et al., 1986; Laurent et al., 1990; Peterson and Herskowitz, 1992; Zhao et al., 2005). This phenotypic difference may reflect the fact that, compared to SNF12, SWI1 and SNF5 are both more closely associated with the catalytic module, the absence of which exerts a more significant effect on the integrity and thus function of the complex. Moreover, overexpression of the catalytic subunit TrSNF2 or TrSWI3 of the same regulatory module within which SNF5 and SNF12 are included also displayed effects different from those of SWI1 or SNF5 on cellulase gene expression. This observation was in accordance with the note that although the integrity of SWI/SNF complex is required for transcription regulation, loss of its subunits can have different effects in the regulation of a subset of genes. Notwithstanding these, the observed defects in relevant physiological processes as well as their effect on induced cellulase gene expression suggest that a functional SWI/SNF exist in T. reesei.
An important question regarding SWI/SNF function is how the complex might be targeted to a specific promoter. Experiments thus far suggest three different mechanisms that might contribute to promoter recruitment of SWI/SNF (Prochasson et al., 2003), one of which poists sequence-specific transcription factors being able to interact with SWI/SNF and may thus recruit the complex to specific genes. Previous studies thus demonstrated that multiple yeast SWI/SNF subunits including SWI1, SNF5 and SNF2, can bind transcription activators and there exists a functional redundancy of the SNF5 and SWI1 activator-interaction domains in facilitating SWI/SNF recruitment (Prochasson et al., 2003). In our present research, SNF12 instead of SWI1 and SNF5 was found to interact with the transcriptional activation domain of the transactivator XYR1 AD in vitro. In this respect, ySNF12 has also been reported to be required for activation with an enhancer element that binds the transcription factors SWI5p and PHO2p but no direct interaction between SNF12 and these activators has been reported (Cairns et al., 1996). On the contrary, various transcription factors have been implicated in interactions with different human SWI/SNF subunits. While BAF250, a yeast SWI1 homolog, directly interacts with the glucocorticoid receptor (Nie et al., 2000), the BAF155 and BAF170 subunits (two yeast SWI3 homologs), along with the BRG1 subunit (a yeast SWI2/SNF2 homolog), directly interact with the zinc finger DNA-binding domain of EKLF (Lee et al., 1999; Kadam et al., 2000). Although it is possible that XYR1 may simultaneously target other subunits within SWI/SNF in vivo, the observation that loss of TrSNF12 abolished the SWI/SNF recruitment indicates the importance of TrSNF12 in mediating XYR1 recruitment of SWI/SNF specifically to cellulase gene promoters for the function of the complex. On this point, one possible explanation for the more severe growth and conidia production phenotypes upon Trswi1, Trswi3 or Trsnf5 deletion is that loss of these subunits could impair SWI/SNF recruitment to more set of genes than loss of TrSNF12 while TrSNF12 were to specifically bring SWI/SNF in contact with XYR1 at cellulase gene promoters. It will be critical in the future to construct activator interaction mutants of TrSNF12 and then use them to verify the relevance of this interaction in vivo.
Regarding SWI/SNF recruitment by transcription factors, two alternative models have been proposed. Whereas transcription factors that have a strong binding affinity for a promoter associate first with an upstream activation sequence (UAS) and then recruit SWI/SNF, activators with relatively weak binding to UAS tend to recruit SWI/SNF off DNA first and then bound SWI/SNF facilitates DNA association of these transcription factors (Burns and Peterson, 1997; Cosma et al., 1999; Gregory et al., 1999). The observation that TrSWI1 binding to cellulase gene promoters strictly depends on XYR1 whereas XYR1 binding to the same DNA sequence showed no significant difference in WT and ΔTrsnf12 strains, suggests that XYR1 follows the first recruitment model of binding in which XYR1 binds strongly to the relative gene promoters and SWI/SNF recruitment is a subsequent event and not associated with binding of XYR1. Again, creating an activator interaction-deficient TrSNF12 would allow us to test more precisely the effects of lack of specific recruitment on cellulase gene expression.
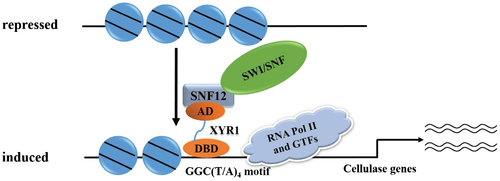
A working model based on these data is summarized in Fig. 10. Under repressing conditions, nucleosome positioning in cellulase gene promoters or even in the open reading frames limits the accessibility of the involved regulatory regions to relative transcription activators as well as the general transcriptional machinery. Upon cellulose induction, increased XYR1 whose transcription activation may also subject to chromatin remodelers, binds to its binding sites and recruits SWI/SNF which aids in repositioning or even losing nucleosomes. These changes in chromatin status may allow for more efficient binding of other transactivators, and in the meantime prevent binding of repressors, e.g., CRE1, thus facilitating the initiation of cellulase gene transcription.
Experimental procedures
Strains and culture conditions
Escherichia coli DH5α cells were used for plasmids construction and E. coli Origami BL21 (DE3) cells were used as a host for the production of the recombinant proteins. Both strains were cultured in lysogeny broth with a rotary shaker (200 rpm) at 37°C.
The S. cerevisiae strain Y2H Gold (MATa, ura3-52, his3-200, ade2-101, trp1-901, leu2-3, 112, gal4Δ, gal80Δ, LYS2::GAL1UAS-Gal1TATA-His3, GAL2UAS-Gal2TATA-Ade2URA3::MEL1UAS-Mel1TATA AUR1-C MEL1) was used as the host for the two-hybrid screen. Yeast cells were routinely cultivated at 30°C in YPD medium (1% of yeast extract, 2% of peptone and 2% of glucose). Synthetic complete (SC) medium lacking tryptophan, leucine, histidine and adenine with 100 ng ml−1 of AbA was used for transformant selection, and growth was allowed for the indicated periods at 30°C. For plate growth assays, serial dilutions of yeast cell suspensions were spotted onto selective plates containing 100 ng ml−1 of AbA.
Trichoderma reesei QM9414 (ATCC 26921) and QM9414Δpyr4 in which the uridine trophic marker gene was deleted in QM9414 (Wang et al., 2019) were used throughout this work as control and parental strains respectively. All T. reesei strains were maintained on malt extract agar supplemented with 10 mM of uridine when necessary. For the transcription and cellulase production analyses, T. reesei strains were pre-grown in 1 L Erlenmeyer flasks on a rotary shaker (200 rpm) at 30°C in 250 ml Mandels-Andreotti (MA) medium with 1% (v/v) of glycerol as the carbon source for 48 h as previously described (Zhang et al., 2013). Mycelia were harvested by filtration and washed twice with medium without a carbon source. Equal amounts of mycelia were then transferred to fresh medium without peptone containing 1% (w/v) of Avicel or other carbon sources as indicated, and incubation was continued for the indicated time periods.
Plasmid construction for the yeast-based screen
Preparation of the cDNA library from mycelia of the strain QM9414 induced on Avicel was carried out as described previously by Cao et al. (2017). The bait plasmid pGBKT7-XYR1 AD was constructed as described previously by Zheng et al. (2017b).
Isolation of Trsnf12 cDNA from the T. reesei cDNA library
The bait plasmid pGBKT7-XYR1 AD was transformed into Y2H Gold to obtain the bait strain, which could not grow on plates lacking tryptophan, leucine, histidine, adenine and with 100 ng ml−1 AbA, indicating that the XYR1 AD itself could not drive reporter gene expression by itself. Ten micrograms of T. reesei cDNA library plasmid was then transformed into the bait strain. Transformants were selected on the above SC plates. Growing colonies were picked and the harbored plasmids were verified by DNA sequencing after retransformation.
Trsnf12, Trswi1 and Trsnf5 gene disruption or Ptcu1-based replacement plasmids and recombinant T. reesei strains construction
To delete Trsnf12, two DNA fragments corresponding to approximately 2.2 kb of Trsnf12 up- and downstream non-coding regions were amplified from QM9414 genomic DNA and ligated into pDONORpyr4 via BP-cloning (Invitrogen) to yield the disruption vector pDONORTrsnf12pyr4, which was used to transform T. reesei QM9414Δpyr4 after linearization with I-SceI. The pDONORpyr4 plasmid was obtained as described previously (Zhang et al., 2013). Similarly, to delete Trswi1 or Trsnf5, approximately 2.2 kb of Trswi1 up- and 2.0 kb of downstream non-coding regions or 2.0 kb of Trsnf5 up- and downstream non-coding regions were amplified from QM9414 genomic DNA and ligated into pDONORpyr4 via BP-cloning to yield the pDONORTrswi1pyr4 and pDONORTrsnf5pyr4 disruption vectors, respectively, which was used to transform T. reesei QM9414Δpyr4 after linearization with I-SceI.
To construct Ptcu1-based promoter replacement vectors for the Trsnf12, Trswi1, Trsnf5, Trsnf2 and Trswi3 genes, the 2.0 kb flanking sequence upstream from the initiation code ATG and an 1.8 kb fragment downstream from ATG of the Trsnf12 gene were amplified from genomic DNA of QM9414, digested with HindIII/AscI and NotI/SpeI, respectively, and ligated into the corresponding sites of the pMDPtcu1-pry4 plasmid (Zheng et al., 2017b) sequentially to obtain pMDPtcu1-Trsnf12. Similarly, two DNA fragments corresponding to approximately 2.0 kb of up- and downstream the ATG codon of the respective genes were amplified from genomic DNA of QM9414, digested with HindIII/AscI and NotI/SpeI for Trswi1, HindIII/AscI and NotI/SpeI for Trsnf5, Trsnf2 and Trswi3, respectively, ligated into the corresponding sites of pMDPtcu1-pry4 digested with the corresponding enzymes and ligated into the corresponding sites of the pMDPtcu1-pry4 plasmid to obtain the pMDPtcu1-Trsnf5, pMDPtcu1-Trswi1, pMDPtcu1-Trsnf2 and pMDPtcu1-Trswi3 plasmids. These plasmids were linearized with HindIII before being transformed into T. reesei QM9414Δpyr4, to obtain Ptcu1-based promoter replacement strains Ptcu1-Trsnf12, Ptcu1-Trswi1, Ptcu1-Trsnf5, Ptcu1-Trsnf2 and Ptcu1-Trswi3 strains.
In order to perform chromatin immunoprecipitation assays of TrSWI1, the pMDPtcu1-Trswi1 plasmid was first digested by AscI and NotI to release the Ptcu1 promoter. The Ptcu1 promoter fused with the protein A coding sequence amplified with fusion extension PCR (Heckman and Pease, 2007) was then insert into the above digested pMDPtcu1-Trswi1 to obtain the pMDPtcu1proA-Trswi1 plasmid, which was linearized with HindIII before being transformed into the QM9414, Δxyr1, OExyr1 (Cao et al., 2017) and ΔTrsnf12 strains, respectively, to obtain the QM9414-proA-Trswi1, Δxyr1-proA-Trswi1, OExyr1-proA-Trswi1 and ΔTrsnf12-proA-Trswi1 strains.
To determine the subcellular localization of GFP-TrSNF12, GFP-TrSWI1 and GFP-TrSNF5, the Trsnf12, Trswi1 and Trsnf5 coding sequences were amplified from QM9414 cDNA, digested with NotI and SpeI, and then ligated into the pMDPtcu1-gfp-TtrpC plasmid (Lv et al., 2015) to generate the pMDPtcu1-gfp-Trsnf12, -gfp-Trswi1 and -gfp-Trsnf5-TtrpC respectively. These plasmids were then individually co-transformed with Ptcu1-mCherry-xyr1-pyr4 (Zheng et al., 2017b) into QM9414Δpyr4.
To analyze the Trsnf12, Trswi1 and Trsnf5 gene complement phenotype in the respective promoter-substituted strains in the presence of copper with the corresponding genes under the control of the constitutive gpd promoter, the gpd promoter and trpC terminator were first amplified from genomic DNA of Aspergillus nidulans, cut with SacI/NcoI and SpeI/HindIII, respectively, and ligated into the pMDP-hph plasmid containing a hygromycin resistance gene cassette as previously described (Xu et al., 2014) to generate the pMDP-gpd-hph plasmid. The Trsnf12, Trswi1 and Trsnf5 coding sequences were then amplified from QM9414 cDNA and digested with NcoI and SpeI, then ligated into pMDP-gpd-hph to obtain the pMDP-gpd-hph-Trsnf12, pMDP-gpd-hph-Trswi1 and pMDP-gpd-hph-Trsnf5 plasmids respectively. These plasmids were transformed into the Ptcu1-Trsnf12, Ptcu1-Trswi1 and Ptcu1-Trsnf5 strains, respectively to obtain the ReTrsnf12, ReTrswi1 and ReTrsnf5 strains.
Trichoderma reesei transformation was carried out essentially as previously described (Zhou et al., 2012). The transformants were selected on minimal medium for either uridine prototroph or for resistance to hygromycin (120 µg ml−1). Anchored PCR was used to verify the correct integration events.
Vegetative growth assays
To assay vegetative growth, equal amounts of growing mycelia were inoculated on minimal media agar plates containing different carbon sources (glucose, glycerol, cellobiose or lactose) or on malt extract agar plates and incubated at 30°C for 3 days.
To determine the growth in liquid culture, equal amounts of mycelia were transferred to fresh MA with 1% (w/v) of glucose or as the sole carbon source. At the indicated time points, mycelia cultured on glucose were filtrated on filter paper, dried at 70°C for 48 h and then weighed.
Fluorescence microscopy
To visualize GFP-TrSNF12, GFP-TrSWI1 and GFP-TrSNF5, the respective recombinant strain spores were inoculated and germinated in MA medium containing either 1% (v/v) of glucose for 16 h or 1% (w/v) of Avicel for 24 h at 30°C. The fluorescence was detected with a Nikon Eclipse 80i fluorescence microscope (Nikon, Melville, NY, USA), and images were captured and processed with the NIS-ELEMENTSAR software.
Enzyme activity and protein analysis
Cellobiohydrolase and β-glucosidase activities were determined by measuring the amount of released p-nitrophenol using p-nitrophenyl-D-cellobioside (pNPC; Sigma) and p-nitrophenyl-β-D-glucopyranoside (pNPG; Sigma) as the substrates respectively. The cellulase activity assays were performed in 200 μl of reaction mixtures containing 50 μl of culture supernatant and 50 μl of the respective substrate plus 100 μl of 50 mM sodium acetate buffer (pH 4.8) and then incubated at 45°C for 30 min. Total secreted and intracellular proteins were determined using the Bradford protein assay with bovine serum albumin (BSA) as a standard. Determination of CMC hydrolytic activities was carried out at 50°C in a 100 μl of reaction mixture containing 50 μl of appropriately diluted culture supernatant and 50 μl of 0.5% (w/v) CMC sodium in 50 mM of sodium acetate buffer (pH 4.8). The FPA assay was performed at 50°C in a 200 μl of reaction mixture including 50 μl of appropriately diluted culture supernatant and 150 μl 50 mM sodium acetate buffer (pH 4.8) with Whatman No. 1 filter paper as substrates. One unit (U) of CMCase or FPA was defined as the release of 1 μmol reducing sugar per minute under the test conditions (Xu et al., 2014; Wang et al., 2018). SDS-PAGE and Western blotting were performed according to standard protocols and CBH1 was immunoblotted using a polyclonal antibody raised against amino acids 426–446 of the protein, as previously described (Zhou et al., 2012).
Quantitative RT-PCR (qRT-PCR)
Total RNAs were extracted using the TRIzol reagent (Invitrogen, Grand Island, NY, USA) and purified using the TURBO DNA-free kit (Ambion, Austin, TX, USA) to eliminate genomic DNA contamination according to the manufacturer's instructions. Reverse transcription was performed using the PrimeScript RT reagent Kit (Takara, Japan) according to the instructions. Quantitative PCR was performed using SYBR green supermix (TaKaRa) on a Bio-Rad myIQ2 thermocycler (Bio-Rad). Data analysis was performed using the relative quantitation/comparative CT (ΔΔCT) method and were normalized to an endogenous control (actin), with expression on glycerol as the reference sample. Three biological replicates were performed for each analysis and the results and errors are the mean and SD, respectively, from the replicates. Statistical analysis was performed using the student's t-test analysis.
Protein production in E. coli and GST pull down assays
For the expression of the TrSNF12 and XYR1 AD in E. coli, the DNA fragment coding for TrFSNF12 was amplified from the T. reesei cDNA and was inserted into the pET22b (+) expression vector after digestion with NdeI and XhoI to obtain the pET22b-TrSNF12 plasmid. Similarly, the XYR1 AD (amino acids 776-860) was amplified from the pGBKT7-XYR1 AD plasmid and ligated into the pGEX4T-1 expression vector after digestion with NotI and BamHI. To purify the GST-XYR1 AD776-860 and TrSNF12-His, the indicated expression constructs were transformed into CaCl2-treated competent E. coli BL21 (DE3) cells. Protein purification was carried out essentially as previously described (Cao et al., 2017). All of the protein preparations were stored at −80°C in the presence of 20% (v/v) glycerol. GST pull-down assay was carried out as previously described (Zheng et al., 2017b). Briefly, purified GST or GST-XYR1 AD776-860 pre-coupled on glutathione beads was incubated with TrSNF12-His and rotated for at least 2 h at room temperature. The supernatant removed and the beads were washed three times with PBST (137 mmol L−1 of NaCl, 2.7 mmol L−1 of KCl, 10 mmol L−1 of Na2HPO4, 2 mmol L−1 of KH2PO4, 0.5% of Triton X-100, pH 7.4). The proteins retained on the beads were resolved by SDS-PAGE and detected by Western blot with anti-His antibody (Sigma).
Chromatin immunoprecipitation (ChIP) analysis
ChIP assays were performed according to a previously described protocol (Li et al., 2010; Cao et al., 2017). Briefly, the mycelia were fixed in minimal medium containing 1% of formaldehyde at 30°C for 10 min with shaking before the cross-linking was quenched via the addition of 25 ml of 1.25 M glycine for 5 min. The mycelia were then collected, ground in liquid N2 and broken in lysis buffer (50 mM of HEPES pH 7.5, 150 mM of NaCl, 1 mM of EDTA, 0.5% of Triton X-100, 0.1% of sodium deoxycholate, 0.1% of SDS, 1 mM of PMSF (phenylmethanesulfonyl fluoride), 1 μg ml−1 of leupeptin and 1 μg ml−1 of pepstatin) with glass beads (0.45 mm). This crude lysate was further sonicated to obtain an average DNA fragment size of approximately 500 bp. Immunoprecipitation was performed by incubating IgG (GE Healthcare) or anti-H4 antibody, with an aliquot of the clarified cell lysates containing equal amounts of protein (2 mg) at 4°C for 5 h. Forty microliters of protein A/G beads pre-coated with 1 mg ml−1 of BSA and 1 mg ml−1 of fish sperm DNA were used per IP. Following immunoprecipitation and extensive sequential washes, the DNA was eluted with elution buffer (100 mM of Tris-HCl (pH 7.8), 10 mM of EDTA, 1% of SDS, 10 mM of NaHCO3 and 100 mM of NaCl) at 65°C for 5 h and recovered by proteinase K treatment of the pelleted samples at 45°C for 1 h, phenol-chloroform extraction and ethanol precipitation. Quantitative PCR was performed on the precipitated chromatin DNAs using a Bio-Rad IQ5 thermocycler (Bio-Rad) and the SYBR Green Supermix (Takara). Relative enrichment of the DNAs was calculated as a percentage of the input DNA.
Acknowledgements
We thank Dr. Robert L. Mach for kindly providing the Δxyr1 strain. This work is supported by grants from the National Natural Science Foundation of China (31670040 and 31770047) and Shandong Technology Innovation Center of Synthetic Biology (sdsynbio-2018PY-01). Cao YL is supported by China postdoctoral Science Foundation (2019M652368).
Author contributions
Y.L.C. and F.L.Z. performed the experiments, W.F.L., W.X.Z. and X.F.M performed data analysis. Y.L.C. and W.F.L. designed the project. Y.L.C. and W.F.L. wrote the manuscript.
Conflict of interest
The authors declare no conflict of interest with the content of this article.
Open Research
Data availability statement
The data support the findings of this study are available from the corresponding author upon reasonable request.