The sigma factor σ54 is required for the long-term survival of Leptospira biflexa in water
Summary
Leptospira spp. comprise both pathogenic and free-living saprophytic species. Little is known about the environmental adaptation and survival mechanisms of Leptospira. Alternative sigma factor, σ54 (RpoN) is known to play an important role in environmental and host adaptation in many bacteria. In this study, we constructed an rpoN mutant by allele exchange, and the complemented strain in saprophytic L. biflexa. Transcriptome analysis revealed that expression of several genes involved in nitrogen uptake and metabolism, including amtB1, glnB-amtB2, ntrX and narK, were controlled by σ54. While wild-type L. biflexa could not grow under nitrogen-limiting conditions but was able to survive under such conditions and recover rapidly, the rpoN mutant was not. The rpoN mutant also had dramatically reduced ability to survive long-term in water. σ54 appears to regulate expression of amtB1, glnK-amtB2, ntrX and narK in an indirect manner. However, we identified a novel nitrogen-related gene, LEPBI_I1011, whose expression was directly under the control of σ54 (herein renamed as rcfA for RpoN-controlled factor A). Taken together, our data reveal that the σ54 regulatory network plays an important role in the long-term environmental survival of Leptospira spp.
Graphical Abstract
Introduction
The genus Leptospira belongs to the phylum Spirochaetes and is comprised of saprophytic and pathogenic species (Ko et al., 2009; Adler and de la Peña Moctezuma, 2010; Picardeau, 2017). Pathogenic Leptospira are the causative agents of leptospirosis, a re-emerging globally important zoonotic disease (Costa et al., 2015). Despite of the development of genetic tools, genetic manipulation of Leptospira, especially for pathogenic Leptospira species, remains to be a major challenge and has greatly hampered the characterization of the pathogenesis as well as the general biology of Leptospira. L. biflexa is a free-living saprophytic spirochete that survives exclusively in the external environment. It has extensive genetic and structural similarities with infectious species of Leptospira (Picardeau et al., 2008). L. biflexa has a faster growth rate and is more amenable to genetic manipulation than pathogenic Leptospira, which makes it a useful system for genetically elucidation of gene functions of Leptospira (Picardeau et al., 2001, 2008; Guegan et al., 2003; Louvel et al., 2006, 2008).
One feature of Leptospira is its ability to survival in a vast array of environments that range from soil and water to the tissues of mammalian hosts (Barragan et al., 2017). Transcriptomic and proteomic analyses of Leptospira spp. have identified global changes in gene expression in response to diverse changes of environmental factors, such as innate immunity (Xue et al., 2010), iron limitation (Lo et al., 2010a), temperature (Lo et al., 2006; Qin et al., 2006), osmolality (Matsunaga et al., 2007), growth phase (Stewart et al., 2016), serum exposure (Patarakul et al., 2010), host-adaption (Caimano et al., 2014; Nally et al., 2017) and chronic infection (Monahan et al., 2008). On the other hand, our understanding of the mechanisms underlying gene regulation in Leptospira is limited. Genome analyses reveal a large number of genes encoding proteins involved in signal transduction and gene regulation in Leptospira species. For example, they have a large number of genes encoding the two- component systems (102 in L. biflexa and 76 in L. interrogans) (Fouts et al., 2016). So far, only a response regulator, HemR, has been characterized, showing that it affects transcriptional activation and repression of genes involved in heme metabolism (Morero et al., 2014). Few other regulators have been studied in Leptospira, including PerR, which regulates oxidative stress response (Lo et al., 2010a) and KdpE, which activates the KdpABC potassium transporter (Matsunaga and Coutinho, 2012). Elegant in-depth work has been conducted on the regulator LexA in L. interrogans (Cuñé et al., 2005; Fonseca et al., 2013; Schons-Fonseca et al., 2016). L. interrogans Copenhageni serovar has two copies of lexA genes: lexA1 and lexA2. LexA activates an SOS response following DNA damage. ChIP-seq data identified 24 LexA1 binding sites in the genome upstream of the genes whose expression increases upon DNA damage (Schons-Fonseca et al., 2016).Alternative sigma factors are a common strategy for bacteria to regulate gene expression in response to environmental and physiological cues (Kazmierczak et al., 2005; Feklistov et al., 2014). In addition to a housekeeping σ70, all leptospiral species have alternative sigma factor 54 (σ54 or RpoN) and extracytoplasmic function (ECF) sigma factors (σE) (Fouts et al., 2016). σ54 is a unique sigma factor that is phylogenetically distinct from other sigma factors and is widely distributed among bacteria (Bush and Dixon, 2012; Yang et al., 2015; Bonocora et al., 2015; Zhang et al., 2016; Siegel and Wemmer, 2016). It recognizes a unique −24/-12 promoter motif instead of the −35/-10 motif recognized by σ70. Its activation absolutely requires the input of free energy (ATP) from an associated activator, referred as enhancer-like binding protein (EBP), to initiate transcription. Each EBP-σ54 pair activates a set of genes in response to different signals (Bush and Dixon, 2012).
σ54 is a well-recognized factor that plays important roles in environmental and host adaptation in many bacteria (Francke et al., 2011). The function of σ54 in any Leptospira species remains unknown heretofore. We recently reported that a transposon mutant lacking production of one of the EBP, EbpA in L. interrogans, is incapable of surviving in environmental water (Hu et al., 2016). Since EbpA activates σ54-dependent genes, such finding suggests that σ54 is important for leptospires to survive in water. However, inactivation of the rpoN gene encoding σ54 in pathogenic leptospires has not been successful (Hu et al., 2016). In fact, no mutant has been generated for any of predicted alternative sigma factors or global regulators in Leptospira species.
In this study, we reported the successful inactivation and complementation of the σ54-coding gene (rpoN) in L. biflexa by allelic exchange. We performed transcriptome analysis and identified several putative σ54-dependent genes, including a novel nitrogen-responsive gene, LEPBI_I1011, whose expression is under the direct control by σ54. We showed that σ54 is important for leptospiral adaptation to the natural environment outside of a host.
Results
Comparison of EBP and σ54 regulatory pathways among Leptospira species
Both saprophytic and pathogenic Leptospira encode a single copy of rpoN gene (encodes for σ54), which is located on the large chromosome of the genome. L. biflexa σ54 sequence shares ∼60% identity at the amino acid level with that of pathogenic and intermediate leptospiral species. Phylogenetic analysis showed that the Leptospira σ54 was evolutionary separated from other bacteria, even from other Spirochaetaceae genera including Borrelia and Treponema (Fig. 1). Leptospira σ54 has all three conserved functional domains, Region I, II and III (Fig. 2A), despite that the overall identities of Leptospira σ54 at the amino acid level are less than 30% with a typical σ54 such as in Enterobacteriaceae.
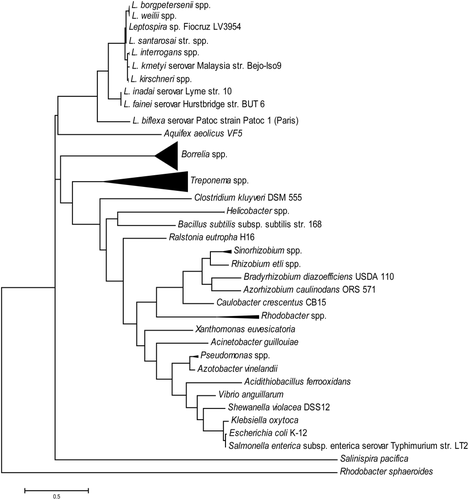
Molecular phylogenetic analysis of Leptospira σ54. The evolutionary history was inferred by using the Maximum Likelihood method based on the JTT matrix-based model (Jones et al., 1992). The tree with the highest log likelihood (-10238.1361) is shown. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using a JTT model and then selecting the topology with superior log likelihood value. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The analysis involved 101 amino acid sequences. Multiple items were selected for the genera of Leptospira, Borrelia and Treponema, while only function characterized RpoN items were selected for other genera. All positions containing gaps and missing data were eliminated. There were a total of 142 positions in the final dataset. Evolutionary analyses were conducted in MEGA7 (Kumar et al., 2016). Triangles represent the diversity within genospecies.
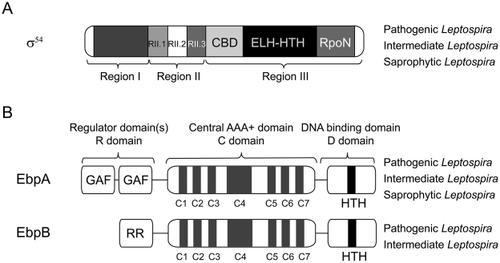
Domain architectures of σ54 and their activators, enhancer-like binding proteins (EBPs) in the Leptospira genus.
A. The Leptospira σ54 contains all three conserved regions (I to III) found in other σ54 (Yang et al., 2015). Region I plays an inhibitory role and contains the contact sites for its cognate activator proteins. Region II is the most variable region among σ54 proteins with high abundance of acidic amino acids, and it is proposed to facilitate template loading (RII.2) and RNA separation from the DNA template (RII.3). The subdomains of Region III, including the RNAP core-binding domain (CBD) and the following extra-long α-helix-helix-turn-helix motif (ELH-HTH) domain, are involved in interacting with the −12 promoter element. The RpoN box, located in the C-terminal of Region III, is responsible for −24 promoter element recognition. DNA cross-linking region [ELH] and helix-turn-helix [HTH] are involved in DNA-binding.
B. The pathogenic Leptospira have two EBPs, EbpA and EbpB, while the saprophytic Leptospira only has EbpA. Both EbpA and EbpB consist of three functional domains: an N-terminal regulatory domain (R domain), a central AAA+ (ATPases Associated with a wide variety of cellular Activities) domain (C domain) and a C-terminal DNA-binding domain (D domain). The R domain does not share a common homolog with other members of the EBP family, and different sensory domains are present depending on the signal to be detected. EbpA is an FhlA (formate hydrogen lyase activator)-like EBP, which includes two GAF (cGMP-specific phosphodiesterases, adenylyl cyclases and FhlA) motifs in the R domain. EbpB is an NtrC-like EBP, which has a conserved response regulator-type R-domain. The central C domain of EbpA and EbpB contain C1 to C7 seven conserved subdomains that are essential for σ54-dependent transcription. The HTH motif in D domain directs the EBP to a specific binding site.
Activation of σ54–controlled genes requires a prokaryotic enhancer binding protein. Both pathogenic and saprophytic Leptospira have the activator EbpA for activation of σ54-controlled transcription initiation (Fouts et al., 2016; Hu et al., 2016) (Fig. 2B). EbpA is an FhlA (formate hydrogen lyase activator)-like EBP (group III), which includes two GAF (cGMP-specific phosphodiesterases, Adenylyl cyclases and FhlA) domains (Hopper and Bock, 1995). In addition, pathogenic Leptospira species have an activator EbpB that is not present in saprophytic Leptospira (Fouts et al., 2016; Hu et al., 2016). EbpB is an NtrC-like EBP (group I), which is activated by phosphorylation at the RR domain by a cognate histidine kinase (Doucleff et al., 2005). Thus, pathogenic and saprophytic Leptospira share an EbpA-σ54 regulatory network, whereas pathogenic Leptospira have an additional EbpB-σ54 network.
Targeted inactivation and complementation of rpoN in L. Biflexia
We first attempted to inactivate rpoN in pathogenic Leptospira. Despite multiple attempts, no mutant could be obtained in. L. interrogans strain Lai 56601, Fiocruz L1–130 or L495. We thus focused on saprophytic Leptospira, and successfully inactivated the rpoN gene (LEPBI_I1650) by allelic exchange in L. biflexa serovar Patoc strain Patoc1 (Fig. 3A). The lack of rpoN expression and the product σ54 in the rpoN mutant were demonstrated by quantitative reverse transcription-PCR (qRT-PCR) (Fig. 3B) and immunoblotting analyses (Fig. 3C). A complemented strain for the rpoN mutant was also achieved by transforming a shuttle vector containing a wild-type rpoN gene driven under the hsp10 promoter of L. biflexa (Fig. 3A), and restoration of σ54 was confirmed in the complementary strain (rpoN/pJJ003) (Fig. 3B and C).
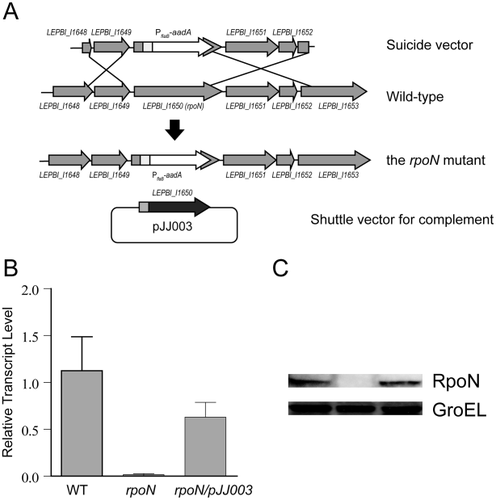
Inactivation and complementation of rpoN (LEBI_1650) in L. biflexa by allele exchange.
A. Schematic representation of rpoN and its neighboring genes in the chromosome of wild-type L. biflexa. The middle 793 bp of the rpoN gene was disrupted by insertion of a 1197 bp flaB driven streptomycin-resistance gene (aadA) cassette from Myxococcus xanthus (Magrini et al., 1998) by homologous recombination. The shuttle plasmid pJJ003 containing the hsp10 promoter that drives the expression of the rpoN gene was used to complement the rpoN mutant (bottom panel).
B. Confirmation of the rpoN disruption and complementation by qRT-PCR analysis of rpoN transcriptional levels.
C. Immunoblot analysis of wild type, rpoN mutant and complemented strains. Whole-cell lysates of L. biflexa equivalent to 108 cells were separated on 12% Tris-glycine gel and then immunoblotted with antisera directed against RpoN and cytoplasmic heat shock protein, GroEL (loading control). The blotted membrane was developed by chemical luminescence.
Transcriptome analysis for the identification of σ54-regulated genes
To identify genes influenced by σ54, we performed whole-transcriptome analysis for wild-type L. biflexa and the rpoN mutant. The results revealed that expression of 20 genes was lower in the rpoN mutant than that in the wild-type strain (with a cut-off of over twofold of changes in expression), suggesting that these genes were positively regulated by σ54 (Table 1). The analysis also identified 36 genes whose expression was negatively regulated by σ54 (Table 2). Among those genes that were σ54-dependent, several genes are predicted involving in nitrogen transport and metabolism, including genes encoding ammonia transporter AmtB1 (LEPBI_I1767), AmtB2 (LEPBI_I2408), nitrogen regulatory protein P-II (LEPBI_I2407), Nark-like nitrate/nitrite antiporter (LEPBI_I2771) and NtrX family nitrogen assimilation regulator (LEPBI_I1654). Further qRT-PCR analysis confirmed that expressions of these genes were significantly downregulated in the rpoN mutant, while the complemented strain could fully restore their expression (Fig. 4). Note that we also examined the patter of gene regulation in the rpoN mutant at both 30°C and room temperature, and a similar pattern was observed.
| Locus tag | Mean fold changea | COGb | Description of gene product | Orthologous in L. interrogans serovar Lai 56601 | Orthologous in L. borgpetersenii serovar Hardjo-bovis str. L550 |
|---|---|---|---|---|---|
| LEPBI_I1650 | 176.64 | COG1508K | RNA polymerase sigma-54 factor | LA_2404 | LBL_1670 |
| LEPBI_I2408 | 10.19 | COG0004P | Ammonium transporter, AmtB2 | LA_3622, LA_3806 | LBL_2706 |
| LEPBI_I2407 | 3.25 | COG0347E | Nitrogen regulatory protein P-II (GlnB) | LA_3807 | LBL_2705 |
| LEPBI_I1011 | 2.92 | – | hypothetical protein | No hit | No hit |
| LEPBI_I1767 | 2.51 | COG0004P | Ammonium ABC transporter permease, AmtB1 | LA_3622, LA_3806 | LBL_2706 |
| LEPBI_I1174 | 2.49 | – | Hypothetical protein | No hit | No hit |
| LEPBI_I3124 | 2.49 | COG2208TK | Serine phosphatase | No hit | No hit |
| LEPBI_I0440 | 2.48 | – | Hypothetical protein | No hit | No hit |
| LEPBI_I1657 | 2.30 | COG0223J | Methionyl-tRNA formyltransferase | LA2396 | No hit |
| LEPBI_I2653 | 2.29 | COG0687E | Putative spermidine/putrescine-binding periplasmic protein PotF/PotD; putative signal peptide | No hit | No hit |
| LEPBI_I2502 | 2.25 | – | Hypothetical protein | No hit | No hit |
| LEPBI_I1652 | 2.21 | COG1925G | Phosphocarrier protein HPr (histidine-containing protein) | LA_2402 | LBL_1668 |
| LEPBI_I1653 | 2.20 | COG5000T | Histidine kinase sensor protein; putative membrane protein | LA_2401 | LBL_1667 |
| LEPBI_II0220 | 2.17 | – | Hypothetical protein | LA_2746, LA_4046 | LBL_1948, LBL_2853 |
| LEPBI_II0102 | 2.14 | – | Hypothetical protein | No hit | No hit |
| LEPBI_I1651 | 2.08 | COG1493T | HPr kinase/phosphorylase | LA_2403 | LBL_1669 |
| LEPBI_I0858 | 2.07 | – | Hypothetical protein | No hit | No hit |
| LEPBI_I2771 | 2.04 | COG2223P | Nitrite extrusion protein 1, NarK | No hit | No hit |
| LEPBI_p0010 | 2.02 | COG2208TK | Putative phosphoserine phosphatase; putative membrane protein | No hit | No hit |
| LEPBI_I1654 | 2.02 | COG2204T | Putative nitrogen assimilation regulatory protein NtrX | LA_2400 | LBL_1666 |
- a. Mean values of two replicates are shown for three oligonucleotides on microarray with a cut-off value of 2.
- b. COG (clusters of orthologous groups) categories.
| Locus tag | Mean fold changea | COGb | Description of gene product | Homologues in L. interrogans serovar Lai | Homologues in L. borgpetersenii serovar Hardjo-bovis str. L550 |
|---|---|---|---|---|---|
| LEPBI_p0016 | 7.27 | – | Hypothetical protein | No hit | No hit |
| LEPBI_II0270 | 6.09 | COG3329R | Putative sodium bicarbonate cotransporter; putative membrane protein | LA_4270 | LBL_0122 |
| LEPBI_pa0017 | 5.79 | – | Heme-binding protein HmuY | LB_192 | LBL_4177 |
| LEPBI_p0015 | 5.73 | COG4558P | ABC-type Fe3+-hydroxamate transport system, periplasmic component | No hit | No hit |
| LEPBI_II0269 | 5.70 | COG0288P | Putative beta-type carbonic anhydrase | No hit | LBL_2497 |
| LEPBI_p0018 | 4.44 | COG4206H | Putative TonB-dependent outer membrane receptor | LB_191 | LBL_4178 |
| LEPBI_p0013 | 4.09 | COG4559P | ABC-type hemin transport system, ATPase | LA_0969 | LBL_1280 |
| LEPBI_p0012 | 3.93 | COG3720P | Hemin degradation protein HemS | No hit | No hit |
| LEPBI_p0014 | 3.93 | COG0609P | ABC-type hemin transport system, permease; putative membrane protein | No hit | No hit |
| LEPBI_II0279 | 3.90 | – | Hypothetical protein; putative signal peptide | No hit | No hit |
| LEPBI_I2760 | 3.32 | COG1629P | Putative TonB-dependent receptor protein | LA_2242 | LBL_1447 |
| LEPBI_I2761 | 3.17 | – | Putative signal peptide | LA_2241 | LBL_1445 |
| LEPBI_II0185 | 3.00 | COG0499H | S-adenosyl-L-homocysteine hydrolase | LB_106 | LBL_4105 |
| LEPBI_I2678 | 2.99 | – | Hypothetical protein | No hit | No hit |
| LEPBI_I2246 | 2.97 | – | Hypothetical protein | No hit | No hit |
| LEPBI_I2762 | 2.66 | COG3182S | Putative iron-regulated membrane protein; putative membrane protein | LA_2440 | No hit |
| LEPBI_I2257 | 2.59 | – | Hypothetical protein | No hit | No hit |
| LEPBI_I0683 | 2.58 | – | Putative lipoprotein; putative signal peptide | No hit | No hit |
| LEPBI_I2431 | 2.54 | – | Hypothetical protein | No hit | No hit |
| LEPBI_II0183 | 2.54 | COG1410E | B12-dependent methionine synthase | LB_108 | LBL_4107 |
| LEPBI_I3034 | 2.45 | COG3329R | Putative permease; putative membrane protein | LA_4270 | LBL_0122 |
| LEPBI_I2424 | 2.42 | COG1136V | ABC-type transport system, ATP binding protein | LA_0274, LA_2982, LA_3713 | LBL_0572, LBL_0299, LBL_2006, LBL_2140 |
| LEPBI_I3255 | 2.32 | COG0300R | SDR family dehydrogenase/reductase | LA_2621 | LBL_1854 |
| LEPBI_I2672 | 2.27 | – | Hypothetical protein | LA_1273 | LBL_2068 |
| LEPBI_I0141 | 2.17 | – | Hypothetical protein | No hit | No hit |
| LEPBI_I1418 | 2.16 | COG0174E | Glutamine synthetase (glutamate–ammonia ligase) | LA_1313 | LBL_1991 |
| LEPBI_I3269 | 2.16 | COG4974L | Putative integrase | No hit | No hit |
| LEPBI_I1664 | 2.14 | COG0335J | 50S ribosomal protein L19 | LA_2387 | LBL_1656 |
| LEPBI_I0682 | 2.08 | – | Hypothetical protein | No hit | No hit |
| LEPBI_I1089 | 2.07 | COG4270S | Hypothetical protein | No hit | No hit |
| LEPBI_I3149 | 2.06 | COG2885M | OmpA-family lipoprotein | LA_0222 | LBL_2925 |
| LEPBI_II0159 | 2.06 | – | Hypothetical protein | No hit | No hit |
| LEPBI_II0050 | 2.05 | – | Hypothetical protein | No hit | No hit |
| LEPBI_I1195 | 2.05 | COG0623I | Putative enoyl-(Acyl-carrier-protein) reductase | No hit | No hit |
| LEPBI_I2927 | 2.03 | – | Hypothetical protein | LA_0268 | LBL_0294 |
| LEPBI_I0937 | 2.01 | COG1004M | UDP-glucose 6-dehydrogenase (UDP-Glc dehydrogenase; UDP-GlcDH) (UDPGDH) | LA_1459 | LBL_2074 |
- a. Mean values of two replicates are shown for three oligonucleotides on microarray with a cut-off value of 2.
- b. COG (clusters of orthologous groups) categories.
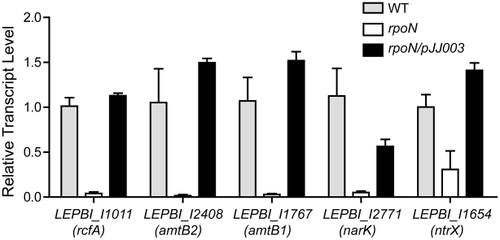
qRT-PCR analyses of σ54-dependent genes in L. biflexa. The L. biflexa wild-type (WT), rpoN mutant (rpoN) and complemented strain (rpoN/pJJ0003) were grown in regular EMJH medium to mid-logarithmic phase. RNA samples were then isolated and subjected to qRT-PCR analysis. The levels of gene expression in each sample were normalized to flaB, and the relative levels to that in wild-type strain (set as value of 1) were reported. Error bars indicate standard deviation from three replicates. The expression levels of all tested candidates in the mutant demonstrated statistical differences (P < 0.05) in student t test compared to expression in wild-type.
The σ54 regulon is important for leptospiral survival under the nitrogen starvation or environmental water conditions
To investigate whether σ54 is important for nitrogen utilization of L. biflexa, wild-type, the rpoN mutant and the complemented strains were cultured either under the nitrogen-rich conditions (regular Ellinghausen-McCullough-Johnson-Harris (EMJH) medium, containing 5 mM NH4Cl) (Fig. 5A), or nitrogen-poor conditions in which NH4Cl in the EMJH medium was replaced with 5 mM of glutamine (Fig. 5B), arginine (Fig. 5C) or alanine (Fig. 5D). The result showed that all L. biflexa strains grew well when glutamine, a known major amide-donor amino acid, was used as a nitrogen source. However, both wild-type and the mutant grew poorly when arginine or alanine was used as a nitrogen source, suggesting that L. biflexa cannot utilize these poor nitrogen sources (Fig. 5C and D).
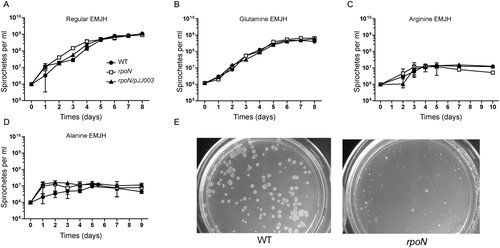
Growth phenotypes of the rpoN mutant under various nitrogen conditions. The L. biflexa wild-type (WT), rpoN mutant (rpoN) and complemented strains (rpoN/pJJ0003) were grown in regular EMJH medium to mid-logarithmic phase and then subcultured into EMJH (A) or modified EMJH with 5 mM glutamine (B), arginine (C), or alanine (D) replacing NH4Cl. All cultures were incubated at 30°C, and the growth was monitored by enumeration under dark-field microscopy daily. The experiment was performed three times with a representative result shown. Error bars indicate standard error from triplicate cultures. (E), σ54 is required to recover from nitrogen starvation. The strains of L. biflexa wild-type and rpoN mutant were first starved for a week in EMJH media without NH4Cl and then plated on solid EMJH media. Plates were incubated for 7 days at 30°C before the images were taken.
We then examined whether there is a difference in survival between wild-type and the rpoN mutant strains under the long-term nitrogen starvation conditions. After spirochetes were incubated under the nitrogen-starvation conditions for one week, cells were plated on regular EMJH solid media for colony count. As shown in Fig. 5E, wild-type cells recovered well, whereas the rpoN mutant could no longer recover and only few tiny colonies were found. This data suggests that the rpoN mutant has a defect in survive under the long-term nitrogen starvation conditions.
Leptospira is known to be capable of surviving in environmental water for a long time. Since the rpoN mutant could not survive during nitrogen starvation, we argued that it should be incapable to survive in water. To this end, wild-type, the rpoN mutant and the complemented strain of L. biflexa were incubated in spring water, and samples were collected weekly and plated on the solid EMJH media for quantitation of cell viability. The rpoN mutant formed tiny colonies, and became no longer detectable at week 4 after incubation in water (Fig. 6), whereas wild-type and the complementary strains grew normally and remained detectable at week 6 (Fig. 6). This result indicates that σ54 is also important to the viability of Leptospira in water.
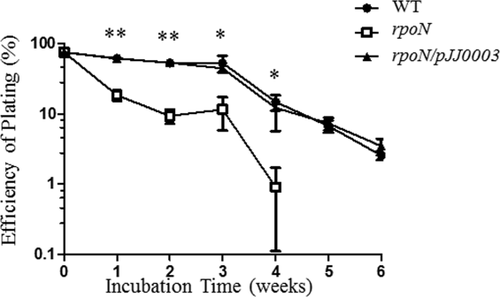
σ54 is required for the long-term survival of L. biflexa in mineral water. The L. biflexa wild-type (WT), rpoN mutant (rpoN) and complemented strains (rpoN/pJJ0003) were grown in regular EMJH medium to mid-logarithmic phase and then harvested by centrifugation. The cells were resuspended in spring waters and incubated at 30°C. Viable cells were counted weekly by plating a defined volume of diluted cultures on solid EMJH media and counting the number of colonies that arose. The experiment was performed three times with a representative result shown. Error bars indicate standard error from triplicate cultures. Student t test was used for the statistical comparisons between wild-type and the rpoN mutant. * P < 0.05, **P < 0.01.
amtB1, amtB2 and narK are induced under the nitrogen starvation condition, and are controlled by σ54 indirectly
To begin elucidating the mechanisms underlying how the RpoN regulon governs the survival of L. biflexa in the nitrogen-limiting mediums and in water, we focused on σ54-regulated genes, amtB1, amtB2 and narK. We examined if the expression of these genes is induced under the nitrogen-limiting conditions (Fig. 7A–C). In wild-type L. biflexa, their expressions were significantly upregulated under the nitrogen limiting conditions. The expression of these genes in the rpoN mutant could not be detected under any of the conditions tested, further supporting the notion that the expression of these genes is absolutely dependent on σ54.
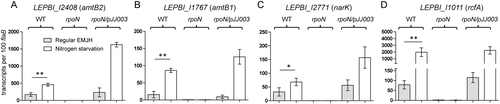
Several σ54-dependent genes are highly regulated in response to nitrogen limitation by qRT-PCR analysis. Wild-type (WT), the rpoN mutant (rpoN), and the complemented (rpoN/pJJ0003) strains were grown in regular EMJH medium to mid-logarithmic phase, harvested and resuspended into standard EMJH or nitrogen-limited (ammonia-free EMJH) medium. All treatments were incubated at 30°C for one week, and RNA samples were then isolated and subjected to qRT-PCR analyses for the expression levels of LEPBI_I2408 (amtB2) (A), LEPBI_I1767 (amtB1) (B), LEPBI_I2771 (narK) (C) and LEPBI_I1011 (rcfA) (D). Values represent the average numbers of each transcript per 100 copies of flaB from three biological replicates. Student t test was used for the statistical analysis. * P<0.05, **P<0.01.
σ54 recognizes a highly conserved −24/-12 promoter sequence (TGGCA < 6bp > TTGCT/A). Further analysis of the upstream sequences of amtB1, amtB2 and narK (including the upstream sequences of the putative operons in which these genes may reside) did not identify a putative σ54-type promoter, a result that is consistent with recent reports of genome-wide analysis of σ54-type promoters in published bacteria genomes (Francke et al., 2011; Bonocora et al., 2015). These data suggest that the expression of amtB1, amtB2 and narK are not directly under the control of σ54.
rcfA encoding a tetratricopeptide repeat protein is induced under the nitrogen starvation condition, and is under the direct control of σ54
Transcriptome analysis revealed that the expression of LEPBI_I1011, which encodes a hypothetical protein containing a tetratricopeptide repeat (TPR, pfam13424) domain, was dramatically downregulated in the rpoN mutant (Table 1). Further qRT-PCR analysis confirmed this finding (Fig. 4). Similar to other predicted nitrogen utilization-related genes, expression of LEPBI_1011 was significantly upregulated in ammonia starvation but downregulated when ammonia is in excess, and such regulation is dependent on σ54 (Fig. 7D). These data suggest that LEPBI_I1011 is a novel protein involved in nitrogen regulation of L. biflexa. We hereby rename it as RpoN-controlled factor A (RcfA).
Promoter analysis of the upstream sequence of rcfA revealed that there is a putative σ54-type promoter sequence (TGGCA < 6bp > TTGCA) that is virtually identical to the consensus sequence of the σ54-type promoter, located 33bp upstream of the ATG translational start codon of RcfA (Fig. 8A). Based on the predicted transcriptional start site, transcription from this promoter will yield a rcfA mRNA containing a 21 bases of untranslated sequence, which includes a putative CGGAGG ribosomal binding site (Shine-Dalgarno sequence).
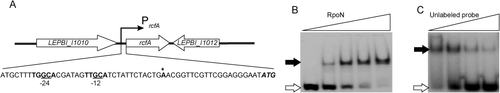
LEPBI_I1011 (rcfA) was directly controlled by σ54 in L. biflexa.
A. Schematic of the gene organization of rcfA and its promoter sequence. The −24 and −12 motifs of the typical σ54 promoter are labeled and underlined. The translational start site, ATG, is indicated in italics. The bold letter with a star indicates the predicted transcriptional start site and the boxed sequence indicates a putative ribosomal binding site (Shine-Dalgarno sequence).
B. The electrophoretic mobility shift assays (EMSAs) were conducted using purified RpoN and DNA fragment containing the promoter region of gene rcfA labeled with 32P. Various amounts of purified RpoN (0, 5, 10, 15 and 25 pmol) were incubated with 0.25 pmol of labeled DNA in a 10 μl reaction. For the competition reaction (C), unlabeled probes (0, 20, 50 and 200-fold) were added to the reaction that contained 0.25 pmol labeled DNA and 15 pmol purified RpoN. All reactions contain 0.5 μg salmon sperm DNA, and were incubated for 30 min at 23°C and then analyzed on non-denatured polyacrylamide gels. Gels were dried, and radioactive signals were visualized by exposure to X-ray film. The free probes are indicated by open arrows and the retarded DNA fragments by solid arrows.
To examine if σ54 of L. biflexa binds to the putative rcfA promoter, we purified recombinant RpoN protein (σ54) of L. biflexa and performed the in vitro electrophoretic mobility shift assays (EMSAs). To this end, a 40 bp oligonucleotide encoding the predicted −24/-12 region of the promoter of rcfA (Fig. 8A) was end-labeled with 32P and incubated with varying amounts of purified RpoN. The results showed that σ54 bound to the rcfA promoter region in a dose-dependent fashion (Fig. 8B). Moreover, the binding of RpoN to labeled probe was inhibited by the addition of 200-fold excess of specific cold competitor DNA (Fig. 8C), and was not affected by the addition of 100-fold excess of non-specific salmon sperm DNA. Noted that no DNA shift was observed in the EMSAs using promoter fragments of LEPBI_I2408, LEPBI_I1767 and LEPBI_I2771 (data not shown). These results suggest that rcfA is directly under the control of σ54.
Since EbpA is the essential activator for genes with σ54-type promoter, we examined EbpA binding to the upstream region of rcfA (-450-50 relative to the ATG start code). The EMSA results demonstrated that EbpA was capable of binding to the upstream region of rcfA in a dose-dependent manner (Fig. 9A). The binding of EbpA to rcfA was specific, as (1) the binding was not affected with addition of nonspecific competitor poly(dI-dC) (Fig. 9B) and (2) EbpA did not bind to the upstream region of glpF that has a σ70-type promoter (Fig. 9C).
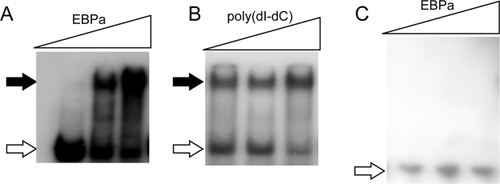
The enhancer-like binding protein EbpA binds to the upstream region of LEPBI_I1011 (rcfA). EMSA was conducted as described in Fig. 8.
A. Various amount of recombinant EbpA (0, 5, 10 pmol) were incubated with 32P labeled DNA fragment containing 450 bp upstream of rcfA (0.05 pmol) in the 10 μl reaction.
B. EbpA binding to upstream of rcfA in the presence of non-specific competitor poly dI:dC (0, 50, 100 ng).
C. EbpA binding to upstream of a σ70 promoter-driven gene, glpF. The free probes are indicated by open arrows and the retarded DNA fragments by solid arrows.
Discussion
Pathogenic leptospiras colonize the renal tubules of wild and domestic animals, and are shed by urine into soil and water. Their ability to survive in the aquatic environment for months is one of the key contributing factors to leptospirosis outbreak (Smith and Turner, 1961; Trueba et al., 2004; Ganoza et al., 2006; Andre-Fontaine et al., 2015; Hagan et al., 2016). However, the molecular mechanisms underlying the leptospiral survival in the environment are largely unexplored. In this study, we showed that the σ54 regulon is required for L. biflexa to survive in mineral water environments. This function of σ54 is through modulation of nitrogen uptake and metabolism, as the σ54-defective mutant is incapable of survival under the nitrogen starvation conditions as well as in water. The gene targets regulated by σ54 identified in this study, either directly or indirectly controlled by σ54, set the foundation for further investigation how the σ54 regulon responds to environmental signals and maintains survival under the nutrient-deprived conditions.
This study was carried out in the saprophytic strain L. biflexa, but the finding also has implication for the function of σ54 in pathogenic Leptospira. First, σ54 is highly conserved among all Leptospira, including saprophytic and pathogenic species. Second, we recently showed that EbpA, one of the EBPs that activate σ54, is also important for environmental survival of L. interrogans (Hu et al., 2016). Third, σ54 of L. biflexa and EbpA of L. interrogans both control the expressions of several nitrogen uptake/metabolism genes (Hu et al., 2016). Obviously, constructing a σ54-defective mutant in pathogenic Leptospira is needed to have a complete understanding of σ54 in Leptospira species. Unfortunately, constructing an rpoN mutant has not been successful despite multiple attempts in several strains of L. interrogans (data not shown). Nevertheless, based on the finding in this study and the previous finding on EbpA of L. interrogans, we postulate that the EbpA-σ54 pathway in both L. biflexa and L. interrogans has a similar role, i.e., modulating genes that are important for Leptospira to survive in the environment.
σ54 is known to be involved in a variety of processes in bacteria, such as nitrogen assimilation, carbon source utilization, certain fermentation pathways, flagellar synthesis and bacterial virulence (Reitzer and Schneider, 2001; Francke et al., 2011). In response to nitrogen limitation, σ54 regulates expression of genes involved in ammonia transporters and assimilation via the NtrB-NtrC system in E. coli (Reitzer and Schneider, 2001). Based on our bioinformatics analysis, no NtrB-NtrC system is present in Leptospira species (data not shown). However, we showed that the expressions of many nitrogen-utilization related genes of L. biflexa including amtB1, amtB2, narK and narX, are also σ54-dependent and are induced under nitrogen starvation. Membrane-bound AmtB belongs to the Ammonia Channel Transporter (Amt) family, which facilitates the uptake of ammonium/ammonia and is important for bacteria growth at low external ammonium concentrations in E. coli and other bacteria (Javelle et al., 2004; van Heeswijk et al., 2013). In ammonia-sufficient conditions, the diffusion of ammonia across the cytoplasmic membrane is sufficient to support bacterial growth, as demonstrated in E. coli (Soupene et al., 1998), Bacillus subtilis (Detsch and Stulke, 2003) and Corynebacterium glutamicum (Siewe et al., 1996; Meier-Wagner et al., 2001). L. biflexa has three copies of amtB genes, LEPBI_I1767 (amtB1), LEPBI_I2408 (amtB2) and LEPBI_I0794 (amtB3). The latter two genes are genetically linked with the gene encoding GlnK-like signal transduction protein. The AmtB-GlnK physical interaction is highly conserved, and the two genes form the conserved gene pair in a diverse range of Eubacteria and Archaea (Thomas et al., 2000). They are responsible for ammonia uptake and modulating the activity of glutamine synthetase (GlnA) for ammonia assimilation. Of note, our transcriptome analysis showed that the expression level of amtB2 is 10-fold higher than amtB1 and amtB3 (data not shown), indicating that AmtB2 may play a major role in ammonia uptake in L. biflexa.
The finding from this study suggests that the reason that the lack of nitrogen source is one of the reasons why the σ54 mutant of L. biflexa cannot sustain viability in the environmental water, as the σ54 mutant is unable to survive in the growth medium that lacks ammonia. This is in contrast to wild-type L. biflexa which remains viable for months in such medium. How does σ54 contribute to the survival of L. biflexa under the nitrogen starvation conditions? One of the possible mechanisms is via regulation of genes involved in nitrogen assimilation, in particular, the glnK-amtB operon. GlnK of E. coli has been shown to play many functions during nitrogen assimilation (Atkinson and Ninfa, 1999; Atkinson et al., 2002; Blauwkamp and Ninfa, 2002). It has a dramatic effect on the ability of E. coli cells to survive during nitrogen starvation (Blauwkamp and Ninfa, 2002). Given that the expression of the glnK-amtB2 operon is drastically downregulated in the σ54 mutant of L. biflexa, we hypothesize that σ54 contributes to the survival of L. biflexa in nitrogen starvation and environmental water conditions via regulation of glnK-amtB operon. Further studies to construct the glnK mutants in L. biflexa and L. interrogans and test their survival ability in environmental water are warranted to test this hypothesis.
We demonstrated in this study that σ54 regulates nitrogen assimilation genes (amtB1, amtB2, narK and ntrX) indirectly. How σ54 regulates expression of these genes remains to be elucidated. One of the genes we identified whose expression is directly controlled by σ54 is rcfA (LEPBI_I1011). Although the function of this 146 amino acid long, TPR domain-containing protein remains undefined, the level of rcfA expression is dramatically upregulated under the nitrogen starvation condition (Fig. 7D), suggesting that RcfA is involved in nitrogen assimilation. TPR-containing proteins often function as a module for protein–protein interactions, and can involve in a variety of cellular functions including gene regulation, signaling, transport, as well as involved in virulence (Cerveny et al., 2013). Whether σ54 regulates other nitrogen-regulated genes via RcfA remains to be tested. Of note, RcfA is highly conserved among saprophytic Leptospira species including L. meyeri, L. terpstrae, L. vanthielii, L. wolbachii and L. yanagawae. Interestingly, we showed that a gene encoding a TPR domain-containing protein, LMANv2_200027, in L. interrogans serovar Manilae strain L495, is also controlled by EbpA directly (Hu et al., 2016). We postulate that these two σ54-controlled TRP proteins may function similarly in response to nitrogen deprivation. In fact, most of the genes identified in this study to be differentially expressed in the rpoN mutant are not unique to saprophytic leptospires, and have homologous in pathogenic leptospiral species (Tables 1 and 2). These results indicate that EbpA-σ54 regulation is similar between saprophytic and pathogenic leptospires, and are important for environmental survival for Leptospira species. Pathogenic leptospires have addition pathway EbpB-σ54, which is lacking in saprophytic leptospires and likely plays a role in mammalian infection (Hu et al., 2016).
In addition to the genes positively regulated by σ54, transcriptome analysis in this study uncovered 36 genes whose expression was negatively regulated by σ54. Interestingly, several genes relate to iron-acquisition such as hemin binding/transport/degradation (LEPBI_p0012, LEPBI_p0013, LEPBI_p0014, LEPBI_pa0017), TonB-dependent outer membrane receptor (LEPBI_p0018, LEPBI_I2760), ABC-type Fe3+-hydroxamate transport system (LEPBI_p0015) and putative iron-regulated membrane protein; LEPBI_I2762). Iron is essential for the growth of both saprophytic and pathogenic Leptospira spp (Cullen et al., 2002; Louvel et al., 2005; Asuthkar et al., 2007; Murray et al., 2009; Lo et al., 2010b). Iron limitation upregulates many genes including genes predicted or demonstrated to be involved in hemin uptake or TonB-dependent membrane receptor (Cullen et al., 2002; Lo et al., 2010b). LEPBI_I2760 was experimentally showed to encode a TonB-dependent membrane receptor protein for ferrioxamines in L. biflexa, and the disruption of LEPBIa2760 resulted in an impaired utilization of desferrioxamine as an iron source (Louvel et al., 2006). Our data suggest that inactivation of σ54 upregulates hemin or other iron-related transport system as a general response to nutrient deprivation. How σ54 indirectly influences these gene expressions remains unclear. In this regard, L. biflexa and L. interrogans genomes have four fur-like genes encoding Fur, Zur and PerR family proteins that are likely involved in modulating expression of genes involved in iron acquisition (Louvel et al., 2005; Lo et al., 2010b). Expression levels of these genes were not affected by σ54 deletion. One possibility is that function, not the level of these proteins, were altered in the deficient mutant, subsequently, led to upregulation of these iron-related genes.
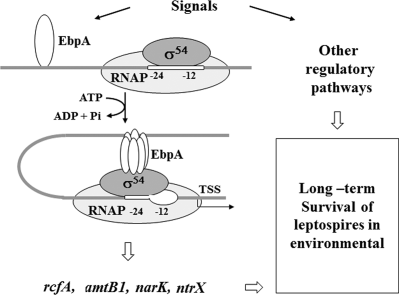
In summary, although a number of reports showing that leptospiras regulate their gene expression in responding to a wide range of environmental and host signals, genetic networks modulating gene expression in response to environmental cues have not been uncovered heretofore. This study identified the EbpA-σ54 regulatory network that modulates expression of genes associated with nitrogen uptake and metabolism, and that plays a key role in the environmental survival of Leptospira. Based on this and our previous finding, we propose the following model: EbpA likely senses environmental stress signals via its GAF motifs, and along with σ54, activates transcription of rcfA and other genes. σ54 indirectly regulates the expression of nitrogen-utilization related genes including amtB1, glnK-amtB2, narK and ntrX (Fig. 10). Expression of these and other σ54-dependent genes is one of the key factors that allow Leptospira to survive in the environment. Given the complex environmental conditions leptospires encounter and the presence of many putative regulatory proteins in Leptospira genomes, further identification of other regulatory systems modulating environmental adaptation of Leptospira are warranted.

Model for the σ54 regulon in L. biflexia. EbpA, the sole EBP in L. biflexa, senses environmental stress signals, and activates transcription of genes from a σ54-type promoter (-24/-12), in an ATP-dependent manner (upon oligomerization). EbpA-σ54 directly regulates expression of rcfA from a σ54–type promoter, and indirectly regulates expression of nitrogen-utilization related genes including amtB1, glnK-amtB2, nark, and ntrX. Expression of these and other σ54–dependent genes allows Leptospira to adapt and survive in nutrient-limiting environmental conditions such as environmental water. RNAP, RNA polymerase; TSS, transcriptional start site.
Experimental procedures
Bacterial strains and culture conditions
Leptospira biflexa serovar Patoc strain Patoc 1 (Paris) was maintained and grown in EMJH medium at 30°C. For the rpoN mutant and its complemented strains, spectinomycin and gentamycin were added to a final concentration of 50 μg ml−1 respectively. For the modified EMJH, NH4Cl (5 mM) in the standard EMJH medium was replaced with the same concentration of glutamine, arginine, or alanine, with the pH adjusted to 7.4. The constructed suicide and shuttle vectors were maintained in E. coli strain DH5α, and E. coli strain β2163 was used as the donor for E. coli- Leptospira conjugation.
Construction of the rpoN mutant and the complementation strains
The RpoN (LEPBI_I1650)-coding gene disruption mutant was created by allelic exchange in L. biflexa by transforming with the suicide vector pLb-RpoN-KO (Fig. 3A). To construct the knockout plasmid, both the upstream and downstream 1700-bp fragments of rpoN were PCR amplified from L .biflexa genomic DNA. The resulting DNA fragments were then cloned upstream and downstream of a spectinomycin-resistance marker (aadA) driven by a constitutively expressed Borrelia promoter flaB via HindIII/MluI and NheI/XmaI restriction sites respectively. The resulting suicide plasmid was confirmed by both restriction enzyme digestion and sequencing followed by electroporation. Positive Leptospira transformants were selected via proper antibiotic resistance, and rpoN inactivation was verified by qRT-PCR and immunoblot analysis.
A shuttle vector, pJJ003, was constructed for the complementation of rpoN (Fig. 3A) based on the E. coli-Leptospira shuttle vector pCJSpLe94 (Picardeau, 2008). To this end, the spectinomycin-resistance marker in pCJSpLe94 was first replaced by a gentamycin-resistance marker originated from plasmid pSL94PfGenta (Poggi et al., 2010) to construct pJJ002. Then, an hsp10-promoter driving rpoN cassette was inserted into plasmid pJJ002. The resulting plasmid, pJJ003, was sequenced before it was transformed into the L. biflexa rpoN mutant by conjugation as described (Picardeau, 2008). The transformants with both gentamycin and spectinomycin resistance were selected and subjected to qRT-PCR and immunoblot analysis to confirm the restoration of RpoN expression.
RNA extraction, microarray construction, scanning and data analysis
The annotation of the L. biflexa strain Patoc 1 (Paris) genome (http://www.ncbi.nlm.nih.gov/genome/?term=NC_010602) was used to design 60-mer oligonucleotides using the web-based application eArray (Agilent). Three different probes were designed for each open read frame, based on the optimization of melting temperature (Tm), secondary structure and homology to other sites in the whole genome. In total, 11,166 oligonucleotides were designed, representing 3722 (out of 3730) putative open reading frames of the L. biflexa genome. They were printed in triplicate on each subarray, which were in turn printed in quadruplicate on the slide. The oligonucleotide synthesis and microarray print, as well as the scanning and data analysis, were completed by MOgene.
For the RNA extraction, both the wild-type and the rpoN mutant strains were cultivated in EMJH medium at room temperature and harvested at the mid-logarithmic growth. Total RNA was extracted from two biological replicates using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Digestion of contaminating genomic DNA in the RNA samples was performed using RNase-Free DNase I (New England Biolabs), and removal of DNA was confirmed by PCR amplification using primers specific for flaB of L. biflexa. cDNA was synthesized and labeled with Cy3 or Cy5 by use of the Amersham Post-Labeling Kit according to the manufacturer's instructions, with minor modifications. Briefly, 5 μg of total RNA was converted to cDNA by use of CyScript RT in the presence of 1 μl of random nonanucleotides (Amersham Biosciences). Each cDNA sample was labeled with Cy3 and Cy5 separately. Cy3- or Cy5-labeled cDNA from the parental strain was then combined with the Cy5- or Cy3-labeled cDNA from the mutants. Labeled probes were purified and then used in the microarray experiment. With two pairs of samples plus dye switching, we made a total of four hybridized slides. Hybridized slides were then scanned on an Axon 4000B microarray scanner using GenePix Pro 6.1 (Molecular Devices). The image was analyzed using the GenePix program, and data were then analyzed with Acuity 4.0 (Molecular Devices) by using the ratio-based normalization method. A 2-fold change cutoff value was used to select candidate genes.
qRT-PCR
RNA samples were extracted from leptospiral cultures grown to middle log phase (around 1 × 108 cells per ml) at 30°C using the RNeasy Mini Kit (Qiagen) according to the manufacturer's protocols. The cDNA was synthesized using the SuperScript III Reverse Transcriptase with random primers (Invitrogen) from 1 μg RNA. qRT-PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems) on an ABI 7000 sequence detection system. The flaB gene of L. biflexa was used as a reference. The relative transcription level was determined by the threshold cycle (2–ΔΔCt) method (Livak and Schmittgen, 2001). All samples were run in triplicate, and the student t test was performed to determine the statistical significance between the expression levels of different groups.
Measurement of cell growth and viability on solid medium
To investigate the growth abilities of Leptospira strains in the mediums with different nitrogen sources, the tested strains were first grown in regular EMJH medium to late log phase. The cells were harvested by centrifugation and washed once with ammonia-free EMJH to avoid carryover of residual nitrogen sources. Then cells were subcultured in the modified fresh EMJH with different nitrogen sources at an initial concentration of 106 spirochetes per ml. Every medium was independently analyzed two times. Those nitrogen sources that supported growth were further analyzed with three independent replicates each time. All cultures were incubated at 30°C and the cell numbers were numerated every 24 hr under a dark-field microscope. The viabilities of leptospires were enumerated by plating diluted culture samples on solid EMJH medium.
Water survival test
The long-term water survival abilities of Leptospira strains were investigated by a modified method (Smith and Turner, 1961; Trueba et al., 2004). Since Leptospira cannot survive in distilled water, the spring waters used in the study were commercially available drinking water in North American (pH 7.2, Kirkland Signature, USA) with trace mineral contents. The waters were filter sterilized to avoid modifying the chemical equilibrium. In brief, leptospiral cells grown to logarithmic phase (3 × 108 cells per ml) were harvested by centrifugation. The supernatants were thoroughly drained, and the cells were resuspended in the original volume (15 ml) of water. All cultures were incubated at 30°C, and the viabilities of leptospires were enumerated every week by plating 100 μl diluted culture samples on solid EMJH medium. The plates were incubated at 30°C for 2 weeks, and the efficiency of plating was determined by dividing the total number of colonies, irrespective of size, by the number of colonies expected based on the cell counting via dark-field microscopy. Culturable cells were also detected by inoculating 0.5 ml culture into 5 ml fresh EMJH and incubating for 4 weeks. The typical gyrations in liquid media were used to identify live leptospires.
EMSA
EMSAs were performed to determine protein: DNA interactions as described previously (Hellman and Fried, 2007). For RpoN binding, a 50-bp putative promoter fragment containing an engineered EcoRI site was PCR amplified. The resulting fragment was purified and digested with EcoRI, and then labeled with 32P using Taq DNA polymerase by filling in the EcoRI sites with [α-32P]dATP. For EbpA binding, a 500-bp DNA fragment upstream of the gene LEBI_I1011 (−450 and +50 bp relative to the ATG start codon) was PCR amplified from the genome of L. biflexa with primers with containing an engineered EcoRI site, and then labeled as above. Labelled fragments (0.2 pmol) and various amounts of purified RpoN or EbpA were mixed in 10 μl binding reactions in the presence of 100 μg ml−1 salmon sperm DNA for 30 min at room temperature. Some reactions included unlabeled promoter competitors or non-specific competitors for competition studies. The reactions were analyzed on non-denatured polyacrylamide gels, and then the gels were dried and exposed in a cassette using an X-ray film for autoradiography.
Protein expression and antisera preparation
For expression and purification of RpoN of L. biflexa, the rpoN gene was PCR amplified from genomic DNA of L. biflexa and cloned into the expression vector pET100 (Invitrogen). The resulting plasmid, pJJ032, was transformed into E. coli BL21DE3 (Novagen), and the C terminal His6 tagged RpoN was expressed with the induction of 0.4 mM IPTG. The recombinant RpoN protein was further purified using Ni-NTA affinity chromatographic column (NEB) according to the manual. To obtain the recombinant protein EbpA, the full-length ebpA gene was PCR amplified by PCR application. The PCR product was cloned into pGEX-4T-2 vectors (Amersham Pharmacia Biotech) via BamHI and NotI (NEB) digestion. The resulting plasmid, pGEX-4T-2ebpA, was transformed into E. coli BL21(DE3) for the expression of glutathione S-transferase (GST) fused recombinant EbpA protein. The protein was expressed under the induction of 0.5 mM IPTG, and purified by glutathione superflow agarose (Pierce) according to the manual. To increase the solubility of the recombinant proteins, all induction was conducted at 16°C. The expressed and purification of recombinant proteins were monitored by SDS polyacrylamide gel electrophoresis.
To obtain the recombinant protein GroEL, the full-length groEL gene was PCR amplified from L. interrogans strain Lai genomic DNA. The product was digested with NdeI and XhoI endonucleases before it was inserted into pET42a and transformed into E. coli BL21DE3 (Novagen). The His6 tagged recombinant GroEL protein was expressed under the induction of 1 mM isopropy-β-Dthiogalactoside (IPTG), and the soluble GroEL was purified using Ni-NTA affinity chromatographic column (NEB) according to the manual.
For antisera production, New Zealand rabbits were immunized intradermally on days 1, 14, 21 and 28 with 2 mg leptospiral RpoN and GroEL respectively, which were pre-mixed with Freund's adjuvant. Fifteen days after the last immunization, the sera were collected to separate the IgGs by ammonium sulfate precipitation plus a DEAE-52 column (Sigma) purification. Phosphate buffer (10 mM, pH 7.4) was used for elution and the titer of the IgGs binding was detected by immunodiffusion test.
Immunoblot analysis
Leptospira strains were inoculated into EMJH medium and grown to late logarithmic phase before they were harvested by centrifugation at 7000 g and washed twice with phosphate buffered saline (PBS, 50 mM, pH7.4). The pellets were collected for immunoblotting using the SuperSignal West Pico chemiluminescent substrate with home-made antibodies according to the manufacturer's instructions (Pierce).
Acknowledgements
This work was supported in part by grants from the National Institute of Allergy and Infectious Diseases, NIH (grants AI083640 to X.F.Y.), the National Natural Science Foundation of China (grants 31270103 and 31070050 [J.J.Z.], 81471907 and 81671974 [J.Y.], 81501713 [W.L.H.]) and Natural Science Foundation of Zhejiang Province, China (grant LY18H190001 [W.L.H.]).
Primers
The primers used in this study were synthesized by Integrated DNA Technologies Inc. The purposes and sequences of the primers are shown in Table 3.
| Purpose | Oligo name | Sequence (5′–3′) |
|---|---|---|
| L. biflexa rpoN upstream amplification for knockout | rpoN upstream F | CGGAAGCTTATTTAAAAGGGGAA |
| rpoN upstream R | GAAACGCGTTTTCTGTCACTCGC | |
| L. biflexa rpoN downstream amplification for knockout | rpoN downstream F | CGGGCTAGCCGAATCGACAATTT |
| rpoN downstream R | ATTCCCGGGCAAGCGTTAGTCGG | |
| Streptomycin-resistance cassette (flaB-aadA) amplification | flaB-aadA F | CGGACGCGTCTTCAAGGAAGATT |
| flaB-aadA R | CGGGCTAGCCTAATTGAGAGAAG | |
| Gentamycin-resistance gene cassette (hsp10-Gen) amplification | Hsp10-Gen F: | CCCGGGCCATGGAATTCTCTAAAAGTATGAATTCC |
| Hsp10-Gen R: | CCCGGGCAGCTGCGTAAGCCGATCTCGGCTTGAACG | |
| rpoN ORF application for complementation | Lb-I1650-F | AAGGCATATGAAACTCGGGGCTTC |
| Lb-I1650-R | GATTGGTACCTTACCCCTTGAGCGAACTG | |
| hsp10 promoter amplification | L.in-pHsp10-F | TTATGGGCCCGAATTCCTACAATTTTAGAATTTG |
| L.in-pHsp10-R | GGTACCATATGGTGATGGTGATGGTGATGAATC | |
| GroEL overexpression in E. coli | pET42a-groEL F | CGCCATATGGCGAAAGATATTGAA |
| pET42a-groEL R | CGCCTCGAGCATCATTCCGCCCATTCC | |
| EBPa overexpression in E. coli | pGEX-4T-2-ebpA F | CGCGGATCCATGTCAGGATATGTGAAG |
| pGEX-4T-2-ebpA R | ATAAGAATGCGGCCGCATAATCGATTTT | |
| RpoN overexpression in E. coli | pET100 Lb rpoN F | CACCATGAAACTCGGGGCTTCACTTTCAC |
| TTACCCCTTGAGCGAACTGATTCGC | ||
| rpoN qRT-PCR | lebi-rpoN-qPCR-F1 | ACTGGTGATGACCCAGGACT |
| lebi-rpoN-qPCR-R1 | CGCCTAATTCATCGAGAAGAGGAT | |
| flaB qRT-PCR | lebi-flaB-qPCR-F | ACACTGCGGCATTGGGATTA |
| lebi-flaB-qPCR-R | AGCATGCTCCATACGGTTGT | |
| rcfA qRT-PCR | lebi-I1011-qPCR-F | CCCTCATTGGCCAGTACGAT |
| lebi-I1011-qPCR-R | ACGGATTTTGATGGCTCGGT | |
| amtB1 qRT-PCR | lebi-I1767-qPCR-F | CCTGGGTTAAATCCAAACCAACC |
| lebi-I1767-qPCR-R | GGCAAATTCCAATCCGATGGTAG | |
| amtB2 qRT-PCR | lebi-I2408-qPCR-F | ACATACCTGCAACTAACACCCAT |
| lebi-I2408-qPCR-R | AAATCAATCGCCTTCCTTCTCCT | |
| narK qRT-PCR | lebi-I2771-qPCR-F | CTGATCTTCGGATTCTTCGTTGC |
| lebi-I2771-qPCR-R | CAGGTGTATCTTGCGTTCCAAAG | |
| ntrX qRT-PCR | lebi-I1654-qPCR-F | GCGGTCAATGCGACGAAAAA |
| lebi-I1654-qPCR-R | CGGCCTGGAAAACAGCAAAC | |
| rcfA promoter region for EMSA | I1011 promoter | TAAGCCCAATATGCTTTTGGCACGATAGTTGCATCTATTCTACTG |
| I1011 promoter C | TTCAGTAGAATAGATGCAACTATCGTGCCAAAAGCATATTGGGCTT | |
| rcfA upstream region for EMSA | I1011UP F | ATGGAATTCCAATTGCCCTCTCACGGGAA |
| I1011UP R | CGTGAATTCAGACTCACCTGCTTTCACAGA |
Author contributions
JJZ and XFY designed the study and wrote the paper. JJZ performed most of the experiments and data analysis. WLH, YY and HL helped with the experiments. MP and JY provided valuable suggestions for the study and the manuscript. All authors read and approved the final manuscript.