Structure of the bacterial cell division determinant GpsB and its interaction with penicillin-binding proteins
Summary
Each bacterium has to co-ordinate its growth with division to ensure genetic stability of the population. Consequently, cell division and growth are tightly regulated phenomena, albeit different bacteria utilise one of several alternative regulatory mechanisms to maintain control. Here we consider GpsB, which is linked to cell growth and division in Gram-positive bacteria. ΔgpsB mutants of the human pathogen Listeria monocytogenes show severe lysis, division and growth defects due to distortions of cell wall biosynthesis. Consistent with this premise, GpsB interacts both in vitro and in vivo with the major bi-functional penicillin-binding protein. We solved the crystal structure of GpsB and the interaction interfaces in both proteins are identified and validated. The inactivation of gpsB results in strongly attenuated virulence in animal experiments, comparable in degree to classical listerial virulence factor mutants. Therefore, GpsB is essential for in vitro and in vivo growth of a highly virulent food-borne pathogen, suggesting that GpsB could be a target for the future design of novel antibacterials.
Graphical Abstract
GpsB is a cell division protein and conserved in many Gram-positive bacteria. Here we describe its function in growth, cell division and cell wall biosynthesis of the important human pathogen Listeria monocytogenes. Analysis of the three-dimensional structure of GpsB now explains how GpsB binds to its interaction partner, the major bi-functional penicillin binding protein PBP A1.
Introduction
Bacteria require a multitude of regulatory factors to co-ordinate cycles of growth and division to ensure genetic stability of the population. Despite the essentiality of the co-ordination process, different bacteria have evolved a variety of regulatory mechanisms and factors. Although the universal tubulin homologue, FtsZ, forms the Z-ring that is required to drive the constriction of the cell division septum (Adams and Errington, 2009), the proteins that regulate FtsZ localisation and dynamics are not conserved. For instance, the Z-ring is anchored to the membrane in Escherichia coli (a proteobacterium) by FtsA and ZipA (Hale and de Boer, 1997; Pichoff and Lutkenhaus, 2005), whereas in Bacillus subtilis (a firmicute), FtsA and the unrelated SepF fulfil this role (Duman et al., 2013). In Streptomyces (from the phylum actinobacteria), FtsA is absent yet there are three orthologues of SepF, and mycobacteria have no FtsA and only one copy of SepF (Gola et al., 2015). Similarly, the modulators of FtsZ in B. subtilis include EzrA and ZapA, in E. coli there is ZapA and ZapB, but there are no homologous gene products in Streptomyces (Adams and Errington, 2009; Jakimowicz and van Wezel, 2012). Consequently, it is important that the cell cycle of relevant bacteria – model organisms, significant pathogens – is better characterised so as to understand the major principles as well as for the development of novel antibiotics.
Among the other regulators of viability, cell growth and division in various Gram-positive bacteria is the DivIVA superfamily (Kaval and Halbedel, 2012; Lin and Thanbichler, 2013), which includes the DivIVA archetypes and the GpsB proteins (Massidda et al., 1998; Claessen et al., 2008; Tavares et al., 2008; Land et al., 2013; Fleurie et al., 2014). Deletion of B. subtilis gpsB is without phenotype under normal conditions, however, ΔgpsB cells lyse and form polar bulges in high salt containing media. Bulging is a result of aberrant cell wall biosynthesis at the cell poles because of the delocalisation of the major bi-functional penicillin-binding protein 1 (PBP1). Bulging can also be observed under standard growth conditions when ΔgpsB is combined with a deletion in ezrA, a negative regulator of Z-ring formation (Claessen et al., 2008). In B. subtilis PBP1 interacts directly with GpsB and EzrA (Claessen et al., 2008), and these interactions determine the localisation of PBP1 by an unknown mechanism. In Streptococcus pneumoniae, depletion of GpsB prevents closure of the divisome in a manner that is independent of PBP1 (Land et al., 2013).
Unlike DivIVA (Lenarcic et al., 2009; Ramamurthi and Losick, 2009), GpsB does not accumulate at curved membranes. Instead, it shuttles dynamically between the lateral sides of the cell cylinder, to which it localises in elongating cells of B. subtilis, and the division septum upon the formation of the divisional Z-ring (Claessen et al., 2008; Tavares et al., 2008). Accumulation of B. subtilis GpsB at the septal ring occurs simultaneously with DivIVA and depends on late-acting division proteins, including DivIC and PBP 2B (Tavares et al., 2008; Gamba et al., 2009), but it is not yet known how GpsB is recruited to the septum and then back to the side wall of the cell. GpsB and DivIVA proteins share a highly conserved N-terminal domain, which in DivIVA is required for binding to curved membranes (Lenarcic et al., 2009; Oliva et al., 2010) as well as for interacting with membrane-bound cell division proteins (van Baarle et al., 2013). By contrast, the C-terminal domains of GpsB and DivIVA are distinct, and this domain is used in DivIVA to interact with soluble cytosolic proteins (Donovan et al., 2012; van Baarle et al., 2013). The crystal structure of the N-terminal domain of DivIVA is a dimeric coiled-coil, and solvent-exposed hydrophobic amino acid side-chains are believed to insert into the membrane (Oliva et al., 2010); the DivIVA C-terminal domain forms a long anti-parallel coiled-coil such that the full-length DivIVA is an extended tetramer (Oliva et al., 2010). By contrast, the structure and function of the two domains of GpsB are unknown.
Here we investigate the role of GpsB in the physiology of Listeria monocytogenes, a human pathogen that causes serious food-borne infections with a mortality rate of 10–30% (Swaminathan and Gerner-Smidt, 2007). We show that GpsB is indispensable for growth and cellular integrity under standard laboratory conditions, leading to a strong attenuation of the ΔgpsB mutant in several infection models. We identify PBP A1 as the functionally relevant interaction partner of GpsB and demonstrate that this interaction is essential for functional PBP A1 activity. We also solve the crystal structures of both the N-terminal and C-terminal domains of GpsB, and confirm that a conserved, contiguous surface patch is used as the predominant PBP A1 binding site in the former. Together, these data confirm the key role of GpsB in growth and virulence and highlight that cell division regulators could be useful new targets for the design of novel antibiotics.
Results
L. monocytogenes GpsB
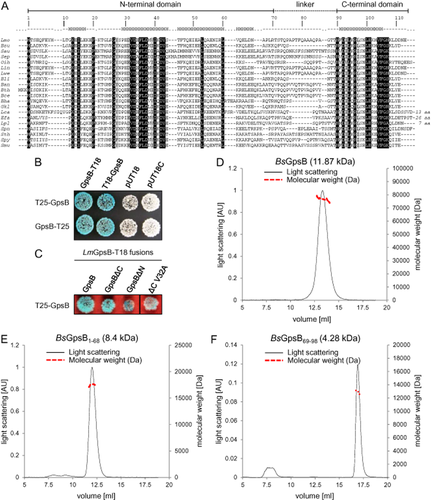
The L. monocytogenes EGD-e lmo1888 gene product shares 56.7% sequence identity with B. subtilis GpsB (BsGpsB) and is thus re-named LmGpsB. LmGpsB contains the highly conserved N-terminal domain (residues 1–69) typical of DivIVA/GpsB proteins (Fig. 1A). The LmGpsB C-terminal region (residues 90–113) is significantly shorter than the coiled-coil C-terminal domain of DivIVA, but is also predicted to contain an amphipathic helix (Fig. S1A–B). The linker region (residues 70–89) is less well conserved than either terminus and is specific to the GpsB family.
Features of L. monocytogenes GpsB.
A. Multiple sequence alignment of selected GpsB proteins. Sequence identity is indicated by black boxes, similarity by grey shading. Numbering is according to L. monocytogenes (Lmo) GpsB. The observed secondary structure is shown above the alignment. Abbreviations: Bsu – Bacillus subtilis, Sau – Staphylococcus aureus, Sep – Staphylococcus epidermidis, Oih – Oceanobacillus ihyensis, Lin – Listeria innocua, Lwe – Listeria welshimeri, Bli – Bacillus licheniformis, Ban – Bacillus anthracis, Bth – Bacillus thuringiensis, Bce – Bacillus cereus, Bha – Bacillus halodurans, Gkl – Geobacillus kaustophilus, Lca – Lactobacillus casei, Efa – Enterococcus faecalis, Lpl – Lactobacillus plantarum, Spn – Streptococcus pneumoniae, Sth – Streptococcus thermophilus, Spy – Streptococcus pyogenes, Smu – Streptococcus mutans.
B. Bacterial two-hybrid experiment analyzing the self-interaction of full-length LmGpsB. The empty pUT18 and pU18C plasmids were included as controls.
C. Bacterial two-hybrid experiment to analyze the effect of truncations and the V32A mutation on LmGpsB self-interaction.
D–F. Dual axis plots of the SEC-MALLS analysis of BsGpsB (D), BsGpsB1–68 (E) and BsGpsB69–98 (F); for clarity only the plots for the BsGpsB proteins are shown, but the LmGpsB proteins behaved similarly. Light scattering of the eluate from the gel filtration column (S200 BsGpsB, S75 BsGpsB1–68, S200 BsGpsB69–98) is plotted as a function of elution volume (x-axis). Light scattering is plotted on the left hand y-axis and molecular masses (right hand y-axis) were calculated by extrapolation from Zimm plots; the monomer molecular weights of each construct are given in parentheses above the SEC-MALLS chromatogram.
LmGpsB strongly self-interacted in a set of bacterial two-hybrid experiments (Fig. 1B). The self-interaction was retained when the C-terminal region was removed (GpsBΔC, residues 1–67), but the self-interaction was weakened somewhat when the N-terminal region was deleted (GpsBΔN, residues 68–113, Fig. 1C). These data indicate that both domains contribute to the self-interaction of GpsB. In fact, full-length GpsB proteins are hexamers in solution as determined by size exclusion chromatography – multi-angle laser light scattering (SEC-MALLS) (Fig. 1D), and equivalent N-terminal fragments – BsGpsB1–68 and LmGpsB1–73 – both form dimers (Fig. 1E). By contrast, the C-terminal domain of BsGpsB (BsGpsB69–98) forms a trimer (Fig. 1F), suggesting that the GpsB hexamers consist of a trimer of dimers.
GpsB localisation in L. monocytogenes
When GpsB-GFP was expressed in L. monocytogenes, the resultant fluorescence signal obeyed a bimodal distribution: cells either displayed bright septa with weaker peripheral fluorescence, or they only had peripheral fluorescence (Fig. 2A). 36% of all cells showed septal GpsB-GFP localisation (76/212 cells), whereas 64% had not accumulated GpsB-GFP signals at the division septa (136/212 cells). Additional fluorescent foci were occasionally observed at one cell pole. Cells without the septal GpsB-GFP signal were significantly shorter (1.48 ± 0.31 μm) than cells with septal GpsB-GFP (2.11 ± 0.36 μm, P < 0.0001, Fig. 2B), and GpsB-GFP stability was confirmed by Western blotting (Fig. 2C). These observations are in good agreement with a previous report that B. subtilis GpsB re-localised from the lateral sides of the cell cylinder in young, shorter growing cells to the division septum as soon as longer, fully grown cells started to divide (Claessen et al., 2008). Western blots of cellular and membrane fractions of L. monocytogenes revealed that GpsB was enriched in membrane fractions (Fig. 2D), and membrane association was dependent on residues L24 and R25 (Fig. 2E). This cellular distribution mirrors that of DivIVA (Fig. 2D), which depends on the equivalent amino acids, F17 and R18 (Oliva et al., 2010).

Subcellular localisation of L. monocytogenes GpsB.
A. Micrograph showing the fluorescence pattern of GpsB-GFP expressed in L. monocytogenes (strain LMS52). LMS52 cells were cultivated in BHI broth at 37°C to mid-log growth phase and fluorescence images were taken (right panel). Phase contrast (left) and merged images (bottom) are included.
B. Frequency plot to illustrate cell length frequency distributions of LMS52 cells with septal or non-septal fluorescence patterns of GpsB-GFP (236 cells measured in total).
C. Western blot to demonstrate full-length expression of GpsB-GFP. Cells of LMS10 (expressing GFP) and LMS52 were included as control, and GFP proteins were visualised using an anti-GFP specific antiserum.
D. Western blots of cellular sub-fractions to demonstrate presence of GpsB in membrane fractions. Cells of strain EGD-e were grown in BHI broth at 37°C to an optical density of OD600 = 1.0, membrane and soluble protein fractions were isolated and tested in Western blots using antisera recognising GpsB (upper panel) or DivIVA (middle panel). Strain LMS10 constitutively expressing GFP as a soluble cytosolic marker protein was included as control (lower panel). (E) Effect of the L24A and R25A mutations on GpsB membrane association. GpsB levels in cellular and membrane fractions of strains EGD-e (wt), LMJR19 (ΔgpsB), LMJR68 (+ L24A) and LMJR4 (+ R25A) were analysed by Western blotting using a GpsB antiserum.
Effects of gpsB deletion on growth of L. monocytogenes
The ΔgpsB mutant (strain LMJR19) did not reveal a growth defect in BHI broth at 30°C (Fig. 3A) but at 37°C it grew with reduced growth rate and only reached half of the final optical density of the wild-type strain (Fig. 3B). Moreover, not all of ΔgpsB mutant cells are viable, as indicated by CFU-based growth measurements (Fig. S2A). This growth defect was rescued upon expression of GpsB-GFP (Fig. S2B). At 42°C, however, the ΔgpsB strain showed a pronounced temperature-sensitive phenotype and did not grow at all (Fig. 3C). Western blotting confirmed the absence of GpsB in strain LMJR19 (ΔgpsB) and IPTG-dependent gpsB expression in the inducible mutant strain LMS56 (Fig. 3B), demonstrating that GpsB is important for growth during standard laboratory conditions and becomes essential at elevated temperatures.
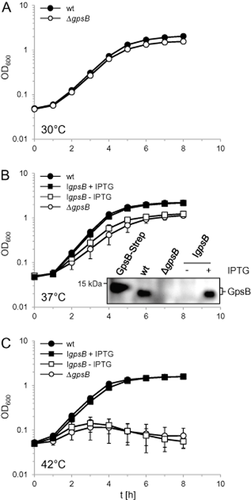
Growth of L. monocytogenes strains lacking gpsB.
A. Growth of the ΔgpsB mutant LMJR19 in BHI broth at 30°C.
B. Delayed growth of L. monocytogenes gpsB mutants in BHI broth at 37°C. Strains EGD-e (wt), LMJR19 (ΔgpsB) and LMS56 (IgpsB, I – denotes IPTG-dependent expression) were grown in BHI broth (± 1 mM IPTG) at 37°C and OD600 was measured. Insert: Lack of GpsB synthesis in gpsB mutant strains. Total cellular protein extracts were analyzed by Western blotting using a polyclonal anti-GpsB antiserum and purified, recombinant LmGpsB-Strep was used as a control. Due to the Strep-Tag its molecular weight is slightly increased (14.1 kDa) in comparison to the untagged GpsB protein (12.9 kDa).
C. Growth defect of L. monocytogenes gpsB mutants in BHI broth (± 1 mM IPTG) at 42°C. Average values and standard deviations in all three experiments are calculated from three independent repetitions (but are too small to be seen in many cases).
Increased autolysis of L. monocytogenes gpsB mutant strains
Phase contrast microscopy revealed that the ΔgpsB mutant produced cells with bent shapes and many phase-bright cells (arrows in Fig. 4A), which represent lysed, dead cells; the ΔgpsB mutant suffers from increased autolysis. Scanning electron microscopy showed that the ΔgpsB mutant generated partially bent, slightly swollen and elongated cell types with the tendency to aggregate (Fig. 4B, Fig. S3). However, neither septum morphology nor cell wall thickness was affected in the ΔgpsB strain as determined by transmission electron microscopy of ultra-thin sections (Fig. S4). By contrast, the ΔgpsB mutant and the inducible gpsB mutant strain LMS56 had increased penicillin sensitivities in comparison to the wild type in the absence of IPTG (Fig. 4C). Penicillin exposure did not simply inhibit growth of the ΔgpsB strains but actually induced bacteriolysis in quantitative autolysis experiments (Fig. 4D). Here penicillin was used to block peptidoglycan biosynthesis so that endogenous autolysis could be measured. Even in the absence of penicillin, the ΔgpsB mutant is more prone to lysis than the wild type, and addition of penicillin increased this effect about twofold. By contrast, penicillin did not induce bacteriolysis of wild-type cells at all (Fig. S5). These observations indicate that the cell wall is somehow weakened in the ΔgpsB mutant, and consistent with this, a twofold increase in sensitivity against the cell wall hydrolase mutanolysin was observed for ΔgpsB cells (Fig. 4E).

The L. monocytogenes ΔgpsB mutant is prone to lysis.
A. Phase contrast images of L. monocytogenes EGD-e (wt) and strain LMJR19 (ΔgpsB). Samples were taken from mid-log cultures, which had been grown in BHI broth at 37°C. Scale bar is 5 μm. Arrows point to lysed phase bright cells.
B. Scanning electron micrographs showing cell morphology of the same set of strains cultivated under the same conditions at a 20 000-fold magnification. Scale bar is 2 μm. Images at a higher magnification are given in Fig. S3.
C. Penicillin susceptibility of L. monocytogenes gpsB mutant strains. L. monocytogenes strains EGD-e (wt), LMJR19, (ΔgpsB) and LMS56 (IgpsB) were used to inoculate BHI plates (containing 1 mM IPTG where indicated) and penicillin G high MIC test strips (0.016–256 μg ml−1, Bestbiondx, Germany) were laid on top of the agar surface afterwards. Zones of growth inhibition became visible after one night of incubation at 37°C. Minimum inhibitory concentrations (in μg ml−1) are given at the bottom of each panel (D and E) Autolysis assay of L. monocytogenes gpsB mutant strains. The same set of strains as in panel C was grown in BHI broth (± 1 mM IPTG) at 37°C to mid-log growth phase (OD600∼0.8). Cells were washed and resuspended in 50 mM Tris.HCl pH 8.0 and penicillin (D) or mutanolysin (E) was added at concentrations of 25 μg ml−1 and 10 U ml−1 respectively. Decrease of optical density (λ = 600 nm) is expressed as relative values. Average values and standard deviations are calculated from experiments performed in triplicate.
Synthetic division defect of a gpsB divIVA double mutant
The similarity in sequence of their N-terminal domains, and their potential for functional redundancy, led us to construct a ΔgpsB ΔdivIVA double mutant (strain LMJR28) to test whether this combination would result in a stronger phenotype than for either single deletion. A slight increase in cell lengths of the ΔgpsB and ΔdivIVA single mutants in comparison with the wild type was observed (Table S1); however, average cell length was increased more than twofold in the ΔgpsB ΔdivIVA strain in comparison with the wild type (Fig. 5A and B, Table S1). Cell lengths of the ΔgpsB ΔdivIVA double mutant showed a pronounced heterogeneity and reached values close to 10 μm (Fig. 5B). The cell width of the ΔgpsB ΔdivIVA double mutant was the thinnest, whereas the width of both single mutants was close to wild type (Table S1). The synergism of the ΔdivIVA and ΔgpsB phenotypes therefore suggests that these proteins have redundant and partially overlapping cell division functions.
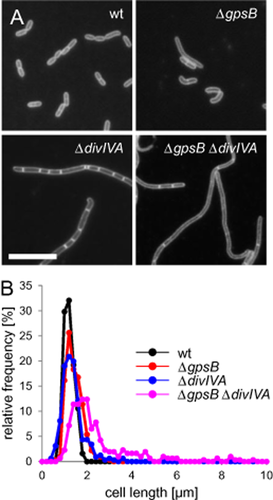
Phenotype of a L. monocytogenes ΔgpsB ΔdivIVA double mutant.
A. Fluorescence micrographs showing Nile red stained cells of L. monocytogenes strains EGD-e (wt), LMJR19 (ΔgpsB), LMS2 (ΔdivIVA) and LMJR28 (ΔgpsB ΔdivIVA). Cells were taken from cultures that were grown in BHI broth at 37°C until mid-logarithmic growth phase. Scale bar is 5 μm.
B. Frequency plot illustrating the distribution of cell lengths of the same set of strains under identical conditions. Approximately 300 cells were measured per strain.
GpsB controls PBP A1 and is essential for functional PBP A1 activity
GpsB is associated with the regulation of PBP1 in B. subtilis (Claessen et al., 2008). In order to test which high molecular weight (HMW) PBP is affected in the absence of GpsB, all five L. monocytogenes HMW PBPs (Rismondo et al., 2015) were introduced as a second IPTG-inducible copy in the ΔgpsB mutant. When the resulting strains were grown on BHI + IPTG plates, overexpression of LmPBP A1 specifically impaired the growth of the ΔgpsB strain (Fig. 6A). This effect was confirmed in liquid culture and was not observed when LmPBP A1 was overexpressed in wild-type cells (Fig. 6B). IPTG-dependent overexpression of LmPBP A1 in both backgrounds was confirmed by SDS-PAGE and subsequent PBP staining using bocillin-fl (Fig. 6C) but did not cause any additional morphological aberrations in the ΔgpsB mutant strain LMJR33 (data not shown). Thus, growth of the ΔgpsB mutant is specifically sensitive to increased LmPBP A1 levels, perhaps because LmGpsB acts as a negative regulator of LmPBP A1 activity.
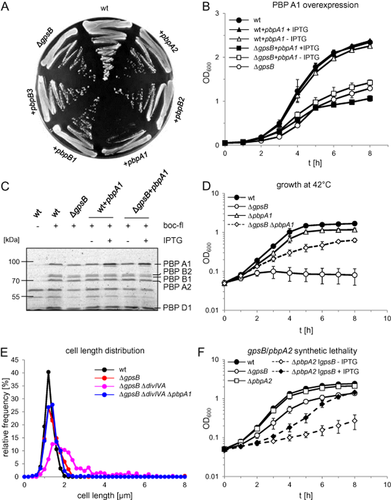
L. monocytogenes GpsB controls function of PBP A1.
A. Overexpression of all HMW PBPs in the L. monocytogenes ΔgpsB background. Strains EGD-e (wt), LMJR19 (ΔgpsB), LMJR31 (ΔgpsB + pbpB3), LMJR32 (ΔgpsB + pbpB1), LMJR33 (ΔgpsB + pbpA1), LMJR34 (ΔgpsB + pbpB2) and LMJR35 (ΔgpsB + pbpA2) were streaked on BHI agar plates containing 1 mM IPTG and grown over night at 37°C.
B. Effect of PBP A1 overexpression on growth of the ΔgpsB mutant in liquid culture. Strains EGD-e (wt), LMJR19 (ΔgpsB), LMJR33 (ΔgpsB + pbpA1) and LMJR39 (wt + pbpA1) were grown in BHI broth ± 1 mM IPTG at 37°C. For better visibility of the effect, growth curves are presented on a linear axis.
C. SDS PAGE showing IPTG-dependent overexpression of PBP A1 in strains LMJR39 (wt + pbpA1) and LMJR33 (ΔgpsB + pbpA1). Strains were grown under the same conditions, and membrane fractions were isolated. PBPs were stained with bocillin-fl and samples were separated by SDS-PAGE (using 8% polyacrylamide gels).
D. Effect of pbpA1 deletion on growth of the ΔgpsB mutant at 42°C. Strains EGD-e (wt), LMJR19 (ΔgpsB), LMS57 (ΔpbpA1) and LMJR38 (ΔgpsB ΔpbpA1) were grown in BHI broth at 42°C.
E. Suppression of the cell division defect of the ΔdivIVA ΔgpsB double mutant by pbpA1 deletion. Cell length distributions of strains EGD-e (wt), LMJR19 (ΔgpsB), LMJR28 (ΔgpsB ΔdivIVA) and LMJR42 (ΔgpsB ΔdivIVA ΔpbpA1) measured during exponential growth in BHI broth at 37°C. Lengths of ∼ 300 cells per strain were measured.
F. Synthetic lethality of gpsB with pbpA2 as demonstrated by IPTG-dependence of the GpsB depletion strain LMJR108, which also lacks PBP A2. Strains EGD-e (wt), LMJR19 (ΔgpsB), LMS64 (ΔpbpA2) and LMJR108 (ΔpbpA2 IgpsB) were cultivated in BHI broth ± 1 mM IPTG at 37°C. Strain LMJR108 was pre-grown in an overnight culture containing IPTG, washed and used to inoculate a depletion culture not containing IPTG. After 1 day of growth, aliquots from such depletion cultures were used to start LMJR108 cultures ± IPTG as shown in this diagram. All growth curves show average values and standard deviations calculated from three independent repetitions.
If LmPBP A1 activity is disregulated in the ΔgpsB mutant, then deletion of pbpA1 should rescue the ΔgpsB phenotype. In fact, a ΔgpsB ΔpbpA1 double mutant (LMJR38) could grow at 42°C, in contrast to the ΔgpsB strain (Fig. 6D). However, the suppression of the ΔgpsB growth defect was not complete (Fig. 6D), indicating that other factors contributed to the ΔgpsB phenotype. Deletion of pbpA1 in the ΔgpsB ΔdivIVA double mutant background also corrected the cell division defect of this strain back to the level of the ΔgpsB single mutant (Fig. 6E), therefore, the ΔgpsB ΔdivIVA division defect is mediated through LmPBP A1. We could not find any differences in GFP-LmPBP A1 localisation patterns between wild type and a ΔgpsB strain (Fig. S6A). In both strains, GFP-LmPBP A1 was present in bright bands at midcell and caused fainter signals at the cell periphery. Fluorescent vancomycin staining did not reveal significant differences between wild type and the ΔgpsB mutant (Fig. S6B), and LmPBP A1 expression levels were similar in both strains (Fig. 6C). Taken together, these data suggest that LmGpsB neither affects localisation nor synthesis of LmPBP A1.
Simultaneous inactivation of the two bi-functional PBPs, LmPBP A1 and LmPBP A2, is not tolerated by L. monocytogenes (Rismondo et al., 2015), providing a means to determine if LmGpsB is required for LmPBP A1 activity. Despite repeated attempts, we were unable to generate a ΔgpsB mutant that also lacked pbpA2, indicating synthetic lethality of both genes. Therefore, a GpsB depletion strain was constructed that also contained a deletion in pbpA2 (strain LMJR108). LMJR108 cells pre-depleted for LmGpsB could grow in the presence of IPTG, but growth was strongly retarded in the absence of the inducer (Fig. 6F). Apparently, LmPBP A2 becomes essential in the absence of LmGpsB, suggesting that LmPBP A1 is non-functional in the ΔgpsB mutant. We assume that the negative impact of GpsB on PBP A1, which we have postulated above, is a prerequisite for correct PBP A1 function.
The interaction of GpsB with PBP A1
Full-length LmGpsB interacted strongly with full-length LmPBP A1 in a bacterial two-hybrid experiment (Fig. 7A). Removal of the C-terminal domain of LmGpsB did not impair LmPBP A1 binding, whereas removal of the N-terminal domain, or introducing a mutation preventing dimerisation of the N-terminal domain (V32A), abrogated binding completely. By contrast, L24A and R25A mutations had no effect (Fig. 7A).
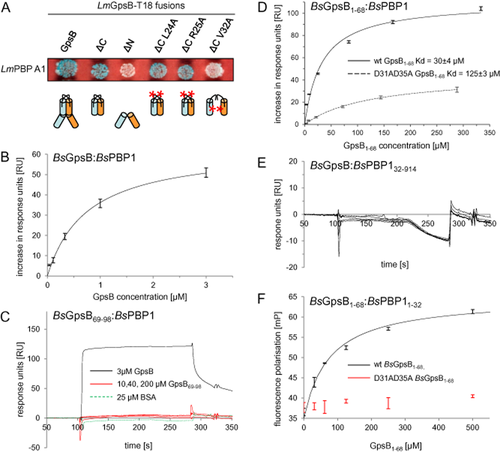
GpsB interacts with PBP A1.
A. Bacterial two-hybrid assay showing the interaction of LmGpsB variants with LmPBP A1. Schematic illustrations of the GpsB variants used are given below. B–E. Surface plasmon resonance of the BsGpsB:BsPBP1 interaction. (B) Binding of full-length BsGpsB fit according to a 1:1 interaction model, gives a Kd of 0.7 ± 0.1 μM. Note a narrow protein concentration range is used for the fit because the fit to a 1:1 interaction model deteriorates on including binding data measured at higher protein concentrations, which may be due to multiple modes of binding of the multivalent BsGpsB hexamer to immobilised BsPBP1 molecules. (C) Raw sensorgrams for full-length BsGpsB (black) and BsGpsB69–98 (red) injected over an SPR chip onto which full-length BsPBP1 had been immobilised. Note that no binding of BSA (green dashes) was observed to the same BsPBP1 SPR chip. (D) SPR analysis of the interaction of BsGpsB1–68 with a BsPBP1-coated chip. The change in response units at various injected concentrations of GpsB1–68 proteins are shown for both the wild type (solid line) and the D31A/D35A mutant (dashed line). Fitting these data to a 1:1 binding model gives a Kd of 30 ± 4 μM for the wild type and 125 ± 3 μM for the D31A/D35A mutant. (E) BsGpsB did not bind demonstrably to SPR chips onto which BsPBP132–914 had been immobilised.
F. Fluorescence polarisation analysis of the interaction of BsGpsB1–68 (black) with the cytoplasmic domain (residues 1–32, C-terminally labelled with fluorescein) of BsPBP1. Fitting the increase in fluorescence polarisation to a 1:1 binding model gives a Kd of 90 ± 10 μM for the interaction with wild type BsGpsB1–68. By contrast, the D31A/D35A mutant (red) does not increase the fluorescence polarisation of the PBP11–32 peptide appreciably even at a concentration of 0.5 mM. Error bars correspond to the standard error from three measurements.
We then used surface plasmon resonance (SPR) to probe the interaction between BsGpsB in solution and BsPBP1 (the orthologue of LmPBP A1) that had been immobilised to the SPR chip surface. Full-length BsGpsB bound with rapid kinetics to full-length BsPBP1 and the binding data could be fitted to a 1:1 model with a Kd of 0.7 ± 0.1 μM (Fig. 7B). By contrast, no binding of BsGpsB69–98 to the chip surface was seen even when the injected protein was at a concentration of 200 μM (Fig. 7C). A Kd of 30 ± 4 μM was obtained for the binding of BsGpsB1–68 to immobilised full-length BsPBP1 (Fig. 7D). The > 40-fold weaker binding of BsGpsB1–68 to BsPBP1 might be a function of the different oligomerisation properties of BsGpsB proteins: full-length, hexameric BsGpsB has the capacity to interact with up to six BsPBP1 molecules on an SPR chip, whereas the truncated N-terminal domain of BsGpsB can only bind to two. Finally, a construct that lacked the entire cytoplasmic domain of BsPBP1 (BsPBP132–914) was immobilised onto an SPR chip surface. Demonstrable binding of BsGpsB to the truncated BsPBP1 protein was not observed (Fig. 7E). The removal of the cytoplasmic domain (amino acids 1–31) from LmPBP A1 also caused a ΔpbpA1-like elongated phenotype in L. monocytogenes (Fig. S7A–B), confirming that the interaction of LmPBP A1 with LmGpsB is critical for LmPBP A1 function. These observations – using different experimental procedures and proteins of different species – demonstrate conclusively that the interaction between GspB and the major bi-functional HMW PBP is limited to the small cytosolic N-terminal domain of the PBP.
Crystal structure of the N-terminal domain of GpsB
In order to understand the molecular basis of the GpsB:PBP interaction, the crystal structure of LmGpsB1–73 was solved (Fig. 8A, Table S2). Consistent with the SEC-MALLS data, LmGpsB1–73 in the crystals is a dimer formed by two identical subunits, each of which contains two α-helices spanning residues G12 to E17 and P29 to N66. The helices assemble into a four-helical bundle, the core of which buries hydrophobic amino acids from helices 1 (I15) and 2 (V32, F35, L36, V39, I40, Y43), and L10 from the coil immediately preceding helix 1 (Fig. 8A). A mutation in the hydrophobic core (V32A) caused almost complete loss of self-interaction in a two-hybrid analysis (Fig. 1C). Further hydrophobic amino acids of a near-perfect heptad sequence of a coiled-coil – F46, I50, L53, L60 and L64 – complete a buried hydrophobic stripe (Fig. 8A). The GSH tripeptide remnant of the thrombin-cleaved His6-tag and the first nine LmGpsB residues splay away from the main body of the protein and few contacts are made between the main body of the protein and the first nine residues (Fig. 8A). The ordering of this part of structure, despite the paucity of contacts to the main body of the protein, is explained by the crystal lattice (Fig. S8A).
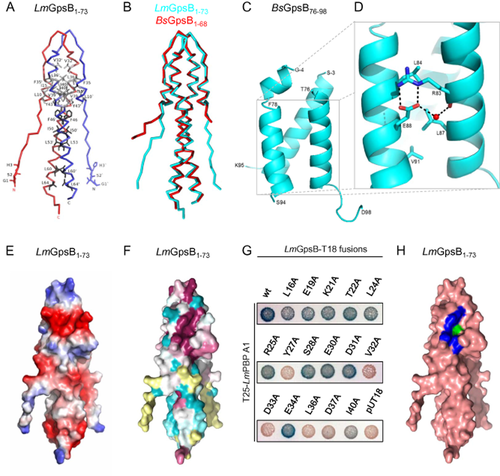
Structures of the GpsB N- and C-terminal domain.
A. Cα trace of LmGpsB1–73 with each molecule in the dimer coloured independently; the positions of the residues that stabilise the four helix bundle of the dimer are highlighted in grey. Additional hydrophobic amino acids that are part of the coiled-coil heptad motif are labelled in black. All panels show the same perspective of LmGpsB1–73.
B. Cα trace of superimposed structures of LmGpsB1–73 (cyan) and BsGpsB1–68 (red).
C. Ribbon diagram of a representative trimer of BsGpsB76–98; terminal amino acids are labelled.
D. Key interactions characteristic of parallel three stranded coiled coils are found in the BsGpsB76–98 trimer; the role of residues drawn in stick representation are described in the text. R83 and E88 in the BsGpsB76–98 correspond to R96 and E101 in L. monocytogenes GpsB (Fig. S1A).
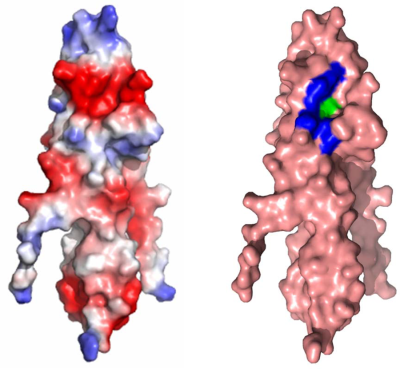
E. Surface electrostatic potential of LmGpsB1–73, with negative charge coloured red and positive charge blue.
F. Surface representation of sequence conservation; the darkest purple shade maps to the most conserved residues in a GpsB alignment and the darkest shade of aquamarine maps to the least conserved. Yellow residues cannot be assigned a reliable conservation score as they only align with a few sequences in the overall alignment.
G. Alanine scanning mutagenesis of LmGpsB to identify residues important for LmPBP A1 binding by bacterial two-hybrid. (H) Surface representation of LmGpsB1–73 in salmon, where blue and green patches represent the residues important for PBP A1 binding (Y27, D33, D37, I40) or self-interaction (V32, L36) respectively.
The structure of BsGpsB1–68 was subsequently also solved (Fig. 8B, Table S2). The two BsGpsB1–68 dimers in the crystallographic asymmetric unit are essentially identical to each other, with monomer:monomer rmsds of 0.65 ± 0.05 Å, which are comparable with the rmsds between BsGpsB1–68 and LmGpsB1–73 monomers of 0.64 ± 0.14 Å. The largest variations in structure between the orthologues are found in the flexible N-terminal regions (Fig. 8B), which participate in crystal contacts in both structures (Fig. S8A–C).
Given that the structure of DivIVA1–65 was used to solve the structure of LmGpsB1–73, it is not surprising that the structures are highly similar: the rmsds in 48 matched Cα atoms between LmGpsB1–73 and DivIVA1–65 monomers range between 0.50 and 1.12 Å, and the 96 matched Cα atoms in the dimers yields an rmsd of 0.96 Å. The two GpsB N-terminal domain structures reported here will serve as models for all members of the GpsB family, since the sequence insertions observed in some orthologues (Fig. 1A) are only of 3 and 4 amino acids in length, corresponding to single turns of an α-helix.
Crystal structure of the C-terminal domain of GpsB
The structure of BsGpsB residues 76–98 (equivalent to LmGpsB residues 89–111) was also solved by X-ray crystallography. Nine copies of the BsGpsB76–98 peptide are present in the asymmetric unit of the crystal lattice, which assemble into three stable trimers based on PDB-PISA (Krissinel and Henrick, 2007) analysis of the intermolecular interfaces. The three trimers superimpose on each other with rmsds of between 0.6 and 0.7 Å on matched Cαs. The trimer is a simple, triple-stranded parallel coiled-coil (Fig. 8C) that includes the highly conserved heptad repeat sequence between K82 and V91. The helical section encompasses residues F78 to F92, and the peptides up- and down-stream respectively, tends to lack regular secondary structure, show variation between chains, and in some chains are completely disordered.
There are over 300 PDB entries (representing ∼ 90 non-redundant structures) that contain homotrimeric, parallel coiled-coils (Testa et al., 2009), the majority of which are proteins that protrude from the surface of the threefold axes of the capsids of viruses and bacteriophages. The core helical region of the trimer can be superimposed on a representative three-stranded parallel coiled-coil [a mutant variant of the leucine-zipper GCN4, PDB code 1GCM (Harbury et al., 1994)] with an rmsd of 1.5 Å on 51 matched Cαs, equivalent to 70% of the sequence of BsGpsB76–98 (Fig. S9A). A conserved RhxxhE sequence motif (h = hydrophobic residue) between residues R83 and E88 of BsGpsB has been described previously in many three-stranded parallel coiled-coils (Kammerer et al., 2005), and the characteristic features of this motif are retained in BsGpsB76–98 (Fig. 8D). First, within a BsGpsB76–98 trimer, there are three bifurcated interhelical salt bridges between R83 and E88 residues; second, an ordered water molecule is observed between the side-chain Oε2 of E88 and the mainchain O of R83; third, the aliphatic portions of R83 and E88 pack against the buried hydrophobic residues (L84, L87 and V91) in the heptad repeat sequence; fourth, the leucines at positions 2 and 5 of the RhxxhE motif (L84 and L87) are the second-most and the most frequently observed hydrophobic amino acids at these locations in the motif, to optimise the packing between helices. These characteristics aid to stabilise the trimer.
None of the arrangements between the trimers in the crystal packing are calculated to be stable by PDB-PISA, consistent with the isolated domain being trimeric in solution (Fig. 1F). However, in the context of the hexameric full-length GpsB, the dimerisation of the N-terminal domains probably provides an additional driving force promoting the association of the C-terminal domain trimers. The formation of the hexamer might therefore require the highly conserved hydrophobic amino acids F78, L81 and F92, which are solvent exposed in isolated trimers but are buried in the crystal lattice (Fig. S9B).
Mutational analysis of structurally relevant GpsB residues
A notable feature of both structures of the N-terminal domain of GpsB is an elliptical-shaped surface cavity, some 7 Å wide, 12 Å long and 5 Å deep. In LmGpsB1–73, the cavity is formed by a series of conserved, mostly negatively charged amino acids including L16, E19, K21, T22, Y27, S28, E30, D31, D33, E34, L36, D37 and I40 (Fig. 8E and F). This structure could drive the interaction with the positively charged amino acids in the cytoplasmic domain of the corresponding PBP. Bacterial two-hybrid revealed that LmGpsB residues Y27, V32, D33, L36, D37 and I40 were essential for the interaction with PBP A1 (Fig. 8G). V32 and L36 were also critical for dimerisation of the N-terminal domain (Fig. 1C and data not shown). Residues Y27, D33, D37 and I40 form a single, contiguous surface patch at the edge of the cavity (Fig. 8H), whereas the base of the cavity is formed predominantly by L36, which thus plays dual roles in stabilising the dimer and in PBP A1 binding.
The effect of gpsB mutations affecting dimerisation (V32A, L36A) and LmPBP A1 binding (Y27A, D33A, D37A, I40A) was analysed in a complementation assay testing restoration of growth of the ΔgpsB mutant at 42°C (Fig. 9A). All mutated LmGpsB variants were expressed as shown by Western blotting (Fig. 9B). Mutations in the dimerisation interface (V32A, L36A) were inactive (Fig. 9A). Alleles with mutations in the LmPBP A1 binding site fell into two classes: D37A and I40A showed intermediate phenotypes, whereas D33A and Y27A were completely inactive (Fig. 9A). D37 and I40 are located on the upper part of the surface cleft, whereas Y27 and D33, and V32 and L36 are oriented towards the bottom or the base of the depression respectively (Figs 8H and 9C). Y27 and D33 were unaffected in self-interaction in bacterial two-hybrid, whereas V32 and L36 impaired GpsB self-interaction (Fig. 1C and data not shown).
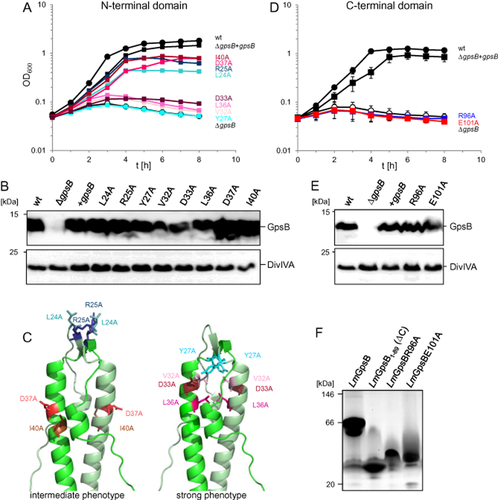
Mutational analysis of structurally relevant amino acids in LmGpsB.
A. Growth of strains LMJR68 (L24A), LMJR4 (R25A), LMJR130 (Y27A), LMJR131 (V32A), LMJR132 (L36A), LMJR133 (D37A), LMJR134 (I40A) and LMJR135 (D33A) in BHI broth containing 1 mM IPTG at 42°C. All strains were ΔgpsB mutants ectopically expressing GpsB mutant proteins with amino acid exchanges in the N-terminal domain of GpsB at positions required for lipid binding (L24, R25), dimerisation (V32, L36) or PBP A1 binding (Y27, D33, D37, I40). Strains EGD-e (wt), LMJR19 (ΔgpsB), LMS56 (ΔgpsB + gpsB) were included as controls. For better clarity, a representative growth curve is shown.
B. Western blots showing GpsB expression in the same set of strains during growth at 37°C. Detection of DivIVA was performed as a loading control.
C. Mapping of the same mutations onto the LmGpsB1–73 crystal structure.
D. Complementation assay comparing growth of strains EGD-e (wt), LMJR19 (ΔgpsB), LMS56 (ΔgpsB + gpsB), LMJR163 (R96A) and LMJR164 (E101A) in BHI broth containing 1 mM IPTG at 42°C. Growth curves show average values and standard deviations calculated from three independent repetitions.
E. Western blot analyzing levels of GpsB proteins in the same set of strains during growth at 37°C; again, DivIVA was used as a control.
F. Blue native PAGE of full-length LmGpsB-Strep and R96A and E101A variants thereof. LmGpsB1–89-Strep lacking the C-terminal domain was included as a negative control.
SPR of the BsGpsB1–68D31A/D35A (corresponding to D33A and D37A in LmGpsB) double mutants against immobilised BsPBP1 both yielded Kds more than fourfold higher than that of the wild-type interaction (Fig. 7D). Furthermore, the D31A/D35A mutation completely abrogated binding of BsGpsB1–68 to a peptide encompassing the cytoplasmic domain of BsPBP1. BsGpsB1–68 bound to a fluorescein-labelled BsPBP11–32 (BsPBP1 residues 1–32) peptide with a dissociation constant of 90 ± 10 μM, measured by fluorescence polarisation. By contrast, the BsGpsB1–68 D31A/D35A mutant did not bind the same peptide even at 500 μM protein concentration (Fig. 7F). By circular dichroism, the D31A/D35A mutation had no discernible effect on the folding of BsGpsB1–68: thermal denaturation curves of the wild type and D31A/D35A BsGpsB1–68 proteins were identical (Fig. S10). The effect of the mutation on the GpsB:PBP interaction is thus not an indirect consequence of destabilising the fold of the mutated GpsB proteins, but primarily due to the loss of specific contacts between the interacting partners. Furthermore, the details of the GpsB–PBP1 interaction identified here are perhaps mimicked by the crystal contacts in the structure of BsGpsB1–68, in which the His6-tag remnant from one BsGpsB1–68 molecule interacts with residues in another BsGpsB1–68 molecule that are essential for the GpsB:PBP interaction (Fig. S8D).
Finally, the structure of BsGpsB76–98 revealed that residues R83 and E88, equivalent to R96 and E101 in LmGpsB, are involved in a network of salt bridges between chains that stabilise the trimeric form of the C-terminal domain (Fig. 8D). Consistent with this key structural role, R96A and E101A gpsB mutant alleles were unable to complement the growth defect of the ΔgpsB mutant at 42°C (Fig. 9D and E). Purified Strep-tagged versions of the R96A and E101A GpsB proteins also ran aberrantly on blue native PAGE gels (Fig. 9F). R96A and E101A are thus critical for the formation of GpsB hexamers, an absolute requirement for GpsB function.
Attenuation of the ΔgpsB mutant in various infection models
The impact of GpsB on the virulence of L. monocytogenes was tested in infection experiments using J774 mouse ascites macrophages at 37°C. This temperature is required for induction of virulence gene expression through the master regulator PrfA, whereas PrfA-dependent virulence genes are silent at 30°C (Johansson et al., 2002). GpsB does not influence phagocytosis by macrophages (t = 0 in Fig. 10A). However, intracellular multiplication of the ΔgpsB mutant lagged more than fourfold behind that of the wild type (t = 6 in Fig. 10A). Cell-to-cell spread of the wild type and the ΔgpsB mutant were compared in a plaque formation assay using 3T3 mouse embryo fibroblasts. Monolayers of fibroblasts were infected and formation of plaques corresponding to zones of killed host cells was visualised on the third day post infection by counterstaining with neutral red. A marginal reduction of plaque size formed by ΔgpsB mutant cells was observed (Fig. 10B). Apparently, the inherent growth defect of the ΔgpsB mutant cannot be complemented by host cell factors and rather leads to reduced intracellular proliferation rates.
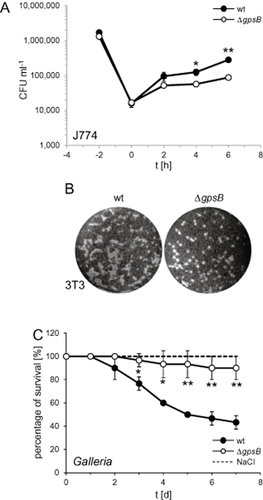
Role of GpsB in virulence.
A. L. monocytogenes strains EGD-e (wt) and the ΔgpsB mutant (LMJR19) were used to infect monolayers of J774.A1 mouse ascites macrophages. Sampling for monitoring of intracellular growth was performed in 2 h intervals. The experiment was performed in triplicate, and standard deviations are shown (but too small to be seen in some cases). Significance levels (t-test) are indicated by asterisks (*P < 0.05; **P < 0.005).
B. Plaque formation assay to analyse cell-to-cell spread. L. monocytogenes strains EGD-e (wt) and LMJR19 (ΔgpsB) were used to infect a confluent layer of 3T3 mouse embryo fibroblasts. Plaques indicate zones of host cell killing and are visualised 3 days post infection by counterstaining with neutral red.
C. Survival curves of Galleria mellonella larvae infected with L. monocytogenes strains EGD-e (wt) and LMJR19 (ΔgpsB). Mock infections using NaCl solution were included as negative control. Results represent average values of three independent experiments with a total of 10 larvae per treatment. Significance levels are labelled as described above.
Further infection experiments were performed using larvae of the wax moth Galleria mellonella as host (Mukherjee et al., 2010). Only 43.3 ± 5.8% of the larvae infected with the wild-type strain EGD-e were still viable 7 days post infection, whereas 90 ± 10% of the larvae infected with the ΔgpsB mutant survived the infection until the seventh day of the experiment (Fig. 10C). Remarkably, this degree of attenuation is similar to that of a mutant lacking the pathogenicity island LIPI-1, which encodes six of the major L. monocytogenes virulence factors prfA, plcA, hly, mpl, actA and plcB and caused survival rates of ∼ 90% in an identical experiment (Mukherjee et al., 2010). Taken together, these results demonstrate that GpsB is required for full pathogenicity of L. monocytogenes.
Discussion
GpsB and its relative, DivIVA, are essential for growth and cell division in diverse Gram-positive bacteria (Fadda et al., 2003; Ramirez-Arcos et al., 2005; Nguyen et al., 2007; Letek et al., 2008; Halbedel et al., 2012; Land et al., 2013). Here we show that GpsB contributes to growth of L. monocytogenes through an interaction with PBP A1 and that this has consequences for virulence of this important human pathogen.
The cellular localisation of GpsB is dynamic and dependent on cell cycle progression. In B. subtilis, septal GpsB localisation depends on early (FtsZ, FtsA) and late cell division proteins (PbpB, DivIC) (Tavares et al., 2008). DivIVA, which assembles at the division site only after constriction has started (Eswaramoorthy et al., 2011), is not required for the septal localisation of GpsB (Tavares et al., 2008; Fleurie et al., 2014). Thus, the septal localisation of GpsB depends only on those divisome components that are essential for Z-ring assembly and constriction. How GpsB is recruited to the septum is unknown. GpsB might interact with a component of the divisome, but also could be a second example of a curvature-sensitive membrane-binding protein with the intrinsic capability to cluster at curved membrane regions.
That the gpsB phenotype can be suppressed by deletion of pbpA1 suggests that LmPBP A1 activity becomes uncontrolled in the absence of LmGpsB, which may act as a negative regulator of the PBP. The genetic relationships identified here are reminiscent of the situation in B. subtilis, where deletion of ponA (encoding PBP1) rescues the lytic phenotype of a gpsB ezrA double mutant (Claessen et al., 2008). Moreover, the second bi-functional PBP (LmPBP A2) becomes essential in the ΔgpsB mutant. Because at least one of the two bi-functional PBPs (A1 or A2) is required for growth of L. monocytogenes (Rismondo et al., 2015), this indicates that LmGpsB is in fact required for proper LmPBP A1 function. As the localisation pattern of LmPBP A1 was LmGpsB-independent, LmGpsB is not a targeting determinant for LmPBP A1.
More likely, the role of LmGpsB is to link LmPBP A1 with other divisome components and/or to link together individual LmPBP A1 molecules as an important prerequisite for efficient biosynthesis of peptidoglycan. The linking of individual LmPBP A1 molecules could affect wall synthesis by spatially co-ordinating PG synthesis activities within the divisome. Tight co-ordination of PG strand synthesis by transglycosylases and strand–strand cross-linking by transpeptidases has been found to be essential in molecular dynamics simulations of cell wall synthesis; in the absence of such co-ordination, the integrity of the network of PG strands in the elongating cell wall breaks down (Nguyen et al., 2015). The linkage of two PBP A1 molecules by GpsB could facilitate spatial co-ordination of PG strand synthesis and PG cross-linking given that the bi-functional PBP A1 has both transpeptidase and transglycosylase activities. In addition, other HMW bi-functional PBPs are known to dimerise, and dimerisation is important for producing nascent PG in vitro with the same degree of peptide cross-linking as seen in vivo (Bertsche et al., 2005). A function of GpsB could thus be to promote dimerisation of its cognate PBP to stimulate the associated transpeptidase activity. Overall, the observed negative genetic effect that GpsB exerts on PBP A1 could be due, therefore, to GpsB mediating a spatial restriction of PBP A1 activity within the divisome. While this GpsB function may not be essential for viability, as long as the second bi-functional PBP (PBP A2) is present, it clearly becomes essential in its absence.
The sequestration of PBP A1 in complex with GpsB would be lost by deletion of either protein, or by mutation of critical interfacing residues. Indeed, in both B. subtilis and in L. monocytogenes, mutation of conserved residues of the negatively charged surface pocket in the N-terminal domain of GpsB resulted in a gpsB null phenotype in vivo and reduced binding constants for the BsGpsB:BsPBP1 interaction in vitro. This binding motif is better conserved among GpsB orthologues than the equivalent residues in DivIVA proteins (Fig. S11), which implies that interaction partners of GpsB are conserved in the different organisms but are different from those of DivIVA. In fact GpsB interacts with PBPs while DivIVA is known to bind to the transmembrane protein MinJ (van Baarle et al., 2013), and interactions between DivIVA and PBPs have not yet been reported. However, the combination of ΔgpsB and ΔdivIVA alleles caused a synergistic deleterious effect on L. monocytogenes cell division. This suggests that both proteins are part of parallel pathways that share an overlapping function during cytokinesis and that they may share a common (but still to be identified) interaction partner. Remarkably, a fundamentally different situation is observed in S. pneumoniae; in this organism, deletion of divIVA cured the cell division defect of a ΔgpsB mutant that led to helical FtsZ rings unable to undergo productive division events (Fleurie et al., 2014). The reason for this diversity is not yet clear, but it could be the consequence of general differences in the interplay of cell growth and cell division between rod-shaped and coccoid bacteria.
GpsB must control processes beyond PBP A1-dependent peptidoglycan biosynthesis in L. monocytogenes, as indicated by the following genetic data: (i) deletion of pbpA1 only partially suppressed the ΔgpsB growth defect at 42°C; (ii) growth of the ΔpbpA1 mutant is only marginally impaired at 42°C (Rismondo et al., 2015), whereas the ΔgpsB mutant cannot grow under this condition; and (iii) mutations in the PBP A1 binding site of GpsB cause a gpsB null phenotype, whereas deletion of the GpsB binding site in PBP A1 only causes an intermediate pbpA1 phenotype. We thus conclude that GpsB binds other, yet to be identified, proteins using an interaction surface that overlaps at the very least with that for binding PBPs. Furthermore, pbpA1 deletion completely cures the synergistic cell division defect of a ΔgpsB ΔdivIVA double mutant, which in turn, suggests that PBP A1 is the main effector of GpsB function with regard to cell division. Hence, GpsB interaction partners other than PBP A1 might be rather relevant for growth at elevated temperatures.
The N-terminal domains of GpsB and DivIVA are highly homologous in structure (Fig. S8E) and share some common functionalities; the N-terminal domains in both proteins are also necessary for the interaction with the membrane, and equivalent residues in the domains play essential roles in this association. However, their C-terminal domains are distinct; DivIVA is an anti-parallel tetramer (Oliva et al., 2010) formed by the close packing of a lengthy C-terminal coiled coil. By contrast, GpsB is hexameric and the C-terminal domain, although a coiled-coil, is a trimer, with a parallel alignment of helices. It follows that the building blocks of the hexamer are three N-terminal domain dimers and two C-terminal domain trimers. The hexamerisation of GpsB must be important for function in vivo as mutations to the R/E salt bridge that disrupt the trimerisation of the C-terminal domain have a gpsB null phenotype, an observation that is consistent with changes in oligomeric state (to n = 2, 4 and 8) when the R/E salt bridge is lost by mutation in other triple-stranded coiled-coils (Kammerer et al., 2005). Perhaps the most logical arrangement of GpsB subunits, which maintains an equivalent distance between covalently linked N and C terminal domains on all subunits, is a tripod-like arrangement (Fig. S9C). In this arrangement, the two C-terminal domain trimers form the base of the tripod and the three N-terminal domain trimers form the legs of the tripod. One such model might assume a relatively straight path between the covalently linked N- and C-terminal domains. However, there is typically at least one proline in the interdomain linker of GpsB proteins, and the linkers sequences display regions of obvious low complexity (Fig. 1A), suggesting that the subunits' dynamic arrangement specifically requires a linker that can follow a bent path.
Finally, inactivation of GpsB induced autolysis in response to penicillin, whereas penicillin did not induce bacteriolysis of ΔpbpA1 cells (Rismondo et al., 2015). We have also demonstrated that GpsB is required for full pathogenicity of L. monocytogenes in animal models. As β-lactam antibiotics generally act bacteriostatically on L. monocytogenes and do not induce bacteriolysis (Hof, 2004; Lemaire et al., 2005; Grayo et al., 2008), a combination of β-lactams and novel GpsB inhibitors could provide an efficient future therapy for the treatment of listeriosis.
Experimental procedures
Bacterial strains and growth conditions
All strains used in this study are listed in Table S3. L. monocytogenes was generally cultivated in BHI broth or on BHI agar plates at 37°C if not stated otherwise. Where required, antibiotics and supplements were added at the following concentrations: erythromycin (5 μg ml−1), kanamycin (50 μg ml−1), Xgal (100 μg ml−1) and IPTG (1 mM). E. coli TOP10 was used as standard cloning host (Sambrook et al., 1989).
General methods, manipulation of DNA and oligonucleotide primers
Transformation of E. coli and isolation of plasmid DNA was performed using standard methods (Sambrook et al., 1989). Preparation of electro-competent L. monocytogenes cells and transformation of L. monocytogenes were carried out as described elsewhere (Monk et al., 2008). Restriction and ligation of DNA was done as described by the manufacturer's instructions. For restriction-free modification of plasmids, an altered version of the original QuikChange mutagenesis protocol was employed (Zheng et al., 2004). All primer sequences are listed in Table S4. Penicillin G test strips (0.016–256 μg ml−1, Bestbiondx, Germany) were used for penicillin sensitivity assays. L. monocytogenes strains were grown in BHI broth and used to swab-inoculate BHI agar plates. Penicillin test strips were placed on top of the agar surface, and the plates were incubated at 37°C for 1 day.
Plasmid and strain construction, protein purification
Construction of plasmids and L. monocytogenes strains as well as all protocols for overproduction and purification of recombinant proteins can be found in the supplementary materials section.
Crystallisation and structure determination of GpsB proteins
Initial crystallisation conditions for LmGpsB1–73, BsGpsB1–68 and BsGpsB76–98 were obtained at 20°C by sitting-drop vapour diffusion; 100 nl of protein and screen solutions were pipetted by a Mosquito (TTP Labtech, Melbourn, UK). An initial crystallisation hit was obtained with LmGpsB1–73 at a concentration of 30 mg ml−1 in a buffer of 2 mM Tris.HCl pH 8.0, 10 mM NaCl versus condition D1 of the Morpheus screen (Molecular Dimensions), which contains a buffer of 0.1 M MES.NaOH, 0.1 M imidazole pH 6.5, 10% PEG 20K, 20% PEG 550 MME and 0.02 M mixture of alcohol additives. For LmGpsB1–73, the crystallisation conditions were optimised in 96 well MRC plates. After 1–2 weeks of growth, LmGpsB1–73 crystals were harvested and mounted in rayon fibre loops in the crystallisation well solution and flash frozen directly in liquid nitrogen prior to data collection using beamline IO4 of the Diamond light source at 100K and a wavelength of 0.9795 Å. The diffraction data were indexed and integrated in XDS (Kabsch, 2010) and scaled in SCALA (Evans, 2006). The LmGpsB1–73 structure was solved by molecular replacement in PHENIX (McCoy et al., 2007) using a search model prepared from the structure of the DivIVA lipid binding domain [PDBid 2WUJ (Oliva et al., 2010)] with CHAINSAW (Stein, 2008). This procedure correctly positioned two protein monomers in the asymmetric unit, consistent with a Matthews' coefficient of 2.08 Å3 Da−1 and a solvent content of 40.8%. The molecular replacement solution was subsequently refined with PHENIX-REFINE (Adams et al., 2010) and missing N- and C-terminal regions of the model were built automatically using ARP-WARP (Langer et al., 2008). Subsequent manual alterations to the atomic model were made in COOT (Emsley et al., 2010), interspersed with rounds of refinement in PHENIX.REFINE (Adams et al., 2010) until convergence was reached. In the Ramachandran plot of the final structure, 99.3% of residues are in the most favoured regions with no outliers.
For BsGpsB1–68, sitting-drop crystallisation screens were set up at a protein concentration of 10 mg ml−1 at 20°C. Crystals from well E9 of the INDEX (Hampton) screen (buffer conditions 0.05 M Bis-Tris.HCl pH 6.5, 0.05 M ammonium sulphate, 30% pentaerythyritol ethoxylate) were harvested in rayon fibre loops and frozen directly in liquid nitrogen. Diffraction data collected from Diamond synchrotron beamline IO4 at 100K and a wavelength of 0.9795 Å were also integrated with XDS (Kabsch, 2010) and scaled with SCALA (Evans, 2006). A search model derived from the co-ordinates of LmGpsB1–73 using CHAINSAW (Stein, 2008) was used in molecular replacement in PHENIX (McCoy et al., 2007), positioning two BsGpsB1–68 dimers in the asymmetric unit, consistent with a Matthews' coefficient of 2.31 Å3 Da−1 and a solvent content of 46.7%. The missing N- and C-terminal regions of the model were automatically built onto the molecular replacement solution using BUCCANEER (Cowtan, 2006), and the model was manually rebuilt in COOT (Emsley et al., 2010) and refined in PHENIX.REFINE (Adams et al., 2010). In the Ramachandran plot of the final structure, 97.3% of residues are in the most favoured regions with no outliers.
BsGpsB76–98 was subjected to spare-matrix crystallisation screening at a concentration of 10 mg ml−1 in 10 mM HEPES.NaOH pH 7.5, 100 mM NaCl buffer and crystallised in several conditions of the PACT screen (Molecular Dimensions); the most consistent quality crystals were obtained in condition E1, which contains 0.2 M NaF and 20% PEG 3350. After optimisation of the precipitant concentration in 96 well MRC plates, the crystals were cryoprotected by step-wise transfer in rayon loops to a cryoprotectant solution containing 0.2 M NaF, 20% glycerol and 24% PEG 3350; the first step involved a transfer to a mixture of 50% well solution and 50% cryoprotectant for 10–30 s, followed by transfer to 100% cryoprotectant for another 10–30 s before mounting in a rayon loop and plunging into liquid nitrogen. Diffraction data were recorded at Diamond beamline IO3 at 100K and a wavelength of 0.61992 Å; to maximise the completeness of the data from these triclinic crystals, three 180° data sets were collected from the same crystal at different crystal to detector distances. The individual datasets were integrated and scaled with the XDS (Kabsch, 2010) INTEGRATE and CORRECT modules, before merging all three datasets with AIMLESS (Evans and Murshudov, 2013). An initial polyalanine model was derived from the diffraction data using the ab initio phasing software ARCIMBOLDO (Sammito et al., 2013). The initial model was rebuilt in COOT (Emsley et al., 2010) against the electron density map calculated from the preliminary model phases, and the model was subsequently subjected to iterated cycles of refinement in PHENIX.REFINE (Adams et al., 2010) and modification in COOT (Emsley et al., 2010). The diffraction data used for model refinement were cut at a resolution of 1.2 Å, although the data signal to noise was still respectable at this resolution and the data completeness decreased rapidly at resolutions higher than 1.2 Å. In the Ramachandran plot of the final structure, 100% of residues are in the most favoured regions. Validation of all structures was performed in MolProbity (Chen et al., 2010), and summaries of the data collection and model refinement statistics are provided in Table S2.
Surface plasmon resonance
All SPR experiments used a Biacore X100 instrument. For the initial immobilisation of BsPBP1 proteins, the Biacore CM5 chip was equilibrated in a buffer of 10 mM HEPES.NaOH pH 7.5, 200 mM NaCl, 0.1% reduced Triton X-100 (immobilisation buffer) at a temperature of 25°C. Carboxyl groups on both flow cells on the chip surface were activated for amine coupling by the manufacturer's standard protocols and then 10 mg ml−1 ampicillin in 0.1 M sodium acetate pH 4.6 was injected over both flow cells at a flow rate of 10 μl min−1 for 9 min, followed by an identical injection of 1 M ethanolamine pH 8.0. The temperature of the chip surface was then adjusted to 35°C and BsPBP1 proteins were then immobilised onto flow cell 2 by several 50–120 s injections (flow rate 10 μl min−1) of 150 nM PBP1 (protein samples were incubated at 37°C for 4 min prior to injection onto the SPR) until 1400 response units of PBP1 had been immobilised. Titrations of BsGpsB over the BsPBP1 surface were undertaken at 25°C in a buffer of 10 mM Tris.HCl pH 8.0, 250 mM NaCl, 0.1% reduced Triton X-100 at a flow rate of 30 μL min−1. For all of the GpsB injections, the response signal on the reference, protein-free surface (coated only with ampicillin [flow cell 1]) was subtracted from the response signal on the PBP1-coated surface (flow cell 2). At the end of each injection of GpsB, the chip surfaces were regenerated by 15–20 s injection with 50 mM HEPES.NaOH pH 7.5, 1 M NaCl, 2% reduced Triton X-100. For the interaction of both full-length BsGpsB and BsGpsB1–68 with immobilised BsPBP1, the kinetics of binding were rapid, with binding reaching equilibrium within the timescale of an injection over a wide range of protein concentrations. The increase in response signal on the BsPBP1 coated surface during an injection could therefore be used as a measure of equilibrium binding to BsPBP1. The increase in response signal per injection was fitted versus injected protein concentration to a 1:1 model using the Biacore X-100 evaluation software. Each SPR titration was performed independently at least twice.
SEC-MALLS
For BsGpsB and BsGpsB1–68, 500 μl samples at concentrations of 1 mg ml−1 or 1.5 mg ml−1 were loaded onto Superdex200 or Superdex75 10/300 GL columns (GE Healthcare) respectively, equipped with a Jasco UV-2077 detector, Wyatt DAWN Heleos II EOS 18-angle laser photometer (with the 13th detector replaced with the QELS in-line dynamic light scattering detector) coupled to a Wyatt Optilab T-rEX refractive index detector. The flow rate was 0.75 ml min−1.
For BsGpsB69–98, a 100 μl sample at 10 mg ml−1 concentration was loaded on a Superdex 200 Increase 10/300 GL column (GE Healthcare) on an Akta Pure operating at a flow rate of 0.5 ml min−1, with column output fed into a DAWN Heleos II MALS detector with laser source at 664 nm and eight fixed angle detectors (Wyatt Technology, Santa Barbara, CA), followed by an Optilab T-rEX differential refractometer using 664 nm LED light source at 25°C (Wyatt Technology).
The running buffer for all SEC-MALLS analyses was 10 mM Tris.HCl pH 8.0 250 mM NaCl. In all cases the molecular mass and concentrations of the peaks eluting from the column were analysed using Astra 6.2 (Wyatt Technology, Santa Barbara, CA).
Circular dichroism
CD spectra were recorded on a JASCO J-810 spectropolarimeter interfaced to a PTC-423S Peltier temperature controller. GpsB proteins were at concentrations of 5 μM in a buffer of 25 mM sodium phosphate pH 7.3, 150 mM NaCl. CD data were collected using a 1 mm path length cuvette, at a wavelength of 222 nm, with a the response time of 8 s, a band width of 2 nm and the temperature was increased at a rate of 1°C per minute. To verify the reversibility of the temperature melts, full wavelength scans were recorded between 195 and 240 nm both before and after the melt; in all cases these CD spectra were superimposed on each other.
Fluorescence polarisation
Fluorescence polarisation was recorded in a PHERAstar FS (BMG Labtech) microplate reader using 386 well microplates. The excitation wavelength was 485 nm, and fluorescence emission was recorded above 520 nm. The focus and gain were adjusted based on a target polarisation of 35 mP in wells containing free fluorescein-labelled peptide. Each data point corresponds to a 20 μl sample with 40 nM labelled peptide in 10 mM Tris.HCl pH 8.0, 250 mM NaCl. Binding data were fit to obtain a dissociation constant in Sigma Plot with the equation P = Pmin + Pmax{[protein]/(Kd+[protein])} where P = polarisation, Pmin = polarisation of free peptide, Pmax = polarisation of peptide:protein complex, Kd = dissociation constant.
Blue native PAGE
NativePAGE Novex 4 to 16% Bis-Tris gels (Invitrogen) were used for separation of purified GpsB proteins by blue native PAGE. Gel runs were performed as specified by the instructions of the manufacturer. The NativeMark™ unstained protein standard (Novex) was used as a molecular weight marker.
Isolation of cellular proteins and Western blotting
Cells were harvested by centrifugation (13 000 r.p.m., 1 min in a table-top centrifuge) and washed once with ZAP buffer (10 mM Tris.HCl pH7.5, 200 mM NaCl). The cell pellet was resuspended in 1 ml ZAP buffer also containing 1 mM PMSF and disrupted by sonication. Cell debris was removed by centrifugation, and the resulting supernatant was considered as total cellular protein extract. In order to separate membrane proteins from the soluble cytosolic proteins, the total cellular protein fraction was ultracentrifuged at 100 000 g for 30 min at 4°C. The supernatant contained the soluble cytoplasmic proteins and the pellet corresponded to the membrane fraction, which was resuspended in 100 μL ZAP buffer. Aliquots of these samples were separated by standard SDS polyacrylamide gel electrophoresis (12% acrylamide), whereas for separation of GpsB, 15% acrylamide gels were used. Gels were either stained using the colloidal Coomassie agent Roti®-Blue (Roth, Germany) or transferred onto positively charged polyvinylidene fluoride (PVDF) membranes using a semi-dry transfer unit. Proteins of interest were immune-stained using polyclonal rabbit antisera recognising DivIVA (Marston et al., 1998), GFP (lab stock) or GpsB (this work) as the primary antibody and an anti-rabbit immunoglobulin G conjugated to horseradish peroxidase as the secondary one. The ECL chemiluminescence detection system (Thermo Scientific) was used for detection of the peroxidase conjugates on the PVDF membrane in a chemiluminescence imager (Vilber Lourmat).
PBP detection
In order to detect PBPs in SDS-PAGE gels, aliquots corresponding to 20 μg of membrane proteins were incubated with 3 μM bocillin-fl (Molecular Probes) for 20 min at 37°C. The binding reaction was stopped by addition of loading dye and incubation for 5 min at 65°C. Samples were separated by SDS-PAGE using 8% polyacrylamide gels, and PBPs were detected using a Fuji raytest FLA 2000 fluorescence scanner.
Bacterial two-hybrid analysis
GpsB self-interactions and interactions of GpsB with PBPs were studied by the bacterial two-hybrid system (Karimova et al., 1998). Plasmids encoding GpsB and PBPs fused to the T18 or the T25 fragment of the Bordetella pertussis adenylate cyclase were co-transformed in E. coli BTH101. Transformants were selected on nutrient agar plates containing ampicillin (100 μg ml−1), kanamycin (50 μg ml−1), Xgal (0.004%) and IPTG (0.1 mM). The plates were photographed after 40 h of growth at 30°C.
Autolysis assays
Listeria monocytogenes strains were grown in BHI broth (containing 1 mM IPTG where required) at 37°C until an optical density of around OD600 = 0.8 was reached. Cells were collected by centrifugation (6000 g, 5 min, 4°C) and resuspended in 50 mM Tris.HCl pH8.0 to an optical density of OD600 = 0.6. Penicillin (25 μg ml−1 final concentration) or mutanolysin (Sigma, 10 U ml−1) was added, and the cells were shaken at 37°C. Autolysis was followed by measuring the decrease in optical density (λ = 600 nm) every 30 min in a spectrophotometer.
Microscopy
Samples (0.4 μl) were taken from exponentially growing bacterial cultures and transferred onto microscope slides that had been covered with a thin agarose film (1.5% in distilled water). The sample droplets were air-dried, covered with a cover lid and analysed by phase contrast or fluorescence microscopy. Membranes were stained by addition of 1 μl of Nile red solution (100 μg ml−1 in DMSO) to 100 μl of culture and shaking for 10 min at 37°C, before the cells were subjected to microscopy. Zones of nascent cell wall biosynthesis were visualised by addition of 3 μl vancomycin-fl (8 mM, Molecular Probes) to 100 μl of culture and shaking for 20 min at 37°C. Images were taken with a Nikon Eclipse Ti microscope coupled to a Nikon DS-MBWc CCD camera and processed using the NIS elements AR software package (Nikon) or ImageJ. Scanning electron microscopy and ultrathin section transmission electron microscopy were performed essentially as described earlier (Rismondo et al., 2015).
Cell culture techniques
Intracellular growth in macrophages was analysed using J774.A1 mouse ascites macrophages (ATCC® TIB-67TM) as described earlier (Halbedel et al., 2014). Cell-to-cell spread was analysed in a plaque formation assay using 3T3 L1 mouse embryo fibroblast-like cells according to protocols published previously (Marquis, 2006; Halbedel et al., 2012).
Insect infection model
Galleria mellonella larvae, purchased from fauna topics (Marbach, Germany), were reared at 32°C in darkness and on an artificial diet (22% maize meal, 22% wheat germ, 11% dry yeast, 17.5% bee wax, 11% honey and 11% glycerine) prior to use. Last instar larvae, each weighing between 250 and 350 mg, were used in all experiments as described previously (Mukherjee et al., 2010). Fresh cultures of bacteria, prepared from an overnight culture, were used in all experiments. Bacteria were grown in BHI broth at 37°C, harvested during exponential growth and washed twice with 1× PBS. The cell pellet was resuspended in 1× PBS, and the bacterial concentration was calibrated based on optical density. Dilutions were prepared in 1× PBS to obtain the required numbers of bacteria for infection. Galleria infections were performed as described previously (Mukherjee et al., 2010). Briefly, the L. monocytogenes wild-type strain EGD-e and the ΔgpsB mutant strain LMJR19 were injected separately (106 CFU/larva) into the hemocoel of the last instar larvae, and the infection was monitored at 37°C for 7 days.
Acknowledgements
We are grateful to Birgitt Hahn, Petra Kaiser and Gudrun Holland for technical assistance. We thank the beamline staff at the Diamond synchrotron for help and access to their facilities, Dr. Arnaud Baslé for help with X-ray data collection and analysis, and Dr. Tom Jowitt (University of Manchester's Biomolecular Analysis Core Facility) and Dr. Owen Davies of Newcastle University for SEC-MALLS. Electrospray ionisation mass spectra were recorded at the Astbury Centre for Structural Molecular Biology and in the Department of Chemistry (CCIAS) at the University of Sheffield. This work has been supported by the DFG (HA 6830/1-1, to SH) and the UK BBSRC (BB/G015902/1 and BB/M001180/1, to RJL).