TbLpn, a key enzyme in lipid droplet formation and phospholipid metabolism, is essential for mitochondrial integrity and growth of Trypanosoma brucei
Summary
Mammalian phosphatidic acid phosphatases, also called lipins, show high amino acid sequence identity to Saccharomyces cerevisiae Pah1p and catalyze the dephosphorylation of phosphatidic acid (PA) to diacylglycerol. Both the substrate and product of the reaction are key precursors for the synthesis of phospholipids and triacylglycerol (TAG). We now show that expression of the Trypanosoma brucei lipin homolog TbLpn is essential for parasite survival in culture. Inducible down-regulation of TbLpn in T. brucei procyclic forms increased cellular PA content, decreased the numbers of lipid droplets, reduced TAG steady-state levels and inhibited in vivo [3H]TAG formation after labeling trypanosomes with [3H]glycerol. In addition, fluorescence and transmission electron microscopy revealed that depletion of TbLpn caused major alterations in mitochondrial morphology and function, i.e., the appearance of distorted mitochondrial matrix, and reduced ATP production via oxidative phosphorylation. Effects of lipin depletion on mitochondrial integrity have previously not been reported. N- and C-terminally tagged forms of TbLpn were localized in the cytosol.
Graphical Abstract
Introduction
Lipins comprise a highly conserved family of phosphatidate phosphatase (PAP) enzymes, catalyzing the dephosphorylation of phosphatidate (PA) to diacylglycerol (DAG) and phosphate (reviewed by [Carman and Han, 2009]). PAP was initially detected in plants (Kates, 1955) and subsequently in a number of animal tissues (Smith et al., 1957). PAP enzymes are divided into two classes: Mg2+-dependent PAP1 enzymes requiring divalent cations for activity and Mg2+-independent PAP2 enzymes lacking cation dependency. In yeast and mammalian cells, PAP1 enzymes are known to be involved in de novo lipid synthesis whereas PAP2 enzymes have important roles in lipid signaling (Brindley, 2004; Carman and Han, 2006). DAG, the product of the reaction catalyzed by PAP enzymes, is used for triacylglycerol (TAG) production and the synthesis of the major glycerophospholipid classes phosphatidylethanolamine (PE) and phosphatidylcholine (PC) via the Kennedy pathway. Conversely, the substrate of the reaction, PA, can be activated to CDP-diacylglycerol (CDP-DAG), a precursor for the synthesis of the minor glycerophospholipid classes phosphatidylserine (PS), phosphatidylinositol (PI) and cardiolipin (CL) (reviewed by [Brindley et al., 2002; Carman and Han, 2006; Pascual and Carman, 2013]). In addition, both DAG and PA are involved in lipid-mediated signaling events, e.g., via the activation of protein kinases C, including membrane trafficking, cytoskeletal rearrangement, cell growth and proliferation, secretion and endocytosis (reviewed by [Brindley, 2004; Testerink and Munnik, 2005; Carman and Han, 2006; Wang et al., 2006; Pascual and Carman, 2013]).
In the yeast Saccharomyces cerevisiae, four genes encoding PAP enzymes have been identified, namely PAH1 (Han et al., 2006) and APP1 (Chae and Carman, 2013) belonging to the class of PAP1 enzymes and DPP1 (Toke et al., 1998) and LPP1 (Toke et al., 1998) belonging to the class of PAP2 enzymes. Whereas PA is the only substrate for PAP1 enzymes (Chae et al., 2012), PAP2 enzymes also accept other substrates, including diacylglycerol pyrophosphate and lyso-PA (Dillon et al., 1997; Chae et al., 2012). Mammalian PAP1 enzymes, also called lipins, are encoded by LPIN1, LPIN2 and LPIN3 and show high amino acid sequence identity to S. cerevisiae Pah1p in the N-terminal part and a haloacid dehalogenase (HAD)-like domain harboring the conserved catalytic motif DXDX(T/V) (Péterfy et al., 2001). The three lipins show distinct but overlapping expression in different tissues, with lipin-1 being the main fat-regulating enzyme in mice. Mammalian lipins, as well as S. cerevisiae Pah1p, are regulated by the phosphorylation status, i.e., dephosphorylation promotes translocation of lipin-1 and Pah1p from the cytosol to the nuclear/endoplasmic reticulum (ER) membrane (Huffman et al., 2002; Santos-Rosa et al., 2005; Harris et al., 2007; Grimsey et al., 2008; Reue and Zhang, 2008; Karanasios et al., 2010; Choi et al., 2011; Harris and Finck, 2011). Phosphorylation and possibly other posttranslational modifications also affect the apparent migration of Pah1p during SDS-polyacrylamide gel electrophoresis (SDS-PAGE): although S. cerevisiae Pah1p has a calculated molecular mass of 95 kDa, it migrates as a 124 kDa protein (Han et al., 2006). In fact, Pah1p was shown to be one of the most heavily phosphorylated proteins in S. cerevisiae, containing more than 30 potential phosphorylation sites (Hsieh et al., 2015). Phosphorylation of Pah1p is mediated by several kinases, regulating enzyme activity, intracellular location and abundance (Choi et al., 2012; Su et al., 2012). Phosphorylation has also been reported for other PAP1 enzymes (Huffman et al., 2002). Conservation of function of PAP1 enzymes between unicellular eukaryotes and mammals was demonstrated by complementation of yeast pah1Δ mutants with mammalian lipin-1 or lipin-2 (Grimsey et al., 2008).
Deletion of S. cerevisiae PAH1 results in increased levels of PA and markedly reduced levels of DAG and TAG. In contrast, only small changes have been observed in the levels of PC, PE, PI, sterol esters and fatty acids during exponential cell growth (Han et al., 2006, 2007). In mammalian cells, lipin-1–deficiency causes generalized lipodystrophy (Reue et al., 2000) and severe myopathy and rhabdomyolysis in childhood (Zeharia et al., 2008). The effects have been related to reduced lipin activity and TAG synthesis, with concomitant PA accumulation contributing to inappropriate activation of signaling pathways (Dwyer et al., 2012).
In the protozoan parasite Trypanosoma brucei, the causative agent of human African sleeping sickness and animal trypanosomiasis (Franco et al., 2014), a lipin homologue (TbLpn; Tb927.7.5450) has been identified based on its amino acid sequence homology to mammalian lipins and yeast Pah1p (Pelletier et al., 2013). TbLpn contains a conserved HAD-like domain with the active site motif DXDXTX. PAP activity of TbLpn was demonstrated in vitro, however, no data have been reported about its function in T. brucei, or any other trypanosomatid. To study the importance of lipid droplet formation and understand the connection between phospholipid and TAG metabolism in this eukaryotic model organism (Smith and Bütikofer 2010; Serricchio and Bütikofer, 2011), we down-regulated the expression of TbLpn in procyclic form T. brucei parasites. The results show that depletion of TbLpn using RNA interference (RNAi) leads to a reduction in TAG and lipid droplet content, increased levels of PA and ultimately parasite death, identifying TbLpn as essential regulator of lipid metabolism in T. brucei.
Results
Characterization of T. brucei lipin
In a previous report, T. brucei lipin (TbLpn; Tb927.7.5450) has been identified and enzymatically characterized in vitro as T. brucei phosphatidate phosphatase (Pelletier et al., 2013). Its physiological role was not investigated. In a protein blast using the deduced amino acid sequence of TbLpn and the deduced proteomes from other protozoan parasites, we identified TbLpn homologs in T. cruzi, Leishmania mexicana, Plasmodium falciparum and Toxoplasma gondii with homologies ranging from 39% to 58% (Supporting Information Fig. S1A). In addition, TbLpn showed homologies to human lipins (36–43%), mouse lipins (35–44%) and S. cerevisiae Pah1p (37%) (Supporting Information Fig. S1B). All parasite sequences contain the extended HAD-like domain and the DXDXTX active site motif within the C-LIP (C-terminal TbLpn) domain. In addition, TbLpn contains a conserved glycine residue (Gly74), which was shown to be essential for mouse lipin-1 and S. cerevisiae Pah1p activity (Gly84 and Gly80 respectively) (Péterfy et al., 2001; Han et al., 2007). The glycine residue is also conserved in putative lipins from other trypanosomatids (Supporting Information Fig. S1A). Algorithmic prediction programs Phobius (Käll et al., 2007) and TMHMM (Sonnhammer et al., 1998) predict no transmembrane domains in TbLpn, which is consistent with an earlier report showing the presence of TbLpn in a cytosolic fraction of T. brucei procyclic forms (Pelletier et al., 2013). To verify its localization, we expressed tetracycline-inducible N- and C-terminally triple c-myc-tagged forms of TbLpn (3xc-myc-TbLpn and TbLpn-3xc-myc respectively) in T. brucei procyclic forms. Analysis by SDS-PAGE and immunoblotting revealed similar apparent molecular masses for 3xc-myc-TbLpn and TbLpn-3xc-myc of approximately 120 kDa (Fig. 1A, C, D), which is considerably higher than the calculated molecular mass from the deduced TbLpn amino acid sequence (92.14 kDa, including the 3xc-myc tag) and the size of TbLpn detected in a previous study (Pelletier et al., 2013) using anti-TbLpn antibody (∼83 kDa). Higher molecular masses than predicted from their primary sequences have also been reported for phosphatidate phosphatases from Escherichia coli (Dillon et al., 1996) and S. cerevisiae (Dillon et al., 1997; Han et al., 2006) and may result from multiple covalent protein modifications, including extensive phosphorylation (Choi et al., 2012; Hsieh et al., 2016). However, it should be noted that S. cerevisiae Pah1p purified from E. coli also migrates with a higher than predicted molecular mass despite not being phosphorylated (Han et al., 2006). In line with these reports, we found using mass spectrometry that immunoprecipitated 3xc-myc-TbLpn contains at least two phosphorylated peptides, LFGSWGK and SVSQISMTRK, with S129 and S498 representing the phosphorylated amino acids (underlined; result from four analyses). These amino acids are among the 15 amino acids of TbLpn identified to be phosphorylated in recent phosphoproteomic analyses of T. brucei procyclic and bloodstream forms (Urbaniak et al., 2013).
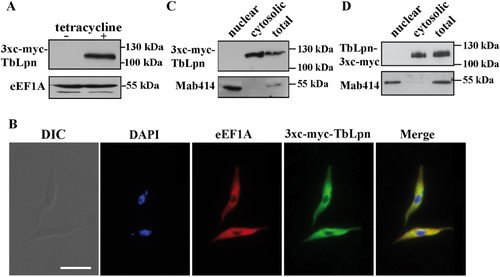
Expression of c-myc-tagged TbLpn and localization by immunofluorescence microscopy.
Panel A. 3xc-myc-tagged TbLpn was expressed in T. brucei procyclic forms under the control of a tetracycline-inducible operator. Proteins from parasites cultured for 2 days in the absence (–) or presence (+) of tetracycline were analyzed by SDS-PAGE and immunoblotting using anti-c-myc antibody. The same blot was also probed with an antibody against eukaryotic elongation factor 1A (eEF1A) to control for equal protein loading. Migration of molecular mass markers is indicated in the margin.
Panel B. T. brucei procyclic forms expressing N-terminal 3xc-myc-tagged TbLpn were analyzed by immunofluorescence microscopy using primary antibodies against c-myc and eEF1A (as cytosolic marker), followed by fluorescent secondary antibodies. DNA was stained with DAPI. DIC, differential interference contrast. Scale bar: 10 µm.
Panels C and D. Protein from T. brucei procyclic forms expressing N-terminal 3xc-myc-tagged TbLpn (C) or C-terminal 3xc-myc-tagged TbLpn (D) before (T) and after fractionation into cytosolic (C) and crude nuclear (N) fractions was analyzed by SDS-PAGE and immunoblotting using anti-c-myc antibody and anti-nuclear pore complex protein antibody Mab414 (as marker for the nuclear fraction). The observed molecular masses for c-myc-tagged TbLpn and nuclear pore complex protein are indicated in the margin.
Analysis of the two differently tagged TbLpn proteins by immunofluorescence microscopy revealed that they co-localized with the cytosolic marker protein eukaryotic elongation factor 1A (Fig. 1B and Supporting Information Fig. S1C). In addition, after crude fractionation of trypanosomes they partitioned in the cytosolic fraction (Fig. 1C and D). This is in contrast to a previous report, in which endogenous TbLpn was detected in a nuclear fraction using a polyclonal antibody against TbLpn (Pelletier et al., 2013). Expression of 3xc-myc-TbLpn had no effect on growth of T. brucei procyclic forms in culture (Supporting Information Fig. S2).
Essentiality of TbLpn in T. brucei procyclic forms
To study the essentiality of TbLpn in T. brucei, we down-regulated its expression in procyclic forms using tetracycline-inducible RNAi against a 452 bp sequence of the ORF. As shown in Fig. 2, growth of parasites in the presence of tetracycline was reduced after 2 days of RNAi as compared to control uninduced cells and completely stopped after 8 days of culture (Fig. 2A). Northern blot analysis (Fig. 2B) and quantitative RT-PCR (Fig. 2C) demonstrated that TbLpn mRNA levels were efficiently ablated after 2 days of RNAi. These results demonstrate that expression of TbLpn is essential for growth of T. brucei procyclic forms in culture.
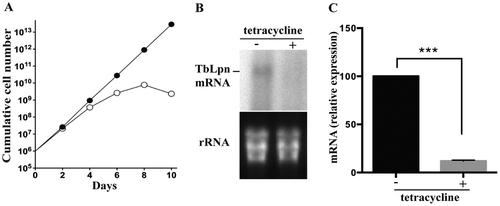
Essentiality of TbLpn in T. brucei procyclic forms.
A. TbLpn RNAi cells were cultured in the absence (black circles) or presence (white circles) of tetracycline for 10 days to maintain or ablate respectively, TbLpn expression. The data points represent mean values from three independent experiments. Standard deviations are smaller than the size of the symbols.
B. Total RNA from TbLpn parasites cultured for 2 days in the absence (–) or presence (+) of tetracycline was separated, blotted and mRNA was analyzed with a [32P]-labeled probe against a fragment of the TbLpn ORF. Bands were visualized by autoradiography (top panel). rRNA was visualized by ethidium bromide staining and used as loading control (bottom panel).
C.Total RNA from parasites incubated in the absence (–) or presence (+) of tetracycline was reverse transcribed into cDNA and analyzed using TbLpn forward and reverse primers. The results represent mean values ± standard deviations from two independent experiments done in triplicates. The asterisk indicates a significant difference between control and TbLpn-depleted cells (***<0.001 paired Student's t-test).
Role of TbLpn in lipid droplet and triacylglycerol formation in T. brucei
To study a possible involvement of TbLpn in the formation of TAGs, which are stored as lipid droplets, TbLpn RNAi cells were cultured for 2 and 4 days in the absence or presence of tetracycline and lipid droplets were stained using the lipophilic fluorescent dye nile red and analyzed by fluorescence microscopy. The results show that compared to control cells the intensity of nile red staining decreased in parasites after RNAi against TbLpn (Fig. 3A, top row of panels). To stimulate de novo formation of lipid droplets, resulting in larger and higher numbers of lipid droplets per cell, T. brucei procyclic forms can be incubated with oleate (Allmann et al., 2014). When we applied this treatment to TbLpn RNAi cells, more lipid droplets were detected in control untreated and induced cells compared to non-oleate-treated parasites (Fig. 3A, bottom row of panels). However, the intensity of nile red staining again decreased after 2 and 4 days of RNAi against TbLpn compared to control uninduced cells (Fig. 3A). Quantification revealed that the average number of lipid droplets per 100 parasites increased from 238 in untreated parasites to 457 in oleate-loaded cells (Fig. 3B). Subsequent depletion of TbLpn decreased the average number of lipid droplets per 100 parasites to 75 and <10 after 2 and 4 days respectively, of RNAi in the absence of oleate and to 287 and 180 after 2 and 4 days respectively, of RNAi in oleate-treated cells (Fig. 3B). The reduction in lipid droplets can also be seen when plotting the actual number of lipid droplets per cell in oleate-loaded parasites before and after induction of RNAi for 2 days (Fig. 3C). The addition of oleate to the culture medium had no effect on growth of parasites (Fig. 4). However, ablation of TbLpn in cells cultured in the presence of oleate resulted in a more severe growth defect (Fig. 4), suggesting that the presence of oleate under conditions when lipid droplet formation is inhibited may be toxic to T. brucei procyclic forms.
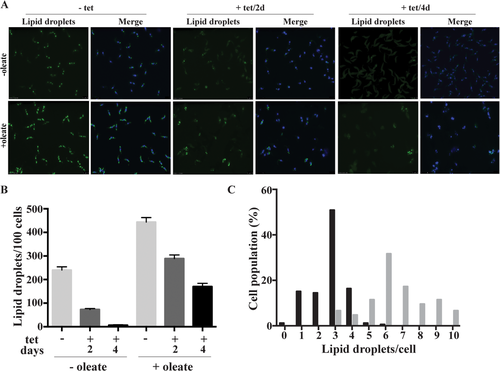
Visualization and quantification of lipid droplets.
TbLpn RNAi parasites were cultured in the absence (–) or presence (+) of oleate for 24 hours to induce lipid droplet formation. Subsequently, tetracycline (tet) was added for 2 or 4 days to down-regulate expression of TbLpn.
A. Lipids droplets were stained with Nile red and visualized by fluorescence microscopy. DNA was stained with DAPI and appears blue in the merged pictures.
B.The number of Nile red-stained lipid droplets in TbLpn RNAi cells cultured in the absence (–) or presence (+) of oleate for 24 h and in the absence (–) or presence (+) of tetracycline (tet) for 2 or 4 days was quantified by counting 100 parasites using imageJ program. The data points represent mean values ± standard deviations from two independent experiments.
C. The number of Nile red-stained lipid droplets per cell in TbLpn RNAi cells cultured in the absence (gray bars) or presence (black bars) of tetracycline for 2 days and in the presence of oleate for 24 h. One hundred parasites at each condition were counted using imageJ program.
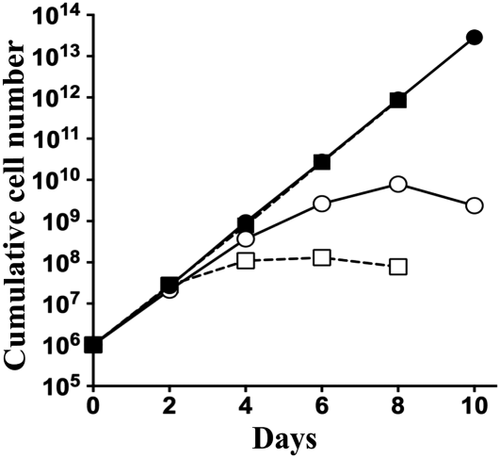
Growth curves of T. brucei procyclic form TbLpn RNAi cells cultured in the presence of oleate.
TbLpn RNAi cells cultured in the absence (black circles) or presence (white circles) of tetracycline and in the absence (solid lines) or presence (dashed lines) of oleate. The data points represent mean values from three independent experiments. Standard deviations are smaller than the size of the symbols.
To corroborate the microscopy results with biochemical analyses, steady-state TAG levels were analyzed by thin layer chromatography (TLC) and de novo formation of TAG was determined by in vivo [3H]glycerol-labeling combined with TLC. Analysis of lipid extracts from TbLpn-depleted parasites cultured in the presence or absence of oleate showed decreased levels of TAG compared to control uninduced cells (Supporting Information Fig. S3). In addition, when parasites cultured in the absence or presence of oleate were depleted of TbLpn by 30 hours of RNAi and subsequently incubated for 6 h with [3H]glycerol, de novo formation of [3H]TAG was clearly reduced compared to uninduced cells (Fig. 5A). De novo formation of [3H]TAG was also increased in parasites incubated with oleate compared to control cells (Fig. 5A; compare top and bottom panels). These results are in good agreement with the observed reduction in the number of lipid droplets per cell after down-regulation of TbLpn and with the increase in lipid droplets after oleate-loading (Fig. 3). Quantification of radioactivity showed that the reduction in [3H]TAG formation was dependent of the time of TbLpn depletion by RNAi, with approximately 40% reduction after 24 hours of RNAi and >80% reduction after 60 hours of RNAi (Fig. 5B). Together, these results demonstrate that RNAi against TbLpn decreases lipid droplet formation, steady-state levels of TAG and de novo [3H]TAG formation in T. brucei procyclic forms.
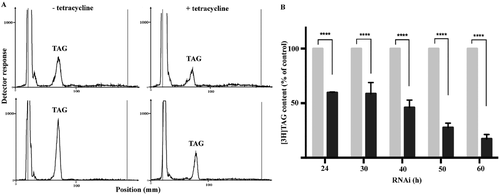
TAG formation and quantification during TbLpn depletion.
A. TbLpn RNAi cells were cultured for 30 h in the absence (–) or presence (+) of tetracycline to induce down-regulation of TbLpn and in the absence (top panels) or presence (bottom panels) of oleate to induce lipid droplet formation. Subsequently, cells were labeled for 6 h with [3H]glycerol and lipids were extracted and analyzed by 1-dimensional TLC using solvent system 2. Radiolabeled lipids were detected using a radioisotope scanner. The scans are representative of 24 h RNAi and 6 h [3H]glycerol labeling experiments.
B. TbLpn RNAi cells were cultured for indicated times in the absence (gray bars) or presence (black bars) of tetracycline to induce down-regulation of TbLpn and labeled for 6 h with [3H]glycerol. After extraction of lipids and separation by 1-dimensional TLC, radiolabeled lipids were quantified using a radioisotope scanner. The data points represent mean values ± standard deviations from three independent experiments. The asterisks indicate significant differences between control and TbLpn-depleted cells (****P < 0.0001; Two-way ANOVA).
Phospholipid analysis of TbLpn-depleted parasites
RNAi-mediated ablation of TbLpn expression is expected to result in increased levels of PA and possibly other lipid classes using PA as substrate. To study phospholipid steady-state levels, TbLpn RNAi cells were cultured in the absence or presence of tetracycline for 60 h and phospholipids were extracted and separated by two-dimensional TLC. Visualization of lipid classes by staining the TLC plates with iodine showed that the PA content in TbLpn-depleted cells is higher compared to control uninduced cells (Supporting Information Fig. S4). Quantification of individual phospholipid classes by lipid phosphorus determination revealed that the PA content of control cells (4 × 108 parasites) was at the detection limit of the assay (Fig. 6). In contrast, in TbLpn-depleted parasites, PA was readily detected (amounting approximately 2.3% of total phospholipid), demonstrating that ablation of TbLpn expression resulted in accumulation of PA by inhibiting its dephosphorylation. The relative amounts of the other phospholipid classes were statistically not different between control and RNAi-induced cells, except for a small decrease in PC in TbLpn-depleted cells (Fig. 6).
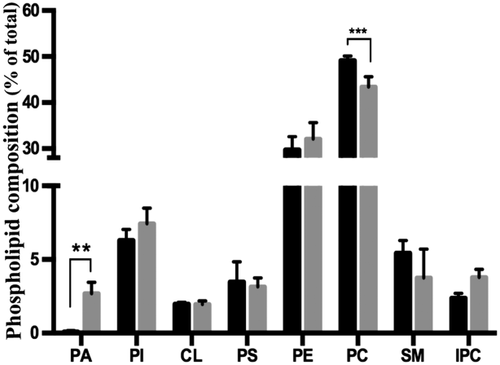
Phospholipid quantification after depletion of TbLpn.
TbLpn RNAi cells were cultured for 60 h in the absence (black bars) or presence (gray bars) of tetracycline. After extraction of lipids and separation by 2-dimensional TLC using solvent systems 3 and 4, phospholipids were quantified by phosphorous assay. The data represent mean values ± SD from four independent experiments. PA, phosphatidate; PI, phosphatidylinositol; CL, cardiolipin; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PS, phosphatidylserine; SM, sphingomyelin; IPC, inositolphosphoryl ceramide. The asterisks indicate significant differences between uninduced (black bars) and induced cells (gray bars) (**P < 0.001; ***P < 0.0001; Two-way ANOVA).
Ablation of TbLpn expression affects parasite organelle morphology
To study if the changes in phospholipid and TAG levels during ablation of TbLpn expression affect organelle morphology, TbLpn RNAi parasites before and after induction of RNAi were analyzed by fluorescence and transmission electron microscopy (TEM). Mitochondrial morphology was assessed using the mitochondrial membrane potential-dependent dye Mitotracker red and the matrix marker Hsp60. Both markers revealed the typical tubular staining of the T. brucei mitochondrion in control cells before addition of tetracycline to induce RNAi. The staining pattern using anti-Hsp60 antibody (Supporting Information Fig. S5A) or Mitotracker (Supporting Information Fig. S5B) remained unaltered after depletion of TbLpn for 4 days. Similarly, no major changes in anti-Hsp60 and Mitotracker staining were seen after depletion of TbLpn for 8 days. Analysis of voltage-dependent anion-selective channel (Vdac), cytochrome c oxidase subunit 4 (Cox4) and heat shock protein 60 (Hsp60) as markers for the outer and inner mitochondrial membrane and the matrix respectively, by SDS-PAGE and immunoblotting using specific antibodies showed similar levels of the proteins in TbLpn-depleted cells compared to control cells (Supporting Information Fig. S5C). No morphological changes in the ER and lysosome were observed after ablation of TbLpn using antibodies against BiP and CatL respectively (Supporting Information Fig. S6A and B).
Examination of TbLpn-depleted parasites by TEM revealed distinct changes between control cells (Fig. 7) and parasites after 4 and 8 days of RNAi (Fig. 8). While in control parasites, lipid droplets were frequently detected (Fig. 7), they were only rarely seen in TbLpn-depleted trypanosomes (Fig. 8A; Supporting Information Figs S7–S9). Instead, in many cases the cytoplasm of RNAi-treated parasites contained numerous, seemingly empty, vacuoles (Fig. 8A–D; Supporting Information Figs S7A and C and S9A, C, D). In TbLpn-depleted trypanosomes, cytoplasmic membrane stacks were found within the cytoplasm (Fig. 8C). Distinct changes were also found in the mitochondrion of RNAi-depleted parasites. In control cultures, the mitochondrial network appeared as a patchy organelle with an intact membrane and an electron dense matrix and occasional cristae (Fig. 7). In RNAi-depleted parasites, closer inspection of the mitochondrion revealed distinct alterations in the mitochondrial matrix with loss of electron dense texture and a reduction, or even complete absence, of cristae (Fig. 8C; Supporting Information Figs S7–S9). In addition, portions of the mitochondrial membrane were altered and became unnoticeable (Fig. 8C; Supporting Information Figs S7B; S8B and C; S9B and D). A small fraction of trypanosomes had undergone more dramatic alterations, including extensive vacuolization, formation of vesicles filled with particulate material and chromatin condensation and fragmentation in the nucleus (Fig. 8D), i.e., events that often are indicative of cell death. In contrast, flagella, endocytic vesicles and Golgi apparatus appeared structurally unaltered. In addition, no alterations in the nuclear membrane were noted.
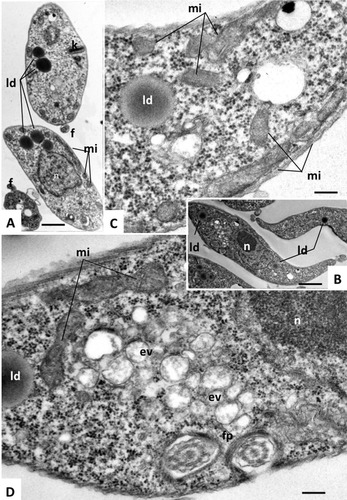
Transmission electron microscopy of T. brucei procyclic forms.
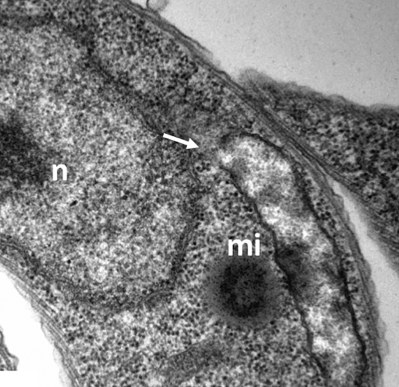
TbLpn RNAi cells cultured in the absence of tetracycline were analyzed by transmission electron microscopy to show normal morphology of parasite organelles. Panels A and B show entire cells, panels C and D are higher magnification views of panel B, showing the mitochondrion (mi), endocytic vesicles (ev), flagellar pocket (fp) and lipid droplets (ld). n = nucleus, f = flagellum. Bars in A = 0.9 µm; B = 0.9 µm; C = 0.2 µm; D = 0.2 µm.
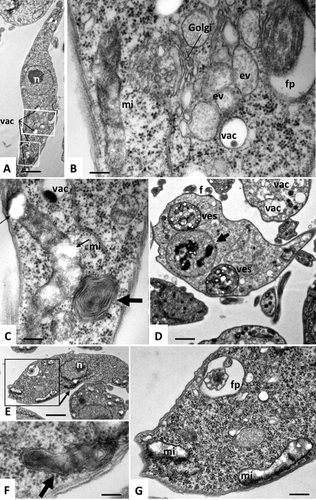
Ultrastructural characteristics of TbLpn-depleted T. brucei procyclic forms.
TbLpn RNAi cells cultured in the presence of tetracycline to induce depletion of TbLpn for 4 days (panels A–D) or 8 days (panels E–G) were analyzed by transmission electron microscopy. Panel A shows a low magnification view, the boxed areas are shown at higher magnification in panels B and C. The boxed area in panel E is shown at higher magnification in panel G. Golgi = Golgi stacks, vac = vacuoles, fp = flagellar pocket, mi = mitochondrion. Note the partial rupture of the mitochondrial membrane and partial depletion of the mitochondrial matrix (thin arrows in panel C) and multiple layer membrane stacks (thick arrow in panel C). Panel D shows a rare parasite exhibiting more dramatic alterations, including the build-up of large vesicles (ves) filled with electron-dense particulate material, and condensed fragmented chromatin within the nucleus (arrow). Bars in A = 0.9 µm; B = 0.15 µm; C = 2 µm, D = 1.1 µm, E = 0.9 µm, F = 0.12 µm, G = 0.24 µm.
Mitochondrial ATP production and mitochondrial membrane potential after TbLpn depletion
To study if mitochondria maintain production of ATP during RNAi against TbLpn, we measured ATP generation via oxidative phosphorylation in isolated crude mitochondria using succinate as substrate (Bochud-Allemann and Schneider, 2002). The results show that after 4 days of down-regulation of TbLpn expression, ATP production was comparable to control parasites cultured in the absence of tetracycline (Fig. 9A). However, after prolonged depletion of TbLpn for 8 days, ATP production via oxidative phosphorylation was reduced by 85% compared to control parasites (Fig. 9A). No changes were noted between control and TbLpn-depleted cells for ATP production via substrate level phosphorylation using α-ketoglutarate as substrate (Fig. 9B). Antimycin and atractyloside were used as inhibitors of ATP production via oxidative and substrate level phosphorylation respectively, in control incubations (Fig. 9A and B).
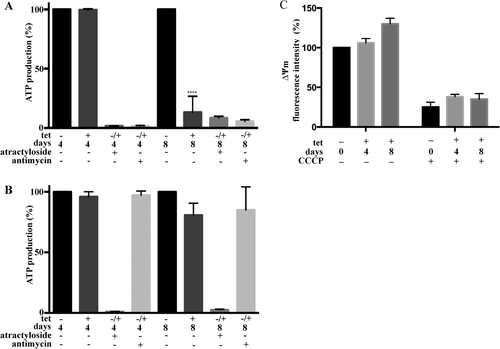
Mitochondrial ATP production and mitochondrial membrane potential after TbLpn depletion.
Mitochondria were isolated using digitonin from trypanosomes cultured in the absence (–) or presence (+) of tetracycline for 4 or 8 days to deplete TbLpn. ATP production via oxidative phosphorylation (panel A) or substrate level phosphorylation (panel B) was measured using succinate or α-ketoglutarate respectively, as substrate. ATP production in the presence of atractyloside and antimycin as inhibitors of the mitochondrial ADP/ATP translocase and respiratory chain complex III respectively, are shown as controls. ATP production in mitochondria from TbLpn-depleted parasites was expressed relative to control mitochondria from cells cultured in the absence of tetracycline and inhibitors. The data represent mean values ± standard deviations from 4 to 7 experiments. Panel C shows quantification of the mitochondrial membrane potential with TMRE staining of trypanosomes after 4 or 8 days of TbLpn depletion. The data represent mean values ± standard deviations from three experiments. The asterisk indicates significant difference between control and TbLpn-depleted cells (****P < 0.0001; **P < 0.05; one-way ANOVA).
To measure the mitochondrial membrane potential ΔΨm after TbLpn depletion, we stained live parasites with the potential-dependent dye TMRE and quantified its uptake into mitochondria by fluorescence intensity measurements. The results show that TMRE fluorescence was unchanged in parasites after 4 days of TbLpn depletion compared to control cells (Fig. 9C). After 8 days of TbLpn knock-down, the mitochondrial membrane potential was slightly elevated compared to control parasites, which may be due to decreased ATP production via oxidative phosphorylation (see Fig. 9) and, as a consequence, hyperpolarization of the inner mitochondrial membrane (Perry et al., 2011). As expected, treatment of parasites with CCCP prior to TMRE staining resulted in reduction of the mitochondrial membrane potential (Fig. 9C).
TbLpn does not interact with 14-3-3 proteins
14-3-3 proteins have been shown to interact with mammalian lipin1 (Péterfy et al., 2010). To study a possible interaction between TbLpn and trypanosomal 14-3-3 protein I and II homologs (Tb927.11.9530 and Tb927.11.6870 respectively) (Inoue et al., 2005), we performed co-immunoprecipitation experiments using T. brucei parasites expressing c-myc-tagged TbLpn. Analysis by SDS-PAGE and immunoblotting of precipitates using antibodies against c-myc or 14-3-3 protein II showed no co-immunoprecipitation of 14-3-3 proteins or c-myc TbLpn respectively (Supporting Information Fig. S10A). In addition, we found no changes in 14-3-3 protein II levels in parasites after down-regulation of TbLpn by RNAi for 4 or 8 days compared to control cells (Supporting Information Fig. S10B). Together, these results indicate that TbLpn and 14-3-3 proteins do not interact in T. brucei procyclic forms.
Discussion
PAP1 enzymes play a central role in lipid metabolism in eukaryotic cells. Both the substrate and the product of the reaction catalyzed by PAP1 enzymes, PA and DAG respectively, directly affect the synthesis of TAGs and phospholipids and, in addition, are involved in transcription, lipid signaling and vesicular trafficking (Brindley et al., 2002) Although a homologue of yeast Pah1p and human lipin has previously been identified in T. brucei (Pelletier et al., 2013), termed TbLpn, its functional roles and importance for parasite viability have not been investigated. We now demonstrate using RNAi to knock-down expression of TbLpn that the enzyme is essential for growth of T. brucei procyclic forms in culture. Parasite growth decreased after 2 days of RNAi against TbLpn and stopped completely after 8 days of TbLpn ablation. Depletion of TbLpn resulted in a decrease in the numbers of lipid droplets, TAG steady-state levels and in vivo [3H]TAG formation after labeling of trypanosomes with [3H]glycerol. In contrast, down-regulation of TbLpn led to an increase in the amounts of PA. These results are entirely consistent with TbLpn catalyzing the dephosphorylation of PA to DAG in T. brucei, resulting in accumulation of the substrate of the reaction, PA and a decrease in the product, DAG, which is a precursor for the formation of TAG and lipid droplets.
Dependence of TAG formation on PAP1 enzymes has also been observed in bacteria, yeast and mammalian cells. In Streptomyces coelicolor, mutations in the Lppα and Lppβ genes led to reduction in de novo TAG biosynthesis (Comba et al., 2013). A similar observation has been made in S. cerevisiae after deletion of Pah1p (Han et al., 2006; Chae et al., 2012). In addition, lipin-1 in mice has been proposed to be the PAP1 enzyme in adipose tissue and skeletal muscle causing impaired formation of TAG, leading to lipodystrophy. The essentiality of TbLpn for parasite growth in culture demonstrates that T. brucei lacks other PAP enzymes able to compensate for loss of TbLpn. This is in contrast to several other organisms in which deletion of genes encoding PAP1 enzymes was compensated, at least in part, by other lipid phosphatases (Dubots et al., 2014; Franco et al., 2014; Hsieh et al., 2015).
It has been shown before that the addition of oleate to T. brucei procyclic forms in culture results in increased levels of TAG and higher numbers of lipid droplets (Allmann et al., 2014), demonstrating that fatty acid carbon can be used for TAG and lipid droplet formation in T. brucei. Oleate-induced formation of lipid droplets has also been reported in mammalian cells and yeast (Singh et al., 2009; Radulovic et al., 2013). We now show that down-regulation of TbLpn decreased lipid droplet formation in both control and oleate-fed trypanosomes, indicating that the TAG pool formed by uptake of exogenous fatty acids is generated via the same pathway as the TAG pool formed using endogenous substrates. At present, the role of lipid droplets in trypanosome biology is unclear. They may be required for rapid lipid turnover, i.e., for the synthesis or remodeling of membrane lipids during cell proliferation, or for energy supply under limited nutrient availability. Although the activities of the first three enzymes of the β-oxidation pathway of fatty acids have been reported in T. brucei parasites isolated from fat tissue (Trindade et al., 2016), formal proof of functional β-oxidation and its role in ATP production in T. brucei is still lacking. Our results do not rule out a role of lipid droplets in energy production in procyclic forms in culture, however, the rapid formation and hydrolysis of TAG observed in our experiments rather suggests that lipid droplets are more likely actively involved in membrane lipid formation or remodeling, i.e., as short-time reservoir for fatty acids.
In a previous study using an antibody raised against a synthetic peptide of the deduced TbLpn amino acid sequence, TbLpn was found to localize in both the cytosol and a crude nuclear fraction (Pelletier et al., 2013). In our work, expression of N- and C-terminally 3xc-myc-tagged forms of TbLpn in T. brucei procyclic forms showed their exclusive presence in the cytosol. At present, it is unclear if the tagged enzymes may not localize in the nucleus because of the presence of the 3xc-myc tags, or if the difference in localization may be related to slightly different protocols used to obtain a crude nuclear fraction. In addition, we cannot exclude that TbLpn translocates to the nucleus as a result of altered culture, i.e., stress, conditions. In contrast to mammalian lipins, TbLpn lacks the conserved sequence motif LXXIL, which is required for interaction with transcription factors and co-activators (reviewed by [Csaki et al., 2013]). In S. cerevisiae, Pah1p has been shown to preferentially localize in the cytosol. However, after dephosphorylation it associated in part with a nuclear/endoplasmic reticulum fraction (Karanasios et al., 2010; Choi et al., 2011). Similarly, dual localization in the cytosol and a nuclear fraction has also been reported for lipin-1 in mammalian cells (Péterfy et al., 2001; Harris et al., 2007; Reue and Zhang, 2008).
It has been shown in 3T3-L1 adipocytes that insulin stimulates lipin-1 phosphorylation and increases its interaction with 14-3-3 proteins (Péterfy et al., 2010). In addition, 14-3-3 proteins have been shown to control lipin-1 subcellular localization, activity and association with other proteins (Péterfy et al., 2010). Whereas mammalian cells may express up to seven isoforms of 14-3-3 proteins, only two homologs have been identified in the T. brucei genome (termed 14-3-3 I and II proteins). They have been shown to form hetero- or homodimers and have a central role in parasite motility, cytokinesis and the cell cycle (Inoue et al., 2005; Inoue et al., 2013). However, their possible association with other proteins has not been studied. Based on our immunoprecipitation experiments and unchanged 14-3-3 protein levels after depletion of TbLpn, it is unlikely that TbLpn interacts with trypanosome 14-3-3 proteins.
Our work also demonstrates that depletion of TbLpn results in increased levels of PA and slightly decreased levels of PC. A similar observation has been reported before in yeast (Park et al., 2015). However, in contrast to S. cerevisiae pah1Δ cells, which also had increased levels of PE and PI (Han et al., 2007), no additional alterations in the phospholipid composition of TbLpn-depleted parasites were observed. This may be due to differences in phospholipid metabolism between yeast and trypanosomes, notably the absence of CDP-DAG-mediated synthesis of PS in T. brucei. In yeast, PS is synthesized from CDP-DAG and serine and subsequently converted to PE via PS decarboxylation (reviewed by [Voelker, 1997]). Thus, an increase in PA may promote production of CDP-DAG and PS, leading to elevated PE levels. In contrast, in T. brucei PE is synthesized exclusively via the Kennedy pathway (Signorell et al., 2008) and, thus, PE levels may not be affected by an increase in PA. In addition, PS is produced via head group exchange with PE and does not involve CDP-DAG (Farine et al., 2017). Together, our observation that an increase in PA in TbLpn-depleted cells has no effect on PE synthesis is consistent with the current knowledge on phospholipid synthesis in T. brucei. Interestingly, depletion of lipin in mammalian cells also had no effect on the overall phospholipid composition (Sembongi et al., 2013). The small decrease in PC in TbLpn-depleted and S. cerevisiae pah1Δ cells (Han et al., 2007) may be due to limited availability of DAG, which serves as precursor for PC production via the Kennedy pathway.
The availability of an RNAi cell line allowed us to study possible morphological changes during progressive depletion of TbLpn. Our data using (immuno-)fluorescence microscopy and TEM revealed that ablation of TbLpn expression resulted in distorted mitochondrial morphology. A similar impact on mitochondrial ultrastructure has been described before in T. brucei parasites with defects in fatty acid synthesis (Guler et al., 2008). In addition, depletion of TbLpn for longer times resulted in decreased ATP production via oxidative phosphorylation, albeit without loss of membrane potential as detected by Mitotracker staining and TMRE incorporation. These late changes coincide with the time at which TbLpn-depleted parasites stop proliferating. Thus, it is not clear if these observations are a cause or a consequence of growth arrest. Interestingly, in yeast the mitochondrial membrane potential of a S. cerevisiae pah1Δ mutant was not significantly different from that of wild type cells and no major changes in oxygen consumption or mitochondrial morphology were reported (Park et al., 2015). However, knock-out cells typically have adapted to the lack of a gene and may not reveal (subtle) functional or morphological changes. In the case of continuous depletion of a gene product, e.g., by knocking down TbLpn expression using RNAi, such alterations may become apparent. A crucial role for PA in controlling mitochondrial structure and function would be consistent with reports showing that mitochondrial PA levels mediate mitochondrial dynamics (Baba et al., 2014) and fusion and fission (Choi et al., 2006; Huang et al., 2011; Adachi et al., 2016). Based on our findings, we hypothesize that accumulation of PA, resulting from depletion of TbLpn, affects the function and structure of the mitochondrion, eventually leading to growth arrest and parasite death.
Experimental procedures
Unless otherwise stated, all reagents were purchased from Sigma Aldrich or Merck. Restriction enzymes were purchased from Thermo Fisher Scientific (Wohlen, Switzerland). Antibiotics and fetal bovine serum (FBS) were obtained from Invitrogen (Basel, Switzerland). Radioactive [1,2,3-3H]glycerol ([3H]glycerol; 1 mCi ml−1, 60 Ci mmol−1) was purchased from American Radiolabeled Chemicals Inc (St. Louis, MO, USA) and dCTP, [α-32P] (3000 Ci mmol−1) was from PerkinElmer Life Sciences (Schwerzenbach, Switzerland). Primers and sequencing services were from Microsynth AG (Balgach, Switzerland).
Trypanosomes and culture conditions
Trypanosoma brucei procyclic form strain 29-13, co-expressing a tetracycline repressor and a T7 polymerase (Wirtz et al., 1999) (obtained from Paul Englund, John Hopkins University School of Medicine), was cultured at 27°C in SDM-79 containing 10% heat-inactivated FBS and 15 μg ml−1 G418 and 25 μg ml−1 hygromycin.
RNAi-mediated gene silencing
Tb927.7.5450 (named TbLpn) was down-regulated in T. brucei procyclic forms by RNA interference (RNAi)-mediated gene silencing using a stem-loop construct containing a puromycin resistance gene (Wirtz et al., 1999). A 452 bp fragment of the TbLpn open reading frame (ORF) was amplified by using primers pah1-RNAi-fw GCGCCCAAGCTTGGATCCTTTGGTTTAGGTTGTTCCGC and pah1-RNAi-rv CTAGGCTCTAGACTCGAGGGTTGCTGATCCTTCTGCTC (restriction sites are underlined) and cloned into pALC14 vector (Niemann et al., 2013). Plasmids were extracted using Plasmid Midi kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions and linearized with NotI then resuspended in buffer (132 mM NaCl, 8 mM KCl, 8 mM Na2HPO4, 1.5 mM KH2PO4, 0.5 mM magnesium acetate, 0.09 mM calcium acetate, pH 7.0), mixed with 10 μg of linearized plasmid and transferred to a 0.2-cm pulse cuvette (Lonza, Köln, Germany). Electroporation was performed in 100 μl nucleocuvettes using Lonza 4D Nucleofector System (pulse code FI-115, ‘Primary Cell P3’ solution). Different dilutions were plated into 24-well plates and stable clones were selected by limiting dilution in the presence of 2 μg ml−1 puromycin.
RNA isolation, Northern blot analysis and quantitative PCR
Total RNA was isolated by using SV RNA extraction kit (Promega, Dübendorf, Switzerland), following the manufacturer's instructions. RNA (10 µg) was separated on formaldehyde-agarose gels (1% (w/v) agarose, 2% (v/v) formaldehyde in 20 mM MOPS (3-[N-morpholino] propanesulfonic acid), containing 8 mM sodium acetate and 1 mM EDTA, pH 7.0) and transferred to Amersham Hybond-N+ nylon membranes (GE Healthcare, Buckinghamshire, UK). Ribosomal RNA was used as loading control on formaldehyde–agarose gels and visualized by ethidium bromide staining. 32P-labeled probes were made from 452 bp ORF PCR products by random priming using a Prime-a-Gene labeling system (Promega, Madison WI, USA). Hybridization was performed overnight at 60°C in hybridization buffer (1 mM EDTA, 0.5 M Na2HPO4, 24 mM H3PO4, pH 7.2, containing 7% [w/v] SDS and 1% [w/v] bovine serum albumin). The membrane was analyzed by autoradiography using BioMax Mass Spectrometry film (GE Healthcare) in combination with TransScreen-HE intensifying screen. Quantitative PCR was performed using Applied Biosystems ViiA7 real-time PCR system. Total RNA (0.5 µg) was used by GoScriptTM reverse transcriptase kit (Promega) to synthesize cDNA. The reaction mixture consisted of 1x Fast Syber Green master mix (Applied Biosystems, Foster City, CA, USA) and 0.9 μM forward GAAAGCAGCGCCTTCAATAC and reverse TACCAAGACCCAAAGGCAAC primers. Gene expression was calculated by using the delta-CT relative quantification method and telomerase as reference gene for normalization (Rao et al., 2013).
Construction of c-myc-tagged TbLpn
To express an N-terminally tagged form of TbLpn in T. brucei procyclic forms, the Tb927.7.5450 ORF was amplified by PCR using TbLpn-N-myc-fw CCTCTTGCACTCGAGATATCTGGTTTTGCAGATTTC and TbLpn-N-myc-rv CTAGGCGGATCCTCACACAGTGTCACCTTGTTG (restriction sites are underlined). The PCR product was digested with XhoI and BamHI, precipitated using ethanol and ligated into an inducible pLEW100-based vector (Wirtz et al., 1999) resulting in plasmid pLDLpn. For C-terminal tagging, the ORF was amplified using primers TbLpn-C-myc-fw CTAGGCGGATCCATGATATCTGGTTTTGCAGAT and TbLpn-C-myc-rv CCTCTTGCACTCGAGCACAGTGTCACCTTGTTGATA (restriction sites are underlined) and processed as described above. Before stable transfection into T. brucei 29-13 procyclic forms, the plasmid was linearized with NotI. Stable clones were selected by limiting dilution using 2 μg ml−1 puromycin.
Crude fractionation of trypanosomes
Fractionation of trypanosomes into a crude nuclear and cytosolic extract was done as described before (Zeiner et al., 2003). Briefly, T. brucei 3xc-myc TbLpn cells (4 × 108) were washed twice in buffer A (150 mM sucrose, 20 mM KCl, 3 mM MgCl2, 20 mM HEPES-KOH, pH 7.9). Nonidet P-40 was added to reach a final concentration of 0.2%, and cells were lysed by vortexing (whole cell extract). Subsequently, the lysate was centrifuged in Labnet PrismTM R refrigerated microcentrifuge at 16,500 g for 10 min using a C2500-R rotor. The supernatant (cytoplasmic fraction) was recovered and the pellet was resuspended in buffer A and centrifuged once more using the same parameters. The supernatant was discarded and the pellet was resuspended in 1 ml buffer A (nuclear fraction).
SDS-PAGE
Proteins were separated using 12% polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA) by semi-dry blotting for 75 min at 2.5 mA/cm2. After 1 hour blocking in Tris-buffered saline (50 mM Tris-HCl, pH 7.5, 150 mM NaCl; TBS) containing 5% (w/v) milk powder, mouse monoclonal anti c-myc antibody 9E10 (diluted 1:1000 in TBS, Santa Cruz Biotechnology) was used and detected by HRP-conjugated secondary antibody rabbit anti-mouse (Dako A/S, Glostrup, Denmark), at a concentration of 1:5000 by using enhanced chemiluminescence (Thermo Fischer Scientific, Rockford, IL, USA). Mouse monoclonal antibody against eukaryotic elongation factor 1A (Upstate, Lake Placid, NY, USA) was used at a concentration of 1:20,000 in TBS. Rat monoclonal antibody against 14-3-3 protein II (a gift from Masahiro Inoue, Kurume University, Department of Infectious Medicine, Kurume, Japan) was used at a concentration of 1:20,000 in TBS and visualized with rat HRP-conjugated secondary antibody (Dako A/S) using SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific). Protein sizes were determined using PageRuler Plus Prestained Protein Ladder (Thermo Fisher Scientific).
(Immuno-) fluorescence microscopy
After collecting and washing parasites in 1 ml cold phosphate-buffered saline (10 mM sodium phosphate, 150 mM NaCl, pH 7.5; PBS), they were allowed to adhere to microscopy slides (Thermo Fischer Scientific) for 15 min. Subsequently, parasites were fixed with 4% (w/v) paraformaldehyde, washed with PBS and permeabilized with 0.2% (w/v) Triton X-100 in PBS. After blocking with 2% bovine serum albumin in PBS for 30 min, the primary antibody in blocking solution was added for 45 min. After washing with PBS, the corresponding secondary antibodies (goat anti-mouse AlexaFluor 594 or goat anti-rabbit AlexaFluor 488 [Thermo Fischer Scientific]) at a dilution of 1:1000 in blocking buffer were added for 45 min. Cells were mounted with Vectashield containing 4′,6-diamidino-2-phenylindole (DAPI, Vector Laboratories, CA, USA). For nile red (9-diethylamino-5H-benzo[alpha]phenoxazine-5-one, Sigma Aldrich) staining, stock solutions (1 mg ml−1 in acetone, protected from light) were diluted 1:50 or 1:1000 in PBS. After washing trypanosomes with PBS and allowing them to adhere to microscopy slides, 150 µl nile red solution was applied for 30 min. After washing with PBS three times for 10 min, cells were mounted with Vectashield containing DAPI. (Immuno-) Fluorescence microscopy was performed with a Leica DMI6000 B inverted microscope using a 63x oil objective. Pictures were acquired, processed and 3D-deconvolved with Leica LAS X software (Leica Microsystems CMS GmbH, Heerbrugg, Switzerland). Antibody signals were detected using excitation wavelengths of 488 nm and 590 nm while nile red fluorescence was recorded at 488 nm.
Lipid analyses
Total parasite lipids were extracted (Bligh and Dyer, 1959) and separated by one-dimensional thin layer chromatography (TLC) on Silica Gel 60 plates (Merck, Darmstadt, Germany) in solvent system 1 composed of chloroform:methanol:acetic acid:water (25:15:4:2; v/v/v/v) for phospholipid (Signorell et al., 2008) or solvent system 2 composed of hexane:ethyl ether:acetic acid (90:15:2; v/v/v) for TAG (Allmann et al., 2014) analysis. After separation, plates were dried and lipids were visualized by exposure to iodine vapor. For in vivo labeling experiments, parasites at mid-log phase were cultured in the presence of [3H]glycerol for indicated times and lipids were extracted as above. After separation by TLC, [3H]-labeled lipids were analyzed by scanning the air-dried plate with a radioisotope detector (Berthold Technologies, Regensdorf, Switzerland). Subsequent quantification of radiolabeled lipids was done using the Rita Control software provided by the manufacturer. On each TLC plate appropriate lipid standards were carried alongside. For lipid phosphorus quantification, total parasite lipids were separated by two-dimensional TLC using solvent system 3 composed of chloroform:methanol:25% NH3:water (45:37:6:4; v/v/v/v) for the first dimension and solvent system 4 composed of chloroform:methanol:acetone:acetic acid:water (40:15:15:12:8; v/v/v/v) for the second dimension (Signorell et al., 2008). After exposure to iodine, lipid spots were scraped from the plates and lipid phosphorus was quantified photometrically (Rouser et al., 1970). Quantification was performed using a series of inorganic phosphate standards. The assay was linear between 0 and 200 nmoles of phosphate per sample (linear correlation coefficients were >0.99 in all experiments).
Transmission electron microscopy
Parasites were washed in cold 100 mM sodium cacodylate buffer (pH 7.2) and resuspended in the same buffer containing 2.5% (v/v) glutaraldehyde. Cells were fixed for 2 hours at room temperature, followed by post-fixation in 2% OsO4 in cacodylate buffer (pH 7.3). After three washes in distilled water, samples were prestained in saturated uranyl acetate solution in distilled water for 30 min at room temperature and extensively washed in distilled water. Dehydration was carried out by stepwise incubation in ethanol (30%–50%–70%–90%–100%). Parasites were then embedded in EPON812 resin as described previously (Hemphill and Croft, 1996). Sections (80 nm) were cut on an ultramicrotome (Reichert & Jung, Vienna, Austria), placed onto 300 mesh formvar-carbon-coated nickel grids (Plano, Wetzlar, Germany) and sections were stained with uranyl acetate and lead citrate (Hemphill et al., 2004). Specimens were viewed on a CM12 transmission electron microscope operating at 60 kV.
ATP production assays
ATP production was measured in digitonin extracts as previously described (Bochud-Allemann and Schneider, 2002). Briefly, purified mitochondrial fractions from 2 × 108 digitonin-solubilized parasites were used. To induce ATP production, 5 mM of substrate (succinate or α-ketoglutarate) and 60 µM ADP were added. In some experiments, the samples were pre-incubated with atractyloside (33.3 µg/ml, final concentration; inhibitor of the ADP/ATP translocase) or antimycin (2 µM, final concentration; inhibitor of respiratory chain complex III). After incubation at 27°C, 60% perchloric acid was added and the samples were left on ice for 10 min and then centrifuged for 5 min at 16,500 g. Aliquots of the supernatants were collected and neutralized with 1 M KOH and the reaction was centrifuged again. Finally, ATP production was determined using Bioluminescence Assay Kit CLS II (Roche, Basel, Switzerland) according to the manufacturer's instructions.
Measurement of mitochondrial membrane potential
Live cells were treated with 200 nM TMRE (tetramethylrhodamine ethyl ester perchlorate) for 30 min at 27°C, washed with PBS and 2 × 105 cells were plated on a dark 96-well plate. Fluorescence intensity was analyzed using a Spark plate reader (Tecan Group Ltd., Männedorf, Switzerland) with 535 nm excitation and 595 nm emission wavelength. CCCP (carbonyl cyanide 3-chlorophenylhydrazone; 25 µM final concentration) was added 10 min prior to TMRE labeling as a control. Data points were measured in triplicate, and three independent experiments were performed.
Mass spectrometry
After immunoprecipitation of c-myc-TbLpn, protein samples were separated by SDS-PAGE, cut into defined bands and sent for mass spectrometry analysis at the Mass Spectrometry and Proteomics Laboratory, University of Bern. The gel pieces were reduced, alkylated and digested by trypsin and the digests were analyzed by liquid chromatography-mass spectrometry/mass spectrometry (PROXEON coupled to a Qexactive HF mass spectrometer (Thermo Fisher Scientific)) with one injection of 5 μl digests (Eng et al., 2012). The database used was T. brucei 427TREU927.
Statistical analyses
Statistical comparison between two groups was performed using Student′s t-test (for real time PCR) or Two-way ANOVA (for TLC scanning results, lipid phosphorus determinations) and one-way ANOVA (for ATP measurements, TMRE quantification) using GraphPad Prism 6.
Acknowledgements
We thank A. González-Salgado, M. Rauch and M. Niemann for technical assistance and support during parts of the study. We also thank M. Inoue (University of Kurume) and A. Schneider (University of Bern) for providing antibodies. P.B. thanks P. Simon for stimulation. The work was supported by Swiss National Science Foundation grants 31003A-169355 to P.B. and 31003A-165782 to A.H. The authors declare no conflict of interest.