The distinct PhoPR mediated responses to phosphate limitation in Bacillus subtilis subspecies subtilis and spizizenii stem from differences in wall teichoic acid composition and metabolism
Summary
The PhoPR-mediated response to phosphate limitation (PHO response) in Bacillus subtilis subsp subtilis is amplified and maintained by reducing the level of Lipid VG composed of poly(glycerol phosphate), a wall teichoic acid (WTA) biosynthetic intermediate that inhibits PhoR autokinase activity. However, the reduction in Lipid VG level is effected by activated PhoP∼P, raising the question of how the PHO response is first initiated. Furthermore, that WTA is composed of poly(ribitol phosphate) in Bacillus subtilis subsp spizizenii prompted an investigation of how the PHO response is regulated in that bacterium. We report that the PHO responses of B. subtilis subsp subtilis and subsp spizizenii are distinct. The PhoR kinases of the two B. subtilis subspecies are functionally equivalent and are activated either by the TagA/TarA or TagB/TarB enzyme product. However, they are inhibited by Lipid VG composed of poly(glycerol phosphate) but not by Lipid VR composed of poly(ribitol phosphate). Therefore, the distinctive PHO responses of these B. subtilis subspecies stem from the differential sensitivity of PhoR kinases to the polyol composition of Lipid V and from the genomic organization of WTA biosynthetic genes and the regulation of their expression.
Graphical Abstract
The PHO responses of B. subtilis subsp subtilis and subsp spizizenii are distinct. The PhoR kinases of both B. subtilis subspecies are activated either by the TagA/TarA or TagB/TarB enzyme product and are inhibited by Lipid V composed of poly(glycerol phosphate) but not by Lipid V composed of poly(ribitol phosphate). Therefore, the distinctive PHO responses stem from differential PhoR sensitivity to differences in wall teichoic acid polyol composition and the regulation of its metabolism.
Introduction
Phosphorous is a major component of lipoteichoic acid (LTA) and wall teichoic acid (WTA), acidic capsular polysaccharides that are located in the cell envelope of bacteria such as Bacillus subtilis (Ward, 1981; Weidenmaier and Peschel, 2008). LTA is a polymer of glycerol phosphate that extends outward into the cell envelope from a glycolipid anchor embedded in the cytoplasmic membrane. It is synthesized extracellularly using the phosphatidyl glycerol head groups of membrane phospholipids as substrate and is involved in processes that determine cell morphology and cell division (Percy and Gründling, 2014; Schneewind and Missiakas, 2014). WTA is a polymer of either glycerol- or ribitol-phosphate synthesized intracellularly on the undecaprenyl phosphate carrier lipid using CDP glycerol or CDP ribitol as substrate (Weidenmaier and Peschel, 2008; Swoboda et al., 2010). This lipid-linked precursor is then exported from the cell and covalently attached to peptidoglycan (Weidenmaier and Peschel, 2008; Swoboda et al., 2010; Kawai et al., 2011). The chemical properties of WTA can be modified through decoration with molecules such as α-glycose, D-alanine or N-acetylglucosamine (Weidenmaier and Peschel, 2008; Percy and Gründling, 2014; Schneewind and Missiakas, 2014). WTA functions in cation binding, cell elongation and division, in targeting autolysins to specific regions of the cell wall and as receptors for bacteriophage attachment (Weidenmaier and Peschel, 2008; Yamamoto et al., 2008; Swoboda et al., 2010; Schneewind and Missiakas, 2014).
The chemical composition of WTA can vary even among different subspecies of some bacteria (Weidenmaier and Peschel, 2008; Swoboda et al., 2010). For example, WTA in B. subtilis subsp subtilis strain 168 (hereafter called B. subtilis 168) is composed of poly(glycerol phosphate) while that in B. subtilis subsp spizizenii strain W23 (hereafter called B. spizizenii W23) is composed of poly(ribitol phosphate) (Weidenmaier and Peschel, 2008; Swoboda et al., 2010). This variation in WTA chemical composition is reflected in different biosynthetic pathways (Fig. 1, Bhavsar and Brown, 2006; Brown et al., 2010; Swoboda et al., 2010). The first three steps are common in the biosynthesis of glycerol-type (TagO, TagA, TagB) and ribitol-type (TarO, TarA, TarB) WTA, and lead to the formation of undecaprenyl pyrophosphoryl-N-acetylglucosamine-N-acetylmannosamine primed with a single glycerol phosphate moiety (Fig. 1B, upper pathway). However, the lower pathway differs in the two subspecies (Fig. 1B, Brown et al., 2010). In B. subtilis 168, the TagF enzyme adds ∼ 30 glycerol phosphate subunits to the TagB enzyme product to form Lipid VG composed of poly(glycerol phosphate) (Fig. 1B). In B. spizizenii W23, the TarK enzyme first primes the TarB enzyme product with a single ribitol phosphate moiety, which is then extended by the TarL polymerase to generate Lipid VR composed of poly(ribitol phosphate) (Fig. 1B, Brown et al., 2010). These differences in biosynthetic pathway are also reflected in distinct genomic organization of the tag and tar genes and in the regulation of their expression (Fig. 1A). In B. spizizenii W23, the genes encoding the TarABKL biosynthetic enzymes are located in one operon (tarABIJKL), which is transcribed from a single promoter (Fig. 1A). However, in B. subtilis 168, the genes encoding the TagABF biosynthetic enzymes are located in two divergently transcribed operons (tagAB and tagDEF), with only the tagAB operon being repressed by activated PhoP∼P (Fig. 1A, Botella et al., 2011). The additional genes in these operons encode enzymes for the synthesis of the CDP glycerol (tagD) and CDP ribitol (tarI and tarJ) substrates and for decoration of the polymer with glucose (tagE).
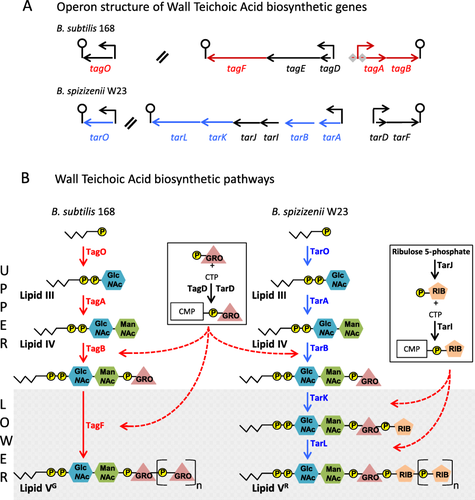
Genomic organization of WTA genes (A) and the WTA biosynthetic pathways (B) in B. subtilis 168 and B. spizizenii W23.
A. Genomic organization of the genes that encode the WTA biosynthetic enzymes in B. subtilis 168 (upper panel) and B. spizizenii W23 (lower panel). Genes are represented by arrows, promoters by bent arrows, and terminators by lollipops. Double slanted lines indicate chromosomal discontinuity while diamonds with minus signs indicate PhoP∼P mediated repression. The genes colored in red are those directly involved in the Lipid VG biosynthesis in B. subtilis 168 and while those in blue are involved in Lipid VR biosynthesis in B. spizizenii W23.
B. The metabolic pathways for biosynthesis of Lipid VG composed of poly(glycerol phosphate) in B. subtilis 168 (left pathway) and Lipid VR poly(ribitol phosphate) in B. spizizenii W23 (right pathway) are shown. Enzymatic steps for biosynthesis of Lipid VG are shown in red (corresponding genes are in red in panel A); those for biosynthesis of Lipid VR are shown in blue while those shown by black arrows are involved in precursor synthesis. The zigzag line with P in a yellow circle represents the lipid carrier undecaprenol phosphate. GlcNAc and ManNAc represent the modified hexoses N-acetylglucosamine and N-acetylmannosamine respectively. GRO in a triangle and RIB in a pentagon represent the glycerol and ribitol WTA subunits. These symbols in square brackets with a subscript ‘n’ represent poly(glycerol phosphate) and poly(ribitol phosphate) respectively. Synthesis of CDP glycerol and CDP ribitol substrate using CTP and glycerol- and ribitol-phosphate are shown in boxes with the broken red arrow indicating the biosynthetic steps in which each substrate is incorporated. The pathways are shown as UPPER and LOWER (shaded) indicating the steps in the pathways that are common (upper) and distinct (lower) between the biosynthetic pathways of the two subspecies.
Given its high phosphorous content, it is not surprising that WTA synthesis is regulated during adaptation to conditions of limited phosphate availability. The main response to phosphate limitation in B. subtilis 168 is mediated by the PhoPR two-component signal transduction system (TCS) (Hulett, 2002; Santos-Beneit, 2015). The constituent genes of the PhoPR regulon encode proteins that execute three adaptive functions (the PHO response): (i) phosphate scavenging; (ii) adjustment of anionic polymer metabolism and (iii) amplification of the response by positive autoregulation of phoPR expression (Hulett, 2002; Allenby et al., 2005; Botella et al., 2011; Salzberg et al., 2015). PhoP∼P mediated activation of alkaline phosphatase and phosphodiesterase (PhoA, PhoB, PhoD, GlpQ) expression functions to release phosphate from macromolecular sources and transport it into the cell using a high affinity ABC-type phosphate transporter (PstSCABABB). Anionic polymer metabolism is adjusted by PhoP∼P mediated repression of tagAB and activation of tuaA-H transcription that lead to a reduction in WTA, and an increase in TUA, content of the cell wall (Fig. 1A). Together these changes reduce the cellular requirement for phosphate while maintaining the anionic character of the cell wall. Recently Myers and colleagues (2016) reported that PhoPR regulon genes phoD and glpQ encode enzymes that cleave poly(glycerol phosphate) endo- and exo-lytically respectively, thereby revealing a capability to recycle and reutilize the phosphorous content of cell wall material that is released during growth. Thus, WTA serves as a phosphorous store that can be mobilized under conditions of limitation (Myers et al., 2016). The involvement of cell wall metabolism in the PHO response was extended by ChIP on chip analysis (Salzberg et al., 2015) which revealed a high level of activated PhoP∼P bound throughout the chromosomal region encoding the tagABDEF, tuaA-H, ggaAB (minor teichoic acid), yqgS (minor lipoteichoic acid synthetase) and dacA (Pbp5, carboxypeptidase) operons (Bisicchia et al., 2010b; Salzberg et al., 2015). Thus, maintenance of anionic polymer homeostasis in the cell envelope is a major function of the PHO response in B. subtilis 168.
The finding that PhoR kinase activity is inhibited by Lipid VG composed of poly(glycerol phosphate (the TagF enzyme product) in B. subtilis 168 (Fig. 1B) revealed that the PHO response is responsive to signals that emanate from WTA metabolism (Botella et al., 2014). Synthesis of WTA and TUA are linked, since the TagO enzyme product is a metabolic precursor of both anionic polymers (Fig. 1B; Botella et al., 2014). Therefore, PhoP∼P directed initiation of TUA synthesis causes a reduction in WTA synthesis by diverting metabolites away from the WTA biosynthetic pathway (Botella et al., 2014). WTA synthesis is also reduced by PhoP∼P mediated repression of tagAB transcription, the genes that encode the first two enzymes in its biosynthesis (Fig. 1B). These features combine to amplify the PHO response after induction by increasing PhoR kinase activity through a reduction in the level of Lipid VG inhibitor (Fig. 1, Botella et al., 2014). Therefore, TUA has a dual role in B. subtilis 168 under phosphate limiting conditions: (i) its synthesis plays a regulatory role, contributing to amplification of the PHO response by lowering the level of the Lipid VG inhibitor and (ii) it maintains anionic polymer homeostasis within the cell envelope by replacement of phosphate-rich WTA with a non-phosphate containing anionic polymer.
The study of Botella and colleagues (2014) has provided insight into the mechanism by which the PHO response is amplified. However, since amplification is dependent on activated PhoP∼P, the mechanism by which the PHO response is initiated remains to be established. A further issue concerns the nature of the PHO response in bacteria that synthesize a poly(ribitol phosphate)-type WTA. Since PhoR autokinase activity is inhibited by Lipid VG composed of poly(glycerol phosphate) in B. subtilis 168, the question arises as to the nature of the PHO response in B. spizizenii W23 that synthesizes WTA composed of poly(ribitol phosphate) (Fig. 1B).
Here we report that the PHO responses of B. subtilis 168 and B. spizizenii W23 are distinct. We propose that PhoR kinase activity is activated in both subspecies by the product of either the TagA/TarA or TagB/TarB enzymes, metabolic intermediates that are common to both WTA biosynthetic pathways (Fig. 1B). However, while Lipid VG composed of poly(glycerol phosphate) inhibits the autokinase activity of both the PhoR168 and PhoRW23 enzymes, neither enzyme is inhibited by Lipid VR composed of poly(ribitol phosphate). Thus the distinct PHO responses of B. subtilis 168 and B. spizizenii W23 derive from the differential sensitivity of their PhoR kinases to the polyol composition of Lipid V and from differences in the genomic organization and regulation of expression of the genes that encode the WTA biosynthetic enzymes.
Results
The PHO responses in B. subtilis 168 and B. spizizenii W23 are distinct
The PHO responses of B. subtilis 168 and B. spizizenii W23 were compared by growing strains BA1546 (PphoAgfp) and MP92 (PphoAgfp) respectively in LPDM (low phosphate defined medium) and measuring promoter activity as described by Botella and colleagues (2014). The PHO response of B. spizizenii W23 is induced just before growth is attenuated by the onset of phosphate limitation, and has a characteristic twin-peak profile (Peaks I and II) during the T0–T120 interval (Fig. 2B). It then declines and falls below the threshold of detection ∼ 300 min after the point of induction. This expression profile is dependent on the presence of a functional phoR gene and does not occur when cultures are grown in HPDM (high phosphate defined medium) (data not shown). A similar twin-peak profile of promoter activity was observed for four other PhoPR regulon genes (PphoBgfp, PpstS-Bgfp, PglpQgfp, PtuaA-Hgfp) when B. spizizenii W23 strains harboring these promoter fusions were grown under similar conditions (Supporting Information Fig. S1). Furthermore, despite the promoters being of different strengths (200–3000 promoter activity units), expression of all fusions falls below the detection threshold ∼ 300 min after the point of induction (Supporting Information Fig. S1). Thus we conclude that the profile of PphoAgfp promoter activity accurately reflects activation of the PhoPR TCS on phosphate limitation in B. spizizenii W23.
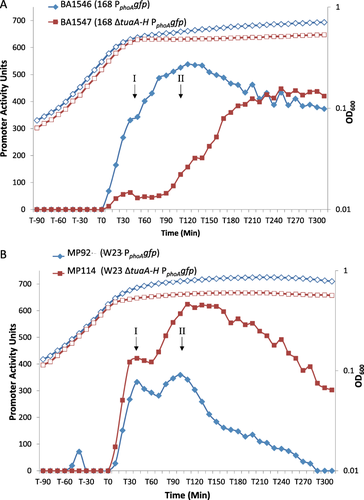
Growth and induction of the PHO response in B. subtilis 168 and B. spizizenii W23. The kinetics of the PHO response in each parental strain (blue lines) is shown by promoter activity of the PphoAgfp transcriptional fusion. The influence of TUA synthesis on the induction profile of the PHO response is also shown (red lines).
A. B. subtilis strains BA1546 (PphoAgfp) (blue diamonds) and BA1547 (ΔtuaA-H PphoAgfp) (red squares).
B. B. spizizenii strains MP92 (PphoAgfp) (blue diamonds) and MP114 (ΔtuaA-H PphoAgf) (red squares). The corresponding growth profiles are shown by open symbols in each panel.
There are three important differences between the PHO responses of B. subtilis 168 (strain BA1546 PphoAgfp) and B. spizizenii W23 (strain MP92 PphoAgfp) (compare blue lines in Fig 2A and B): (i) there is a single peak of activity with a maximum at ∼ T100 in B. subtilis 168 whereas there are two peaks of activity (I and II) with maxima at ∼ T40 and T100 respectively in B. spizizenii W23; (ii) when phosphate limitation conditions persist, the PHO response is maintained in B. subtilis 168 (≥ 65% of maximal activity at T300) but falls below the threshold of detection at T300 in B. spizizenii W23 (≤ 0.3% of maximal activity at T300) and (iii) maximum promoter activity of PphoAgfp is higher in B. subtilis 168 (∼ 550 units) than in B. spizizenii W23(∼ 350 units). The distinctive PHO responses of each Bacillus subtilis subspecies were observed invariantly throughout this study (> 10 experimental repeats, each with three technical replicates).
In summary, the PHO responses in B. subtilis 168 and B. spizizenii W23 are distinct, showing differences in the kinetics of induction and in the magnitude and persistence of the response on prolonged phosphate limitation.
TUA synthesis has opposite effects on the PHO responses of B. subtilis 168 and B. spizizenii W23
A signature finding of Botella and colleagues (2014) is that the PHO response in B. subtilis 168 is amplified during the T0–100 interval by the onset of TUA synthesis. By diverting metabolites away from the WTA pathway, TUA synthesis causes a reduction in the level of Lipid VG (TagF enzyme product), which is an inhibitor of PhoR autokinase activity (Figs. 1A and 2A, Botella et al., 2014). Consequently, amplification of the PHO response is delayed when TUA synthesis is precluded by deletion of tuaA-H (compare blue and red lines, Fig. 2A). To establish the impact of TUA synthesis on the PHO response in B. spizizenii W23, strain MP114 (W23 ΔtuaA-H PphoAgfp) was constructed and grown under phosphate limiting conditions. Results show that prevention of TUA synthesis also has a major impact on the PHO response in B. spizizenii W23 (Fig. 2B, compare blue and red lines). The magnitude of the response in strain MP114 is greater than in parental strain MP92 throughout the period of the experiment (T0–300), although the twin – peak profile is still evident during the initial T0–100 interval (Fig. 2B, compare red and blue lines). Furthermore, prevention of TUA synthesis in B. spizizenii W23 (MP114 ΔtuaA-H PphoAgfp) causes the PHO response to persist on prolonged phosphate limitation (Fig. 2B, red line). The response is maintained at a high level at ∼ T300 in strain MP114 (ΔtuaA-H), the time at which expression in parental strain MP92 has fallen below the threshold of detection (compare red and blue lines respectively, Fig. 2B). In contrast, prevention of TUA synthesis in B. subtilis 168 causes a delay in induction of the PHO response and a reduction in its magnitude during the T0–T120 period (compare red lines and blue line in Fig. 2A, Botella et al., 2014). However, it eventually reaches a high level after an extended period (T300) of phosphate limitation (Fig. 2A).
In summary, TUA synthesis has opposite effects on the PHO responses in these two subspecies of B. subtilis. In B. subtilis 168, the magnitude of the PHO response is amplified and increased by TUA synthesis during the initial period (T0–T100). In contrast, in B. spizizenii W23 the magnitude of the PHO response is lowered (T0–T100) and eventually turned off (T100–300) by TUA synthesis. Thus the PHO response is induced by different mechanisms in these two subspecies of B. subtilis.
The distinct PHO responses in B. subtilis 168 and B. spizizenii W23 are not caused by differences in the orthologous PhoR kinases
The distinct PHO responses of B. subtilis 168 and B. spizizenii W23 could emanate from differences in their respective PhoR kinases or from differences in WTA composition and metabolism. To distinguish between these possibilities, strains of B. subtilis 168 and B. spizizenii W23 were constructed that expressed only the orthologous phoR from the other subspecies. Thus, B. subtilis strain MP1 (phoP168 phoRW23) expresses PhoR from B. spizizenii W23 while B. spizizenii strain MP104 (phoPW23 phoR168) expresses PhoR from B. subtilis 168. It is important to note that the heterologous phoR in MP1 and MP104 is located and expressed in precisely the same manner as the endogenous phoR gene. Western blot analysis confirmed that the PhoR proteins are expressed in the heterologous host and that each is induced on phosphate limitation, although the level to which they accumulate is somewhat lower than that observed in the natural host (Fig. 3A and B lower panels). The extent to which the phoPR operon is autoinduced on phosphate limitation was established by monitoring accumulation of the PhoP protein in strains MP1 (168 phoP168 phoRW23) and MP104 (W23 phoPW23 phoR168) (Fig. 3A and B upper panel). Results show that the PhoP protein is induced and accumulates in strain MP1 (168 phoP168 phoRW23) to approximately the same level as it does in parental strain B. subtilis 168 (Fig. 3A, upper panel). This signifies that the heterologous PhoRW23 kinase is capable of producing a normal PHO response in the B. subtilis 168 subspecies. However, the level of PhoP protein in strain MP104 (W23 phoPW23 phoR168) is lower than in parental strain B. spizizenii W23 signifying a reduced PHO response in this strain (Fig. 3B, upper panel).
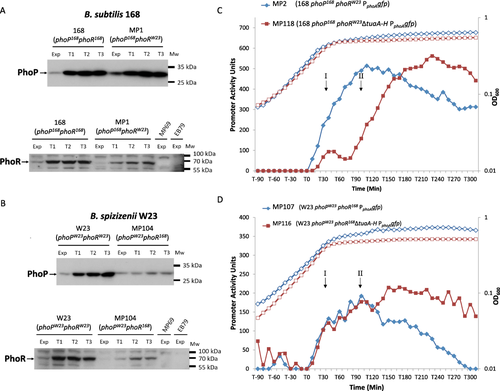
Expression of PhoP and PhoR proteins (A, B) and PHO response induction (C, D) in strains of B. subtilis 168 and B. spizizenii W23. Strains of B. subtilis 168 and B. spizizenii W23 were constructed that expressed the PhoR kinase from the other subspecies (blues lines). Expression of the PhoP and PhoR proteins during growth (Exp) and phosphate limitation (T1 – T3) was established by western blot analysis: in (A) and (B), the upper panels shows the level of PhoP protein detected using a polyclonal antibody raised against PhoP168, while the lower panels show the level of PhoR protein detected using a polyclonal antibody raised against PhoR168. The Mr size ladder is shown by bars. Approximately 30 µg of cell extract was loaded onto each lane.
A. 168 (phoP168 phoR168), parental B. subtilis 168; MP1 (phoP168::phoRW23 ΔphoR168), B. subtilis 168 expressing PhoRW23. Strains EB69 and MP79 are the corresponding control strains with phoR deleted.
B. Strain W23 (phoPW23 phoRW23), parental strain B. spizizenii W23; Strain MP104: (phoPW23::phoR168 ΔphoRW23), B. spizizenii W23 expressing PhoR168. Strains EB69 and MP79 are the control strains with phoR deleted.
C. Growth (open symbols) and the PHO response (closed symbols) in strains of B. subtilis 168 expressing the heterologous PhoRW23 kinase from B. spizizenii W23: blue diamonds MP2 (phoP168::phoRW23 ΔphoR168 PphoAgfp) and red squares MP118 (phoP168::phoRW23 ΔphoR168 ΔtuaA-H PphoAgfp).
D. Growth (open symbols) and the PHO response (closed symbols) in strains of B. spizizenii W23 expressing the heterologous PhoR168 kinase from B. subtilis 168: blue diamonds MP107 (phoPW23::phoR168 ΔphoR W23 PphoAgfp) and red squares MP116 (phoPW23::phoR168 ΔphoRW23 ΔtuaA-H PphoAgfp).
To establish the kinetics of PHO response induction in strains of B. subtilis 168 and B. spizizenii W23 expressing a heterologous PhoR kinase, strains MP2 (phoP168 phoRW23 PphoAgfp) and MP107 (phoPW23 phoR168 PphoAgfp) were constructed. The results show that the PHO response in strain MP2 (phoP168 phoRW23 PphoAgfp) is almost identical to that observed in parental strain BA1546 (phoP168 phoR168 PphoAgfp) (compare blue lines in Figs. 2A and 3C). Furthermore, the profile of the PHO response in strain MP107 (phoPW23 phoR168 PphoAgfp) is similar to that in parental strain MP92 (phoPW23 phoRW23 PphoAgfp) although the magnitude of the response is somewhat lower and the twin-peak profile is not so evident (compare blue lines in Figs. 2B and 3D). This is consistent with the reduced levels of PhoPW23 and PhoR168 proteins observed in the western blot analysis (Fig. 3B, upper and lower panels). Thus the characteristics of the PHO responses in B. subtilis strain MP2 (phoP168 phoRW23 PphoAgfp) and B. spizizenii strain MP107 (phoPW23 phoR168 PphoAgfp) are typical of the host subspecies and not of the heterologous PhoR kinase.
To establish the impact of preventing TUA synthesis on the PHO response in strains expressing a heterologous kinase, the tuaA-H operon was deleted in strains MP2 and MP107 to generate B. subtilis strain MP118 (phoP168 phoRW23 ΔtuaA-H PphoAgfp) and B. spizizenii strain MP116 (phoPW23 phoR168 ΔtuaA-H PphoAgfp) respectively. The results show that amplification of the PHO response in strain MP118 (phoP168 phoRW23 ΔtuaA-H PphoAgfp) during the T0–T90 period is reduced and delayed in precisely the same manner as is observed in strain BA1547 (phoP168 phoR168168 ΔtuaA-H PphoAgfp) (compare red lines in Figs. 2A and 3C). Thus, prevention of TUA synthesis has the same effect on the PHO responses of B. subtilis 168 strains that express either the homologous or the heterologous PhoR kinase. Prevention of TUA synthesis in a B. spizizenii W23 strain expressing the PhoR168 kinase does not affect the PHO response during the initial period (T0–120) but causes the response to be maintained in a manner similar to that observed in strain MP114 (ΔtuaA-H PphoAgfp) (compare red lines in Figs. 2B and 3D).
These results show that the distinct PHO responses observed in B. subtilis 168 and B. spizizenii W23 do not stem from differences in their respective PhoR kinases. Furthermore, the finding that the PHO responses are identical in strains of B. subtilis 168 that express either the PhoR168 or PhoRW23 kinase shows that both kinases are activated and inhibited by the same mechanism. Thus, PhoRW23 can be inhibited by Lipid VG composed of poly(glycerol phosphate) when it is expressed in B. subtilis 168 but not by Lipid VR composed of poly(ribitol phosphate) when it is expressed in B. spizizenii W23. In summary, we conclude that differences in WTA polyol composition (i.e., Lipid VG and Lipid VR) and metabolism contribute to the distinct PHO responses observed in B. subtilis 168 and B. spizizenii W23.
The PhoR kinase of B. spizizenii W23 is activated by an intermediate in WTA metabolism
The results presented in Fig. 2B indicate that PhoRW23 kinase is activated by an intermediate in WTA biosynthesis in B. spizizenii W23, the level of which is lowered by the onset of TUA synthesis. This observation was further explored by establishing if the PHO response is impacted by changes in expression of the WTA biosynthetic enzymes (TarA, B, I, J, K, L). In strain MP162 (PxyltarABIJKL PphoAgfp) the genes encoding the WTA biosynthetic enzymes (tarABIJKL operon, Fig. 1A) are expressed using a xylose inducible promoter while the PHO response can be monitored using the resident PphoAgfp transcriptional fusion. Microscopy of MP162 cultures confirmed xylose inducible control of tarABIJKL operon expression: cells were rod-shaped when grown in the presence of xylose and aberrantly shaped (bulged and of increased width) when grown in the absence of xylose (Supporting Information Fig. S2). Strain MP162 was cultured overnight in 0.3% xylose and inoculated into LPDM medium (1:100 dilution) containing xylose at concentrations between 0% and 0.05%. Therefore, the maximum carryover of inducer is 0.003%, but is probably lower due to xylose utilization in the overnight culture. The growth of all cultures was the same as parental strain MP92 (PphoAgfp) and representative growth curves are shown (Fig. 4). There is a peak of PphoAgfp promoter activity (Peak I) in all cultures (0%–0.05% xylose) of strain MP162 (PxyltarABIJKL PphoAgfp) with the maximum occurring ∼ 40 min period after the onset of phosphate limitation (Fig. 4A). Crucially, the magnitude of Peak I (∼ 300 units) is independent of xylose inducer concentration and is very similar to that observed (∼ 350 units) in parental strain MP92 (PphoAgfp) (dark blue line Fig. 4A). In contrast, the magnitude of Peak II is directly proportional to xylose inducer concentration (Fig. 4A): a progressive increase in xylose inducer concentration (0.01%, 0.015%, 0.03% and 0.05%) leads to a corresponding progressive elevation in the magnitude of Peak II (0, ∼100, ∼200 and ∼230 units respectively) (Fig. 4A). The direct proportionality between inducer concentration and the magnitude of Peak II is highly reproducible and was observed in at least three separate experimental repeats, each with three technical replicates. The direct proportionality between the level of tarABIJKL expression and the magnitude of Peak II supports the view that an intermediate in WTA biosynthesis activates PhoR autokinase activity. However, it is evident that Peak I and Peak II of PhoR autokinase activity are generated by different mechanisms: generation of Peak II is dependent on the expression level of WTA biosynthetic enzymes while generation of Peak I is not.

The dependence of the PHO response on expression of the tarABIJKL operon in B. spizizenii W23. The PHO response in strains of B. spizizenii W23 with expression of the tarABIJKL operon under xylose inducible control. All growth profiles were similar and a representative profile in shown (open symbols) in both panels.
A. Strain MP162 (PxyltarABIJKL PphoAgfp) was grown in the presence of various levels of xylose inducer. Expression of the PHO response is shown for cultures grown with 0.01% xylose, filled red squares; 0.015% xylose, filled green triangles; 0.03% xylose, blue stars and 0.05% xylose filled orange circles.
B. Strain MP164 (PxyltarABIJKL ΔtuaA-H PphoAgfp) grown in the presence of various levels of xylose inducer. Expression of the PHO response is shown for cultures grown with 0% xylose, filled green triangles; 0.02% xylose, blue stars and 0.05% xylose, filled orange circles. The PHO response in parental strains MP92 (W23 PphoAgfp), filled blue diamonds and MP114 (ΔtuaA-H PphoAgfp), filled red squares are shown for comparison.
If PhoRW23 autokinase activity is stimulated by a WTA biosynthetic intermediate in B. spizizenii W23, then we predict that reducing tarABIJKL expression in strain MP114 (ΔtuaA-H PphoAgfp) should (i) suppress the elevation of the PHO response caused by preclusion of TUA synthesis (ΔtuaA-H) in direct proportion to the reduction in xylose concentration and (ii) reduce the magnitude of Peak 2 while leaving that of Peak 1 unaffected. These predictions were tested by culturing strain MP164 (ΔtuaA-H PxyltarABIJKL PphoAgfp) in LPDM with different concentrations of xylose inducer. The PHO responses of parental strains MP92 (PphoAgfp) and MP114 (ΔtuaA-H PphoAgfp) are presented for comparison (Fig. 4B, blue diamonds and red squares respectively). The results show that with addition of 0.05% xylose inducer, the PHO response is very similar to that observed in parental strain MP114 (ΔtuaA-H PphoAgfp) (compare orange circles with red squares, Fig. 4A). However, lowering the level of xylose inducer to 0.02% or to 0% (i.e., ≤ 0.003% with xylose carryover) causes a progressive reduction in the magnitude of Peak 2 (Fig. 4B). In fact, the profile of the PHO response in strain MP164 (ΔtuaA-H PxyltarABIJKL PphoAgfp) with 0% xylose is very similar to that observed in parental strain MP92 (PphoAgfp) (compare greens triangles and blue diamonds, Fig. 4B). Crucially, the magnitude of Peak I is unaffected by alteration of tarABIJKL expression in strains MP162 (Fig. 4A) and MP164 (Fig. 4B). These results are highly reproducible and were observed in at least three separate experimental repeats, each with three technical replicates.
In summary, we conclude that PhoRW23 autokinase activity is activated by an intermediate in the WTA biosynthetic pathway in B. spizizenii W23. They also show that the increases in activator WTA intermediate that produce Peaks I and II are generated by different mechanisms: the increase that generates Peak II is dependent on the level to which WTA biosynthetic enzymes are expressed while the increase that generates Peak I is not.
The PhoR168 kinase of B. subtilis 168 is also activated by an intermediate in the WTA biosynthetic pathway
The results presented indicate that the activity of both PhoR kinases is controlled by a similar mechanism. The very high level of homology between the two PhoR kinases, particularly within the internal PAS domain that is proposed to sense the Lipid VG WTA intermediate, supports this view (Supporting Information Fig. S3, Botella et al., 2014). Furthermore, the results (Figs. 2 and 4) indicate that PhoRW23 is activated by an intermediate in WTA biosynthesis. Therefore, we sought to establish if PhoR168 can also be activated by an intermediate in WTA synthesis. To achieve this, strains MP176 (phoPW23phoR168 PxyltarABIJKL PphoAgfp) and MP180 (phoPW23phoR168 ΔtuaA-H PxyltarABIJKL PphoAgfp) were constructed in which PhoR168 is expressed heterologously in B. spizizenii W23 (a precise replacement of the endogenous phoR gene) and the WTA biosynthetic enzymes are expressed under xylose inducible control (PxyltarABIJKL). If PhoR168 autokinase activity is activated by a WTA biosynthetic intermediate, then we predict (i) that lowering tarABIJKL expression in strain MP176 (phoPW23 phoR168 PxyltarABIJKL PphoAgfp) should reduce the magnitude of Peak II PphoAgfp expression with the magnitude of Peak I being unaffected and (ii) that lowering tarABIJKL expression in strain MP180 (phoPW23 phoR168 ΔtuaA-H PxyltarABIJKL PphoAgfp) should lower the elevated level of Peak II while again leaving Peak I unaffected. The results obtained (Fig. 5) confirm both predictions. Lowering the level of tarABIJKL expression in both strains of B. subtilis W23 expressing the heterologous PhoR168 kinase lowers the magnitude of peak II while leaving the magnitude of peak I largely unaffected (Fig. 5A and B). Crucially, the increase in the magnitude of Peak II in strain MP180 (phoPW23 phoR168 ΔtuaA-H PxyltarABIJKL PphoAgfp) caused by prevention of TUA synthesis (ΔtuaA-H) can be lowered by a reduction in expression of WTA biosynthetic enzymes (tarABIJKL) (Fig. 5B). These expression profiles are highly reproducible and have been observed in at least three experimental repeats, each with three technical replicates.
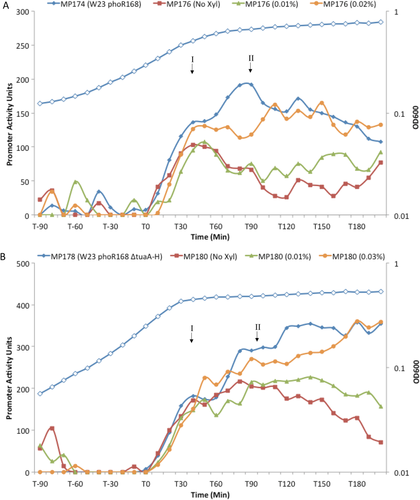
Dependence of the PHO response on tarABIJKL expression in strains of B. spizizenii W23 that express the PhoR168 kinase. The PHO response in B. spizizenii W23 strains MP176 and MP180 that express the tarABIJKL operon under xylose inducible control and also express the heterologous PhoR168 kinase. Growth of all strains was similar and a representative profile in shown (open symbols) in both panels.
A. Strain MP176 (phoPW23::phoR168 ΔphoRW23 PxyltarABIJKL PphoAgfp) was grown in the presence of various levels of xylose inducer. Expression of the PHO response (PphoAgfp expression) is shown for cultures grown with no xylose, filled red squares; 0.01% xylose, filled green triangles and 0.02% xylose, filled orange circles. The PHO response in parental strain MP174 (W23 phoPW23::phoR168 ΔphoRW23 PphoAgfp) is shown for comparison (filled blue diamonds).
B. Strain MP180 (phoPW23::phoR168 ΔphoRW23 ΔtuaA-H PxyltarABIJKL PphoAgfp) grown in the presence of various levels of xylose inducer. Expression of the PHO response (PphoAgfp expression) is shown for cultures grown with 0% xylose (filled red squares); 0.01% xylose, filled green triangles and 0.02% xylose, filled orange circles. The PHO response in parental strain MP178 (W23 phoPW23::phoR168 ΔphoR ΔtuaA-H PphoAgfp) is shown for comparison (filled blue diamonds).
In summary, these results show that PhoR168 is activated by an intermediate in WTA when it is expressed in B. spizizenii W23. This supports the view that both PhoR kinases are activated by the same WTA biosynthetic intermediate. Since only the intermediates formed by the TagO/TarO, TagA/TarA or TagB/TarB enzymes are common to both WTA biosynthetic pathways (Fig. 1B), it is likely one of these is the activator of PhoR autokinase activity in both B. subtilis subspecies.
Phosphate limited cells of B. subtilis 168 and B. spizizenii W23 display different profiles of WTA gene expression
The results presented above show that activation of the PhoPR TCS in both Bacillus subspecies is influenced by changes in WTA metabolism during the onset of phosphate limitation. Furthermore, differences in WTA composition and metabolism confer distinctive features on the PHO responses of B. subtilis 168 and B. spizizenii W23. Therefore, we sought to establish how expression of each WTA biosynthetic gene changes when cells become phosphate limited. The initiation points of transcription of the tarABIJKL and tarDF operons were established and their promoters identified (Supporting Information Fig. S4, data not shown). The activity of neither promoter changed significantly on phosphate limitation, consistent with the absence of recognizable PhoP∼P binding sequences (Supporting Information Fig. S4, data not shown). Furthermore, no inter-cistronic promoters were found within the tarABIJKL operon (data not shown).
The mRNA level of each WTA biosynthetic gene was established (by RTqPCR) in phosphate limited cells of both subspecies (Fig. 6, red column) and expressed relative to that in phosphate replete cells (Fig. 6, blue column, set at unity). These profiles are typical of at least two experimental repeats, each with three technical replicates. That the cultures were sampled during phosphate-replete and phosphate–limiting conditions respectively was confirmed by the absence of detectable phoA transcript in exponentially growing cells (blue) and a ≥ 2,000-fold induction in phosphate limited cells (red) (Fig. 6A and B). These data also confirm that tuaA-H expression (encoding the enzymes for TUA synthesis) is regulated in B. spizizenii W23 in a manner similar to that in B. subtilis 168. Promoter activity (PtuaA-Hgfp) is induced at the onset of phosphate limitation (Supporting Information Fig. S1), leading to a ≥ 500-fold increase in the tuaA transcript level (Fig. 6B), profiles that are consistent with the location of multiple PhoP∼P binding sequences within the tuaA-H promoter region (data not shown).
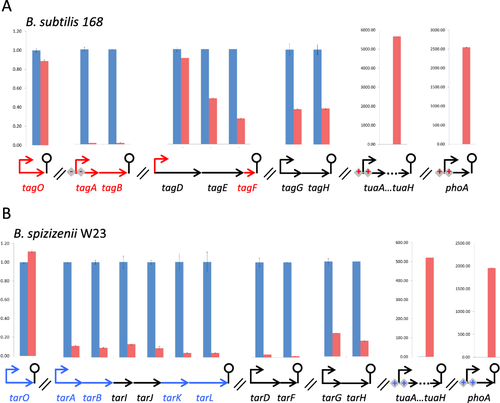
Comparison of mRNA levels for genes that encode the WTA biosynthetic enzymes and for selected PhoPR regulon genes in cells of B. subtilis 168 and B. spizizenii W23 grown under phosphate replete and phosphate limiting conditions. The level of transcript for each gene involved in WTA synthesis, and for selected PhoPR regulon genes (tuaA-H and phoA), was established in (A) B. subtilis 168 and in (B) B. spizizenii W23 cultures growing under phosphate replete (blue columns) and limiting (red columns) conditions. The level of each transcript in phosphate limited cells was expressed relative to that present in phosphate replete cells, which was set at 1. RNA was prepared from exponentially growing cells (blue, phosphate replete) and from cells at 2 h after the onset of phosphate limitation (red, phosphate limited). The values presented for each transcript were determined using the same RNA preparation as template and these profiles and values were observed in at least 3 experimental repeats. The genetic context of each gene is given beneath the columns and symbols are the same as those in Fig. 1. Genes encoding enzymes that are directly involved in Lipid V synthesis are shown by red arrows (A, B. subtilis 168) and blue arrows (B, B. spizizenii W23).
Uniquely, the level of tagO and tarO mRNA does not change detectably on phosphate limitation in B. subtilis 168 or in B. spizizenii W23 respectively (Figs. 1B and 6). However, the mRNA levels of tarA, tarB, tarK and tarL, the four genes directly involved for Lipid VR synthesis, and of tarI and tarJ the genes involved in synthesis of CDP ribitol substrate, are reduced ∼7–20-fold on phosphate limitation in B. spizizenii W23 (Fig. 6B). Specifically, the mRNA level of each gene relative to that in phosphate replete cells is tarA (14%); tarB (10%); tarK (5%), tarL (5%), tarI (14%) and tarJ (10%). A more complex profile of changes occurs in the corresponding WTA biosynthetic genes of B. subtilis 168 on phosphate limitation. Of the three genes directly involved in Lipid VG synthesis, the mRNA level of tagA and tagB genes are each reduced ≥ 100-fold reflecting PhoP∼P-mediated repression while the level of tagF is reduced only ∼ fourfold (to ∼ 27%) (Fig. 6A). Furthermore, the level of tagD mRNA that encodes the enzyme for synthesis of the CDP glycerol substrate is only slightly reduced (to ∼ 91%) on phosphate limitation in B. subtilis 168.
In summary, expression of the gene (tagO/tarO genes) that commits undecaprenol phosphate to anionic polymer synthesis does not change significantly on phosphate limitation in either B. subtilis subspecies. In B. spizizenii W23, expression of the genes that encode the remaining enzymes for WTA synthesis are uniformly reduced by ∼7–20-fold on phosphate limitation, reflecting that they are all co-transcribed. However, in B. subtilis 168 cells, the changes that occur in WTA gene expression on phosphate limitation are more complex reflecting differences in their genomic organization and regulation of expression.
Discussion
The PHO responses of B. subtilis 168 and B. spizizenii W23 were compared to establish if they are affected by differences in WTA chemical composition and metabolism between these two subspecies. This was prompted by the finding that PhoR autokinase activity is inhibited by Lipid VG in B. subtilis 168, a WTA metabolite composed of poly(glycerol phosphate) (Botella et al., 2014). The fundamental finding of this study is that the PHO responses of B. subtilis 168 and B. spizizenii W23 are distinct. We show that the variation in response derives from differences in the polyol composition of WTA and in the genomic organization and expression of WTA genes, but remarkably, not from the PhoR sensor kinases. We propose a unified model for control of PhoPR activity that accommodates the distinct PHO responses observed in B. subtilis 168 and B. spizizenii W23.
The PHO responses of B. subtilis 168 and B. spizizenii W23 are distinct
The PHO responses of B. subtilis 168 and B. spizizenii W23 are distinct in several respects. The most striking difference is seen in the period immediately after induction, when the response is amplified in B. subtilis 168 but has a characteristic double peak with lower activities in B. spizizenii W23 (Fig. 2). Furthermore during prolonged phosphate limitation, the PHO response is maintained in B. subtilis 168 but decreases in B. spizizenii W23, falling below the threshold of detection at ∼ T300 minutes (Fig. 2, Botella et al., 2014). The distinct nature of each PHO response is also revealed in strains that are unable to synthesize TUA (ΔtuaA-H): this causes the response to be delayed and attenuated in B. subtilis 168 but to be increased and prolonged in B. spizizenii W23 (compare red-lines, Fig. 2A and B). These results show that the PHO responses in B. subtilis 168 and B. spizizenii W23 are controlled by different mechanisms.
The PhoR kinases of B. subtilis 168 and B. spizizenii W23 are functionally equivalent and do not play a role in generating distinct PHO responses
Variation in the PHO responses of B. subtilis 168 and B. spizizenii W23 might be expected to emanate from differences in their PhoPR TCS. However, the PhoP and PhoR proteins of B. subtilis 168 and B. spizizenii W23 are highly homologous with 98.75% and 96.2% identity respectively at the amino acid level (Supporting Information Fig. S2). The near identity of the internal PAS domains of PhoR is noteworthy (Supporting Information Fig. S2), since this region is proposed to sense the inhibitory Lipid VG WTA metabolite in B. subtilis 168 (Botella et al., 2014). The contribution of the PhoR kinases to variation of the PHO responses was examined experimentally in strains of B. subtilis 168 (MP1 and MP118) and B. spizizenii W23 (MP107 and MP116) that express only the PhoR kinase of the other subspecies (Fig. 3A and B). Our results show that the PHO response of each of these strains is characteristic of the host and not of the subspecies from which the PhoR kinase is derived (Fig. 3C and D). For example, the PHO response in strain MP1 (phoP168 phoRW23) expressing the PhoRW23 kinase has a profile that is indistinguishable from that of parental strain BA1546 (phoP168 phoR168) (compare blue lines in Figs. 2A and 3C). Similarly, the effect of preventing TUA synthesis on the PHO response in strain MP118 (ΔtuaA-H phoP168 phoRW23) expressing the PhoRW23 kinase is indistinguishable from its effect on that of parental strain MP1547 (ΔtuaA-H phoP168 phoR168) (compare red lines in Figs. 2A and 3C). Therefore, the PhoR kinases of B. subtilis 168 and B. spizizenii W23 are functionally equivalent and do not contribute to variation of the PHO responses. Importantly, because PHO responses typical of the host are observed when either PhoR168 or PhoRW23 is expressed, we infer that control of PhoR activity (activation and repression) must be similar in both subspecies. We propose that the activation signal emanates from WTA metabolism since the onset of TUA synthesis has an impact (albeit different) on the PHO responses of both subspecies (Fig. 2). Furthermore, since TUA synthesis has the same impact on the PHO responses of B. subtilis strains expressing PhoR168 (BA1547) or PhoRW23 (MP118) we infer that Lipid VG can inhibit PhoRW23 autokinase activity, further emphasizing the mechanistic similarities of the two PhoR kinases (Fig. 3C and B). However, the effect of TUA synthesis on the PHO response of B. spizizenii W23 is opposite to that in B. subtilis 168, reducing it during the early stages and causing it to be eventually turned off (Fig. 2B). This indicates that TUA synthesis lowers the level of the WTA intermediate that activates PhoR and also that Lipid VR composed of poly(ribitol phosphate) does not inhibit PhoR autokinase activity in B. spizizenii W23.
In summary, we conclude that the PhoR168 and PhoRW23 kinases are functionally equivalent and do not contribute to variance in the PHO responses of B. subtilis 168 and B. spizizenii W23. We infer that both kinases are activated by a similar mechanism involving a WTA biosynthetic metabolite, and are inhibited by Lipid VG composed of poly(glycerol phosphate) but not by Lipid VR composed of poly(ribitol phosphate). This differential sensitivity of PhoR activity to inhibition by Lipid VG but not by Lipid VR indicates that WTA polyol composition plays a major role in the variance of the PHO responses of B. subtilis 168 and B. spizizenii W23.
The WTA metabolite produced by either the TagA/TarA or TagB/TarB WTA enzymes is an activator of PhoR autokinase activity in both B. subtilis 168 and B. spizizenii W23
The structural and functional equivalence of the B. subtilis 168 and B. spizizenii W23 PhoR kinases (Fig. 3, Supporting Information Fig. S2) indicates that both are activated by the same mechanism. Several lines of evidence support the view that the WTA biosynthetic intermediate produced by either the TagA/TarA or TagB/TarB enzymes is an activator of PhoR autokinase activity. The onset of TUA synthesis during phosphate limitation affects the PHO response of both subspecies in different ways: it amplifies and increases the response in B. subtilis 168 but lowers that of B. spizizenii W23 (Fig. 2). TUA synthesis amplifies the PHO response in B. subtilis 168 by diversion of metabolites away from the WTA biosynthetic pathway thereby lowering the level of the Lipid VG inhibitor (Fig. 7; Botella et al., 2014). Assuming a similar reciprocal relationship between WTA and TUA synthesis in B. spizizenii W23, we infer that the onset of TUA synthesis lowers the level of a WTA metabolite that activates PhoR autokinase activity. This conclusion was tested experimentally in B. spizizenii W23 by establishing how alteration of WTA biosynthetic enzyme expression (PxyltarABIJKL) affects the PHO response. The results show that the magnitude of Peak II in strain MP162 is directly proportional to the level of tarABIJKL expression (Fig. 4A). Furthermore, the increase in the magnitude of Peak II in strain MP164 (ΔtuaA-H) caused by the inability to synthesize TUA can be lowered by reducing tarABIJKL expression (Fig. 4B). Together these results indicate that an intermediate in WTA biosynthesis activates PhoRW23 in B. spizizenii W23, and because of their functional equivalence, also PhoR168 in B. subtilis 168 (Figs. 3 and 5). Since only the products of the TarO, TarA and TarB enzymes are common to the WTA biosynthetic pathways of both B. subtilis subspecies (Fig. 1B), this suggests that one of these is an activator of PhoR autokinase activity. However, the TagO enzyme product can be excluded for the following reasons: (i) Botella et al., (2014) have shown that the TarO product does not inhibit ‘PhoR autokinase activity; (ii) the level of tagO transcript does not change in either subspecies when cells become phosphate limited (Fig. 6) and (iii) if PhoR autokinase activity was activated by the TarO enzyme product, then a reduction in tarABIJKL expression (Fig. 4A, strain MP162 PxyltarABIJKL PphoAgfp) should increase the accumulation of TarO product and hence increase PphoAgfp expression. However, the opposite effect is observed, i.e., a reduction of tarABIJKL expression reduces the magnitude of peak 2 of the PHO response in MP162 (Fig. 4A and B).
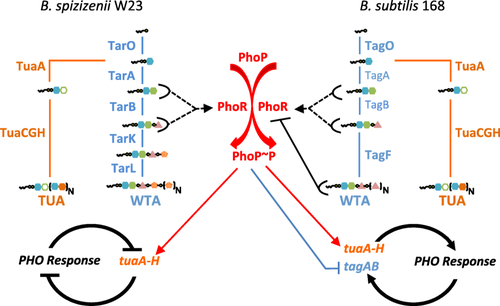
Unified model for the PHO responses in B. subtilis 168 and B. spizizenii W23. A schematic of a unified model for the PHO responses of B. spizizenii W23 (left-hand side) and B. subtilis 168 (right-hand side). Activation of PhoP (PhoP∼P) by PhoR is common to both subspecies and is shown in the center (red). The enzymes and metabolic steps in WTA biosynthesis are shown in blue with vertical blue lines indicating conversion from one intermediate to the next. The structure of each WTA intermediate is shown (symbols the same as in Fig. 1). The enzymes and some steps in TUA synthesis are shown in orange. Transcriptional activation of the tuaA-H operon is shown by thin solid red arrows, and transcriptional repression of tagAB is shown by thin solid blue lines with a bar. Half ovals (black) with attached black lines signify interaction of that metabolic intermediate with PhoR kinase: solid black line with bar signifies inhibition of PhoR autokinase activity by TagF product (Botella et al., 2014) while broken lines with an arrow signifies a proposed interaction between the TagA/TarA or TagB/TarB metabolites to activate PhoR autokinase activity (this study). The effect of these regulatory circuits on the PHO response is indicated by thick black solid lines: attenuation and turn off (B. spizizenii W23, thick line with bar) or amplification and maintenance (B. subtilis 168, thick line with arrow).
In summary, these results suggest that the product of either the TagA/TagB or TarA/TarB enzymes activates PhoR autokinase during the PHO responses of B. subtilis 168 and B. spizizenii W23.
Activation of the PHO response occurs in two distinct steps
Evidence presented here and in Botella and colleagues (2014) indicates that onset of the PHO response occurs in two distinct steps (Fig. 4, Peaks I and II). Insight into the distinction between them is provided by changes that occur in the PHO response of B. spizizenii W23 when expression of WTA enzymes (tarABIJKL) is varied (Fig. 4A and B). The magnitude of Peak II varies in direct proportion to enzyme expression whereas that of Peak I is independent of enzyme expression. Thus the increased levels of PhoR activator (i.e., the product of either the TarA or TarB enzyme) that generate Peaks I and II must be produced by different mechanisms. We hypothesize that Peak I may be generated by a surge in WTA metabolites due to a reduction in cell wall synthesis when growth is lowered on the onset of phosphate limitation. Peak II may be generated by a level of WTA biosynthetic enzyme that is inappropriately high for the reduced growth rate of phosphate limited cells. This causes an increase in the level of TarA/TarB activator and hence increased PhoR autokinase activity. This is supported by the observation that expression of all WTA biosynthetic enzymes is normally reduced 7–20-fold in phosphate limited cells of B. spizizenii W23 (Fig. 6). The onset of TUA synthesis contributes to the magnitude of both peaks in B. spizizenii W23 by diversion of TagO metabolites away from the WTA biosynthesis, thereby reducing the level of the TarA or TarB activator and causing attenuation and ultimately cessation of the PHO response.
Onset of the PHO response in B. subtilis 168 may also occur in two steps, although they are more difficult to discern because WTA metabolites both activate (TagA or TagB enzyme product) and inhibit (TagF enzyme product) PhoR autokinase activity (Botella et al., 2014, this work). Two peaks of PhoR autokinase activity are evident in the PHO response of B. subtilis when TUA synthesis is precluded (Fig. 2A). Furthermore, the PHO response is triggered but not further increased when both tagAB expression is maintained and TUA synthesis is precluded (see Fig. 3D, Botella et al., 2014). This shows that triggering the PHO response in B. subtilis 168 is separate from the process that amplifies and adjusts it to the prevailing conditions. Thus activation of the PHO responses in both subspecies appears to occur in two steps: a (trigger) step to initiate the response and a second step to adjust the response to the prevailing conditions.
Unified model of the PHO responses in B. subtilis 168 and B. spizizenii W23
A unified model for the different PHO responses of B. subtilis 168 and B. spizizenii W23 is shown in Fig. 7. We propose that PhoR is activated on phosphate limitation in both subspecies by a WTA metabolite synthesized by either the TagA/TarA or TagB/TarB enzymes. The reduction in growth rate on phosphate limitation causes the level of activator to increase by (1) decreased utilization of WTA intermediates (Peak I) and (2) levels of WTA biosynthetic enzymes that are inappropriately high for this condition (Peak II). Once the PHO response is triggered, synthesis of WTA is then adjusted to the phosphate limited state (Fig. 7). In B. spizizenii W23, the level of WTA metabolites is lowered by the onset of TUA synthesis mediated by activated PhoP∼P and by reduced expression of WTA biosynthetic enzymes (reduced 7–20-fold, Fig. 6). Together these function as a negative feedback mechanism to attenuate and eventually turn off the PHO response (Fig. 7, left-hand side). In B. subtilis 168, the levels of WTA metabolites are also lowered by the onset of TUA synthesis mediated by activated PhoP∼P (Fig. 7 right-hand side). However, because Lipid VG inhibits PhoR autokinase activity, the onset of TUA synthesis lowers the level of this inhibitor and, therefore, functions as a positive autoregulatory mechanism to amplify the PHO response (Fig. 7 right-hand side, Botella et al., 2014). PhoP∼P mediated repression of tagAB also functions as a positive autoregulatory mechanism in that the level of Lipid VG is lowered by a reduction of TagA and Tag B enzyme levels (Fig. 7 left-hand side, Botella et al., 2014). Together, these regulatory mechanisms function to amplify and maintain the PHO response in B. subtilis 168 while the phosphate limitation condition persists. Since PhoR autokinase activity is both activated (TagA or TagB enzyme product) and inhibited (TagF enzyme product) by intermediates in WTA metabolism, the balance between production and utilization of these intermediates will have a profound influence on the induction kinetics of the PHO response in B. subtilis 168.
Concluding remarks
Several features of this study are intriguing. The first concerns the advantages and disadvantages of maintaining the PHO response during prolonged phosphate limitation as observed in B. subtilis 168 or attenuating it after a 4–5 hour period as in B. spizizenii W23. When the response is maintained, the full battery of phosphate scavenging enzymes is continually produced for a prolonged period. This may place a heavy burden on cellular resources and may not provide additional benefit over an extended period if the level of scavenging enzymes in the vicinity of the bacterium may become saturated. However, the cellular requirement for phosphate is reduced under this condition since the cell wall anionic polymer produced does not contain phosphate. In contrast, attenuating the response lowers the burden of scavenging enzyme production, but the cellular requirement for phosphate gradually increases by reverting to WTA synthesis. These alternate strategies may provide an opportunity for each B. subtilis subspecies to adapt to different ecological niches.
A second intriguing feature is the functional equivalence of the PhoR kinases in the two subspecies, particularly the observation that PhoRW23 can be inhibited by Lipid VG composed of poly(glycerol phosphate) despite being expressed in a bacterium that synthesizes Lipid VR composed of poly(ribitol phosphate). This may be rationalized by proposing that the primary constraint on PhoR is to detect the WTA intermediate that activates autokinase activity. Our evidence suggests that the activation signal may be the TagB/TarB enzyme product, a WTA intermediate that contains a terminal glycerol phosphate moiety attached to two modified sugars (Figs. 1B and 4). If the activation signal is comprised of a terminal glycerol phosphate juxtaposed to some feature of the modified sugars (N-acetylglucosamine or N-acetylmannosamine), then the PhoR kinases of both B. subtilis 168 and B. spizizenii W23 may retain the ability to detect it. In B. subtilis 168, therefore, the terminal glycerol phosphate moiety may be detected by PhoR168 and be an agonist when juxtaposed to a modified sugar (TagB enzyme product) but be an antagonist when juxtaposed to another glycerol phosphate (Lipid VG, Botella et al., 2014). Likewise in B. spizizenii W23, the terminal glycerol phosphate moiety may be detected by PhoRW23 and function as an agonist when located adjacent to the modified sugar. However, PhoRW23 recognition may be abolished on addition of the first ribitol phosphate moiety by TarK (Fig. 1B).
This study also reveals that extrapolation of physiological function to orthologous TCS, even of highly homologous TCS in closely related subspecies, must be done with some caution. The PhoP and PhoR proteins in B. subtilis 168 and B. spizizenii W23 are highly homologous at the DNA (95.7% and 93.6% respectively) and protein (98.75% and 96.2% respectively) levels and the PhoR kinases are functionally equivalent. However, the kinetics of the PHO response are significantly different, being influenced by the polyol composition of WTA, the genomic organization of WTA biosynthetic genes and the regulation of their expression. These features combine to produce different PHO responses, despite the high level of identity between the two PhoPR TCS. The tag and tar genes are located at equivalent positions on the chromosomes of B. subtilis 168 and B. spizizenii W23 and behave as a non-homologous pseudo allelic pair in genetic crosses (Young et al., 1989). Young and colleagues (1989) have shown that inter-strain exchange of these WTA biosynthetic gene cassettes can occur. When viewed in this context, this study shows how significant variation in the bacterial response to phosphate limiting conditions can be achieved through horizontal gene transfer, in this case of the pseudo-allelic cassettes encoding the WTA biosynthetic genes. This illustrates how the versatility of TCS for adaptation to a particular condition can extend beyond diversification of the sensor and output domains of kinases and response regulators respectively, to alteration of TCS function through acquisition and exchange of genetic functions through horizontal gene transfer.
Experimental procedures
Strains, plasmids and growth conditions
Bacterial strains and plasmids used in this study are listed in Supporting Information Tables S1 and S2 respectively. Oligonucleotides were obtained from IDT Belgium and are listed in Supporting Information Table S3. Escherichia coli strain TG-1 (Sambrook et al., 1989) was used for propagating plasmids. Strains were grown in Luria-Bertani (LB) medium with the addition of antibiotics at concentrations described by Noone and colleagues (2014). The high phosphate defined medium (HPDM) and low phosphate defined medium (LPDM) used are described in Botella and colleagues (2011). OD600 was measured using a UVmini-1240 UV-VIS spectrophotometer (Shimadzu Scientific Instruments).
Strain and plasmid construction
To construct plasmid pMP2, two DNA fragments were synthesized by PCR using Phusion polymerase with chromosomal DNA from B. subtilis 168 as template and primer pairs OM1F/OM1R and OM2F/OM2R. A third DNA fragment was synthesized by PCR with chromosomal DNA from B. spizizenii W23 as template using primer pair OM3F/OM3R. These 3 fragments were joined using overlapping PCR with primer pair OM1F/OM2R and cloned into pDG646 to give plasmid pMP2. Plasmid pMP2 was transformed into B. subtilis 168 strains BSB168, BA1556, EB159 and BA1687 to generate strains MP1, MP2, MP117 and MP118 respectively.
To construct plasmid pMP26, a 1 kilobase DNA fragment located upstream (generated by primer pair OM38F/OM13R) and a second located downstream (generated by primer pair OM14F/OM39R) of the phoR gene of B. spizizenii W23 were amplified by PCR. These two DNA fragments were then joined to a DNA fragment encoding a kanamycin resistance gene (generated using Kan_Fwd/Kan_Rev primer pair) by overlapping PCR. In this construct, the fragments flanked the antibiotic resistance cassette and were in the same relative orientation as on the chromosome. The resultant DNA fragment was then cloned into pDG1727 to generate plasmid pMP26. Plasmid pMP26 was transformed into B. spizizenii W23 strain to generate strain MP69 (ΔphoR::kan).
To construct plasmid pMP30, two DNA fragments of the amyE gene of B. spizizenii W23 were amplified: a 661bp fragment (primer pair OM44F/OM16R) from the promoter proximal region of the gene (called amyE front) and a 1221bp fragment (primer pair OM17F/OM53R) from the promoter distal region of the gene (called amyE back). A DNA fragment encoding a transcriptional fusion of the promoter of the phoA gene from B. spizizenii W23 was fused to the reporter gfp gene and the chloramphenicol resistance gene (PphoAW23gfp cat) was amplified using the primer pair OM18F/OM18R and plasmid pMP29 as template. The OM18F/OM18R primers have sequences that generate an overlap with the fragments encoding the amyE front and back regions, allowing all three fragments to be joined by overlapping PCR using primer pair OM44F/OM53R. This we then cloned into plasmid pDG1727 to generate plasmid pMP30. Plasmid pMP30 was transformed into parental strain B. spizizenii W23 and strains MP69, MP113 and MP115 to generate strains MP92, MP94, MP114 and MP116 respectively.
Plasmid pMP32 was constructed by PCR amplification of a 1 kilobase DNA fragment of the polA gene of B. spizizenii W23 using primer pair OM50F/OM50R and cloning it upstream of the spectinomycin resistance gene in pDG1727, thereby generating plasmid pMP32. To construct plasmid pMP33, two fragments were synthesized by PCR: a fragment homologous to the phoP gene of B. spizizenii W23 was synthesized using primer pair OM46F/OM46R and a fragment homologous to the phoR from B. subtilis 168 was synthesized using primer pair OM47F/OM54R. These two DNA fragments were joined by overlapping PCR using primer pair OM46F/OMOM54R and cloned in plasmid pMP32 at a position upstream of the spectinomycin resistance gene, thereby generating plasmid pMP33. B. spizizenii W23 strains MP104 (ΔphoRW23::phoR168 spec) and MP107 (ΔphoRW23::phoR168 spec ΔamyE::PphoAgfpMut3 cat) were constructed by transformation of plasmid pMP33 into strains MP69 and MP94 respectively.
To construct plasmid pMP36, DNA fragments of 1 kilobase in size from upstream of the B. spizizenii W23 tuaA gene (using primer pairs OM62F/OM62R) and downstream of the tuaH gene (using primer pairs OM63F/OM63R) were synthesized by PCR. These two DNA fragments were joined to a DNA fragment encoding the erythromycin resistance gene (erm), generated by primer pair MLS_Fwd/MLS_Rev and using plasmid pDG646 as template, by overlapping PCR in the same relative orientation as on the chromosome. The resultant DNA fragment was cloned into plasmid pDG1515 (encoding the tetracycline resistance gene tet) to give plasmid pMP36. Plasmid pMP36 was transformed into the parental and MP104 strains of B. spizizenii W23 to generate strains MP113 (ΔtuaA-H::erm) and MP115 (ΔtuaA-H::erm ΔphoRW23::phoR168 spec) respectively.
To construct plasmid pMP38, two DNA fragments of the amyE gene of B. spizizenii W23 were amplified: a 661bp fragment (primer pair OM44F/OM16R) from the promoter proximal region of the gene (called amyE front) and a 1221bp fragment (primer pair OM17F/OM53R) from the promoter distal region of the gene (called amyE back). A third DNA fragment of 1928bp in size that encoded a promoterless gfpMUT4 gene, ligation independent cloning sequences (LIC) and a chloramphenicol resistance gene (cat) of 1928bp in size was amplified using primer pairs OM18F/OM18R using plasmid pBP122 as template (Bisicchia et al., 2010a). This fragment (gfp-LIC-cat) has sequences that overlapped those of the amyE front and amyE back homology fragments (OM18F/OM18R) allowing these fragments to be joined by overlapping PCR using primer pair OM44F/OM53R. It was then cloned in pDG1727 to give plasmid pMP38.
To construct plasmids pMP39 to pMP46, DNA fragments containing the promoters of the phoB (primer pair OM66F/OM66R), phoD (primer pair OM67F/OM67R), pstS (primer pair OM70F/OM70R), glpQ (primer pair OM69F/OM75R), phoPR (primer pair OM68F/OM68R), tarA (primer pair OM71F/OM71R), tarD (primer pair OM73F/OM73R) and tuaA (primer pair OM72F/OM72R) genes from B. spizizenii W23 were synthesized by PCR using the primer parirs described. These fragments contained LIC sites that allowed them to be cloned into plasmid pMP38 thereby generating plasmids pMP39 to pMP46 respectively. Each plasmid was then separately inserted into parental B. spizizenii W23 strain to generate strains MP121, MP122, MP123, MP124, MP125, MP126, MP127 and MP128 respectively.
Plasmid pMP54 was constructed to place expression of the tarABIJKL operon of B. spizizenii W23 under the control of the xylose inducible Pxyl promoter. This was achieved by integration into the chromosome by a single cross-over event at the homologous locus. A DNA fragment (474bp) encoding the promoter of the B. spizizenii W23 tarABIJKL operon was amplified by PCR using primer pair OM86F/OM86R and cloned into plasmid pSOUR to generate plasmid pMP54. Plasmid pMP54 was then transformed into B. spizizenii W23 strain MP153 to generate strain MP161 (ΔxylRAB::kan PxyltarABIJKL spec).
To construct plasmid pMP56, DNA fragments of 1 kilobase in size were synthesized by PCR using chromosomal DNA from B. spizizenii W23 as template. One DNA fragment (generated using the OM87F/OM87R primer pair) was located upstream of the xylR gene while the second fragment (generated using the OM88F/OM80R primer pair) was located downstream of the xylB gene. A third DNA fragment was synthesized, using primer pair Kan_Fwd/Kan_Rev and pDG780 as template, encoded a kanamycin resistance gene. The fragments have homologous ends allowing them to be joined by overlapping PCR so that the two chromosomal fragments flank that encoding the kan gene. This fragment was then cloned into pUC19 to generate plasmid pMP56. The parental B. spizizenii W23 strain and strains W23, MP92, MP113 and MP114 were then transformed with plasmid pMP56 to generate strains MP153, MP154, MP155 and MP156 respectively.
Plasmid pMP67 was constructed to change the antibiotic cassette in plasmid pMP33 by sequence and ligation-independent cloning (SLIC) as described by Li and Elledge (2007). A linear DNA fragment was generated using plasmid pMP33 as template and primer pair OM103F/OM103R that did not encode the spectinomycin cassette but contained end sequences that are homologous to a DNA fragment encoding the erythromycin gene erm that was generated by PCR using the primer pair MLS_Fwd/MLS_Rev with plasmid pDG646 as template. Both DNA fragments were then treated with T4 DNA polymerase, allowed to anneal and transformed into competent E. coli cells to generate plasmid pMP67 (erm). Plasmid pMP67 was transformed into strains MP69 and MP94 to generate strains MP171 and MP172 respectively.
Strains MP173 and MP174 were constructed by transformation of strains MP171 and MP172 respectively with chromosomal DNA from strain MP161 (ΔxylRAB::kan).
Strains MP162, MP163, MP164, MP175, MP176, MP179, MP180 were constructed by transformation of strains MP154, MP155, MP156, MP173, MP174, MP177 and MP178 respectively with chromosomal DNA from strain MP161 (PxyltarABIJKL spec).
To construct plasmids pMP58 to pMP61, DNA fragments were synthesized using PCR that encoded the promoters of the tarB (using primer pair OM98F/OM98R), tarI (using primer pair OM99F/OM99R), tarL (using primer pair OM100F/OM100R) and tarF (using primer pair OM101F/OM101R) genes of B. spizizenii W23. Each was inserted into plasmid pMP38 by ligase independent cloning (LIC) generating plasmids pMP58 to pMP61. Each plasmid was then transformed separately into the parental strain of B. spizizenii W23 to generate strains MP166, MP167, MP168 and MP169 respectively.
Plasmid pMP69 was constructed to change the antibiotic cassette in plasmid pMP36 by sequence and ligation-independent cloning (SLIC) as described by Li and Elledge (2007). A linear DNA fragment was generated using plasmid pMP36 as template and primer pair OM105F/OM105R that did not encode the erythromycin cassette but contained end sequences that are homologous to a DNA fragment encoding the bleomycin/phleomycin cassette that was generated by PCR using the primer pair Bleo_Fwd/Bleo_Rev and plasmid pMPA65 as template. Both DNA fragments were then treated with T4 DNA polymerase, allowed to anneal and transformed into competent E. coli cells to generate plasmid pMP69 (phleo). Plasmid pMP69 was then transformed into strains MP173 and MP174 to generate strains MP177 and MP178 respectively.
Measurement of promoter activity
Promoter activity was measured as previously described by Botella and colleagues (2010; 2011).
RNA extraction and RTqPCR
RNA was extracted and transcripts were quantified using RTqPCR as previously described by Salzberg and colleagues (2013).
Western analysis
Western analysis was performed as described in Noone and colleagues (2012).
Transformation of B. subtilis 168 and B. spizizenii W23
B. subtilis 168 (strain BSB168, Jules et al., 2009) was transformed according to the procedure of Anagnostopoulos and Spizizen (1961). The procedure used to transform B. spizizenii W23 was a modification of that described by Jarmer and colleagues (2002). The transformation medium used was that described by Jarmer and colleagues (2002), a modification of Spizizen minimal salts medium. An overnight culture of B. spizizenii W23, grown in the modified Spizizen minimal salts medium, was diluted 1/20 into 1 ml of fresh medium to which 3 µg of circular plasmid DNA was added. The culture was then grown at 37°C for 7 h with aeration at 200 rpm. Transformants were selected on Luria Bertani agar (LB) with addition of antibiotics at concentrations described by Noone and colleagues (2014) as appropriate.
Acknowledgements
The authors would like to thank past and present members of the laboratory for the gift of strains. This work was supported by grants from Science Foundation Ireland, Principal Investigator Awards 08/IN.1/B1859 and 12/1A/1570 to KMD. The authors declare no conflict of interest.
Author Contributions
MP and KMD conceived and designed the studies. MP performed the majority of the experiments as part of his PhD thesis at the University of Dublin, Trinity College. DN performed some additional experiments and provided support and advice throughout. MP, DN and KMD analyzed and interpreted the data. All authors read and approved the final manuscript.





